Abstract
Johnius taiwanensis is a newly described species from the Family Sciaenidae (Perciformes). The species is commonly found in shallow coastal waters along both sides of the Taiwan Strait, on the west sides of Zhejiang, Fujian, Guangdong and Hong Kong and east side of Taiwan, and has been misidentified for decades. We studied the reproductive biology of J. taiwanensis from Fujian coastal waters, southern China, using gonadosomatic index (GSI) and gonad histology analyses. Monthly sampling from July 2016 to October 2017 was conducted and a total of 638 specimens were collected, ranging from 7.3 to 19.0 cm standard length (SL). Gonad histology suggested that the spawning activity of J. taiwanensis females and males lasted from April to October, and the peak spawning months for females was July to September. Mature females and males were 12.5 and 11.8 cm SL, respectively, while the estimated sizes at 50% maturity were 12.0 cm and 10.9 cm SL, respectively. Vitellogenic stage oocytes (O3) and post-ovulatory follicles (POF) or hydrated oocytes (HO) were observed, and POF and O3 in ovaries indicated that J. taiwanensis spawns multiple times each spawning season. HO or both HO and POF were observed in ovaries collected from one same location in May 2017 and August 2016.
Keywords: Gonad histology, Gonadosomatic index, Johnius taiwanensis, Sciaenidae, Spawning season
BACKGROUND
The Family Sciaenidae consists of approximately 300 species from 70 genera throughout Atlantic, Indian and Pacific Oceans, in coastal marine, brackish and freshwater; many associate with muddy or sandy bottoms (Chao et al. 2015; Lin et al. 2019). Sciaenids are important food fishes with global capture fishery production of approximate 1.7 million tons annually over the past decade (http://www.fao.org/fishery/statistics/global-capture-production/en, accessed on 23 August 2019). Some sciaenids form large aggregations for spawning, feeding and over-wintering, which makes them vulnerable to overfishing (Liu and Sadovy de Mitcheson 2008). In recent years, small- and medium-sized croakers enter fisheries and become predominate in Chinese and adjacent waters, e.g., the genera Collichthys, Johnius and Pennahia (Yamaguchi et al. 2006; Li 2010; Tuuli et al. 2011; Wang et al. 2011; Xie et al. 2012).
The genus Johnius is endemic to Indo-West Pacific, with about 35 species, mainly occurring in shallow tropical and subtropical coastal waters, and Johnius species usually have small-to medium-sized bodies and a small mouth, sub-terminal to inferior positioned (Trewavas 1977; Sasaki 1989 2001). Eight Johnius species are confirmed in Chinese waters, including the most recently described species, J. taiwanensis (Chao et al. 2019). Johnius taiwanensis inhabits shallow coastal waters of southeast China, along both sides of the Taiwan Strait, on the west sides of Zhejiang, Fujian, Guangdong and Hong Kong and east side of Taiwan (Chao et al. 2019) (Fig. 1). It is a common species but has been misidentified for years as J. belangerii, J. macrorhynus, J. sina or Wak sina (Chu et al. 1963; Yu and Shen 1987; Shen 1993; Lin et al. 2007). Johnius taiwanensis can be caught by trawler and hook-and-line almost year-round and is an important local food fish.
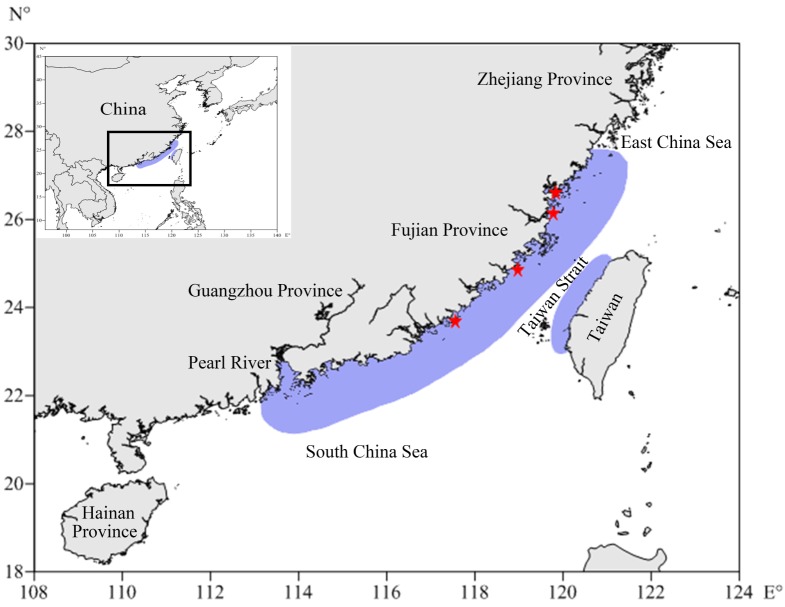
Fig. 1.
Distribution of Johnius taiwanensis (light purple areas) in Chinese waters. Red stars: the sampling sites from north to south, Ningde, Fuzhou, Quanzhou and Zhangzhou in Fujian waters.
As a newly described species, no studies have been conducted on the biology or ecology of J. taiwanensis. The present study examines the reproductive cycle of J. taiwanensis in Fujian waters, southern China, using the gonadosomatic index (GSI) and gonad histology analyses, to evaluate its spawning seasonality and sizes at maturity, and to discuss the results in the context of fisheries.
MATERIALS AND METHODS
Fish sampling
Johnius taiwanensis was collected by hook-and-line in Zhangzhou waters (117°33'50"E, 23°41'20"N), and by trawler in Quanzhou (118°57'42"E, 24°51'10"N), Fuzhou (119°46'12"E, 26°07'16"N) and Ningde (119°50'25"E, 26°36'50"N) waters, Fujian Province, southern China (Fig. 1). Specimens were randomly collected monthly from July 2016 to October 2017 with a continuous. Measurements of total length (TL 0.1 cm), standard length (SL 0.1 cm), whole body weight (BW 0.1 g) and gonad weight (GW 0.1 g) were conducted in the laboratory.
Gonad histology
Middle portions of paired gonad lobes from males and females were fixed in Dietrich’s fixative for at least one week. Gonad tissue was transferred to 70% alcohol prior to histological procedures. The gonad tissue was dehydrated in a series of alcohols (from 75 to 100%), cleared in toluene, and embedded in paraffin wax. Gonad tissue were sectioned at 6-7 μm, stained with haematoxylin and eosin, and mounted on slides.
Sexual maturation stages
Gonad sections were examined under microscopy. Maturity stages of ovaries were determined based on the most advanced oocytes, and the occurrence of hydrated oocytes (HO), post-ovulatory follicles (POF) and/or atretic oocytes. The relative proportion of primary and secondary spermatocytes (1SC/2SC) and spermatids (ST) in testes, and the appearance of sperm (SP) in sperm duct were used to determine the maturity stages of testes. Each gonad was allocated to one of the five maturity stages: immature/resting female (F1) and immature/resting male (M1), developing female (F2) and developing male (M2), maturing female (F3) and maturing male (M3), ripe female (F4) and ripe male (M4), and spent female (F5) and spent male (M5) (Grier 1981; Wallace and Selman 1981; Yamaguchi et al. 2006; Tuuli et al. 2011) (Table 1).
Table 1.
Descriptions of sexual maturation stages for females and males of Johnius taiwanensis
| Sexual maturation stages | Gonadal characters |
| Females | |
| Immature/resting (F1) | The most advanced oocytes are primary growth stage oocytes (O1). O1 closely packed and predominate. |
| Developing (F2) | The most advanced oocytes are at cortical alveolar stage oocytes (O2), together with O1. |
| Maturing (F3) | The most advanced oocytes are at vitellogenic stage oocytes (O3), but prior to the nucleus migratory stage. Zona radiata is thicker than those of O1 and O2. Yolk globules begin to fuse. |
| Ripe (F4) | The most advanced oocytes are O3 with the nucleus migratory or a single yolk mass originated from the yolk globules. Hydrated oocytes (HO) or post-ovulatory follicles (POF) may be present in some ovaries. |
| Spent (F5) | O1 dominate together with the occurrence of atretic O3 (AO3). |
| Males | |
| Immature/resting (M1) | Only spermatogonia (SG), primary and secondary spermatocytes (1SC/2SC) are present. No sperm in sperm duct. |
| Developing (M2) | Large amount of 1SC/2SC with the appearance of spermatids (ST). No sperm in sperm duct. |
| Maturing (M3) | Large amount of ST at the peripheral and central tubules. Sperm duct has sperm, but not full. |
| Ripe (M4) | Large amount of SP at the central tubules. Sperm duct is full of sperm with large amount of ST. |
| Spent (M5) | The lumen of tubules and sperm duct are empty or with residual sperms. SG and 1SC/2SC can be observed at the peripheral tubules. |
Spawning seasonality
The spawning months were determined using both the average monthly GSI and gonad histology analyses. The GSI was calculated using the formula: GSI (%) = GW /(BW – GW) × 100. The GSI is an easy, cheap and quick method to evaluate the spawning seasonality by identifying the peak month(s) on GSI value. Evaluating spawning seasonality through gonad histological examination gives a more detailed description of gonad developmental stages and sexual maturation stages with a time consume.
Size at maturity
The minimum body sizes for female and male maturity were estimated based on GSI data and gonadal histological results. The GSI values of mature females (F3, F4 and F5) and mature males (M3, M4 and M5), determined by gonadal histology, were plotted against their body sizes (SL); the SL with the largest and most persistent increase in GSI% was considered to be the minimum sizes for female and male maturity.
To determine the body sizes at 50% sexual maturation for females and males, all individuals collected over the spawning months were assigned to body size classes (SL) of 1.0 cm, and the percentage of mature females and males in each size class were calculated. A maturity curve was plotted against SL classes to estimate the size at 50% sexual maturation.
Statistical analysis
Differences in body sizes of females and males were analyzed by statistical significance, set to < 0.05.
RESULTS
Biological parameters
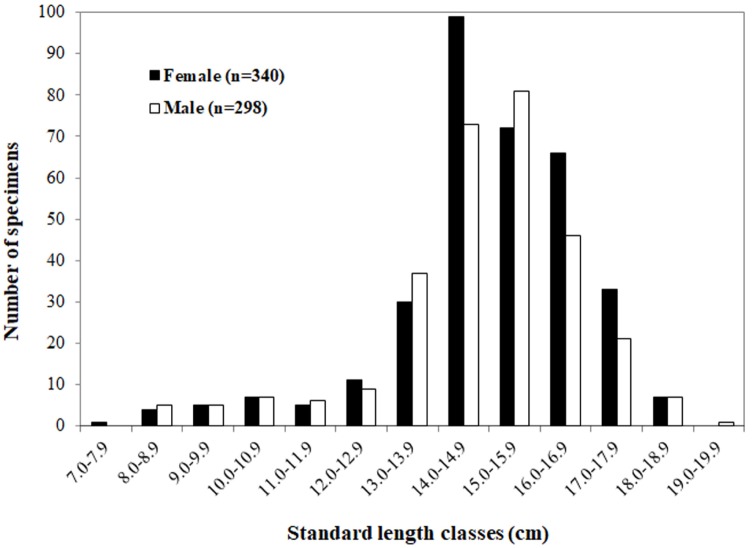
A total of 638 individuals were collected in July 2016-October 2017; at least 30 individuals for most of the months. Body sizes ranged from 7.3 to 18.5 cm SL (14.9 ± 1.9, mean ± S.D., n = 340) for females and from 8.0 to 19.0 cm SL (14.8 ± 1.9, n = 298) for males; there was no significant difference in SL between females and males (p = 0.313) (Fig. 2). Sex ratio of female: male showed monthly variation between 0.69:1.00 in September and 2.50:1.00 in June, but there was no significant difference (p = 0.569). The relationship of TL (cm) to SL (cm) and BW (g) to SL (cm) were: TL = 1.1366 × SL + 0.9558 (R2 = 0.9432, n = 638) (Fig. 3a) and BW = 0.02 × SL3.0198 (R2 = 0.9533, n = 638) (Fig. 3b), respectively.
Fig. 2.
Body size (standard length in cm) distributions for females (n = 340) and males (n = 298) of Johnius taiwanensis collected between July 2016 to October 2017 in Fujian waters, China.
Fig. 3.
Relationships of (a) total length (TL in cm) to standard length (SL in cm) and (b) body weight (BW in g) to standard length (SL in cm) of Johnius taiwanensis collected between July 2016 and October 2017 in Fujian waters, China.
Sexual maturation stages
All five sexual maturation stages for females were observed: immature/resting (F1), developing (F2), maturing (F3), ripe (F4) and spent (F5) (Table 1; Fig. 4). In males, developing (M2), maturing (M3), ripe (M4) and spent (M5) males were determined; no immature/resting males (M1) were found in the present study (Table 1; Fig. 5).
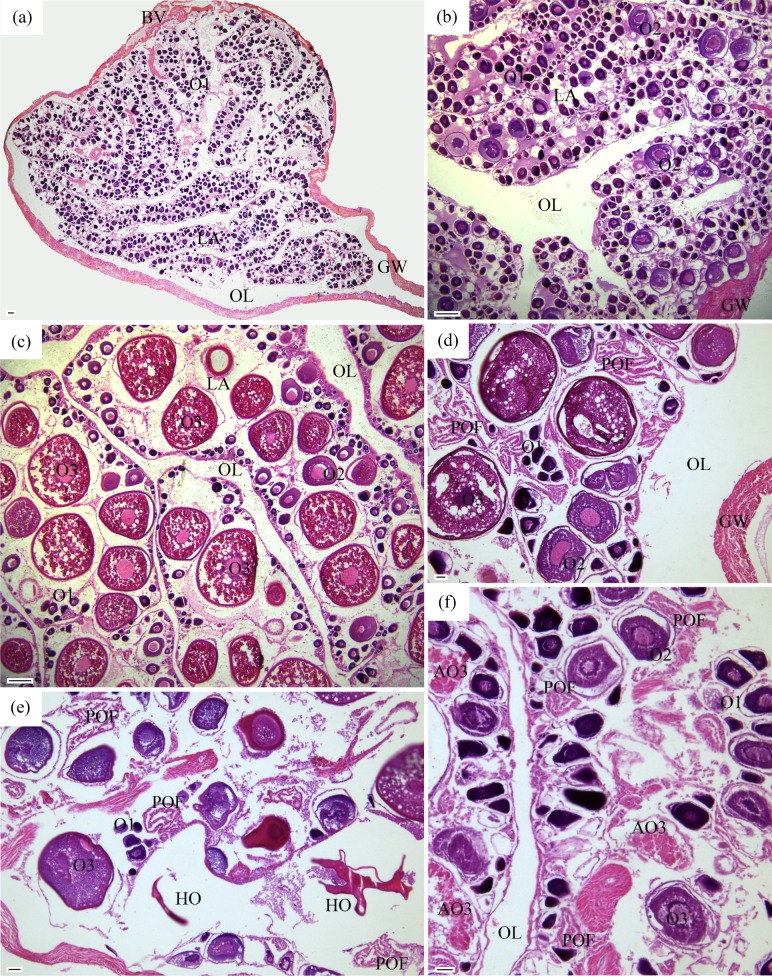
Fig. 4.
Sexual maturation stages in females of Johnius taiwanensis. (a) Immature/resting (F1) (17.2 cm SL, November 2016); (b) developing (F2) (15.2 cm SL, April 2017); (c) maturing (F3) (16.6 cm SL, September 2016); (d) ripe (F4) (16.8 cm SL, July 2016); (e) ripe (F4) (16.5 cm SL, August 2016); (f) spent (F5) (14.2 cm SL, July 2016). AO3, atretic vitellogenic stage oocyte; BV, blood vessels; GW, gonadal wall; HO, hydrated oocyte; LA, lamellae; O1, primary growth stage oocyte; O2, cortical-alveolar stage oocyte; O3, vitellogenic stage oocyte; OL, ovarian lumen; POF, post-ovulatory follicle. Scale bars: a-c = 200 μm; d-f = 50 μm.
Fig. 5.
Sexual maturation stages in males of Johnius taiwanensis. (a) Developing (M2) (13.1 cm SL, July 2016); (b) maturing (M3) (14.4 cm SL, September 2017); (c) ripe (M4) (17.8 cm SL, September 2017); (d) spent (M5) (15.1 cm SL, November 2016). GW, gonadal wall; SC, spermatocytes; SD, sperm duct; SP, sperm; ST, spermatids. Scale bars = 50 μm.
Ovaries with evidence of HO or both HO and POF were found in May (2017) and August (2016), collected in Dongshan Bay (117°33'50"E, 23°41'20"N) of Zhangzhou waters, indicating very recent spawning activity. Ovaries had O3 and POF or O3, HO and POF, confirming that J. taiwanensis is a multiple spawner.
Spawning seasonality
The monthly average GSI% of females and males showed similar and distinct seasonal variation patterns, with females generally having higher GSI values than males (Fig. 6). In females, the average GSI peaks were in July and August, with GSI over 5.5%, indicating that these were the peak spawning months for females. From November to April, the average GSI% of females were low, less than 1.5%. In males, the average GSI peaks were in May and August with GSI over 1.3%, indicating that this was the peak spawning season for males. Similar to females, the average GSI% of males were low from November to next April, less than 0.6%.
Fig. 6.
Monthly gonadosomatic index (GSI%, mean ± S.D.) in females and males of Johnius taiwanensis collected between July 2016 and October 2017.
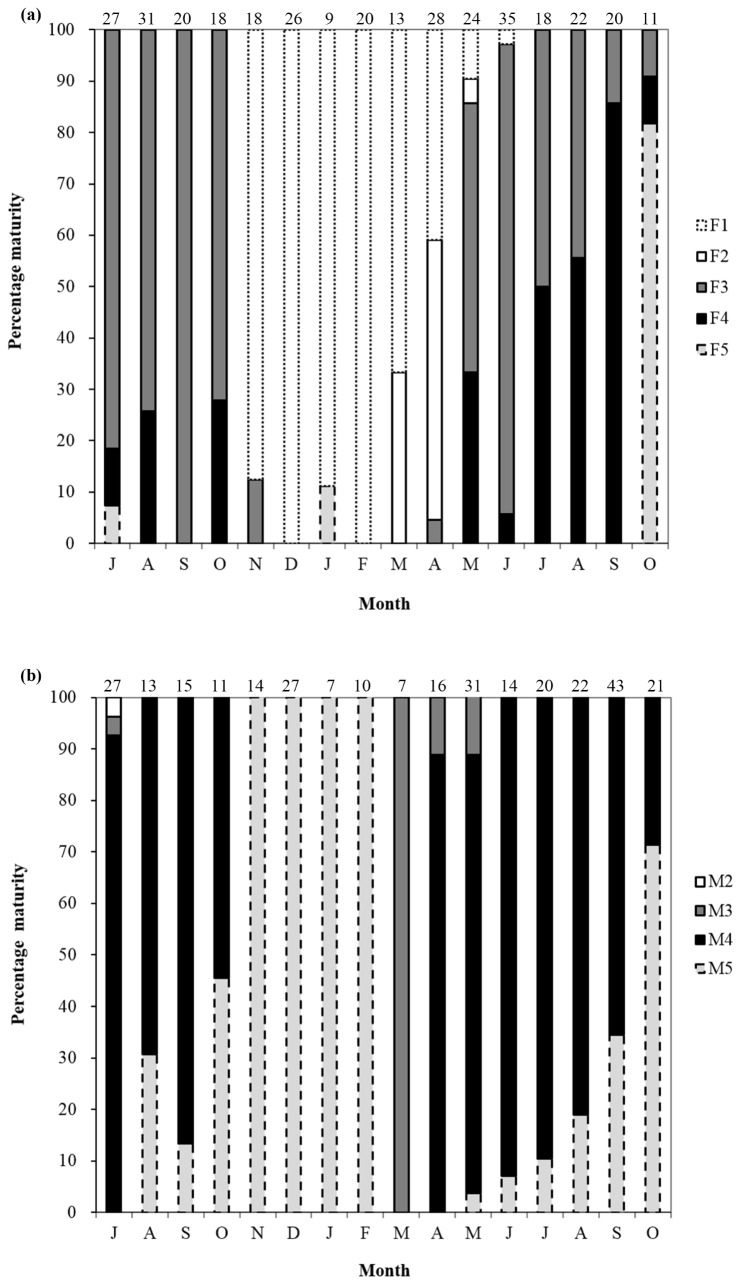
Based on gonadal histology, the spawning seasons for females and males were revealed to last longer than those determined by GSI values above. In females, F1 and F2 were found from November to June, dominating from November to next April (Fig. 4a b; Fig. 7a). The proportion of F2 increased in March and April, followed by the appearance of F3 in April and the increase of F3 in May-August, indicating that this was the ovary development phase of female sexual maturation (Fig. 4c; Fig. 7a). The proportions of F3 and F4 remained high between May and October, indicating the female spawning season in May−October. In July−September, the proportions of F4 were more than 50%, indicating the peak spawning season (Fig. 4d e; Fig. 7a). The proportion of F5 and F1 were high after October, indicating the end of the spawning season (Fig. 4f; Fig. 7a).
Fig. 7.
Monthly female (a) and male (b) sexual maturation stages of Johnius taiwanensis based on gonad histology. F1, immature/resting female; F2, developing female; F3, maturing female; F4, ripe female; F5, spent female; M2, developing male; M3, maturing male; M4, ripe male; M5, spent male. The criteria for sexual maturation stages were described in tables 1 and 2. Numbers on the top of bars represent sample sizes.
M1 was not found in males. M2 was only found in July (Fig. 5a; Fig. 7b). M3 was absolutely dominant in March (100%), and decreased with the dramatically increase of M4 proportions in April and May (Fig. 5b c; Fig. 7b). M4 remained high between April and October, indicating that the male spawning season was April−October, longer than that of females. High proportions of M4 (> 50%) in April-September indicated the peak spawning season. M5 were dominant from October to next February, indicating the end of the spawning season (Fig. 5d; Fig. 7b).
Size at maturity
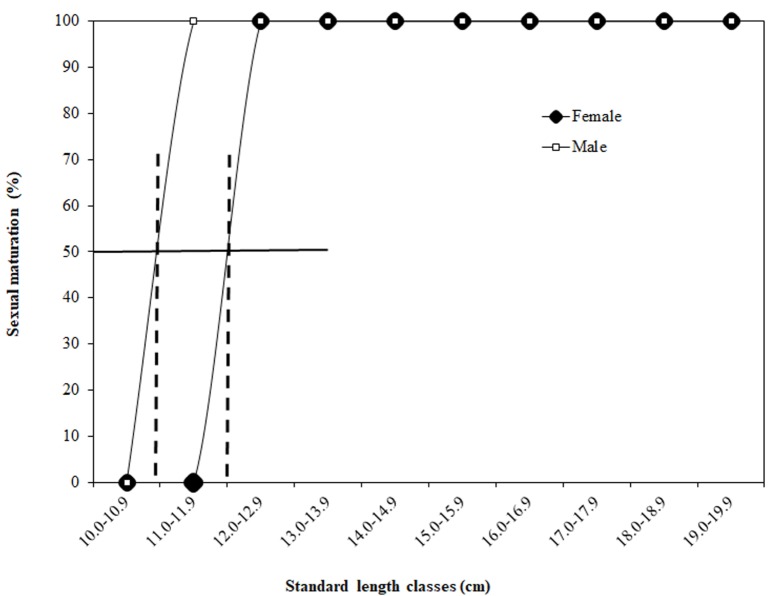
The estimated minimum body sizes for female and male maturity were 12.5 and 11.8 cm SL, respectively (Fig. 8a b). The estimated body sizes at 50% sexual maturation for females and males were 12.0 and 10.9 cm SL, respectively, based on all females and males collected during the spawning months from April to October (Fig. 9).
Fig. 8.
Relationship of the gonadosomatic index (GSI%) and standard length (SL) for (a) mature females (n = 204) and (b) mature males (n = 260) of Johnius taiwanensis. F3, maturing female; F4, ripe female; F5, spent female; M3, maturing male; M4, ripe male; M5, spent male. Vertical lines represent the minimum sizes with large increase in GSI%: 12.5 cm SL in (a) and 11.8 cm SL in (b).
Fig. 9.
Sizes at 50% sexual maturation for females and males of Johnius taiwanensis, based on all females (n = 204) and males (n = 198) collected during the spawning months from April to October. 12.0 cm and 10.9 cm SL were extrapolated directly for females and males, respectively.
DISCUSSION
In Chinese waters, 27 sciaenid species are confirmed from 14 genera (Argyrosomus, Atrobucca, Bahaba, Chrysochir, Collichthys, Dendrophysa, Johnius, Larimichthys, Megalonibea, Miichthys, Nibea, Otolithes, Pennahia, Sciaenops), including S. ocellatus, which was introduced from the USA for aquaculture purposes in the 1990s and has invaded Chinese waters (Liao et al. 2009; Chao et al. 2019). Most of these sciaenid species are small- and medium-sized, with only domestic and local commercial importance. The maximum size of J. taiwanensis in the present study was a male of 19.0 cm SL, collected in Zhangzhou waters (Fig. 1; Fig. 2). It is confirmed to be the largest individual recorded to date because the maximum size among holotype and paratype specimens was 18.6 cm SL (Chao et al. 2019).
In Chinese and adjacent waters, the spawning seasonality of sciaenids has been examined in a few species based on either gonad external morphology only, or GSI% and/or gonad histology analyses (Chu 1960; Chu and Wu 1985; Kakuda and Nakai 1981; Li et al. 2000; Li 2010; Lin et al. 2008; Ni 2018; Tuuli et al. 2011; Wang et al. 2011 2012; Wu 1981; Xu 2014; Yamaguchi et al. 2006). The results showed that the spawning activity of most species examined lasted 3−8 months, e.g., Chrysochir, Collichthys, Dendrophysa, Johnius, Larimichthys, Nibea, Otolithes and Pennahia species (Table 2). To the north, the spawning season in the East China Sea was mainly dominant in Spring (April and May), e.g., for L. crocea and L. polyactis, while to the south in the Taiwan Strait and northern South China Sea, the spawning seasons lasted longer, from Spring to Autumn, mainly dominant in May−October, e.g., for C. aureus, C. lucidus, D. russelii, J. belangerii, J. taiwanensis, L. crocea and P. macrocephalus. In the present study, the spawning period of J. taiwanensis lasted from April to October in Fujian waters, China, with the peak in July−September for females, based on gonadal histology analyses.
Table 2.
Spawning seasons of sciaenid species in Chinese and adjacent waters
| Species | Spawning season (peak season) | Methodology | Area | Reference |
| Chrysochir aureus | June to December (July to September) | GSI and gonadal histology | Southwestern waters of Taiwan | Ni 2018 |
| Collichthys lucidus | January and April | Gonad external morphology | Fujian waters, China | Wang et al. 2011 |
| March to December | Gonad external morphology | Pear River Estuary of Guangdong, China | Li et al. 2000 | |
| Dendrophysa russelii | March to September (March to May) | GSI and gonadal histology | Liusha Bay of Guangdong, China | Xu 2014 |
| Johnius belangerii | June to September (July and August) | Gonad external morphology | Zhoushan waters of Zhejiang, China | Wang et al. 2012 |
| Year-round (April and August) | Gonad external morphology | Fujian waters, China | Wang et al. 2011 | |
| May to July | Gonad external morphology | Pear River Estuary of Guangdong, China | Li et al. 2000 | |
| March to October (March) | GSI and gonadal histology | Liusha Bay of Guangdong, China | Xu 2014 | |
| J. taiwanensis | April to October (July to September) | GSI and gonadal histology | Fujian waters, China | The present study |
| Larimichthys crocea | October to December | Gonad external morphology | Hong Kong waters | Chu 1960 |
| October to December | Gonad external morphology | Liusha Bay of Guangdong, China | Li et al. 2000 | |
| April to June | - | East China Sea | Chu and Wu 1985 | |
| L. polyactis | April to June | GSI and gonad histology | Bohai and the northern yellow Sea | Wu 1981 |
| April and May | Gonad external morphology | Southern Yellow Sea | Lin et al. 2008 | |
| March to May | Gonad external morphology | East China Sea | Lin et al. 2008 | |
| Nibea albiflora | May to August (June and July) | GSI and gonadal histology | Seto Inland Sea, Japan | Kakuda and Nakai 1981 |
| Otolithes ruber | April to July (April to July) | GSI and gonadal histology | Southwestern waters of Taiwan | Ni 2018 |
| Pennahia anea | March to June (May) | GSI and gonadal histology | Hong Kong waters, China | Tuuli et al. 2011 |
| P. argentata | April to September (May to August) | GSI and gonadal histology | Ariake Sound waters, Japan | Yamaguchi et al. 2006 |
| P. macrocephalus | May to October (July to September) | GSI and gonadal histology | Southwestern waters of Taiwan | Li 2010 |
| April and August | Gonad external morphology | Fujian waters, China | Wang et al. 2011 |
-: no data.
The minimum sizes at maturity or the sizes at 50% maturity for females and males are the two criteria commonly used for evaluating the maturity sizes of the fish. For most studies on sciaenid species, mature females are generally larger than mature males. For instance, the estimated minimum sizes of mature females and males of P. anea are 12.5 cm and 11.9 cm SL, respectively, and the size at 50% maturity for females was 12.3 cm SL (Tuuli et al. 2011). For P. macrocephalus, the sizes at 50% maturity for females and males are 15.9 cm and 14.8 cm SL, respectively (Li 2010). For C. aureus, the sizes at 50% maturity for females and males are 26.3 cm and 21.0 cm SL, respectively (Ni 2018). For O. ruber, the sizes at 50% maturity for females and males were 21.8 cm and 19.9 cm SL, respectively (Ni 2018). In a few species, mature females were smaller than mature males. For instance, in J. belangerii, the sizes at 50% maturity for females and males were 12.5 cm and 13.6 cm SL, respectively (Wang et al. 2012). In the present study, the estimated minimum sizes for female and male maturity of J. taiwanensis were 12.5 cm and 11.8 cm SL, respectively, mature females larger than mature males. The estimated body sizes at 50% sexual maturation for females and males of J. taiwanensis were 12.0 cm and 10.9 cm SL, respectively, smaller than the minimum sizes for female and male maturity.
Johnius taiwanensis is a multiple spawners in a spawning season based on gonadal histology, just like other sciaenid species reported. The evidence includes the appearance of different developmental stages of oocytes from primary growth to vitellogenic in ovaries and sometimes beyond vitellogenic such as HO and POF (Kakuda and Nakai 1981; Li 2010; Ni 2018; Tuuli et al. 2011; Wu 1981; Xu 2014; Yamaguchi et al. 2006; the present study).
Spawning grounds of some sciaenid species in Chinese waters were associated with estuaries. For B. taipingensis, the spawning grounds were associated with the Yangtze River, the Min River and the Pearl River Estuaries (Sadovy and Cheung 2003). For L. crocea, the spawning grounds were also associated with the river mouths along the Chinese coast (Liu and Sadovy de Mitcheson 2008). The appearance of HO or both HO and POF in ovaries of J. taiwanensis was confirmed based on gonadal histology, indicating the samples were from its spawning ground. The spawning ground was subsequently confirmed in Dongshan Bay (117°33'50"E, 23°41'20"N) of Zhangzhou waters, associated with the Zhangjiang River Estuary.
CONCLUSIONS
Johnius taiwanensis is one of the few remaining wild-caught croakers commonly found in the Taiwan Strait. In the present study, the spawning seasonality, the minimum sizes for female and male maturity and the sizes at 50% maturity of J. taiwanensis were determined. The spawning season of J. taiwanensis lasted from April to October, peaking in July-September for females. Currently, the nationwide management for domestic marine fisheries has a fishing moratorium in May-August, adjusting slightly for fishing gears. Therefore, J. taiwanensis can be partly protected under the current management measures. Future studies should further investigate the locations of J. taiwanensis spawning grounds.
Acknowledgments
The present study was supported by the National Programme on Global Change and Air-Sea Interaction (GASI-02-PAC-YDaut), and Fundamental Research Funds for Central Universities from Xiamen University (grant no. 20720170077). The authors would like to thank Nian-Ping Cai, Wei-Di Yang, Ying Su, Yuyan Jia, Baian Lin, Xiao-Bing Jiang and Yan Jiang for field and laboratory support. We also thank Prof. Ning Labbish Chao for specimen identification and Dr. Claire Gorman for providing the distribution map.
Footnotes
Authors’ contributions: LLZ and ML designed the study. LLZ wrote the first draft of the manuscript and performed data analyses. LLZ, LPF and JJL conducted the field collection. LLZ and QX conducted laboratory measurement, dissection and gonadal histology. ML and LPF revised the manuscript. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no competing interests.
Availability of data and materials: All the data and materials are provided within the manuscript.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Chao NL, Chang CW, Chen MH, Guo CC, Lin BA, Liuo YY, Shen KN, Liu M. 2019. Johnius taiwanensis, a new species of Sciaenidae from the Taiwan Strait, with a key to Johnius species of Chinese waters. Zootaxa 4651(2):259–270. doi:10.11646/zootaxa.4651.2.3. [DOI] [PubMed]
- Chao NL, Frédou FL, Haimovici M, Peres MB, Polidoro B, Raseira M, Subirá R, Carpenter K. 2015. A popular and potentially sustainable fishery resource under pressure-extinction risk and conservation of Brazilian Sciaenidae (Teleostei: Perciformes). Global Ecology and Conservation 4:117–126. doi:10.1016/j.gecco.2015.06.002.
- Chu CY. 1960. The yellow croaker fishery of Hong Kong and preliminary notes on the biology of Pseudosciaena crocea (Richardson). Hong Kong University Fisheries Journal 3:111– 164.
- Chu Y, Lo Y, Wu H (eds). 1963. A study on the classification of the Sciaenoid fishes of China, with description of new genera and species. China: Shanghai Science and Technology Press. (in Chinese with English abstract)
- Chu Y, Wu H. 1985. Sciaenidae. In: Chu Y (ed) The Fishes of Fujian Province. China: Fujian Science and Technology Press, pp. 131–133. (in Chinese)
- Grier H. 1981. Cellular organization of the testis and spermatogenesis in fishes. American Zoology 21:345–357.
- Kakuda S, Nakai K. 1981. On the maturity and spawning of Nibea albiflora. B Jap Soc Sci Fish 47(1):17–25. doi:10.2331/suisan.47.17.
- Li JZ. 2010. Reproductive biology of big head pennah croaker (Pennahia macrocephalus) sampled from the southwestern waters off Taiwan. Master thesis, National Kaohsiung Marine University. (in Chinese with English abstract)
- Li Y, Chen G, Sun D. 2000. Analysis of the composition of fishes in the Pearl River estuarine waters. Journal of Fisheries of China 24(4):312–317. doi:10.3321/j.issn:1000-0615.2000.04.004.
- Liao YC, Chen LS, Shao KT. 2010. The predatory Atlantic red drum, Sciaenops ocellatus, has invaded the western Taiwanese coast in the Indo-West Pacific. Biol Invasions 12(7):1961–1965. doi:10.1007/s10530-009-9642-x.
- Lin LS, Cheng JH, JiangYZ, Yuan XW, Li JS, Gao TX. 2008. Spatial distribution and environmental characteristics of the spawning grounds of small yellow croaker in the southern Yellow Sea and the East China Sea. Acta Ecologica Sinica 28(8):3485–3494.
- Lin YC, Mok HK, Huang BQ. 2007. Sound characteristics of big-snout croaker Johnius macrorhynus (Sciaenidae). J Acoust Soc Am 121(1):586–593. doi:10.1121/1.2384844. [DOI] [PubMed]
- Lin YJ, Qurban MA, Shen KN, Chao NL. 2019. Delimitation of tigertooth croaker Otolithes species (Teleostei: Sciaenidae) from the western Arabian Gulf using an intergrative approach, with a description of Otolithes arabicus sp. nov. Zool Stud 58:10. doi:10.6620/ZS.2019.58-10. [DOI] [PMC free article] [PubMed]
- Liu M, Sadovy de Mitcheson Y. 2008. Profile of a fishery collapse: why mariculture failed to save the large yellow croaker. Fish Fish 9:219–242. doi:10.1111/j.1467-2979.2008.00278.x.
- Ni HY. 2018. Reproductive biology of Chrysochir aureus and Otolithes ruber in the waters off southwestern Taiwan. MSc Dissertation, National Kaohsiung Sun Yat-sen University. (in Chinese with English abstract)
- Sadovy Y, Cheung WL. 2003. Near extinction of a highly fecund fish: the one that nearly got away. Fish Fish 4:86–99. doi:10.1046/j.1467-2979.2003.00104.x.
- Sasaki K. 1989. Phylogeny of the Family Sciaenidae, with notes on its zoogeography (Teleostei, Perciformes). Memoirs of the Faculty of Fisheries Hokkaido University 36(1-2):1–137.
- Sasaki K. 2001. Sciaenidae. In: Carpenter KE and Niem VH (eds) FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. Volume 5: Bony Fishes Part 3 (Menidae to Pomacentridae). Rome, FAO, pp. 3117–3174.
- Shen SC. 1993 (ed). Fishes of Taiwan. Department of Zoology, National Taiwan University. (in Chinese)
- Trewavas E. 1977. The sciaenid fishes (croakers or drums) of the Indo-West-Pacific. Trans Zool Soc London 33:253–541. doi:10.1111/j.1096-3642.1977.tb00052.x.
- Tuuli CD, Sadovy de Mitcheson Y, Liu M. 2011. Reproductive biology of the greyfin croaker Pennahia anea in the northern South China Sea. Ichthyol Res 58(4):302–309. doi:10.1007/s10228-011-0228-0.
- Wallace RA, Selman K. 1981. Cellular and dynamic aspects of oocyte growth in teleosts. Amer Zool 21(2):325–343.
- Wang JQ, Zhang YZ, Huang LM, Li J, Xie YJ. 2011. Fishery biology of main economic fishes in Fujian coastal waters. Journal of Jimei University (Natural Science) 16(3):161–166. (in Chinese with English abstract)
- Wang K, Zhang SY, Wang ZH, Zhao J, Xu M. 2012. A preliminary study on fishery biology of Johnius belangerii off Ma’an Archipelago. Journal of Fisheries of China 36(2):228–237. (in Chinese with English abstract)
- Wu P. 1981. Histological observations on the maturity of female gonad of the small croaker, Pseudosciaena polyactis Bleeker. Journal of Fisheries of China 5(2):161–169. (in Chinese with English abstract)
- Xie YJ, Li J, Huang LM, Li WW, Zhang YZ. 2012. Temporal and spatial variations of sciaenid fish resources in Fujian coastal waters in 2006 and 2007. Journal of Oceanography in Taiwan Strait 31(3):403–411. doi:10.3969/J.ISSN.1000-8160.2012.03.015. (in Chinese with English abstract)
- Xu M. 2014. The biology research of Johnius belangerii and Dendrophysa russelii in Liusha Bay. MSc Dissertation, Guangdong Ocean University. (in Chinese with English abstract)
- Yamaguchi A, Todoroki T, Kume G. 2006. Reproductive cycle, sexual maturity and diel-reproductive periodicity of white croaker, Pennahia argentata (Sciaenidae), in Ariake Sound, Japan. Fish Res 82:95–100. doi:10.1016/j.fishres.2006.08.012.
- Yu LC, Shen SC. 1987. Study on sciaenoid fishes from the adjacent waters of Taiwan. Annual of Taiwan Museum 30:65–134.