Abstract
CD200 and its receptor, CD200R, constitutes an endogenous inhibitory signaling, and is being increasingly recognized in studies of various central nervous system (CNS) disorders. Emerging data have demonstrated that neuronal CD200 binds to CD200R to modulate immune responses to pathogenic stimuli. However, on which component of the immune response that CD200-CD200R signaling acts is not well understood. In this review, we focused on cellular expression of the signaling, the effects on immune cell activation, and the function in pathological procedures of neurodegenerative diseases, in both clinical and experimental disease models. Essential functions of CD200-CD200R interaction and the treatment relevance have been elaborated. Immune responses to diseases under the control of CD200-CD200R axis were also discussed in the review.
Keywords: Brain, CD200, CD200R, immune responses, neuron
Introduction
Immune responses to brain tissue damage are triggered by the recognition of non-self or altered self-molecular patterns by professional cells including microglia, neurons, astrocytes, and oligodendrocytes [1,2]. The recognition leads to activation of immune cells that is regulated by endogenous inhibitory pathways including CD200 signaling. The cluster of Differentiation-200 (CD200), a 41-47 KDa protein [3-11] characterized by two immunoglobulins superfamily (IgSF) domains [11], one transmembrane region, and a small cytoplasmic domain, is suggested to be devoid of intracellular signaling function [12]. However, primarily expressed in the somas, axons, dendrites and synapses of neurons, and in endothelial cells, CD200 is an important inhibitory ligand to interact with immune cells [10]. Genes encoding CD200 are located on chromosome 3, precisely 3q12-13. The homology between human and mouse CD200 is 77.6% for protein and 81.7% for DNA, which in the case of human vs. rat is 77.2% (protein) and 80.7% (DNA) [13]. CD200 receptor (CD200R) also has two IgSF domains but with a longer cytoplasmic tail [7,21], constituting a cellular signaling domain [14]. CD200R is mainly expressed by myeloid cells [20,26,30], but also present on thymocytes [15], T and B cells [8,24]. CD200R family include CD200R1, R2, R3 and R4 in mouse; and CD200R1 and R2 in human [31,32]. However, it was found that CD200 only binds to CD200R1 but is not the ligand for other CD200R isoforms [16,17]. CD200R interacts with CD200 ligand through its N-terminal Ig V-type domain, forming an endogenous inhibitory signaling for immune responses [18]. The human CD200R gene spans a region of 52 kb consisting of nine exons and encodes a 348-amino-acid cell-surface protein [14]. In contrast to murine CD200R protein, the human membrane-bound and soluble CD200R proteins have an insertion of 23 amino acids at position 23, encoded by exon 2, which generates a putative dihydroxyacid dehydratase domain [14]. Despite these differences, CD200-CD200R signaling plays a pivotal role in modulating immune responses in both murine and human upon inflammatory stimuli.
Molecular mechanisms of CD200-CD200R signaling
CD200R does not contain any immunoreceptor tyrosine-based inhibitory motifs (ITIMs) which are usually present in a large number of inhibitory receptors and which mediate their inhibitory roles through the recruitment of protein tyrosine phosphatases such as Src homology 2 domain-containing phosphatase (SHP) 1, SH2, or the inositol phosphatase (SHIP) upon phosphorylation [19]. Instead, the molecular signaling mechanism of CD200R following activation involves direct interaction of the adaptor protein downstream to tyrosine kinase (Dok2), with the membrane distal tyrosine residue located within a phosphotyrosine-binding (PTB) domain recognition motif (NPxY) [20]. This interaction leads to binding and recruitment of RAS p21 protein activator (RasGAP) which is an SH2 domain containing protein [21,22]. The formation of the Dok2-RasGAP complex inhibits Ras activation (Figure 1), leading to inhibition of other downstream inflammatory signals through inhibition of principal mitogen activated protein kinases including Phosphoinositide 3-kinase (PI3K) and Extracellular Signal-regulated Kinase (Erk) [10,23-25]. According to Snelgrove et al. [26] the interaction between CD200 and CD200R induces phosphorylation of tyrosine residues, initiating a signaling cascade which recruits SHIP and RasGAP [27,28]. Dok2 appears to be regulated by Dok1 through Crk Like (CrkL)-RasGAP suppression; both Dok2 and Dok1 are recruited during CD200-CD200R interaction that leads to recruitment of RasGAP and SH2-containing inositol phosphatase [29]. As shown in Figure 1, Dok1 activation is initiated through binding to one of the three phosphotyrosine residues located on the cytoplasmic amino acid chain of CD200R. This Dok1-phosphotyrosine binding then suppresses Dok2’s effect on Ras through activation of CrkL [30]. It has been demonstrated that knockdown of Dok2 but not Dok1 ameliorated the increase in IL-8 production following CD200R activation in U937 cells [29]. The regulatory effect of Dok2 by Dok1 was also confirmed by using macrophages with Dok1 knockdown, which shows increased phosphorylation of Dok2 and enhanced recruitment of RasGAP [14]. Thus, the activation and recruitment of Dok2, and the subsequent activation of RasGAP are the key events downstream to the CD200-CD200R interaction that induce immune regulatory function in immune cells [29].
Figure 1.
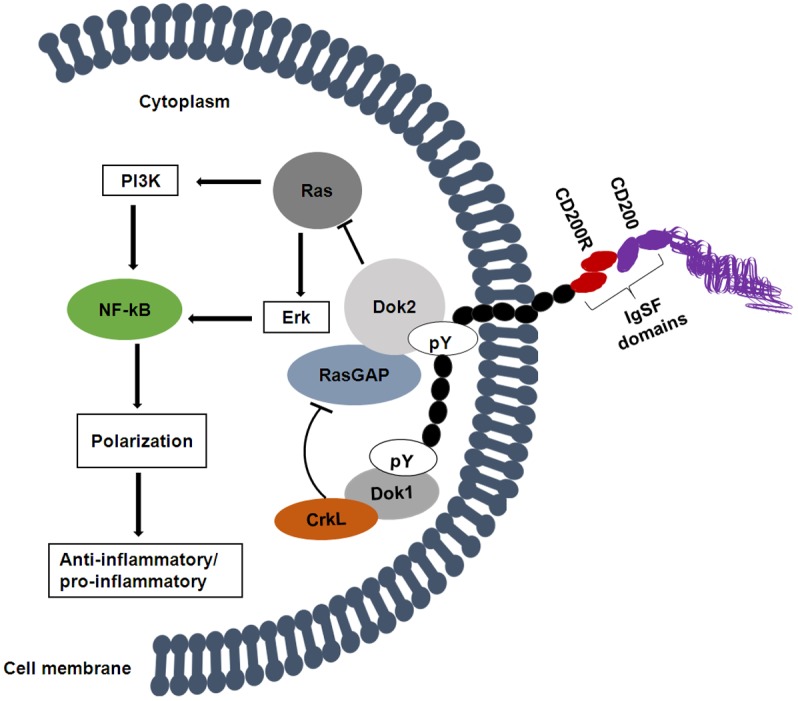
Presumptive mechanism of CD200-CD200R interactions underlying the activation of immune cells (monocytes, lymphocytes, etc.). The primary mechanism involves activation of Dok2 and RasGAP, leading to the inhibition of Ras activation and suppression of downstream effects on PI3K and Erk. Resultantly an increase of multiple anti-inflammatory signals occurs due to the inhibition of NF-kB. Activation of RasGAP can be inhibited by the combination of Dok1 and CrkL, leading to activation of NF-kB and the pro-inflammatory phenotype. pY: Phosphotyrosine. This illustration is based on the findings from [7,22,29,69,85,86].
Neuronal CD200 signaling
CD200 is mainly expressed by neurons and vascular endothelia cells [31-33], although relatively weaker expression was also found in astrocytes and oligodendrocytes [12,25,36], as well as in peripheral thymocytes [3,24] and NK cells [34]. Neurons express the highest level of CD200 compared to other cell types [35], and execute their functions on immune cells where CD200 receptor (CD200R) is expressed, including macrophages and dendritic cells [39,49], B and T cells [39,45]. CD200 gene in the human brain is likely regulated differently compared with rodents [13]; however the characterization of human CD200 localization [14,15] has shown a similar distribution on neuronal cell membrane as that of rodents [13,35]. Microglial CD200R expression has been controversial, as low levels of CD200R have been detected in microglia from multiple sclerosis [35] and Alzheimer’s disease (AD) [36] animal models. In another report CD200R was only present in macrophages but not microglia [37,38]. In vitro studies showed that isolated microglia from AD human brains had low levels of CD200R mRNA and protein compared with that in blood-isolated macrophages [36], suggesting that monocytic cells are more sensitive to CD200-CD200R signaling. Our recent study examined CD200R expression in infiltrating immune cells in mouse brains subjected to experimental ischemic stroke model, and found microglia express near null CD200R; while robust signals of the receptor were found in lymphocytes with modest expression on monocytes [39]. The vast amount of infiltrating CD200R+ leukocytes in the ischemic brain indicates the enhanced activation of these immune cells, which could be attributed to the loss of neuronal CD200 after stroke.
CD200R may not be the sole receptor for neuronal CD200. The addition of CD200 to primary neuronal cultures promoted neuronal survival and neurogenesis, an experiment performed through CD200 binding to the fibroblast growth factor receptor (FGFR) instead of CD200R1 [40]. Since the ultimate anti-inflammatory effect of FGFR activation was suggested being mediated by CD200 upregulation [28,29], further research is warranted to investigate whether the protective effects of neuronal CD200 are derived from either a direct interaction with CD200R on immune cells or indirectly by binding to other putative receptors.
CD200 signaling with aging
CD200 levels in neurons reduce with age [41,42]. Frank and his collaborators [41] compared the gene-expression profiles of the hippocampus from young (3 months) and aged (24 months) male F344xBN F1 rats. Their data showed a decrease in CD200 mRNA in the older animals accompanied by a significant increase in mRNA levels of inflammatory markers including the major histocompatibility complex (MHCII), Cluster of Differentiation-86 (CD86), and interferon gamma (IFN-γ). Microglial activation in the hippocampus of aged and β-amyloid (Aβ)-treated rats was accompanied by decreased expression of neuronal CD200, providing evidence that neurons can downregulate the microglial activation through CD200 signaling [43]. Data from this report also showed that the expression of CD200 was increased by IL-4 supplement, suggesting that IL-4 is also involved in the neuronal CD200 signaling to downregulate the activation of aged microglia under inflammatory conditions.
With the aim of characterizing CD200 and CD200R expression in elderly human brains, Walker et al. [13] found that many neurons in the cortex and hippocampus did not show CD200 immunoreactivity. Quantitative studies on human AD brain tissues from averagely 84.4 year old patients (16 females and 15 males) showed a significant decrease in CD200 protein and mRNA in the hippocampus and inferior temporal gyrus, but not in the cerebellum [34]. A significant decrease in CD200R mRNA expression in AD hippocampus and inferior temporal gyrus, but not in cerebellum, was also detected. Low expression of CD200R by microglia was confirmed at the mRNA and protein level using cultured human microglia compared to blood-derived macrophages [34], and the treatment of cultured microglia and macrophages with IL-4 and IL-13 significantly increased the expression of CD200R. These data indicate that the CD200-CD200R axis may be deficient in AD brains and mechanisms aimed at increasing levels of CD200 and CD200R could be neuroprotective in human neurodegenerative diseases.
Ischemic stroke
Ischemic stroke, the leading cause of death and long-term disability in the USA [44,45], accounts for > 80% of all stroke cases [46]. Post-stroke inflammation is a fundamental pathophysiological process that is tightly controlled by endogenous regulatory pathways including CD200-CD200R signaling. In the acute phase of brain ischemia, neuronal CD200 expression is markedly decreased in the brain due to the degeneration of CD200-expressing cells [11,47]; however, increased expression of CD200 was found 5 days after stroke [11,47,48]. It was suggested that this unstable expression during ischemia disrupts the CD200-CD200R axis and triggers microglial activation [49]. Yang et al. [50] reported that the expression of CD200 was decreased within 48 h after permanent middle cerebral artery occlusion (pMCAO) accompanied by a decrease of neuron-specific enolase (NSE) expression. The loss of CD200 caused by neuronal death could be one of contributing factors in immune cell activation after cerebral ischemia.
We have found that CD200-CD200R inhibitory axis could be a critical regulator of peripheral immune response after brain ischemia, impacting animal survival rate and their susceptibility to post-stroke infection, as the loss of CD200R signaling leads to exacerbated leukocyte infiltration in the brain, greater spontaneous bacterial colonization of the lung, and worse stroke outcomes [39]. CD200R may also co-exist with CD200 in immune cells and functions through an autocrine mechanism. Using rat MCAO model, Matsumoto et al. [11] found that macrophage-like cells expressing CD200 have suppressive effects on activation of myeloid cells including microglia by interacting with CD200R. In this study CD200-mRNA and protein were detected in the ischemic core as well as the contralateral region, and in isolated spherical Iba1+ macrophage-like cells. Similarly, it was reported that microglial CD200 interacts with CD200R to maintain microglia in an alternatively activated state, whereas interactions between neuronal CD200 and microglial CD200R keep microglial quiescent [51].
Interestingly, physical exercise and stem cell treatment have been found to boost the CD200-CD200R signaling in experimental stroke studies. Sun et al. [52] found that treadmill exercise significantly increased CD200 and CD200R levels in the ipsilateral hippocampus and cortex, and facilitated sensorimotor cognitive functional recovery after tMCAO. In addition, neural stem/progenitor cell proliferation, differentiation, and migration were enhanced in the ipsilateral subventricular and subgranular zones. Human placenta amniotic membrane-derived mesenchymal stem cells (AMSCs) transplanted into ischemic rat brains dramatically inhibited the expression of pro-inflammatory cytokines and increased CD200 expression in neurons, as compared with the sham-treated group [53]. The reason why stem cells promote CD200-CD200R signaling in stroke is not known, which might be delivered by the intrinsic protective mechanism conveyed by the new-born cells.
Alzheimer’s disease
Alzheimer’s disease (AD) is morphologically distinguished by the presence of senile plaques in the brain, composed mainly of different species of fibrillar amyloid-β (Aβ) produced by the cleavage of the Aβ precursor protein (APP), and neurofibrillary tangles composed of various isoforms of hyperphosphorylated and truncated tau proteins [54-57]. CD200 levels have been found decreased in AD brains mainly in hippocampus and inferior temporal gyrus [36,58]. Data obtained from brain tissues of AD patients showed that there is not only a deficit of CD200 but also of CD200R [36]. It has been established that aggregated Aβ plaques activates microglia to a pro-inflammatory state, augmenting phagocytic and lysosomal activity, and capable of producing a wide range of neurotoxic mediators [43,58,69,70]. Lyons et al. [59] examined the impact of CD200 deficiency on Aβ-induced changes in microglia and reported that the effect of Aβ was surprisingly reduced in microglia prepared from the CD200-deficient mice. It was previously shown that CD200FC administration reduced pro-inflammatory cytokine production in the presence of Aβ in microglia and attenuated the decrease in long-term potentiation (LTP) [60]. In summary, these data clearly showed that CD200-CD200R signaling is critical for Aβ removal and could be associated with other intrinsic molecules, and probably related to microglial phagocytosis.
Parkinson’s disease
In one study using Parkinson’s disease (PD) model created in rodents by 6-hydroxydopamine injection into substantia nigra (SN) neurons, blocking of CD200R resulted in significantly more severe PD-like movement dysfunction and a significantly greater loss of tyrosine hydroxylase-positive dopaminergic neurons in SN [37]. A 4-fold greater number of activated microglia was noticed in rats treated with CD200R blocking antibody compared with control animals, accompanied by 3 to 4-fold higher levels of TNF-α and IL-6 in the SN. Blocking CD200-CD200R interaction by anti-CD200R antibody resulting in microglial activation and intensified neurodegeneration in PD was also confirmed in monocyte-derived macrophages (MDMs) from 32 individuals with advanced PD [61], demonstrating an essential role of this CD200-CD200R axis in the regulation of PD.
Multiple sclerosis
Data of CD200 signaling in multiple sclerosis (MS) have been mainly from studies with Experimental Autoimmune Encephalomyelitis (EAE) which showed disruption of CD200-CD200R interaction due to neuronal damage leading to microglia activation [62-65]. According to Lyons et al. [59], both the severity and disease progression during the chronic phase of EAE in mice were ameliorated by CD200-Fc injection. Decreased CD200 mRNA expression has been reported in the rim and center of MS lesions, as well as in adjacent normal white matter compared with matched controls [66].
In both EAE and experimental autoimmune uveoretinitis (EAU) mice with CD200 deficiency, variations in peripheral immune cell infiltration were observed. The infiltration of monocyte-derived macrophages is increased after EAU or EAE induction [67-69], and blockade of CD200R leads to an increase in T-lymphocyte and monocyte-derived macrophage infiltration in both models [70-72]. Interestingly, the increase in these monocyte-derived macrophages and T-lymphocyte were reversed following administration of CD200-Fc [65,70]. The involvement of CD200-CD200R in immune cell infiltration could be related to the expression of CD200 in capillary endothelial cells as immune regulatory agents [73-75]. Another explanation is that T-cell infiltration may be favored by an enhanced microglial activation state, as already shown in CD200-deficient mice, due to the release of an increased number of chemokines, including CCL-2, CCL-5, or CXCL-10 [74]. These reports reinforced the rational that CD200-CD200R is an important immunoregulatory signaling in autoimmunity, and suggested that targeting CD200-CD200R can dampen neuroinflammation and demyelination, and halt the progression of MS.
CD200-CD200R signaling in other diseases
Tumorigenesis
A number of groups have confirmed that high expression of CD200 in tumor cells is an independent prognostic factor predicting reduced overall survival rate in a number of hematological malignancies including multiple myeloma, acute myeloid leukemia (AML), and chronic lymphocytic leukemia (CLL) [76-78]. How CD200 signaling suppresses anti-tumor responses remains elusive although the malignity of CD200-expressing tumors may suggest a direct inhibitory signal delivered by the tumor against the anti-tumor response [79]. Bisgin et al. [80] examined the immunohistochemical expression and localization of CD200 and CD200R1 in rectal cancer patients; the results showed a strikingly high level of CD200 in tumor cells and high CD200R1 expression in normal mucosal epithelium and stromal cells. Kawasaki et al. [81] hypothesized that cancer stem cells might be able to evade the immune system by generating a tolerogenic response facilitated by CD200. CD200 expression increases on a breast cancer cell line transplanted to immune competent mice in contrast to immune deficient mice [82], suggesting a CD200 mediated anti-inflammatory profile of CD200 positive cancer cells. In a word, CD200 signaling seems to benefit cancer by diminishing immune responses.
Allergy
Mast cells and basophils play a crucial role in allergic reactions in body tissues and blood respectively. It has been confirmed that CD200-CD200R interaction reduces their degranulation and attenuates the allergic inflammation [12,27]. Administration of intratracheal CD200 recombinant to experimental asthmatic rats was reported to inhibit airway hyper-responsiveness by local alterations of T cell response and cytokine secretion [83].
CD200-CD200R signaling as a therapeutic target
The therapeutic potential of CD200 has been explored both in vitro and in vivo. Studies using neuron-microglia co-cultures suggested that CD200-CD200R interaction may be one of the mechanisms by which IL-10 protects neurons from inflammatory damage caused by microglia-induced cytotoxicity, as IL-10 increased the expression of CD200 in neurons [58,84,85]. An up-regulatory effect of IL-4 on CD200 expression has also been reported [43,86]. IL-4-deficient mice showed low expression of CD200 in neurons, but IL-4 intracerebroventricular administration was able to reverse CD200 expression to normal levels [86]. Likewise, adeno associated viral gene-delivery of IL-4 in APP-PS1 mice restored CD200 expression in the hippocampus [84,87], and treatment of cultured microglia from aged rodents with IL-4 resulted in the production of CD200 [13,51]. These data indicate that it is feasible to manipulate CD200-CD200R signaling.
In AD brains, microglia are primarily classically activated but enhancing CD200R expression can promote alternative activation to confer neuroprotection. Up to date, it is not known yet how much of CD200 expression is sufficient to activate CD200R. In human brains the level of CD200R expression appears to be several orders of magnitude lower than that of CD200 [13,36]; therefore, boosting CD200R may be more effective especially in diseases where the CD200R levels remain low.
Cannabinoids participate in the control of brain immune responses as well as in the protection of the CNS against injury [88-92]. Treatments with the endocannabinoid compound N-arachidonoylethanolamine (AEA) induced the recovery of CD200 and CD200R gene expression that was reduced in the Theiler’s murine encephalomyelitis virus induced demyelinating disease (TMEV-IDD) model of MS [85]. This was accompanied by decreased inflammatory mediators and reduced microglial reactivity. Endocannabinoids may regulate CD200-CD200R axis through activating cannabinoid receptors expressed on immune cells including microglia, astrocytes, and neurons [91-94]. The endogenous ligand 2-arachidonoylglycerol (2-AG) binds and activates cannabinoid 2 (CB2) receptor [91,95]. Neurons and glial cells produce 2-AG [96-99] suggesting CB2 receptors and 2-AG could play a role in the regulation of the CD200-CD200R axis especially in diseases where CB2 receptors are more likely to be expressed by immune cells.
Studies of manipulation of the CD200-CD200R axis have tested the effect of anti-CD200 monoclonal antibodies, and also soluble CD200R or fragments of CD200 [39,71,100]. A therapeutic trial based on the use of CD200 blocking antibodies in chronic lymphocytic leukemia (CLL) patients with ClinicalTrials.gov Identifier NCT00648739 [101] has recently been conducted. Of the 23 patients with advanced CLL enrolled, 21 patients received > 1 treatment cycle with samalizumab, a novel recombinant humanized monoclonal antibody that targets CD200. Treatment produced dose-dependent decreases in CD200 expression on CLL cells and decreased turnover of circulating CD200+CD4+ T cells. This first-in-human investigation showed a good and safe treatment protocol that was associated with reduced tumor burden in a majority of patients [101], and support further development of samalizumab therapy for immune responses. Targeting CD200-CD200R signaling to modulate the immune response represents a promising therapeutic strategy for various inflammatory diseases. Table 1 summarizes commonly used animal models for CD200 signaling study in neuroinflammatory diseases.
Table 1.
Summary of experimental CD200-CD200R axis
| Disease | Animal model | Tissue | Function of CD200 signaling | Intervention of CD200-CD200R signaling | Ref. |
|---|---|---|---|---|---|
| Ischemic stroke | CD-1 mice | Cortex | Restored neural progenitor cell proliferation, differentiation, and migration. | Intracerebroventricular injection of recombinant CD200 protein after pMCAO. Decrease of Iba-1, IL-1β, TNF-α, and IL-10. | [50] |
| Sprague-Dawley rats | Ipsilateral hippocampus and cortex | Sequential treadmill exercise for 4 weeks, with different speed and running times. | [52] | ||
| Alzheimer’s | APP-PS1 mice | Hippocampus | Restored neural progenitor cell proliferation and differentiation in the subgranular and granular cell layers of dentate gyrus and reduce diffuse but not thioflavin-s+ plaques. | AAV2/1-CD200 bilateral injection into the CA1 region of hippocampus at 6 months of age. | [84] |
| AAV-IL-4 bilateral injection into mouse hippocampus at 3 months of age. | [87] | ||||
| Parkinson’s | Sprague-Dawley rats | Substantia nigra | Function loss due to depletion of dopaminergic neurons and decrease in tyrosine hydroxylase-immunoreactive neurons. | Injection into the right striatum with CD200R-blocking antibody, and then with 6-hydroxydopamine the next day injected unilaterally into the right ascending medial forebrain bundle. | [37] |
| EAE | C57BL/6 mice | Spinal cord | Attenuated the course of EAE and decreases axonal loss and demyelination. | Subcutaneous administration of CD200-Fc from onset of EAE to day 30. | [65] |
| GL261 glioma tumors | C57BL/6 mice | Brain | Negatively regulated immune response by activating CD200R on myeloid-derived suppressor cells promoting tumorigenesis. | Intradermal injection with CD200R antagonist peptide. | [105] |
Summary
The neurobiological roles of CD200-CD200R signaling in CNS disorders have not been well understood. Probably owing to the differences between rodents and humans in the expression of CD200 and CD200R, data from literature about the pathway have been controversial. The development of humanized transgenic mice that express the human genes under their native promoters have been reported [102-104], which appears to be promising in the research of the field. As most of the experimental studies have involved application of CD200 protein or CD200R-blocking antibodies, an approach that has been questioned for human therapy, the development of suitable gene vectors that can target at neural cells, or embryonic stem cells could be more relevant. The research of CD200-CD200R signaling in neuroinflammatory diseases, including ischemic stroke, is still in its infancy; further studies at both the cellular and molecular levels are warranted to better understand the inhibitory signaling.
Acknowledgements
This work was supported by funding from National Institutes of Health: R01 NS093042/NS108779 (Fudong Liu).
Disclosure of conflict of interest
None.
References
- 1.Elward K, Gasque P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 2.Hauwel M, Furon E, Canova C, Griffiths M, Neal J, Gasque P. Innate (inherent) control of brain infection, brain inflammation and brain repair: the role of microglia, astrocytes, “protective” glial stem cells and stromal ependymal cells. Brain Res Brain Res Rev. 2005;48:220–233. doi: 10.1016/j.brainresrev.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Barclay AN, Ward HA. Purification and chemical characterisation of membrane glycoproteins from rat thymocytes and brain, recognised by monoclonal antibody MRC OX 2. Eur J Biochem. 1982;129:447–458. doi: 10.1111/j.1432-1033.1982.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 4.McMaster WR, Williams AF. Monoclonal antibodies to Ia antigens from rat thymus: cross reactions with mouse and human and use in purification of rat Ia glycoproteins. Immunol Rev. 1979;47:117–137. doi: 10.1111/j.1600-065x.1979.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 5.Barclay AN, Clark MJ, McCaughan GW. Neuronal/lymphoid membrane glycoprotein MRC OX-2 is a member of the immunoglobulin superfamily with a light-chain-like structure. Biochem Soc Symp. 1986;51:149–157. [PubMed] [Google Scholar]
- 6.Clark MJ, Gagnon J, Williams AF, Barclay AN. MRC OX-2 antigen: a lymphoid/neuronal membrane glycoprotein with a structure like a single immunoglobulin light chain. EMBO J. 1985;4:113–118. doi: 10.1002/j.1460-2075.1985.tb02324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 8.Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102:173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slepko N, Levi G. Progressive activation of adult microglial cells in vitro. Glia. 1996;16:241–246. doi: 10.1002/(SICI)1098-1136(199603)16:3<241::AID-GLIA6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Manich G, Recasens M, Valente T, Almolda B, Gonzalez B, Castellano B. Role of the CD200-CD200R axis during homeostasis and neuroinflammation. Neuroscience. 2019;405:118–136. doi: 10.1016/j.neuroscience.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Takahashi H, Imai Y, Tanaka J. Expression of CD200 by macrophage-like cells in ischemic core of rat brain after transient middle cerebral artery occlusion. Neurosci Lett. 2007;418:44–48. doi: 10.1016/j.neulet.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Holmannova D, Kolackova M, Kondelkova K, Kunes P, Krejsek J, Ctirad A. CD200/CD200R paired potent inhibitory molecules regulating immune and inflammatory responses; Part II: CD200/CD200R potential clinical applications. Acta Medica (Hradec Kralove) 2012;55:59–65. doi: 10.14712/18059694.2015.56. [DOI] [PubMed] [Google Scholar]
- 13.Walker DG, Lue LF. Understanding the neurobiology of CD200 and the CD200 receptor: a therapeutic target for controlling inflammation in human brains? Future Neurol. 2013;8 doi: 10.2217/fnl.13.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihrshahi R, Brown MH. Downstream of tyrosine kinase 1 and 2 play opposing roles in CD200 receptor signaling. J Immunol. 2010;185:7216–7222. doi: 10.4049/jimmunol.1002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rygiel TP, Meyaard L. CD200R signaling in tumor tolerance and inflammation: a tricky balance. Curr Opin Immunol. 2012;24:233–238. doi: 10.1016/j.coi.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH, Barclay AN. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 17.Hatherley D, Cherwinski HM, Moshref M, Barclay AN. Recombinant CD200 protein does not bind activating proteins closely related to CD200 receptor. J Immunol. 2005;175:2469–2474. doi: 10.4049/jimmunol.175.4.2469. [DOI] [PubMed] [Google Scholar]
- 18.Hatherley D, Barclay AN. The CD200 and CD200 receptor cell surface proteins interact through their N-terminal immunoglobulin-like domains. Eur J Immunol. 2004;34:1688–1694. doi: 10.1002/eji.200425080. [DOI] [PubMed] [Google Scholar]
- 19.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith MJ, Hardy WR, Murphy JM, Jones N, Pawson T. Screening for PTB domain binding partners and ligand specificity using proteome-derived NPXY peptide arrays. Mol Cell Biol. 2006;26:8461–8474. doi: 10.1128/MCB.01491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 22.Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 23.Songyang Z, Yamanashi Y, Liu D, Baltimore D. Domain-dependent function of the rasGAP-binding protein p62Dok in cell signaling. J Biol Chem. 2001;276:2459–2465. doi: 10.1074/jbc.M005504200. [DOI] [PubMed] [Google Scholar]
- 24.Lock P, Casagranda F, Dunn AR. Independent SH2-binding sites mediate interaction of Dok-related protein with RasGTPase-activating protein and Nck. J Biol Chem. 1999;274:22775–22784. doi: 10.1074/jbc.274.32.22775. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarty K, Heumann R. Prospective of Ras signaling in stem cells. Biol Chem. 2008;389:791–798. doi: 10.1515/BC.2008.104. [DOI] [PubMed] [Google Scholar]
- 26.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J Immunol. 2004;173:6786–6793. doi: 10.4049/jimmunol.173.11.6786. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Phillips JH. Identification of tyrosine residues crucial for CD200R-mediated inhibition of mast cell activation. J Leukoc Biol. 2006;79:363–368. doi: 10.1189/jlb.0705398. [DOI] [PubMed] [Google Scholar]
- 29.Mihrshahi R, Barclay AN, Brown MH. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J Immunol. 2009;183:4879–4886. doi: 10.4049/jimmunol.0901531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng KY, Yin T, Machida K, Wu YI, Mayer BJ. Phosphorylation of Dok1 by Abl family kinases inhibits CrkI transforming activity. Oncogene. 2015;34:2650–2659. doi: 10.1038/onc.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89:687–699. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- 32.Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, Mills KH, Lynch MA. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem. 2005;280:9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- 34.Rijkers ES, de Ruiter T, Baridi A, Veninga H, Hoek RM, Meyaard L. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol Immunol. 2008;45:1126–1135. doi: 10.1016/j.molimm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol. 2009;68:159–167. doi: 10.1097/NEN.0b013e3181964113. [DOI] [PubMed] [Google Scholar]
- 36.Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, Zhang YJ, Ding JQ, Chen SD. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J Neuroinflammation. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank MG, Fonken LK, Annis JL, Watkins LR, Maier SF. Stress disinhibits microglia via down-regulation of CD200R: a mechanism of neuroinflammatory priming. Brain Behav Immun. 2018;69:62–73. doi: 10.1016/j.bbi.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritzel RM, Al Mamun A, Crapser J, Verma R, Patel AR, Knight BE, Harris N, Mancini N, Roy-O’Reilly M, Ganesh BP, Liu F, McCullough LD. CD200-CD200R1 inhibitory signaling prevents spontaneous bacterial infection and promotes resolution of neuroinflammation and recovery after stroke. J Neuroinflammation. 2019;16:40. doi: 10.1186/s12974-019-1426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pankratova S, Bjornsdottir H, Christensen C, Zhang L, Li S, Dmytriyeva O, Bock E, Berezin V. Immunomodulator CD200 promotes neurotrophic activity by interacting with and activating the fibroblast growth factor receptor. Mol Neurobiol. 2016;53:584–594. doi: 10.1007/s12035-014-9037-6. [DOI] [PubMed] [Google Scholar]
- 41.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- 43.Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Executive summary: heart disease and stroke statistics--2016 update: a report from the American heart association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 45.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sierra A, Navascues J, Cuadros MA, Calvente R, Martin-Oliva D, Ferrer-Martin RM, Martin-Estebane M, Carrasco MC, Marin-Teva JL. Expression of inducible nitric oxide synthase (iNOS) in microglia of the developing quail retina. PLoS One. 2014;9:e106048. doi: 10.1371/journal.pone.0106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F. Regulation of microglial activation in stroke. Acta Pharmacol Sin. 2017;38:445–458. doi: 10.1038/aps.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Ye H, Cai X, Sun W, He B, Yang Z, Xu P. Bone marrow-mesenchymal stem cells modulate microglial activation in the peri-infarct area in rats during the acute phase of stroke. Brain Res Bull. 2019;153:324–333. doi: 10.1016/j.brainresbull.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Zhang XJ, Zhang C, Chen R, Li L, He J, Xie Y, Chen Y. Loss of neuronal CD200 contributed to microglial activation after acute cerebral ischemia in mice. Neurosci Lett. 2018;678:48–54. doi: 10.1016/j.neulet.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Yi MH, Zhang E, Kang JW, Shin YN, Byun JY, Oh SH, Seo JH, Lee YH, Kim DW. Expression of CD200 in alternative activation of microglia following an excitotoxic lesion in the mouse hippocampus. Brain Res. 2012;1481:90–96. doi: 10.1016/j.brainres.2012.08.053. [DOI] [PubMed] [Google Scholar]
- 52.Sun H, Li A, Hou T, Tao X, Chen M, Wu C, Chen S, Zhu L, Liao H. Neurogenesis promoted by the CD200/CD200R signaling pathway following treadmill exercise enhances post-stroke functional recovery in rats. Brain Behav Immun. 2019;82:354–371. doi: 10.1016/j.bbi.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Kong T, Park JM, Jang JH, Kim CY, Bae SH, Choi Y, Jeong YH, Kim C, Chang SW, Kim J, Moon J. Immunomodulatory effect of CD200-positive human placenta-derived stem cells in the early phase of stroke. Exp Mol Med. 2018;50:e425. doi: 10.1038/emm.2017.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aso E, Ferrer I. CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci. 2016;10:243. doi: 10.3389/fnins.2016.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 56.Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu YT, Beiser AS, Breteler MMB, Fratiglioni L, Helmer C, Hendrie HC, Honda H, Ikram MA, Langa KM, Lobo A, Matthews FE, Ohara T, Peres K, Qiu C, Seshadri S, Sjolund BM, Skoog I, Brayne C. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327–339. doi: 10.1038/nrneurol.2017.63. [DOI] [PubMed] [Google Scholar]
- 58.Hernangómez M, Carrillo-Salinas FJ, Mecha M, Correa F, Mestre L, Loría F, Feliú A, Docagne F, Guaza C. Brain innate immunity in the regulation of neuroinflammation: therapeutic strategies by modulating CD200-CD200R interaction involve the cannabinoid system. Curr Pharm Des. 2014;20:4707–4722. doi: 10.2174/1381612820666140130202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyons A, Minogue AM, Jones RS, Fitzpatrick O, Noonan J, Campbell VA, Lynch MA. Analysis of the impact of CD200 on phagocytosis. Mol Neurobiol. 2017;54:5730–5739. doi: 10.1007/s12035-016-0223-6. [DOI] [PubMed] [Google Scholar]
- 60.Lyons A, Downer EJ, Costello DA, Murphy N, Lynch MA. Dok2 mediates the CD200Fc attenuation of Abeta-induced changes in glia. J Neuroinflammation. 2012;9:107. doi: 10.1186/1742-2094-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo XG, Zhang JJ, Zhang CD, Liu R, Zheng L, Wang XJ, Chen SD, Ding JQ. Altered regulation of CD200 receptor in monocyte-derived macrophages from individuals with Parkinson’s disease. Neurochem Res. 2010;35:540–547. doi: 10.1007/s11064-009-0094-6. [DOI] [PubMed] [Google Scholar]
- 62.Sospedra M, Martin R. Immunology of multiple sclerosis. Semin Neurol. 2016;36:115–127. doi: 10.1055/s-0036-1579739. [DOI] [PubMed] [Google Scholar]
- 63.Marik C, Felts PA, Bauer J, Lassmann H, Smith KJ. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130:2800–2815. doi: 10.1093/brain/awm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bogie JF, Stinissen P, Hellings N, Hendriks JJ. Myelin-phagocytosing macrophages modulate autoreactive T cell proliferation. J Neuroinflammation. 2011;8:85. doi: 10.1186/1742-2094-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Bando Y, Vargas-Lowy D, Elyaman W, Khoury SJ, Huang T, Reif K, Chitnis T. CD200R1 agonist attenuates mechanisms of chronic disease in a murine model of multiple sclerosis. J Neurosci. 2010;30:2025–2038. doi: 10.1523/JNEUROSCI.4272-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koning N, Bo L, Hoek RM, Huitinga I. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann Neurol. 2007;62:504–514. doi: 10.1002/ana.21220. [DOI] [PubMed] [Google Scholar]
- 67.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 68.Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. 2002;161:1669–1677. doi: 10.1016/S0002-9440(10)64444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Copland DA, Calder CJ, Raveney BJ, Nicholson LB, Phillips J, Cherwinski H, Jenmalm M, Sedgwick JD, Dick AD. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am J Pathol. 2007;171:580–588. doi: 10.2353/ajpath.2007.070272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT, Khoury SJ. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol. 2007;170:1695–1712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meuth SG, Simon OJ, Grimm A, Melzer N, Herrmann AM, Spitzer P, Landgraf P, Wiendl H. CNS inflammation and neuronal degeneration is aggravated by impaired CD200-CD200R-mediated macrophage silencing. J Neuroimmunol. 2008;194:62–69. doi: 10.1016/j.jneuroim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 72.Banerjee D, Dick AD. Blocking CD200-CD200 receptor axis augments NOS-2 expression and aggravates experimental autoimmune uveoretinitis in Lewis rats. Ocul Immunol Inflamm. 2004;12:115–125. doi: 10.1080/09273940490895326. [DOI] [PubMed] [Google Scholar]
- 73.Ko YC, Chien HF, Jiang-Shieh YF, Chang CY, Pai MH, Huang JP, Chen HM, Wu CH. Endothelial CD200 is heterogeneously distributed, regulated and involved in immune cell-endothelium interactions. J Anat. 2009;214:183–195. doi: 10.1111/j.1469-7580.2008.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun. 2013;34:86–97. doi: 10.1016/j.bbi.2013.07.174. [DOI] [PubMed] [Google Scholar]
- 75.Cohen M, Ben-Yehuda H, Porat Z, Raposo C, Gordon S, Schwartz M. Newly formed endothelial cells regulate myeloid cell activity following spinal cord injury via expression of CD200 ligand. J Neurosci. 2017;37:972–985. doi: 10.1523/JNEUROSCI.2199-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, Darley RL. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21:566–568. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- 77.McWhirter JR, Kretz-Rommel A, Saven A, Maruyama T, Potter KN, Mockridge CI, Ravey EP, Qin F, Bowdish KS. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Natl Acad Sci U S A. 2006;103:1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, Mohler T, De Vos J, Rossi JF, Goldschmidt H, Klein B. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108:4194–4197. doi: 10.1182/blood-2006-06-029355. [DOI] [PubMed] [Google Scholar]
- 79.Coles SJ, Wang EC, Man S, Hills RK, Burnett AK, Tonks A, Darley RL. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25:792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bisgin A, Meng WJ, Adell G, Sun XF. Interaction of CD200 overexpression on tumor cells with CD200R1 overexpression on stromal cells: an escape from the host immune response in rectal cancer patients. J Oncol. 2019;2019:5689464. doi: 10.1155/2019/5689464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawasaki BT, Farrar WL. Cancer stem cells, CD200 and immunoevasion. Trends Immunol. 2008;29:464–468. doi: 10.1016/j.it.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Gorczynski RM, Chen Z, Diao J, Khatri I, Wong K, Yu K, Behnke J. Breast cancer cell CD200 expression regulates immune response to EMT6 tumor cells in mice. Breast Cancer Res Treat. 2010;123:405–415. doi: 10.1007/s10549-009-0667-8. [DOI] [PubMed] [Google Scholar]
- 83.Lauzon-Joset JF, Langlois A, Lai LJ, Santerre K, Lee-Gosselin A, Bosse Y, Marsolais D, Bissonnette EY. Lung CD200 receptor activation abrogates airway hyperresponsiveness in experimental asthma. Am J Respir Cell Mol Biol. 2015;53:276–284. doi: 10.1165/rcmb.2014-0229OC. [DOI] [PubMed] [Google Scholar]
- 84.Varnum MM, Kiyota T, Ingraham KL, Ikezu S, Ikezu T. The anti-inflammatory glycoprotein, CD200, restores neurogenesis and enhances amyloid phagocytosis in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2015;36:2995–3007. doi: 10.1016/j.neurobiolaging.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hernangomez M, Mestre L, Correa FG, Loria F, Mecha M, Inigo PM, Docagne F, Williams RO, Borrell J, Guaza C. CD200-CD200R1 interaction contributes to neuroprotective effects of anandamide on experimentally induced inflammation. Glia. 2012;60:1437–1450. doi: 10.1002/glia.22366. [DOI] [PubMed] [Google Scholar]
- 86.Lyons A, McQuillan K, Deighan BF, O’Reilly JA, Downer EJ, Murphy AC, Watson M, Piazza A, O’Connell F, Griffin R, Mills KH, Lynch MA. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav Immun. 2009;23:1020–1027. doi: 10.1016/j.bbi.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 87.Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24:3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kitchigina VF. [Endogenous cannabinoid system of the brain as the target for influences at neurodegenerate diseases] . Zh Vyssh Nerv Deiat Im I P Pavlova. 2016;66:387–413. [PubMed] [Google Scholar]
- 89.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 90.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 91.Quartu M, Poddighe L, Melis T, Serra MP, Boi M, Lisai S, Carta G, Murru E, Muredda L, Collu M, Banni S. Involvement of the endocannabinoid system in the physiological response to transient common carotid artery occlusion and reperfusion. Lipids Health Dis. 2017;16:14. doi: 10.1186/s12944-016-0389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benito C, Romero JP, Tolon RM, Clemente D, Docagne F, Hillard CJ, Guaza C, Romero J. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. 2007;27:2396–2402. doi: 10.1523/JNEUROSCI.4814-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brusco A, Tagliaferro P, Saez T, Onaivi ES. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008;62:944–949. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- 95.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 96.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 97.Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- 98.Walter L, Stella N. Endothelin-1 increases 2-arachidonoyl glycerol (2-AG) production in astrocytes. Glia. 2003;44:85–90. doi: 10.1002/glia.10270. [DOI] [PubMed] [Google Scholar]
- 99.Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- 100.Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahadevan D, Lanasa MC, Farber C, Pandey M, Whelden M, Faas SJ, Ulery T, Kukreja A, Li L, Bedrosian CL, Zhang X, Heffner LT. Phase I study of samalizumab in chronic lymphocytic leukemia and multiple myeloma: blockade of the immune checkpoint CD200. J Immunother Cancer. 2019;7:227. doi: 10.1186/s40425-019-0710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheung C, Gonzalez FJ. Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J Pharmacol Exp Ther. 2008;327:288–299. doi: 10.1124/jpet.108.141242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hosur V, Low BE, Avery C, Shultz LD, Wiles MV. Development of humanized mice in the age of genome editing. J Cell Biochem. 2017;118:3043–3048. doi: 10.1002/jcb.26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moertel CL, Xia J, LaRue R, Waldron NN, Andersen BM, Prins RM, Okada H, Donson AM, Foreman NK, Hunt MA, Pennell CA, Olin MR. CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy. J Immunother Cancer. 2014;2:46. doi: 10.1186/s40425-014-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


