Abstract
Background: Studies reported that evaluating the interleukin serum level of MS and NMO patients is helpful for differentiating these two diseases from each other. This study aimed to compare the level of IL-6 and IL-17 in MS and NMO patients and healthy subjects. Methods: This study is a case control study that evaluated the serum level of IL-6 and IL-17 in MS and NMO patients in comparison to controls in patients who referred to Kashani hospital clinics. The level of serum IL-6 and IL-17 were measured by ELISA test in all patients. Participants were divided in to three groups include MS patients, NMO patients and controls and the level of IL-6 and IL-17 were compared in this three groups. Results: Mean of serum level of IL-6 in the NMO group was significantly lower than MS and healthy subject (P=0.02 for NMO and MS, P=0.001 for NMO and healthy subjects) but there was no significant difference between MS and healthy subjects (P=0.09). The mean of serum level of IL-17 in the MS and NMO were significantly higher than healthy subjects (P<0.001 for both). Also the mean of serum level of IL-17 in the MS was significantly higher than NMO (P=0.01). A positive significant correlation between age and serum level of IL-6 in all subjects (r=0.23, P=0.01). There was a positive significant correlation between age and serum level of IL-17 in MS and NMO patients (r=0.28, P=0.012). Conclusion: Using IL-17 and IL-6 were inflammatory markers to diagnosis of NMO, MS and healthy subjects.
Keywords: Multiple sclerosis, neuromyelitis optica (NMO), interleukin
Introduction
Multiple sclerosis [1] is the most common inflammatory demyelinated central nervous system (CNS) disease and the third common disease that causes major disability [2]. There are evidences that the main mechanism of these diseases is autoimmune inflammatory reaction mediated by some components of immune system [3-6]. MS is more popular in young women and the prevalence in different countries is between 30-190 per 100,000 [7]. Neuromyelitis optica (NMO) is another inflammatory CNS disorder with presentation of optic neuritis and myelitis that resemble those of MS [8-10].
Although NMOSD is different from MS regarding to neuroimaging, pathological and immunological features, sometimes make correct initial diagnosis is difficult due to similarities of presentations [11].
However, early diagnosis and appropriate treatment with immunosuppressive drugs is in paramount of importance in order to prevent severe disability in NMOSD patients. Furthermore, it should be noticed that some commonly medical therapy used in MS such as olimod, interferons and natalizumab may result aggressive disease activity in NMOSD. As a result, early differentiation between NMOSD and MS has become increasingly important [12,13].
Whereas serum autoantibody NMO-IgG is known as a specific biomarker for NMOSD diagnosis with high sensitivity and specificity, many of NMOSD patients are seronegative [14,15]. Though considering other biomarkers for distinguish between NMOSD and MS patients particularly when neuroimaging findings are not characteristic seems reasonable [15].
Although the immunological differences between MS and NMO have recognized by several studies, a clear definition remains controversial [15].
Over last decades, several studies have been done to illustrate the serum or cerebrospinal fluid (CSF) cytokines and chemokines profiles among patients with NMOSD and MS. Matsushita was suggested over expression of a cluster of Th-17 and Th-1 related proinflammatory cytokines in NMO rather than MS [16]. There are some more studies supporting the idea that increased level of Th-17 and Th-2 related molecules especially IL-6 may be essential factors for developing NMO [17-20]. Furthermore, biomarkers as CSF glial fibrillary acidic protein (GFAP) are related to clinical severity of NMO relapse [21].
According to these studies, there is a probable correlation between the CSF or serum concentration of IL-6 and IL-17 in the pathogenesis of NMOSD [17-22].
Therefore, evaluation of these biomarkers may be useful to differentiate between these two demyelinating disorders and it might make a contribution to potential therapeutic targeting program in the future. This study was done to compare the serum levels of IL-6 and IL-17 in acute relapse phase of MS, NMOSD and healthy subjects in order to find any difference for the early differentiation of these diseases.
Methods
Study design
This study is a case control study that evaluated the serum level of IL-6 and IL-17 in MS and NMO patients in comparison to controls in patients who referred to Kashani hospital clinics in Isfahan University of Medical Science (IUMS) and neurologist office cooperates in this study in 2016-2017. Inclusion criteria were as followed: 1) age more than 18, 2) presence of primary symptoms of MS or NMO, 3) receiving no corticosteroid at least in recent one month and 4) patient’s willingness to participate in this study. Exclusion criteria were as followed: 1) having underlying disease includes diabetes, vasculitis, inflammation and rheumatologic diseases and 2) patient’s unwillingness to continue this study. This study was approved by IUMS Ethical Committee.
About 101 patients with MS or NMO were selected based on inclusion and exclusion criteria. Patients were divided in to NMO or MS group based on their clinical manifestations and then followed for one year to approve this diagnosis. Also 39 healthy individuals were selected from patients who referred to neurology clinic because of other complaints that had no history of underlying disease and were homogenous in age and gender as a control group. At first demographic data includes age and gender were collected by introducing patents. Then, blood sampling was done for each participant from peripheral vein (7 cc) and then kept in laboratory in standard situation. At the time of analysis, the serum of these blood samples, were separated. Then, the level of serum IL-6 and IL-17 were measured by ELISA test (Boster Biological Thechnology Co., Ltd., Wuhan, China). All patients were followed for certain diagnosis of MS or NMO and the proper diagnosis of MS or NMO were registered for patients. Finally, participants were divided in to three groups include MS patients, NMO patients and controls and the level of IL-6 and IL-17 were compared in this three groups.
Three groups were matched in the age and gender based on randomized.
All data were entered to SPSS version 22 (SPSS crop., Chicago, IL, USA) and then analyzed. For reporting quantitative and qualitative data we used mean ± standard deviation and number or percent, respectively. For comparing quantitative and qualitative variables between groups independent t test, Chi Square and one-way ANOVA were used and also Pearson correlation was used to correlate between quantitative variables. A two-sided α level of 0.05 was used to assess statistical significance.
Results
In this study, 70 MS patients (13 males and 57 females with mean ages of 30.20 ± 1.42 years), 31 NMO patients (12 males and 19 females with mean ages of 29.64 ± 1.47 years) and 39 healthy subjects (12 males and 27 females with mean ages of 30.53 ± 4.30 years). There was no significant difference between groups based on age and gender (P>0.05), so groups were matched in the age and gender (Table 1).
Table 1.
Variables of study between groups
| Variables | Groups | P-value | ||
|---|---|---|---|---|
|
| ||||
| MS | NMO | Healthy subjects | ||
| Gender (m/f) | 13/57 | 12/19 | 12/27 | 0.09 |
| Age (years) (mean ± SD) | 30.20 ± 1.42 | 29.64 ± 1.47 | 30.53 ± 4.30 | 0.35 |
| IL-6 (pg/dl) | 12.73 ± 11.42 | 7.30 ± 4.94 | 16.07 ± 6.70 | 0.003 |
| IL-17 (pg/dl) | 11.54 ± 2.70 | 10.21 ± 2.76 | 4.80 ± 1.43 | <0.001 |
The means of serum level of IL-6 in the MS, NMO and healthy subject groups were 12.73 ± 11.42, 7.30 ± 4.94 and 16.07 ± 6.70 pg/dl, respectively, therefore there was a significant difference between groups based on serum level of IL-6 (P=0.003) that mean of serum level of IL-6 in the NMO group was significantly lower than MS and healthy subject (P=0.02 for NMO and MS, P=0.001 for NMO and healthy subject) but there was no significant difference between MS and healthy subjects (P=0.09) (Figure 1).
Figure 1.
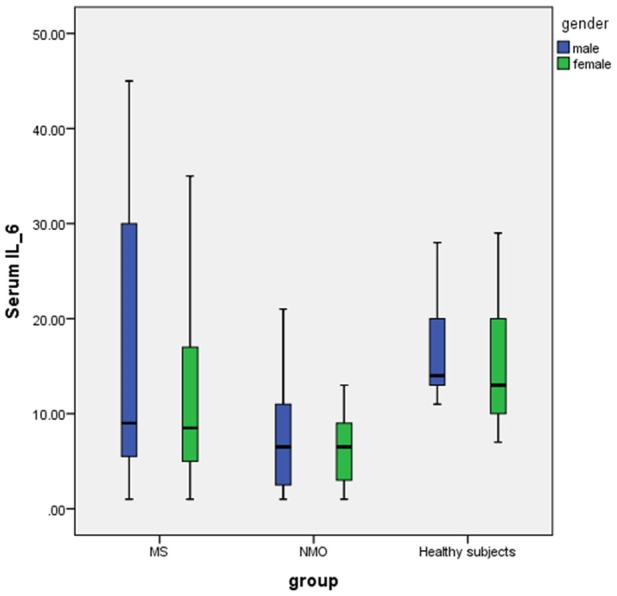
Boxplot of serum level of IL-6 levels in MS, NMO and healthy subject based on gender. The mean of serum level of IL-6 in NMO patients was significantly lower than MS and healthy subjects (One-way ANOVA).
The means of serum level of IL-17 in the MS, NMO and healthy subject were 11.54 ± 2.70, 10.21 ± 2.76 and 4.80 ± 1.43 pg/dl, respectively that this difference was significant between groups (P=0.001). The mean of serum level of IL-17 in the MS and NMO were significantly higher than healthy subjects (P<0.001 for both). Also the mean of serum level of IL-17 in the MS was significantly higher than NMO (P=0.01) (Figure 2).
Figure 2.
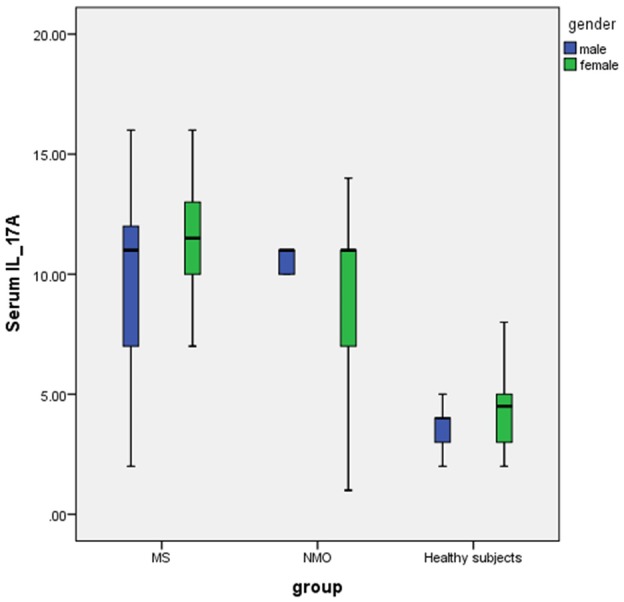
Boxplot of serum level of IL-17 levels in MS, NMO and healthy subject based on gender that the mean of IL-17 in healthy subjects was significantly lower than NMO and MS patients based one-way ANOVA.
Also there was no significant between males and females based on serum level of IL-6 (P=0.19) and IL-17 (P=0.24).
Pearson correlation showed there was a positive significant correlation between age and serum level of IL-6 (r=0.23, P=0.01) (Figure 3) but there was no significant correlation between age and serum level of IL-17 in all subjects (r=-0.08, P=0.33). Also there was a positive significant correlation between age and serum level of IL-17 in MS and NMO patients (r=0.28, P=0.012) (Figure 4).
Figure 3.
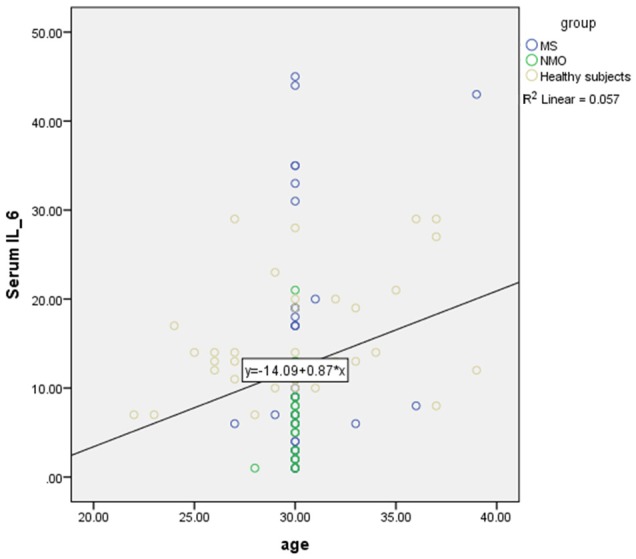
Positive significant correlation or direct relation between age and serum level of IL-6 in MS, NMO and healthy subjects based on Pearson correlation.
Figure 4.
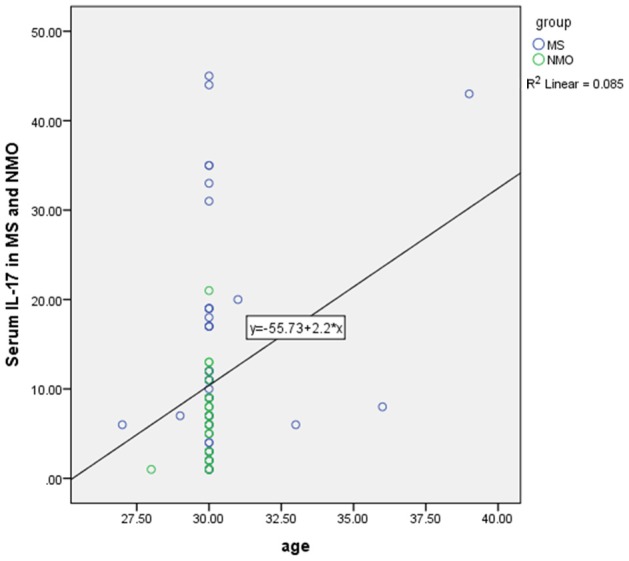
Positive significant correlation between age and serum level of IL-17 in MS and NMO patients based on Pearson correlation.
Discussion
According our result, serum level of IL-6 in the patients with NMO was lower than MS and control. Also the serum level of IL-17 in patients with NMO or MS was higher than control and serum level of IL-17 in MS patients was higher than NMO cases. In addition, there was a positive significant correlation between age and serum level of IL-6.
Although NMO and MS were once supposed to be a single autoimmune disease, they are different entities in various respects, and must be treated with different therapies. For instance, an increasing interest is in finding a biomarker to help early distinguish between MS and NMOSD.
One of the suggestive factor in genesis of both MS and NMOSD has been T and B cell interaction. it is considered that Th17 cells would have an important effect in the initial phase of MS [23].
Th17 cells have ability to disrupt the BBB and access the CNS and play a role in development of CNS autoimmunity. In addition, Th17 cells produce a large number of pro-inflammatory cytokines include mainly IL-17 [23].
IL-17, increases T cells activity. It can amplify endothelial inflammation by enforce endothelium to release pro-inflammatory mediators including IL-1, IL-6, TNFα and chemokines [24]. Finally, the role of IL-17 is causing local tissue inflammation by induction release of pro-inflammatory cytokines and neutrophils stimulating cytokine [25].
Based on IL-17 characteristics, it has been considering a strong association between IL-17 and the initial phase of MS disease [26].
Increased frequency of Th17 cells in the peripheral blood and cerebrospinal fluid (CSF) of some RRMS, especially during the acute episode has been reported [27].
In addition, there is some evidence implicating significant reduction in Th17 cell counts and IL-17 after IVMP pulse therapy in MS patients [28].
Along with these findings, Ghaffari et al has reported high level IL-17 concentrations in newly diagnosed MS patients compare to healthy and treated MS patients [29].
On the other hand, high level of IL-17 were considered both in the CSF [18] and in the blood [22] of NMO patients.
Uzawa A, did not find an increase of IL-17 in CSF of NMO but find increase CSF level of IL-6 [30].
Different factors are involved for differentiation of B cells to producing immunoglobulin. IL-6 is one of B cell stimulating factor which differentiated B cell to plasma cell and leads to producing immunoglobulin [31,32]. IL-6 is recognized as an important cytokine in inflammatory diseases of the central nervous system (CNS) is produced by different cells including activated monocytes, T cells, fibroblasts, endothelial cells, astrocytes and glial cells [31,33].
IL-6 can increases the survival of plasmablasts that producing anti AQP4-IgG and result further tissue lesion [34].
Along with this characteristic, one study has showed higher proportion of IL-6 and IL-17 cell subsets in NMOSD patients than healthy controls and concluded that expansion of peripheral IL-6 and IL-17 TFH cells may have a role in the severity of NMOSD [35].
Although, one study found the level of IL-17 level was not higher in NMO than in MS [36]. Matsushita et al, compared NMO, RRMS, and PPMS and reported an increased expression of Th17 in NMOSD. In this study, of the total 73 patients, the CSF level of IL-17 and IL-6 were significantly higher in NMO patients than in RRMS at relapse [16].
In the study of Uzawa et al on 17 NMO patients and 21 MS patients, the CSF IL-6 levels were significantly higher in NMO patients in comparison to MS patients [16]. In contrast in the study of Li et al, memory Th17 and IL-17 level were much higher in both NMO or MS and were related to clinical feature which has been decreased following steroid therapy [19].
Conclusion
In the concluded of our result and other studies, IL-6 and IL-17 have important role in the neuroinflammatory diseases such as MS and NMO. There are limited evidences that reported there is a correlation between Th17 in NMO and MS diseases. In addition, most of studies that evaluated interleukins in MS and NMO patients, analyzed CSF and limited studies measured the serum level of these interleukins
Disclosure of conflict of interest
None.
References
- 1.Sanderson PA, Critchley HO, Williams AR, Arends MJ, Saunders PT. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update. 2017;23:232–254. doi: 10.1093/humupd/dmw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salhofer-Polanyi S, Windt J, Sumper H, Grill H, Essmeister M, Diermayr G, Zebenholzer K, Leutmezer F, Zulehner G, Vass K. Benefits of inpatient multidisciplinary rehabilitation in multiple sclerosis. NeuroRehabilitation. 2013;33:285–292. doi: 10.3233/NRE-130956. [DOI] [PubMed] [Google Scholar]
- 3.Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation. 2015;22:400–404. doi: 10.1159/000439278. [DOI] [PubMed] [Google Scholar]
- 4.Farrokhi M, Dabirzadeh M, Dastravan N, Etemadifar M, Ghadimi K, Saadatpour Z, Rezaei A. Mannose-binding lectin mediated complement pathway in autoimmune neurological disorders. Iran J Allergy Asthma Immunol. 2016;15:251. [PubMed] [Google Scholar]
- 5.Etemadifar M, Ghadimi M, Ghadimi K, Alsahebfosoul F. The serum amyloid β level in multiple sclerosis: a case-control study. Caspian J Neurol Sci. 2017;3:214–221. [Google Scholar]
- 6.Rafiee Zadeh A, Ghadimi K, Mohammadi B, Hatamian H, Naghibi SN, Danaeiniya A. Effects of estrogen and progesterone on different immune cells related to multiple sclerosis. Caspian J Neurol Sci. 2018;4:83–90. [Google Scholar]
- 7.Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci. 2001;22:117–139. doi: 10.1007/s100720170011. [DOI] [PubMed] [Google Scholar]
- 8.Frohman EM, Racke M, van den Noort S. To treat, or not to treat: the therapeutic dilemma of idiopathic monosymptomatic demyelinating syndromes. Arch Neurol. 2000;57:930–932. doi: 10.1001/archneur.57.7.930. [DOI] [PubMed] [Google Scholar]
- 9.Rafiee Zadeh A, Askari M, Azadani NN, Ataei A, Ghadimi K, Tavoosi N, Falahatian M. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 1. Int J Physiol Pathophysiol Pharmacol. 2019;11:95–104. [PMC free article] [PubMed] [Google Scholar]
- 10.Rafiee Zadeh A, Ghadimi K, Ataei A, Askari M, Sheikhinia N, Tavoosi N, Falahatian M. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 2. Int J Physiol Pathophysiol Pharmacol. 2019;11:105–114. [PMC free article] [PubMed] [Google Scholar]
- 11.Dhib-Jalbut S. Pathogenesis of myelin/oligodendrocyte damage in multiple sclerosis. Neurology. 2007;68:S13–S21. doi: 10.1212/01.wnl.0000275228.13012.7b. [DOI] [PubMed] [Google Scholar]
- 12.De Jager PL, Rossin E, Pyne S, Tamayo P, Ottoboni L, Viglietta V, Weiner M, Soler D, Izmailova E, Faron-Yowe L. Cytometric profiling in multiple sclerosis uncovers patient population structure and a reduction of CD8low cells. Brain. 2008;131:1701–1711. doi: 10.1093/brain/awn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Boxel-Dezaire AH, Hoff SC, van Oosten BW, Verweij CL, Dräger AM, Adèr HJ, van Houwelingen JC, Barkhof F, Polman CH, Nagelkerken L. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann Neurol. 1999;45:695–703. doi: 10.1002/1531-8249(199906)45:6<695::aid-ana3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, Mandler RN, Weinshenker BG, Pittock SJ, Wingerchuk DM, Lucchinetti CF. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 15.Cree B. Neuromyelitis optica: diagnosis, pathogenesis, and treatment. Curr Neurol Neurosci Rep. 2008;8:427–433. doi: 10.1007/s11910-008-0066-2. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita T, Tateishi T, Isobe N, Yonekawa T, Yamasaki R, Matsuse D, Murai H, Kira J. Characteristic cerebrospinal fluid cytokine/chemokine profiles in neuromyelitis optica, relapsing remitting or primary progressive multiple sclerosis. PLoS One. 2013;8:e61835. doi: 10.1371/journal.pone.0061835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uzawa A, Masahiro M, Kuwabara S. Cytokines and chemokines in neuromyelitis optica: pathogenetic and therapeutic implications. Brain Pathol. 2014;24:67–73. doi: 10.1111/bpa.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, Minohara M, Murai H, Mihara F, Taniwaki T. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128:988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 19.Uzawa A, Mori M, Ito M, Uchida T, Hayakawa S, Masuda S, Kuwabara S. Markedly increased CSF interleukin-6 levels in neuromyelitis optica, but not in multiple sclerosis. J Neurol. 2009;256:2082–2084. doi: 10.1007/s00415-009-5274-4. [DOI] [PubMed] [Google Scholar]
- 20.Cong H, Jiang H, Peng J, Cui S, Liu L, Wang J, Zhang X. Change of Th17 lymphocytes and Treg/Th17 in typical and atypical optic neuritis. PLoS One. 2016;11:e0146270. doi: 10.1371/journal.pone.0146270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang KH, Ro LS, Lyu RK, Chen CM. Biomarkers for neuromyelitis optica. Clin Chim Acta. 2015;440:64–71. doi: 10.1016/j.cca.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Wang H, Long Y, Lu Z, Hu X. Increased memory Th17 cells in patients with neuromyelitis optica and multiple sclerosis. J Neuroimmunol. 2011;234:155–160. doi: 10.1016/j.jneuroim.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Rostami A, Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J Neurol Sci. 2013;333:76–87. doi: 10.1016/j.jns.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Christensen JR, Börnsen L, Ratzer R, Piehl F, Khademi M, Olsson T, Sørensen PS, Sellebjerg F. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17-and activated B-cells and correlates with progression. PLoS One. 2013;8:e57820. doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babaloo Z, Babaie F, Farhoodi M, Aliparasti M, Baradaran B, Almasi S, Hoseini A. Interleukin-17A and interleukin-17F mRNA expressions in peripheral blood mononuclear cells of patients with multiple sclerosis. Iran J Immunol. 2010;7:202. [PubMed] [Google Scholar]
- 28.Liu M, Hu X, Wang Y, Peng F, Yang Y, Chen X, Lu Z, Zheng X. Effect of high-dose methylprednisolone treatment on Th17 cells in patients with multiple sclerosis in relapse. Acta Neurol Scand. 2009;120:235–241. doi: 10.1111/j.1600-0404.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 29.Ghaffari SA, Nemati M, Hajghani H, Ebrahimi H, Sheikhi A, Jafarzadeh A. Circulating concentrations of interleukin (IL)-17 in patients with multiple sclerosis: evaluation of the effects of gender, treatment, disease patterns and IL-23 receptor gene polymorphisms. Iran J Neurol. 2017;16:15. [PMC free article] [PubMed] [Google Scholar]
- 30.Uzawa A, Mori M, Arai K, Sato Y, Hayakawa S, Masuda S, Taniguchi J, Kuwabara S. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16:1443–1452. doi: 10.1177/1352458510379247. [DOI] [PubMed] [Google Scholar]
- 31.Barros P, Cassano T, Hygino J, Ferreira T, Centurião N, Kasahara T, Andrade R, Linhares U, Andrade A, Vasconcelos C. Prediction of disease severity in neuromyelitis optica by the levels of interleukin (IL)-6 produced during remission phase. Clin Exp Immunol. 2016;183:480–489. doi: 10.1111/cei.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplin AI, Deshpande DM, Scott E, Krishnan C, Carmen JS, Shats I, Martinez T, Drummond J, Dike S, Pletnikov M. IL-6 induces regionally selective spinal cord injury in patients with the neuroinflammatory disorder transverse myelitis. J Clin Invest. 2005;115:2731–2741. doi: 10.1172/JCI25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narikawa K, Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. CSF chemokine levels in relapsing neuromyelitis optica and multiple sclerosis. J Neuroimmunol. 2004;149:182–186. doi: 10.1016/j.jneuroim.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Uzawa A, Mori M, Kuwabara S. Neuromyelitis optica: concept, immunology and treatment. J Clin Neurosci. 2014;21:12–21. doi: 10.1016/j.jocn.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro C, Fernandes G, Kasahara TM, Barros PO, Dias ASO, Araújo ACRA, Ornelas AMM, Aguiar RS, Alvarenga R, Bento CAM. The expansion of circulating IL-6 and IL-17-secreting follicular helper T cells is associated with neurological disabilities in neuromyelitis optica spectrum disorders. J Neuroimmunol. 2019;330:12–18. doi: 10.1016/j.jneuroim.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Dai Y, Qiu W, Lu Z, Peng F, Wang Y, Bao J, Li Y, Hu X. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci. 2011;18:1313–1317. doi: 10.1016/j.jocn.2011.01.031. [DOI] [PubMed] [Google Scholar]


