Abstract
The brain is the command center of the body that regulates the vital functions of circulation, respiration, motor function, metabolic activities, or autonomic nervous system outcomes. The brain coordinates these continuous activities at the expense of huge energy utilization. This energy demand is achieved by active transport of nutrients across the endothelial blood-brain barrier (BBB). This review discusses the barrier interfaces in the CNS that include the BBB, blood-spinal cord barrier, the epithelial choroid plexus, and the epithelial arachnoid. While transporting of nutrients across the BBB is a normal physiological function, the trafficking of xenobiotics and inflammatory cells/agents across these interfaces is harmful to brain cells. This leads to production of waste metabolites in the brain. Clearance of these waste metabolites maintains the normal brain homeostasis, while aggregation is detrimental to neurological complications. Since the CNS lacks lymphatic system, the CSF serves as the clearance path for water-soluble peptides/solutes, but not large size waste metabolites like Aβ protein. In particular, this review will focus on the mechanisms of waste metabolites clearance paths in the CNS. This will include the recently discovered waste metabolites movement from interstitial space (IS) directly into perivascular clearance (PVC), or via IS-CSF-PVC, and its exchange from PVC to circulation. Concluding remarks will discuss the therapeutic approach to improve the clearance mechanisms for ameliorating neurological diseases.
Keywords: Central nervous system (CNS) clearance, cerebrospinal fluid (CSF), interstitial fluid (ISF), perivascular space, blood-brain barrier (BBB)
Brain barrier interfaces
The brain is the command center of the body. It controls many functions of cardiovascular and systemic circulation, respiratory center, motor activities, metabolic function, renal and gastrointestinal excretion, and autonomic nervous system. Coordination of these numerous activities is carried out by the release of endocrinal chemical messengers known as hormones. The brain does this by receiving signals from sensory nerve cells, integrating and processing the information in interneurons, and sending out the message to the effector tissue organs through motor neurons. Relaying of the message from the central nervous system to different parts of the body through the peripheral nervous system is connected by the brainstem via the spinal cord. Constant supply of nutrients/minerals/ions across the selectively permeable blood-brain interface known as the blood-brain barrier (BBB) meets the energy demand of the brain. The localization of tight junction proteins, protein/nutrient/ion transporters, multidrug resistant efflux receptors, or enzymes at the BBB selectively maintain the ionic/nutrients homeostasis in the brain by dumping toxic agents into the circulation [1], as illustrated in Figure 1. Thus, small size molecules like glucose, amino acids, or essential minerals/ions are transported across the BBB by carrier-mediated transporters, whereas receptor mediated transporters translocate the large size peptides and proteins [1,2].
Figure 1.
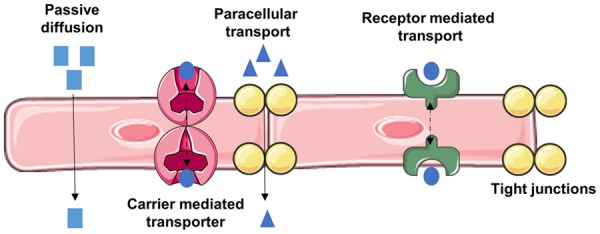
Routes of transport across the BBB. Passive diffusion favors lipophilic molecules; Carrier mediated transporters bi-directionally transport molecules influx and/or efflux through BBB endothelial cell layer; receptor mediated transport requires binding of ligand for transport of macromolecules such as glucose, peptides and proteins across BBB endothelium (transcytosis); paracellular pathway through tight junctions allows diffusion of for small molecules and cell trafficking.
In addition to endothelial BBB and blood-spinal cord barrier (BSCB) interfaces, there are two other epithelial barriers that can render entry of molecules into the brain. The interface between the blood and ventricular cerebrospinal fluid (CSF) is known as the epithelial choroid plexus (CP), and the interface between the blood and CSF subarachnoid is known as the epithelial arachnoid villi [3,4]. The main function of CP is to act as the secretory source of cerebrospinal fluid for maintaining the fluid volume and ionic balance in the CNS. The concept is essentially justified by the fact that CP serves as the drainage sink in the brain. Recent findings reveal that this epithelial barrier is also involved in immune cell trafficking [5] and pathogens entry interface [6,7]. As such CP is implicated to involve in the development of neurological diseases [8]. But most recent report by Uchida et al. (2019) showed the impairment of tight junction protein claudin-11 expression at the BBB, blood-spinal cord barrier (BSCB), and epithelial arachnoid barrier in multiple sclerosis without affecting the epithelial CP [9]. On the basis of this finding, they indicated that the CP barrier is unlikely to involve in the development of neurological diseases. However, impairment of CP directly or indirectly involves in the development of neurological diseases still remains to be investigated. Choroid plexus is also implicated for supply of nutrients and signaling molecules to brain parenchyma, as such, it may play a role in brain development and aging [10], however this argument remains for open discussion. Another recent finding implicates CP as a potential BBB bypass route for drug delivery in the CNS [11]. This concept is based on the rationale that since carrier-mediated influx transporters or receptors are expressed in CP epithelium, these molecules can be designed for drug penetration across CP. The rationale uses the same principle that these molecules have been employed for drug delivery across BBB. The function of the second epithelial barrier, known as the arachnoid villi interface between the blood and the CSF, acts as the main drainage channel of water-soluble peptides/solutes from CSF subarachnoid to sagittal sinus for clearance. Intrigu-ingly, micropinocytosis and vacuolization of endothelial cells present in the stromal central core of the villi seem to facilitate a bidirectional active transport across the arachnoid villi barrier [12].
Out of these two endothelial (BBB and BSCB) and two epithelial (CP and arachnoid villi) barrier interfaces in the brain, the BBB is the primary transport/trafficking route of nutrients, micro-organisms, immune cells, and xenobiotic substances into the brain [13-16]. The myeloid cells in the brain respond to inflammatory events in the form of phagocytosis, proteolytic degradation, and autophagy, which produce harmful waste metabolites [17,18]. Such harmful metabolites include components of degenerating neurons and extracellular vesicles that are entrapped in the interstitial space (IS) [19-21]. Proper clearance of these waste metabolites from interstitial fluid/CSF maintains the normal homeostasis function of the brain, while aggregation leads to neurological disorders. Here, we focus on the clearance mechanisms of these waste metabolites from the brain with emphasis on perivascular clearance.
Interstitial space waste metabolites
The main donors of fluid in the interstitial space are blood circulation and cellular secretion. The total volume of the interstitial fluid (ISF) is believed to be contributed by passive diffusion of fluid from the circulation. The ISF contains cellular metabolites, cellular shedded microvesicles and exosomes under normal conditions. When it turns into neuropathologic conditions like Alzheimer’s disease, entangled proteins like amyloid-β are widely observed in ISF as well [21]. Therefore, understanding the mechanisms of these waste metabolites movement from ISF to terminal clearance path is exceedingly important for improving neurological diseases. The drainage of water-soluble metabolites from ISF to CP, and to CSF compartments is a major route for clearance of small-size and hydrophilic substances.
There are two schools of thought on the mechanisms of ISF movement, bulk flow and diffusive movement [22]. Cserr et al. (1977 and 1981) showed that a bulk flow of ISF-independent diffusion was responsible for clearance of waste metabolites in the CNS [23,24]. Their conclusion was based on the findings that deposition of different molecular size tracers in rat brain interstitial space were drained at nearly identical half-life rates with varying diffusion coefficients. It was estimated that the rate of this bulk flow was 0.1-0.3 μL/g brain/min in rat brain [24,25]. Recently, the hypothesis of ISF bulk flow mechanisms has been challenged by other critiques. Sykova and Nicholson (2008) and Bakker et al. (2016) pointed out that the movement of ISF in the interstitial spaces was attributed to diffusive movement rather than bulk flow mechanisms [26,27]. Their argument was that first, the interstitial narrow spaces between cells are too limited to allow significant ISF bulk flow. Second, the extracellular matrix of the interstitial space contains repeated long chain polysaccharides like glycosaminoglycans, which are negatively charged, hydrophilic, and can easily trap water molecules and positive ions like sodium. These hindrance factors will not only deter the bulk flow of interstitial fluid, but also limit the movement of large size waste metabolites in the interstitial space. Thus, the question of whether the ISF move through bulk flow or passive diffusion mechanisms remains unclear. Based on the emerging multiphoton imaging on time-dependent interstitial bio-distribution of small, medium, and large size molecular weight fluorescent tracers, we will discuss our thoughts in the glymphatic section below.
Clearance of waste metabolites in cerebrospinal fluid (CSF)
The cellular metabolites, extracellular vesicles, degraded peptides, and other degenerated cellular components contained in the interstitial fluid are mostly collected in the choroid plexuses (CP). The C3 compartment CP is localized in the cerebrum and C4 is localized in the cerebellum regions of the brain. The C3 and C4 are connected by an aqueduct. The interstitial fluid drainage sink (CP) and ventricular cerebrospinal fluid (CSF) is separated by the epithelial choroid plexus barrier, through which collected interstitial fluid is excreted into the CSF [28]. The CSF flow passes through the interventricular foramina of the third ventricle to the fourth ventricle via an aqueduct. From the fourth ventricle, the fluid then enters the subarachnoid space (SAS) through a median and two lateral apertures [29,30]. The CSF containing the water-soluble waste metabolites, but not cells, ultimately moves through the subarachnoid space to reach to the perivascular space [31,32]. The water-soluble metabolites in the CSF subarachnoid space are absorbed into sagittal venous sinuses through arachnoid granulations. The evidence came from the work of Weed et al. (1914), who found that non-toxic tracers injected into CSF crossed arachnoid granulations along sagittal sinus and eventually penetrated into dura wall of the sinus [33,34]. The absorption of CSF dye arachnoid granulations was further confirmed by electron microscopy studies, which showed that the presence of pressure-sensitive vacuolation cycle of pores indicate the one-way valves transcellular flow [35]. The CSF contents in the sagittal sinus, most likely at the confluence of sinus exit along the myelin sheath of olfactory bulbs through cribriform plate [36], which is then believed to be delivered into the deep cervical lymph nodes through nasal lymphatics [37]. This drainage path from subarachnoid space to nasal lymphatic system was further confirmed in seven different species using microfils tracers [38]. Recently, these lymphatic vessels have been investigated in rodents by using specific antibodies [39,40].
In summary, the schematic presentation in Figure 2 illustrates the intracranial CSF flow. It is important to emphasize here that the exchange of waste metabolites from subarachnoid to superior sagittal venous sinus may account only for the water-soluble small size metabolites. This is because the granulation at the arachnoid microvilli barrier restricts the clearance of large size waste metabolites. As such, the clearance mechanisms of large size waste metabolites like Aβ aggregation from the interstitial space or from the CSF remains to be investigated. To this end, emerging findings such as the glymphatic hypothesis and our most recent published article on perivascular clearance mechanisms revealed the clearance mechanisms of large size waste metabolites from the interstitial space or from the CSF circulation.
Figure 2.
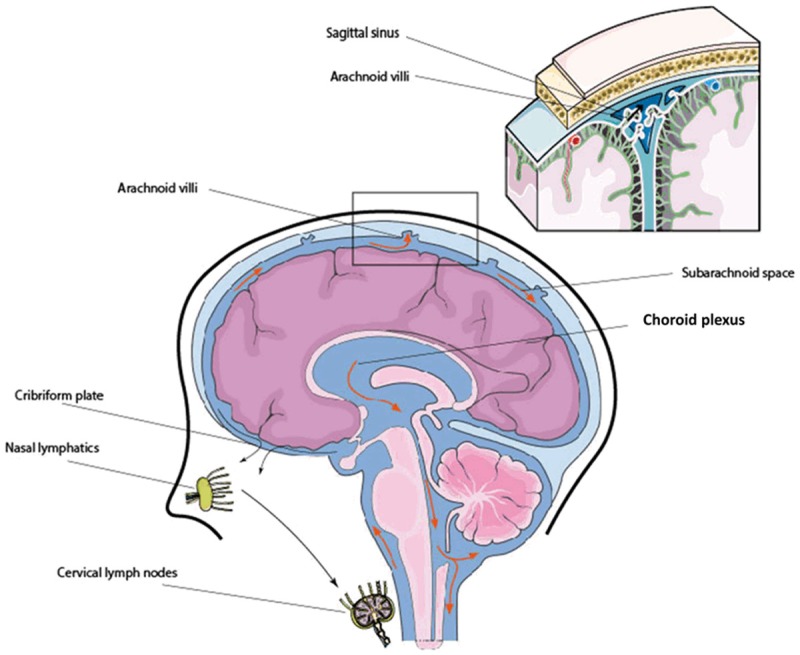
Schematic presentation of the intracranial CSF flow. CSF is produced by the choroid plexus and flows from the third ventricle to the fourth ventricle through the cerebral aqueduct. After circulating over hemispheres, CSF is absorbed into superior sagittal sinus, transverse sinus, and sigmoid sinuses via arachnoid villi, as well as efflux from the CNS along olfactory nerves through cribriform plate to nasal lymphatics and eventually to cervical lymph nodes.
Glymphatic system
The glymphatic hypothesis proposed by Iliff, Nedergaard et al. (2012) revealed the continuous exchange of interstitial fluid (ISF) and cerebrospinal fluid (CSF) in the brain compartments [31]. According to this hypothesis, the exchange is facilitated by aquaporin-4 (AQP4) water channels that are expressed in highly-polarized astrocytic end feet. In that, CSF from subarachnoid spaces fluxed into deep brain tissues along pial and penetrating arterioles and into the brain parenchyma towards peri-venous spaces, which is facilitated by the convective function of astrocytic AQP4 [31,41]. Figure 3 illustrates the convective interstitial flow towards peri-venous spaces surrounding deep veins. They showed that water-soluble waste metabolites in ISF were collected and carried towards peri-venous space, from where metabolites were drained to lymph nodes and systemic circulation.
Figure 3.
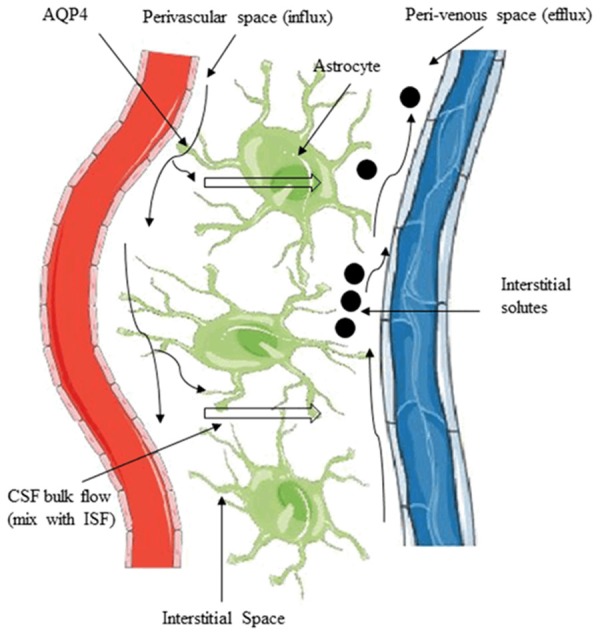
Movement of solutes in ISF and CSF through the glymphatic pathway. CSF from subarachnoid space fluxes into the brain from perivascular spaces and exchanges with ISF. The exchange is facilitated by aquaporin-4 (AQP4) water channels that highly expressed in the perivascular astrocyte end-foot. The bulk movement of CSF into the brain drives the convective flow of ISF and interstitial solutes to the peri-venous route.
The evidence was shown by imaging the bio-distribution of small size (Texas Red: 3 kD), medium size (Ovalbumin, 647 kD) and large size (FITC-d2000 kD) molecular weight tracers injected in mice through cisterna magna. It was observed that the tracers rapidly entered the cortical pial arteries, then fluxed into perivascular spaces along the penetrating arterioles, which was distinguished in Tie2-GFP:NG2-DsRed double reporter mice. The small size tracer was found to exit primarily along the central deep veins and lateral-ventral caudal rhinal veins, while the medium and large size tracers accumulated at perivascular and peri-arterial spaces respectively in a time-dependent manner. Using AQP4 knockout mice, this same group of investigators validated the proof-of-concept that astrocytic AQP4 was in part responsible for the clearance of radiolabeled Aβ1-40 peptide because they observed 55% reduction of Aβ1-40 peptide clearance in AQP4 knockout mice compared with wild-type [31,41-43]. This idea of the glymphatic hypothesis is further embraced by the independent studies of other investigators [44]. However, on the basis of time-dependent movement of tracers as stated above, the movement of medium to large size metabolites in interstitial fluid favors diffusion rather than the bulk flow mechanism of the glymphatic hypothesis.
The reproducibility of the bulk flow mechanism is questioned by several most recent publications. Some investigations argued that clearance of waste metabolites in interstitial fluid is a passive diffusion and not a bulk flow convective transport [45,46]. This argument is based on the size of metabolites. A model of diffusive and convective transport in brain extracellular space was designed to illustrate the validation in support of this argument. Findings from this model stated that diffusion alone [47] or combined effects of diffusion and macroscopic fluid motion [48] is responsible for transport of waste metabolites in brain parenchyma rather than bulk flow. The combined effects appear to reconcile the conflicting transport mechanisms of solutes in ISF or in perivascular space. This is in parallel with the findings that cyclic changes in arterial pressure produces mixing of flow in opposite directions in different spaces of the arterial wall [49]. It is likely that convection can assist the movement of solutes into and out of the ISF without producing the net flow of fluid as required by the glymphatic system. The diffusive molecular flow along the peri-arterial sheaths into subarachnoid CSF [50], and the diffusive drainage of waste metabolites from brain parenchyma into the basement membrane of capillaries [32] also argue against the glymphatic hypothesis.
We discuss here that the transport of small size water-soluble waste metabolites could be mediated by glymphatic function. The missing piece is the accumulation of large size metabolites at the perivascular space, where the glymphatic system does not account for clearance mechanisms. This concept is clinically important because aggregation of entangled proteins at perivascular spaces is a hallmark of certain neurological diseases like Alzheimer’s disease and cerebral amyloid angiopathy [51-53]. Unravelling the clearance mechanisms of these entangled proteins from perivascular to blood circulation would be novel for ameliorating the relevant neurological diseases. Our recent findings describe this novel clearance mechanism at the perivascular space, which is discussed in the perivascular clearance section.
Meningeal lymphatic vessels
The lymphatic system is critical for maintenance of physiological homeostasis and immune surveillance. It is a conventional belief that the central nervous system (CNS) lacks lymphatic vessels. Recently, Aspelund et al., 2015 and Louveau et al., 2015, independently reported the existence of dura lymphatic vessels, known as the meningeal lymphatic vessels in the meninges of the brain [39,40]. They showed this evidence by positive stain of lymphatic endothelial cells marker hyaluronan receptor 1 (Lyve-1) and vascular endothelial growth factor receptor 3 (VEGFR3) marker along the superior sagittal and transverse sinuses.
Aspelund et al., 2015, concluded that dural lymphatic vessels absorb CSF from the adjacent subarachnoid space and from brain ISF through the glymphatic system, in which CSF is then transported into deep cervical lymph nodes via foramina at the base of the skull [39]. They validated this drainage route by showing nearly complete absence of tracer accumulation in cervical lymph nodes in transgenic mice deficient of dural lymphatic vessels compared with wild-type after injection of tracer into the CSF. Louveau et al., 2015, found that the meningeal lymphatic cells were able to transport fluid and immune cells from the CSF and clear them to the deep cervical lymph nodes [40]. Their discovery of meningeal lymphatic function in the CNS becomes highly significant in the context of immune cell surveillance since the development of many neurological diseases involve inflammatory processes. The findings of Aspelund et al. (2015) seem to connect with the glymphatic hypothesis, but the CSF drainage route in the meningeal lymphatic system appears to exit at the deep cervical lymph nodes, similar to conventional CSF exit. Here, we illustrate the possible connection of CSF flow, glymphatic and meningeal lymphatic system clearance routes, Figure 4. The glymphatic system depicts the exchange of CSF/ISF waste metabolites at the perivascular space towards peri-venous space, while the meningeal lymphatic system accounts for cervical lymph nodes clearance route.
Figure 4.
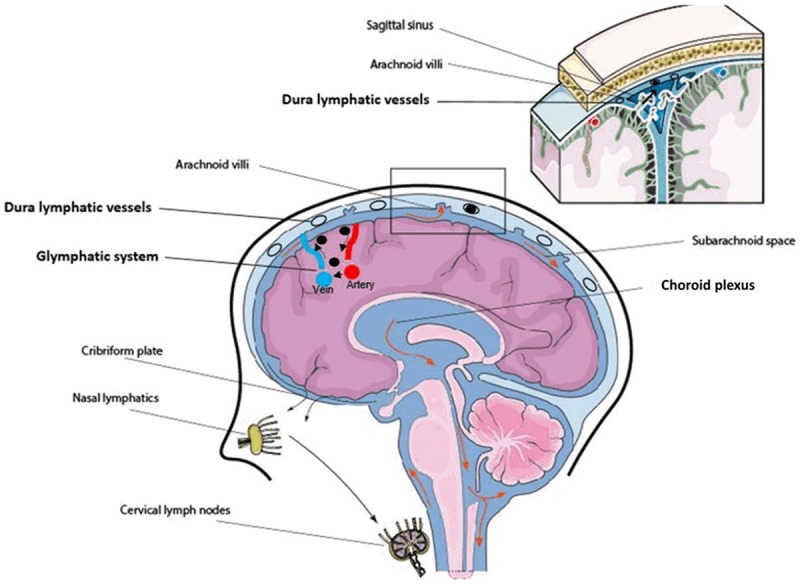
Connection of CSF circulation, glymphatic system and dura lymphatic vessels in the context of clearance. The glymphatic system (black arrow depicted flow direction) resides inside the brain is responsible for small size water-soluble waste metabolites clearance (black dots). Lymphatic vessels (black circle) present in dura are postulated to involve in waste metabolites clearance by granting access for waste metabolites that are drained via the glymphatic system.
It is possible that waste metabolites from peri-venous space may gain access to meningeal lymphatics through the subarachnoid or along the drainage veins that can merge into dura sinus. It may still be too early to embrace the meningeal lymphatic vessel as clearance route of CNS waste metabolites because there is no direct evidence to strongly support this argument. In fact, multiple paths of CSF drainage in the CNS that are functionally and anatomically close to lymphatic vessels are known [54-56]. However, the exact connection between those routes and lymphatic vessels is largely unknown. This is because the precise location of lymphatic vessels, whether within subarachnoid, inside the dura or bathed between these two layers needs to be unraveled [57].
Since meningeal lymphatic vessels are not directly connected to CSF compartments, the primary route of CSF waste metabolites absorption from subarachnoid into sagittal sinus will be across the arachnoid villi. The arachnoid microvilli barrier is permeable to small size water-soluble metabolites, as such the clearance mechanisms of large size waste metabolites remains to be investigated further. Thus, promoting the mechanisms of perivascular clearance becomes clinically significant since entangled proteins like Aβ or neurofibrillary tau-phosphorylated protein are commonly aggregated at the perivascular space.
Perivascular space clearance mechanisms
Perivascular space (PVS) is referred to the space surrounding the penetrating arterioles when diving down into deep brain tissues from pial arteries [58,59]. The fine structure of PVS consists of vascular endothelial and glial cells. The glial layer of the astrocyte end-feet forms the outer wall, which becomes continuous with the vascular membrane and basal lamina as it penetrates into the deep brain [60,61]. The PVS channels are known to involve in CSF/ISF waste metabolites movement, as indicated by distribution of horseradish peroxidase following lateral ventricle or subarachnoid space injection in cats and dogs [62]. Recently, a dynamic exchange of fluorescently labeled tracer between CSF/ISF and the perivascular was shown in NG2-DS transgenic mice by using two-photon microscopy imaging [31]. In fact, in the 1970’s the PVS was implicated as a conduit of waste metabolites sink similar to the choroid plexuses because injection of horse radish peroxidase (HRP) tracer into striatum of rat was found to aggregate around the PVS [23].
The assumption of PVS serving as a conduit sink in the CNS is significantly in line with the accumulation of waste metabolites around vasculatures that are clinically observed in certain neurological diseases. The classic examples of such waste metabolites deposition around the perivascular space are the neuropathological observations of phosphorylated tau protein in cerebral amyloid angiopathy (CAA) and aggregation of amyloid beta protein in Alzheimer’s disease [51-53,63]. These observations clearly suggest that PVS may be directly involved for the possible clearance of large size waste metabolites like phosphorylated tau protein and Aβ protein, which are transported by CSF, meningeal lymphatics and glymphatic function. Intriguingly, the ApoE family of molecules (ApoE2, E3) when mediated by low-density receptor protein (LRP1) seem to actively involved in the clearance of Aβ protein through BBB trans-vascular transcytosis [57,64,65]. In fact, experimental observations have shown that the trans-vascular clearance from brain to blood circulation across the BBB is believed to account for more than 80% of amyloid-beta clearance [66-68]. However, such findings are based on clearance of small Aβ peptide in normal physiological condition. In neuropathological conditions, aggregation of phosphorylated tau protein and Aβ protein at the perivascular space still remains to be the hallmark of neurological diseases. The formation of this hallmark phenomenon needs further investigation to understand whether the aggregation of entangled proteins at the perivascular space is due to onsite formation or translocation from the ISF. Since the aggregated proteins in AD are mostly originated from neuronal cell components, it is likely to translocate from the interstitial fluid rather than onsite formation. The rationale is that unlike the astrocyte end-feet, the location of neurons is not directly in contact with perivascular endothelial cells.
Thus, we posit here two fundamental questions for further investigation. What is the driving force that regulates the movement of these waste metabolites towards the perivascular space, and how do we improve the clearance of these metabolites from perivascular space into the circulation in a timely manner? Understanding the improved mechanisms of the intertwined processes will be significant to alleviate this neurological disease. To this end, our recent findings gain clinical significant in understanding the underlying cellular and molecular mechanisms of waste metabolites movement towards perivascular space. We have shown that large size waste metabolites injected into the CSF directly or into the cortical interstitial fluid diffused towards perivascular-peri-venous space instead of clearing through the conventional CSF subarachnoid and sagittal sinus exchange [69]. We indicated that the hindrance for this exchange is due to the presence of the arachnoid villi barrier between the two compartments, which allows the absorption of mostly small size water-soluble metabolites/solutes. As a result, these stagnated waste metabolites are forced to move towards perivascular space, and we postulated that the driving force that regulate the movement appears to be the dilative reactivity of arterial endothelial cells and smooth muscle cells interactions.
The rationale is that reactivity of vascular smooth muscle cells was indicated to govern the cerebral arterial pulsation driven perivascular CSF flow locally [70]. This dynamic pulsatile pattern of neighboring blood vessels may exert significant attraction on fluid movement towards perivascular space. In this, the bioavailability of nitric oxide (NO), a potent vasodilator becomes essential to regulate dilative reactivity of cerebral arterial endothelial cells and smooth muscle cells interactions. Thus, we examined the proof-of-concept that activation of brain endothelial specific nitric oxide synthase (eNOS) by low dose ethanol could produce favorable physiological levels of NO for increasing the cellular reactivity and increase diffusive movement of waste metabolites towards perivascular space. The rationale is that moderate level of ethanol has been shown to augment endothelial-SMCs dilative activity in placenta and pulmonary arterial vessels [71-73]. In deed our recent findings revealed that a very low dose ethanol (5.0 mM, equivalent of 0.02% blood alcohol level) significantly enhanced the diffusive movement of waste metabolites from ISF/CSF to perivascular-peri-venous space [69]. We found that dilative reactivity of brain endothelial and smooth muscle cell was the underlying cellular and molecular mechanisms for this improved diffusive clearance of waste metabolites at the perivascular-peri-venous space. Figure 5 illustrates the underlying mechanisms of waste metabolites clearance at the perivascular-peri-venous space.
Figure 5.
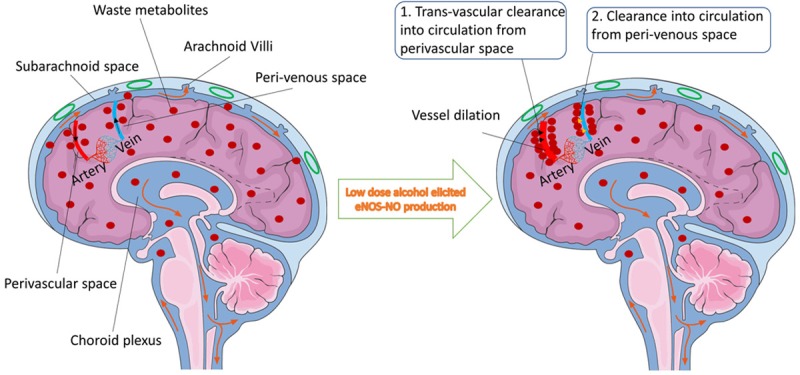
Schematic presentation of waste metabolites clearance at the perivascular-peri-venous space. Figure indicates the CSF subarachnoid flow (orange arrow) and the perivascular-peri-venous clearance path (black arrow) of large size waste metabolites (red dots) in neurodegenerative brain. Waste metabolites like β-amyloid proteins are aggregated more inside the interstitial space due to lack of lymphatic system in the brain. Less metabolites are drained into the CSF flow due to less vessel dilative reactivity (Left). In the presence of low dose alcohol (Right), dilative arterial vessel reactivity promotes the dynamic movement of large size waste metabolites from interstitial fluid and interstitial-CSF subarachnoid to the perivascular-peri-venous route. Reactivity of endothelial and smooth muscle cells in arteries is mediated by alcohol-elicited eNOS activation and NO production. From perivascular and/or peri-venous spaces, large size metabolites are exchanged into circulation through two postulated mechanisms: (1) trans-vascular clearance to circulation from the perivascular space, and (2) diffusion into circulation from the peri-venous space.
Our findings also validated the exchange between perivascular-peri-venous and blood circulation by detecting fluorescently labeled large size waste metabolite in systemic blood plasma after CSF/parenchyma injection. The question of whether the maximum diffusion into the blood circulation takes place at the perivascular space or at the peri-venous space, and/or both requires future studies. Other studies indicated that peri-venous flow may gain access to meningeal lymphatic vessels or large caliber draining veins, which may then merge to form the dura sinus [31,42], but it has no direct evidence. This rationale is unlikely because lymphatic vessels appear to localize in dura leaflets or inside surface of the dura that are separated from the CSF, as such it will not have access to waste metabolites. Based on tissue organ environmental considerations, Rapper et al. (2016) concluded that unlike the function of the peripheral lymphatic system, the meningeal lymphatics may not involve in the CSF waste metabolites drainage system [49]. In summary, the movement of Aβ protein from ISF/CSF subarachnoid to perivascular space is regulated by dilative reactivity of brain arterial smooth muscle and endothelial cells interactions. This clearance path can be significantly promoted by low dose ethanol via eNOS-induced NO production.
Concluding remarks
Efficient clearance of interstitial waste metabolites in CNS is essential for normal maintenance of brain homeostasis. We describe the up-to-date emerging discoveries of various possible clearance systems operating in the brain apart from the conventional cerebrospinal fluid (CSF) clearance path. This includes glymphatic system, meningeal lymphatic vessels, diffusive movement of waste metabolites from ISF/CSF to perivascular space, and perivascular clearance of waste metabolites across the BBB trans-vascular vessels. Each of these individual discoveries does carry significant impact on current scientific knowledge gaps towards the understanding of waste metabolites clearance and immune cells surveillance function in the CNS. Apart from the focal finding of astrocytic aquaporin-4 mediated clearance path, the glymphatic system does reveal the movement of waste metabolites like amyloid-beta towards the perivascular space. Whether the movement of these waste metabolites from ISF/CSF to perivascular space is regulated by diffusion or a bulk flow mechanisms needs further verification. However, aggregation of waste metabolites at the perivascular space prompted the clearance path through the BBB trans-vascular or re-entry into the capillary basement membranes [74], which is relevant for eventual prevention of neurological disease like Alzheimer’s disease. Thus, improving the newly discovered CNS clearance path (s) in the absence of traditional lymphatic drainage system assumes great therapeutic relevance for many neurological diseases.
Acknowledgements
This work was supported by NIH grant 1R21AA022734-01A1, R21 AA020370-01A1 (to JH).
Disclosure of conflict of interest
None.
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Strazielle N, Ghersi-Egea JF. Physiology of blood-brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol Pharm. 2013;10:1473–1491. doi: 10.1021/mp300518e. [DOI] [PubMed] [Google Scholar]
- 3.Zlokovic BV, Segal MB, Davson H, Lipovac MN, Hyman S, McComb JG. Circulating neuroactive peptides and the blood-brain and blood-cerebrospinal fluid barriers. Endocrinol Exp. 1990;24:9–17. [PubMed] [Google Scholar]
- 4.Segal MB, Zlokovic BV. The blood-brain barrier, amino acids and peptides. Dordrecht, Boston and London: kluwer academic publishers; 1990. [Google Scholar]
- 5.Kivisakk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwerk C, Tenenbaum T, Kim KS, Schroten H. The choroid plexus-a multi-role player during infectious diseases of the CNS. Front Cell Neurosci. 2015;9:80. doi: 10.3389/fncel.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daum RS, Scheifele DW, Syriopoulou VP, Averill D, Smith AL. Ventricular involvement in experimental Hemophilus influenzae meningitis. J Pediatr. 1978;93:927–930. doi: 10.1016/s0022-3476(78)81213-x. [DOI] [PubMed] [Google Scholar]
- 8.Kaur C, Rathnasamy G, Ling EA. The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol. 2016;75:198–213. doi: 10.1093/jnen/nlv030. [DOI] [PubMed] [Google Scholar]
- 9.Uchida Y, Sumiya T, Tachikawa M, Yamakawa T, Murata S, Yagi Y, Sato K, Stephan A, Ito K, Ohtsuki S, Couraud PO, Suzuki T, Terasaki T. Involvement of claudin-11 in disruption of blood-brain, -spinal cord, and -arachnoid barriers in multiple sclerosis. Mol Neurobiol. 2019;56:2039–2056. doi: 10.1007/s12035-018-1207-5. [DOI] [PubMed] [Google Scholar]
- 10.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci. 2015;16:445–457. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Praetorius J, Damkier HH. Transport across the choroid plexus epithelium. Am J Physiol Cell Physiol. 2017;312:C673–C686. doi: 10.1152/ajpcell.00041.2017. [DOI] [PubMed] [Google Scholar]
- 12.Yamashima T. Functional ultrastructure of cerebrospinal fluid drainage channels in human arachnoid villi. Neurosurgery. 1988;22:633–641. doi: 10.1227/00006123-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Campos-Bedolla P, Walter FR, Veszelka S, Deli MA. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch Med Res. 2014;45:610–638. doi: 10.1016/j.arcmed.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol. 2008;6:625–634. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang SL, Yue Z, Arnold DM, Artiushin G, Sehgal A. A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell. 2018;173:130–139. e10. doi: 10.1016/j.cell.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J. Myeloid cells in the central nervous system. Immunity. 2017;46:943–956. doi: 10.1016/j.immuni.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alirezaei M, Kemball CC, Whitton JL. Autophagy, inflammation and neurodegenerative disease. Eur J Neurosci. 2011;33:197–204. doi: 10.1111/j.1460-9568.2010.07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei Y, Han H, Yuan F, Javeed A, Zhao Y. The brain interstitial system: anatomy, modeling, in vivo measurement, and applications. Prog Neurobiol. 2017;157:230–246. doi: 10.1016/j.pneurobio.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cserr HF, Cooper DN, Milhorat TH. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp Eye Res. 1977;25(Suppl):461–473. doi: 10.1016/s0014-4835(77)80041-9. [DOI] [PubMed] [Google Scholar]
- 24.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240:F319–328. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 25.Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984;246:F835–844. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- 26.Bakker EN, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AW, Weller RO, Carare RO. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol. 2016;36:181–194. doi: 10.1007/s10571-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keep RF, Jones HC. A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res Dev Brain Res. 1990;56:47–53. doi: 10.1016/0165-3806(90)90163-s. [DOI] [PubMed] [Google Scholar]
- 29.Bradley WG Jr, Kortman KE, Burgoyne B. Flowing cerebrospinal fluid in normal and hydrocephalic states: appearance on MR images. Radiology. 1986;159:611–616. doi: 10.1148/radiology.159.3.3704142. [DOI] [PubMed] [Google Scholar]
- 30.Feinberg DA, Mark AS. Human brain motion and cerebrospinal fluid circulation demonstrated with MR velocity imaging. Radiology. 1987;163:793–799. doi: 10.1148/radiology.163.3.3575734. [DOI] [PubMed] [Google Scholar]
- 31.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 33.Weed LH. Studies on cerebro-spinal fluid. No. II: the theories of drainage of cerebro-spinal fluid with an analysis of the methods of investigation. J Med Res. 1914;31:21–49. [PMC free article] [PubMed] [Google Scholar]
- 34.Weed LH. Studies on cerebro-spinal fluid. No. IV: the dual source of cerebro-spinal fluid. J Med Res. 1914;31:93–118.11. [PMC free article] [PubMed] [Google Scholar]
- 35.Levine JE, Povlishock JT, Becker DP. The morphological correlates of primate cerebrospinal fluid absorption. Brain Res. 1982;241:31–41. doi: 10.1016/0006-8993(82)91225-2. [DOI] [PubMed] [Google Scholar]
- 36.Nagra G, Koh L, Zakharov A, Armstrong D, Johnston M. Quantification of cerebrospinal fluid transport across the cribriform plate into lymphatics in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1383–1389. doi: 10.1152/ajpregu.00235.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015;14:977–979. doi: 10.1016/S1474-4422(15)00221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asgari N, Berg CT, Mørch MT, Khorooshi R, Owens T. Cerebrospinal fluid aquaporin-4-immunoglobulin G disrupts blood brain barrier. Ann Clin Transl Neurol. 2015;2:857–863. doi: 10.1002/acn3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife. 2017:6. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holter KE, Kehlet B, Devor A, Sejnowski TJ, Dale AM, Omholt SW, Ottersen OP, Nagelhus EA, Mardal KA, Pettersen KH. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A. 2017;114:9894–9899. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin BJ, Smith AJ, Verkman AS. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol. 2016;148:489–501. doi: 10.1085/jgp.201611684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asgari M, de Zelicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep. 2016;6:38635. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp Neurol. 2015;273:57–68. doi: 10.1016/j.expneurol.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 51.Merlini M, Wanner D, Nitsch RM. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer’s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol. 2016;131:737–752. doi: 10.1007/s00401-016-1560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rangel A, Race B, Phillips K, Striebel J, Kurtz N, Chesebro B. Distinct patterns of spread of prion infection in brains of mice expressing anchorless or anchored forms of prion protein. Acta Neuropathol Commun. 2014;2:8. doi: 10.1186/2051-5960-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO. Failure of perivascular drainage of beta-amyloid in cerebral amyloid angiopathy. Brain Pathol. 2014;24:396–403. doi: 10.1111/bpa.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Squier W, Lindberg E, Mack J, Darby S. Demonstration of fluid channels in human dura and their relationship to age and intradural bleeding. Childs Nerv Syst. 2009;25:925–931. doi: 10.1007/s00381-009-0888-5. [DOI] [PubMed] [Google Scholar]
- 55.Johnston M, Armstrong D, Koh L. Possible role of the cavernous sinus veins in cerebrospinal fluid absorption. Cerebrospinal Fluid Res. 2007;4:3. doi: 10.1186/1743-8454-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zenker W, Bankoul S, Braun JS. Morphological indications for considerable diffuse reabsorption of cerebrospinal fluid in spinal meninges particularly in the areas of meningeal funnels. An electronmicroscopical study including tracing experiments in rats. Anat Embryol (Berl) 1994;189:243–258. doi: 10.1007/BF00239012. [DOI] [PubMed] [Google Scholar]
- 57.Raper D, Louveau A, Kipnis J. How do meningeal lymphatic vessels drain the CNS? Trends Neurosci. 2016;39:581–586. doi: 10.1016/j.tins.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krahn V. The pia mater at the site of the entry of blood vessels into the central nervous system. Anat Embryol (Berl) 1982;164:257–263. doi: 10.1007/BF00318509. [DOI] [PubMed] [Google Scholar]
- 59.Weller RO. Microscopic morphology and histology of the human meninges. Morphologie. 2005;89:22–34. doi: 10.1016/s1286-0115(05)83235-7. [DOI] [PubMed] [Google Scholar]
- 60.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- 62.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 63.Rehm J, Hasan OSM, Black SE, Shield KD, Schwarzinger M. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11:1. doi: 10.1186/s13195-018-0453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, Ichida JK, Zlokovic BV. Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Y, Liu X, Ma X, Garcia R, Belfield K, Haorah J. Alcohol promotes waste clearance in the CNS via brain vascular reactivity. Free Radic Biol Med. 2019;143:115–126. doi: 10.1016/j.freeradbiomed.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 70.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Acevedo CG, Carrasco G, Burotto M, Rojas S, Bravo I. Ethanol inhibits L-arginine uptake and enhances NO formation in human placenta. Life Sci. 2001;68:2893–2903. doi: 10.1016/s0024-3205(01)01070-0. [DOI] [PubMed] [Google Scholar]
- 72.Venkov CD, Myers PR, Tanner MA, Su M, Vaughan DE. Ethanol increases endothelial nitric oxide production through modulation of nitric oxide synthase expression. Thromb Haemost. 1999;81:638–642. [PubMed] [Google Scholar]
- 73.Greenberg SS, Xie J, Wang Y, Kolls J, Shellito J, Nelson S, Summer WR. Ethanol relaxes pulmonary artery by release of prostaglandin and nitric oxide. Alcohol. 1993;10:21–29. doi: 10.1016/0741-8329(93)90049-t. [DOI] [PubMed] [Google Scholar]
- 74.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]


