Abstract
Background: The molecular mechanism of bladder cancer is yet not fully understood. Aim of this study was to compare the levels of BLCA-4 nuclear matrix protein in the urine of patients with bladder cancer and non-affected individuals. Materials and method: The current cross sectional study was conducted on 45 patients with bladder cancer and 45 patients without bladder cancer who were referred to Alzahra Hospital of Isfahan, Iran in 2017. BCLA-4 Urinary Marker was measured in urine of the patients and individuals. Also correlation between the urine levels of BCLA-4 and other variables were evaluated. Results: The urine levels of BLCA-4 in the patients with bladder cancer was significantly higher than non-bladder cancer group (P<0.001). There was no significant relationship between urine levels of BLCA-4 with tumor stage and size (P>0.05). Conclusion: The present study indicated that high urine levels of BLCA-4 was presented in patients with bladder cancer and this tumor marker has a high capability for early diagnosis of the disease, which can be used for screening and follow-up of bladder cancer.
Keywords: BLCA-4, bladder, urine, malignancy
Introduction
Bladder cancer is the fourth most common cancer among men and the ninth most common among women. Transitional Cell Carcinoma (TCC) is the most common bladder malignancy (90%) [1]. Despite the recent advances, the molecular mechanisms of bladder cancer is yet not fully understood [2,3]. Screening in the population at risk has become an important issue in reducing mortality [4]. The importance of early diagnosis of bladder cancer in the early stages is such that it can increase the survival rate of 5 years to 94% [5]. Gold standard cystoscopy is used to follow up patients after surgery [6]. However, due to several factors, such as cost and invasion, cystoscopy is not ideal for control and follow-up [2,4].
Although it has been the preferred non-invasive method for diagnosing bladder cancer for decades, new biomarkers had to be discovered due to its low sensitivity, especially in low-grade tumors [7]. Progress made in identifying specific changes in the nuclear structure of bladder cancer cells opened new diagnostic perspectives [5,7]. The members of nuclear matrix protein family BLCA-1 and BLCA-4 are currently under evaluation as bladder cancer urinary markers [8,9]. They are involved in tumor cell proliferation, survival, and angiogenesis [2,3,9]. After research conducted by different research teams on BLCA-4 nuclear matrix protein, it was proposed as the most sensitive (96.4%) and most promising (100%) marker of bladder cancer. This protein increases the levels of IL-1α, IL-8 and thrombomodulin [10]. Also NMP22 is a biomarker that was approved and recommended by Food and Drug Administration (FDA) to follow up of patients with family history of bladder cancer [11].
Given the importance of using the appropriate biomarker in this study, the mean level of BLCA-4 nuclear matrix protein in the urine of patients with bladder cancer and non-affected individuals were determined and compared.
Method
This cross sectional study was done on 45 patients with bladder cancer and 45 individuals without bladder cancer who were referred to Alzahra Hospital and private clinic of Isfahan, Iran in 2017. All patients had written informed consent to including in the study. The protocol of present study was approved in the ethical committee of Isfahan University of Medical Sciences.
Inclusion criteria of bladder cancer patients were included new cases of bladder cancer accompanied with pathology confirmation. Individuals suspected were patients without bladder cancer or patients with benign tumor who under diagnosis test and pathology and workup tests were negative for bladder cancer.
Exclusion criteria were patients with chronic diseases such as liver, lung, heart and kidney failures that affect protein metabolism, taking immunosuppressive drugs, patients with multiple cancers at once and impairment in sampling or laboratory response. All subjects had informed consent for participation to study.
Consecutive sampling method was used in this study, where the qualified patients visiting the Urology Clinic of Alzahra Hospital and the private clinic of the faculty members of the university were selected, and sampling continued until the sample size was achieved.
Initially, demographic and initial data were obtained from patients, including age, gender, and clinical stage of cancer and tumor size. In this study, BCLA-4 Urinary Tumor Marker BioAssay ELISA Kit was used, which was a 1.5 hour solid-phase competitive ELISA designed for the quantitative determination of BCLA-4 Urinary Tumor Marker in Human Serum, plasma, tissue homogenates, cell culture supernatants and body fluids. This kit was intended for research use only and may not be used for therapeutic or diagnostic applications. To this end, the patients were referred to the laboratory and all stages of urine sampling were performed in one laboratory and carried out by the same laboratory team. The level of BCLA-4 bladder cancer nuclear matrix protein was measured and recorded. Then, values were investigated and compared by category, gender, primary or recurrent cancer, and recurrence, tumor size, and the clinical stage of the disease. The staging tumor was included based on the American Joint Committee on Cancer (AJCC) TNM system. T0 and TIS are noninvasive carcinoma without metastasis to lymph nodes and distant sites. T1-T4 are invasive carcinoma to inner layers that T1 is speared to connective tissue layer, T2 is speared to inner and outer layer of muscle, T3 is speared to fatty tissue layer and T4 is speared to pelvic organs so T1 to T4 can be along with metastasis to distant layers and lymph nodes [12]. When data collection completed, all data entered into SPSS (Version 24). The required analyses were conducted in the descriptive statistics section using statistical indices of percentage, mean, and standard deviation, and, in the analytic statistics section, Independent t test (to compare of quantitative variables between the two groups), Chi Square (to compare of qualitative variables between the two groups), one-way ANOVA (to compare of quantitative and qualitative variables) and Pearson correlation (correlation between quantitative variables) were used. P-value under 0.05 was considered significant threshold.
Results
In this study patients were divided into bladder cancer (35 males and 10 females with mean aged 70.78±9.16 years) and non-bladder cancer (30 males and 15 females with mean aged 67.80±11.77) groups. The bladder cancer and non-bladder cancer group were matched based on age and gender (P>0.05).
In the patient group, 23 (51.1%), 16 (35.6%), and 6 (13.3%), were in stage T1-Ta, T2-T4 and Tis, respectively. The mean of tumor size in the bladder cancer group was 2.52±1.55 cm.
The mean of urine BLCA-4 level in the bladder cancer and non-bladder cancer group were 765.55±180.33 mg/dl and 209.77±47.78 mg/dl, respectively (Figure 1), and the urine BLCA-4 level in the bladder cancer group was significantly higher than non-bladder cancer group (P value <0.001) (Table 1). There was no significant relationship between urine BLCA-4 with tumor stage (P=0.11) and gender (P=0.53). Also there was no significant correlation between BLCA-4 with age and tumor size (P>0.05).
Figure 1.
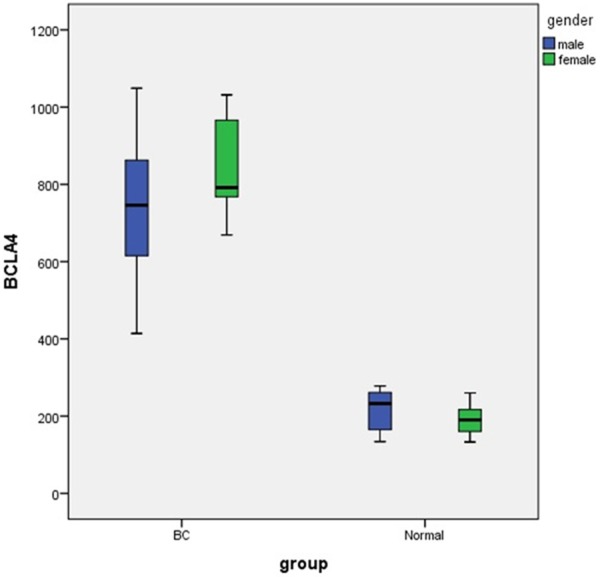
Urine levels of BCLA-4 in patients with bladder cancer and healthy subjects.
Table 1.
Demographic Characteristic of patients included in study
| Variable | Groups | P | ||
|
| ||||
| Bladder cancer | Non-bladder cancer | |||
|
| ||||
| Quantitative Variables | ||||
|
| ||||
| Mean ± SD | Mean ± SD | |||
|
| ||||
| Age | 70.78±9.16 | 67.80±11.77 | 0.18 | |
| Tumor Size | 2.52±1.55 | - | - | |
| BLCA-4 | 765.55±180.33 | 209.77±47.78 | <0.001 | |
|
| ||||
| Qualitative Variables | ||||
|
|
||||
| N (%) | N (%) | |||
|
| ||||
| Gender | Male | 35 (77.8) | 30 (66.7) | 0.17 |
| Female | 10 (22.2) | 15 (33.3) | ||
| T stage | T1, Ta | 23 (51.1) | - | - |
| T2-T4 | 16 (35.6) | - | ||
| Tis | 6 (13.3) | - | ||
Discussion
Although there have been many advances in tumor diagnosis, cancer treatment and radiotherapy, little progress has been made in the prognosis and quality of life of cancer patients in the last decade [13]. Due to the increased mistakes in routine therapies and the inadequate response to them, new molecules involved in cancer were investigated [14].
BLCA-4 is a nuclear transcription factor in bladder tumors and benign bladder areas [7]. It is one of the six tumor markers in the diagnosis of bladder cancer [9]. BLCA-4 protein level in urine was tested using ELISA [15]. Preliminary studies indicated a sensitivity of 89%-96% with a specificity of 100% for bladder cancer [9]. BLCA-4 has probably a potential role in tumor growth or development [1]. In this study, the relationship between urine level of BLCA-4 and bladder cancer or non-bladder cancer. In this study, there was no significant relationship between serum BLCA-4 levels and the age of the patients, which is well-documented in other studies, for example, in the study by Qian Zhao, who examined this tumor marker. Lack of significant relationship between the ability to use this tumor marker in people of all ages and the serum level of this tumor marker was not related to the individual’s gender, as demonstrated in the studies by Justin Parker and Badrinath R. Konety [16-18].
The relationship between different stages of the disease and the tumor marker level was not significant, which indicated an increase in the tumor marker level in all stages of the disease. In the study by C. C. Feng, there was no significant relationship between the stage of the disease and BLCA-4. In a similar study, Konety et al. concluded that there is no significant difference between the stage of the disease and BLCA-4 [18].
In their study, Qian Zhao et al. concluded that the tumor size is not related to the BLCA-4 level. However, no further studies were conducted to determine the relationship between tumor size and BLCA-4 levels. In the present study, BLCA-4 levels and tumor size were not significantly correlated.
The important point in the present study was a significant correlation between BLCA-4 levels in a comparison made between the patients and the healthy non-bladder cancer group. In the study by Qiliang Cai, and other similar review studies, there was a significant correlation between the two groups indicating a higher serum BLCA-4 level in the patients. In the study by Konety, it was shown that there is a significant relationship between these two groups, and higher tumor marker levels were seen in patients [16-18].
Conclusion
The present study indicated that high BLCA-4 levels in patients’ urine can be due to the presence of a tumor, and this tumor marker has a high capability for diagnosis of the disease, which can be used for patients’ screening and follow-up. So we need to future studies to provide our results.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Case RA, Hosker ME, McDonald DB, Pearson JT. Tumours of the urinary bladder in workmen engaged in the manufacture and use of certain dyestuff intermediates in the British chemical industry. I. The role of aniline, benzidine, alpha-naphthylamine, and beta-naphthylamine. Br J Ind Med. 1954;11:75–104. doi: 10.1136/oem.11.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, Fraumeni JF Jr. Urinary tract infection and risk of bladder cancer. Am J Epidemiol. 1984;119:510–515. doi: 10.1093/oxfordjournals.aje.a113768. [DOI] [PubMed] [Google Scholar]
- 4.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J, Roupret M. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Witjes JA, Hendricksen K. Intravesical pharmacotherapy for non-muscle-invasive bladder cancer: a critical analysis of currently available drugs, treatment schedules, and longterm results. Eur Urol. 2008;53:45–52. doi: 10.1016/j.eururo.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sukhun S, Hussain M. Molecular biology of transitional cell carcinoma. Crit Rev Oncol Hematol. 2003;47:181–193. doi: 10.1016/s1040-8428(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 8.Whelan P, Britton JP, Dowell AC. Three-year follow-up of bladder tumours found on screening. Br J Urol. 1993;72:893–896. doi: 10.1111/j.1464-410x.1993.tb16292.x. [DOI] [PubMed] [Google Scholar]
- 9.Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003;61:109–118. doi: 10.1016/s0090-4295(02)02136-2. discussion 118. [DOI] [PubMed] [Google Scholar]
- 10.Schwalb DM, Herr HW, Fair WR. The management of clinically unconfirmed positive urinary cytology. J Urol. 1993;150:1751–1756. doi: 10.1016/s0022-5347(17)35886-x. [DOI] [PubMed] [Google Scholar]
- 11.Santoni M, Catanzariti F, Minardi D, Burattini L, Nabissi M, Muzzonigro G, Cascinu S, Santoni G. Pathogenic and diagnostic potential of BLCA-1 and BLCA-4 nuclear proteins in urothelial cell carcinoma of human bladder. Adv Urol. 2012;2012:397412. doi: 10.1155/2012/397412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Downs TM, Efstathiou JA, Friedlander T, Greenberg RE. NCCN guidelines insights: bladder cancer, version 5.2018. J Natl Compr Canc Ne. 2018;16:1041–1053. doi: 10.6004/jnccn.2018.0072. [DOI] [PubMed] [Google Scholar]
- 13.Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002;21:1237–1256. doi: 10.1002/sim.1099. [DOI] [PubMed] [Google Scholar]
- 14.Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg. 2005;79:16–20. doi: 10.1016/j.athoracsur.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Feng CC, Wang PH, Guan M, Jiang HW, Wen H, Ding Q, Wu Z. Urinary BLCA-4 is highly specific for detection of bladder cancer in Chinese Han population and is related to tumour invasiveness. Folia Biol (Praha) 2011;57:242–247. doi: 10.14712/fb2011057060242. [DOI] [PubMed] [Google Scholar]
- 16.Cai Q, Wu Y, Guo Z, Gong R, Tang Y, Yang K, Li X, Guo X, Niu Y, Zhao Y. Urine BLCA-4 exerts potential role in detecting patients with bladder cancers: a pooled analysis of individual studies. Oncotarget. 2015;6:37500–37510. doi: 10.18632/oncotarget.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Q, Shen WH, Chen ZW, Zhou ZS, Ji HX. High expression level of BLCA-4 correlates with poor prognosis in human bladder cancer. Int J Clin Exp Pathol. 2012;5:422–427. [PMC free article] [PubMed] [Google Scholar]
- 18.Konety BR, Nguyen TS, Brenes G, Sholder A, Lewis N, Bastacky S, Potter DM, Getzenberg RH. Clinical usefulness of the novel marker BLCA-4 for the detection of bladder cancer. J Urol. 2000;164:634–639. doi: 10.1097/00005392-200009010-00004. [DOI] [PubMed] [Google Scholar]


