Abstract
The highly lipid-rich blubber in the hypodermis is a specialized structure that functions in thermoregulation, energy storage, buoyancy control, locomotion, and streamlining the body shape in marine mammals. The key objective of this study was to investigate blubber development in the East Asian Finless Porpoise (Neophocaena asiaeorientalis sunameri) across the ontogenetic (fetuses, calves, and adults) and reproductive states (adults, pregnant, and lactating). Blubber samples were collected from East Asian Finless Porpoises (EAFP) that were accidentally caught in the fishing nets in the Bohai/Yellow Sea from late April to mid May of 2015. The mean blubber depth was significantly thinner in fetuses across the ontogenetic groups and significantly thicker in pregnant and lactating vs. adult females across the reproductive states. Across the four regions in each group, we did not find significant variations in blubber depth. However, the correlations between body length and weight vs. blubber depth was significant. Histological observation identified three layers of stratified blubber with a significantly smaller adipocyte cell size in fetuses and a significantly higher area ratio of structural fiber in the middle and inner layers across the ontogenetic groups. Across the reproductive states, we did not observe a statistically significant difference in the adipocyte cell size or area ratio of the structural fiber. Our results suggest that prenatal blubber growth is characterized by an increase in the adipocyte cell count, while postnatal growth is the result of an increase in cell size. They also indicate that ontogeny can affect blubber depths and cellular measurements in the EAFP.
Keywords: Adipocyte, Blubber, Finless porpoise, Ontogeny, Reproductive state
BACKGROUND
Cetaceans have a special hypodermis called “the blubber” (Parry 1949). It is a layer of fatty tissues between the underlying muscles and epidermis (Hamilton et al. 2004). Blubber is a multifunctional and very dynamic structure. It provides biomechanical assistance throughout locomotion and streamline the body surface to further increase the efficiency of locomotion (Pabst 2000; Hamilton et al. 2004). It also acts as a buoyancy adjuster (Kipps et al. 2002). Most importantly, blubber is the primary structure for energy storage. The stored energy is utilized during energy crises and replenished when food is abundant (Ryg et al. 1988; Struntz et al. 2004). High lipid storage in blubber also provides thermal insulation to the animals by reducing heat flow from the body to external environment (Dunkin et al. 2005). Due to such crucial physiological functions, blubber measurements in conjunctions with other parameters are used to investigate the health status of cetaceans (Miller et al. 2011; Nabi et al. 2017). Moreover, the various functions of blubber vary in several regions of the body; for example, the energy storage site in ringed seals (Pusa hispida) is mainly the posterior region (Ryg et al. 1988) while in harbor porpoises (Phocoena phocoena), it is thoracic-abdominal region, and the hydrodynamic function occurs in the tailstock region (Koopman 1998). Furthermore, histological and biochemical studies of blubber in bottlenose dolphins (Tursiops truncates) and harbor porpoises suggest that fat deposition and mobilization primarily occur in the middle and inner layer of the thoracic-abdominal region, whereas the metabolically inert outer layer mainly functions as a thermal insulator (Koopman et al. 2002; Struntz et al. 2004; Samuel and Worthy 2004).
The bottlenose dolphins showed significant blubber stratification in the structural fiber density, adipocyte size, and in the number of adipocytes (Struntz et al. 2004). Various factors such as sex, age, water temperature, geographic location, nutrition, and reproductive status affects blubber morphology (Struntz et al. 2004; Dunkin et al. 2005). For instance, the blubber of juvenile and adult bottlenose dolphins has considerably higher mean adipocyte size and lipid content compared to fetuses (Struntz et al. 2004). Similarly, dolphins had highest lipid content and adipocyte pregnancy (Montie et al. 2008).
The East Asian finless porpoise (Neophocaena asiaeorientalis sunameri) is a marine subspecies of the narrow-ridged finless porpoise (Neophocaena asiaeorientalis), and one of the smallest odontocete, dwelling in the coastal waters of the Bohai Sea, the Yellow Sea, and the northern part of the East China Sea (Wang et al. 2008 2010; Xiao et al. 2018). An ultrasound study of East Asian finless porpoise (EAFP) showed three layers stratified (outer, middle and inner layers) blubber. In this subspecies, dorsal blubber depth steadily increases from the head region, peaked near the umbilical area, and declines near the anal region. However, its thickness both in the ventral and lateral parts is comparatively identical in the abdomen and thorax regions. Furthermore, mean blubber depths posterior to the head (or neck) varied significantly across the life history categories such as neonates, juvenile males, adult males, and lactating groups. In neonates, blubber depth is the thinnest across the ontogenetic development at almost all sites. In lactating females, blubber is thicker at the dorsal thoracic and abdominal sites compared to juveniles, while at ventral and lateral abdominal sites, juvenile has slightly thicker blubber than lactating females (Zeng et al. 2015). Similarly, the inner layer also shows significant ontogenetic variation at some positions (Zeng et al. 2015; Chen et al. 2018). Therefore, it would be interesting to know if the ultrasonographic findings on blubber stratification and distribution can be confirmed by histological observations. What is the physiological or ecological significance of ontogenetic and reproductive state differences in the EAFP blubber? And what is the main contributor to the blubber depth changes, the adipocyte size or the cell numbers? Through histological investigations on the blubber of this animal, our specific objectives were therefore to 1) investigate the morphological changes in blubber with ontogeny and different reproductive states and 2) to try to understand the ecological adaptation of this animal by changing their blubber depth.
MATERIALS AND METHODS
Animals
We collected and examined blubber tissuesed from twenty EAFPs (13 females and 7 males). Detail information on the animals is listed in table 1. All the EAFPs were caught accidentally in gill nets in the Bohai/Yellow Sea in Penglai, Shandong province, China from late April to mid May of 2015. All the animals appeared to be in good conditions, with few indications of fishing gear entanglement with no scavenger damage (Zeng et al. 2015). Porpoise carcasses were discovered within 12 hours of death, and damage to the blubber from the transport process was considered negligible. Once the animals were receipted, three measures—body size, standard length, body mass, and girth length— were collected from each animal as soon as possible (American Society of Mammalogists 1961). Porpoises were classified into six life history categories (1, fetus; 2, calf 3, sub-adult; 4, adult; 5, pregnant; 6, lactating). Fetuses were described by their presence in the uterus. Pregnancy and lactation state were confirmed by examining the reproductive tract and mammary glands (Akin et al. 1993; Jefferson et al. 1994). The calf, sub-adult, and adult states were based on body length (Gao and Zhou 1993).
Table 1.
Detailed information of the study animals
| Animal ID | Sex | Weight (Kg) | Total body length (Cm) | Life history category |
| PL20150428F07 | Female | 6.6 | 80.5 | Fetus |
| PL20150430F11 | Female | 7.0 | 77.5 | Fetus |
| PL20150427M13 | Male | 26.4 | 110 | Calf |
| PL20150429M16 | Male | 21.2 | 109 | Calf |
| PL20150504M24 | Male | 22 | 107 | Calf |
| PL20150421M04 | Male | NC | 105 | Calf |
| PL20150515F35 | Female | 21 | 105.6 | Calf |
| PL20150423F02 | Female | 20 | 104 | Calf |
| PL20150425F04 | Female | 22.3 | 110 | Calf |
| PL20150422M06 | Male | 21.2 | 128 | Sub-adult |
| PL20150501F13 | Female | 33.4 | 131 | Sub-adult |
| PL20150503F16 | Female | 22 | 131 | Sub-adult |
| PL20150430M20 | Male | 75 | 191.6 | Adult |
| PL20150516M36 | Male | 51.8 | 156 | Adult |
| PL20150430F10 | Female | 41.4 | 162.8 | Adult |
| PL20150510F23 | Female | 52 | 153 | Pregnant |
| PL20150428F06 | Female | 64.6 | 163 | Pregnant |
| PL20150430F09 | Female | 60.6 | 50 | Pregnant |
| PL20150516F36 | Female | 60 | 169.2 | Lactating |
| PL20150517F37 | Female | 61 | 171 | Lactating |
Sample collection
The body was divided into four regions: neck, axillary, umbilicus, and anus region. In each region, three positions, dorsal, ventral, and lateral positions were selected. As a result, the thickness of blubber was measured in a total of 12 body positions (Fig. 1). Because of bilateral symmetry, the blubber was surgically removed from left side only. From each animal, one blubber sample from the umbilical region (Fig. 1) was used for histological analysis.
Fig. 1.
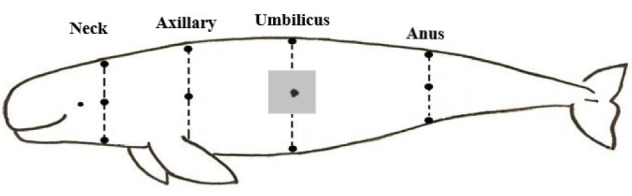
Positions of blubber thickness measured in the East Asian finless porpoises. The three dots in each dotted line represent the dorsal, lateral and ventral region, respectively. Grey square indicates the position for collection of histological samples.
Analysis
Blubber thickness was measured by a steel caliper immediately after the animal was dissected. To conduct histological sectioning on bubbler, a bubbler cube (1 cm3) was made from the lateral position of the umbilicus region. The bubble cubes were placed on a flat table and only tissues between the skin and muscle were counted and fixed together in 10% neutral buffered formalin (NBF) before histological analysis. Experiments were carried out by making the tissue into paraffin sections. Briefly, the blubber was removed from the NBF, then the routine gradient alcohol dehydration was performed and the blubber was embedded in paraffin, the embedded paraffin was cutted into 10 μm sections and stained with hematoxylin and eosin (H&E) (Chen and Farese 2002; Struntz et al. 2004). Slides were viewed under a microscope (Zeiss Axiophot Microscope, Shanghai Co., Ltd) and color images were obtained using a microscope digital camera at 100×magnification. The boundaries between three blubber layers were visible on the H&E slide with the naked eye (Struntz et al. 2004). The blubber layers limits were visible on the H&E slide, because the eosin stain intensity changed along with the density of structural fibers, and therefore it was easy for the three layers to be distinguished (Struntz et al. 2004; Montie et al. 2008). Consequently, the boundary between the outer and the middle layer was indicated by the lower intensity of eosin stain and the boundary between the middle and the inner layer was indicated by the higher intensity of eosin stain (Montie et al. 2008; Gómez-Campos et al. 2015). Five images (689.54 × 523.05 μm) were captured from each blubber layer, and the cell number, average cell size, and the structural fibers area of each image were analyzed with Image-Pro Plus software (Media Cybernetics, Inc. Rockville, MD 20850, USA). The number of adipocytes was counted by marking one in each cells; cells less than were not taken into account. The area of the adipocytes was measured using an area tool in the Image Pro Plus software and the average cell size was the adipocytes area divided by cell counts. The area tool was used to determine the structural fibers area by converting the image to a standard value grey scale because the fibers appeared black and the cells were white (Struntz et al. 2004; Montie et al. 2008).
Statistics
The animals were broadly divided into two groups; ontogenetic (fetus, calf, sub-adult, and adult) and reproductive states (adult, pregnant, and lactating). For statistical analysis, both the sub-adults and adults in males and females were grouped together. To measure the variation in blubber depth at each region and the mean blubber depth of all regions across the ontogenetic and reproductive states, one-way ANOVA followed by Tukey’s test using Graph pad prism, version 7.01 (Graph pad software Inc., San Diego, CA, USA) was used. Similarly, histological parameters such as adipocyte cell size and area ratio of structural fibers in each group across the layers and in each layer across the ontogenetic and reproductive states, one-way ANOVA followed by Tukey’s test was used. The relationship between mean body length and weight vs. blubber depth were analyzed using a Pearson correlation. Normality was assessed using the Shapiro-Wilk test. A p < 0.05 indicated the significant difference.
RESULTS
In this study, we measured the blubber depth of each animal at four regions (Fig. 1) and mean blubber depth across the ontogenetic (fetus/n = 02; male calf/n = 04; female calf/n = 03; adult male/n = 03, and adult female/n = 03) and reproductive states (adult female/n = 03, pregnant/n = 03, and lactating/n = 02). The adipocyte cell size and area ratio of structural fibers across the three layers in each group and across the ontogenetic and reproductive states were analyzed.
Blubber depths
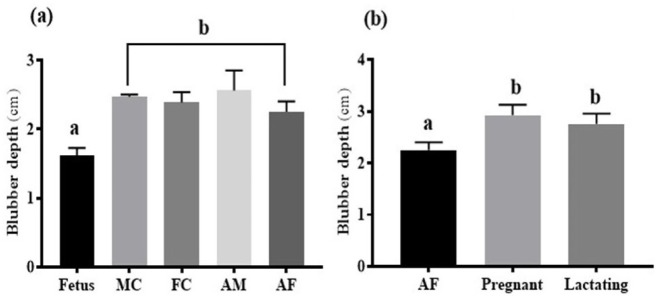
Across the ontogenetic groups, we observed significantly thinner overall mean ± SD blubber depths in fetuses (1.61 ± 0.11) compared to all other age groups using one-way ANOVA (p = <0.0001; F = 19.55; d.f. = 19; Fig. 2a). The overall mean ± SD blubber depth across the reproductive states showed significantly thicker blubber in pregnant (2.92 ± 0.19) and lactating (2.76 ± 0.19) groups vs. adult female (2.25 ± 0.15) by one-way ANOVA (p = 0.0015; F = 14.66; d.f. = 11; Fig. 2b). However, no significant difference was reported between a pregnant and lactating group (Fig. 2b).
Fig. 2.
Overall mean blubber depth across age groups (a) and reproductive states (b). Blubber depths across the age groups and reproductive states followed by the same letters were not significantly different.
Across the four regions in each group, we did not observe significant variations among fetus (p = 0.1398; F=3.29;d.f.=7),MC(p=0.9889;F=0.03;d.f.=15), FC (p = 0.6915; F = 0.51; d.f. = 7),AM (p = 0.4579; F = 0.95; d.f. = 11), AF (p = 0.7485; F = 0.41; d.f. = 11), pregnant (p = 0.1461; F = 2.47; d.f. = 10), and lactating (p = 0.8535; F = 0.25; d.f. = 7) groups. However, we found a significant correlation between body length vs. blubber depth (p = 0.0411; r = 0.608) and body weight vs. blubber depth (p = 0.0013; r = 0.8656; Fig. 3).
Fig. 3.
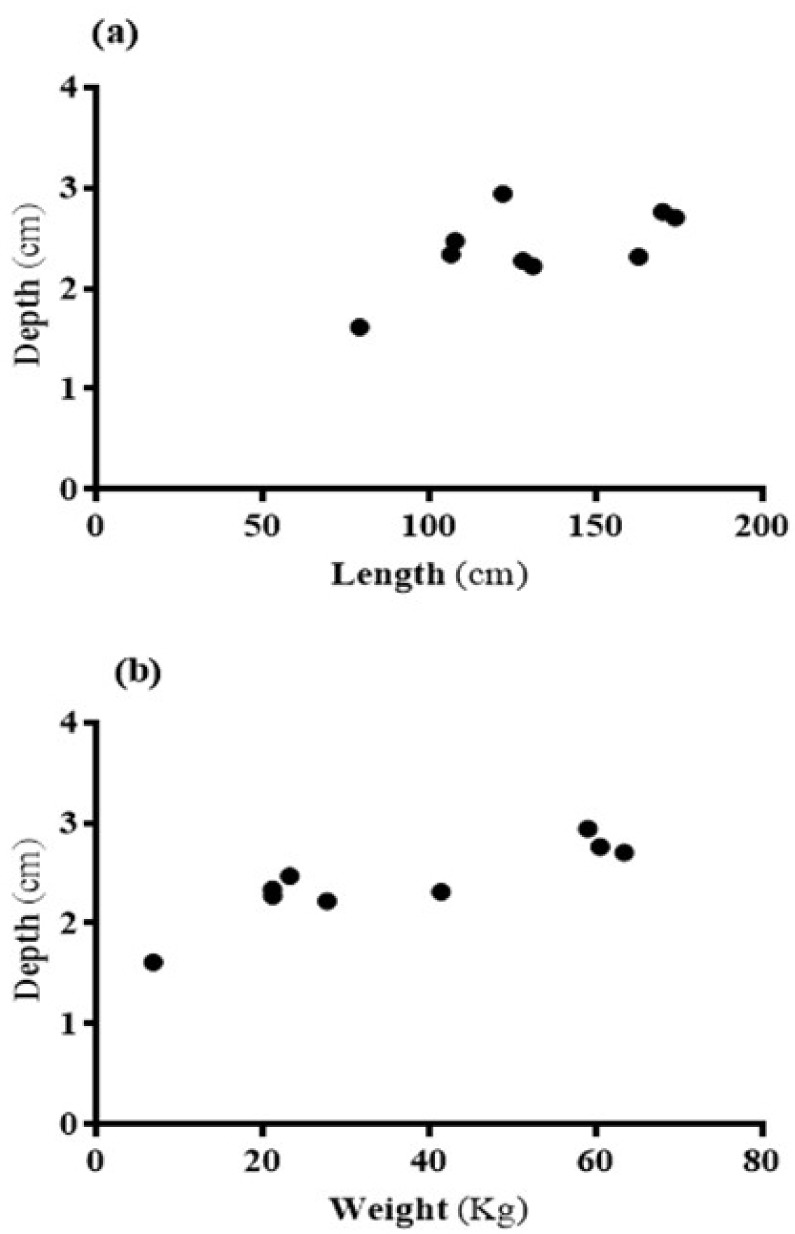
Correlations between body length (a) and weight (b) vs. blubber depth. Both the body length and weight vs blubber depth showed statistically significantly positive correlation.
Blubber stratification
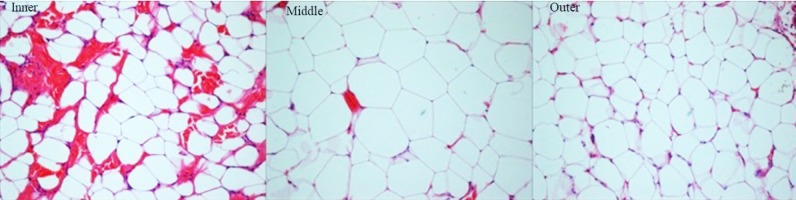
Histological observation showed three visually distinct layers (outer, middle, and inner) of stratified blubber. Most of the blubber was comprised of a white thick middle layer followed by a light pink thin inner layer close to musculatures, while the outer thin layer close to the dermis was light gray (Fig. 4). All the details of cellular measurements across the blubber layers both in the ontogenetic and reproductive groups are summarized in tables 2-5. The adipocyte cell size by one-way ANOVA varied significantly across the blubber layers in the ontogenetic (Table 2) and reproductive states (Table 3). The adipocyte cell size in all the groups except MC, pregnant, and lactating was significantly smaller in the outer layer (one-way ANOVA) compared to both the middle and inner layers (Tables 2, 3). On the other hand, the adipocyte cell size in the middle layer was significantly greater in the female calf and adult female compared to the inner layer (Table 2). In the outer and inner layers, the shapes of adipocytes were polyhedral to ovoid and more tall than wide. Unlike adipocyte cell size, the area ratio of structural fiber also varied significantly across the blubber layers and was highest in the outer layer, as indicated by the density of red color fibers (except the pregnant group; Tables 2, 3). Only the FC and AF (Table 2) showed a significantly higher area ratio of the structural fibers in the inner layer compared to the middle layer.
Fig. 4.
Cellular measurements across the layers.
Table 2.
Cellular measurements across the layers in ontogenetic groups
| Groups |
Mean ± SD |
F |
d.f. |
p |
|
Adipocyte Size (µm2) | ||||
| Fetus | O*: 9.57 ± 1.06a | 17.35 | 5 | 0.0225 |
| M*: 17.33 ± 1.76b | ||||
| I*: 18.17 ± 1.87b | ||||
| Male calf | O: 28.65 ± 10.95 | 2.20 | 5 | 0.2575 |
| M: 96.62 ± 38.56 | ||||
| I: 72.51 ± 40.29 | ||||
| Female calf | O: 17.49 ± 1.47a | 152 | 5 | 0.0010 |
| M: 69.41 ± 2.06bc | ||||
| I: 47.68 ± 4.51bd | ||||
| Adult male | O: 44.34 ± 4.03a | 52.42 | 5 | 0.0046 |
| M: 247.6 ± 32.94b | ||||
| I: 181.7 ± 11.38b | ||||
| Adult female | O: 54.26 ± 8.75a | 152.4 | 5 | 0.0010 |
| M: 149.2 ± 1.88bc | ||||
| I: 87.78 ± 3.32bd | ||||
|
Area Ratio of Structural Fibers (%) | ||||
| Fetus | O: 0.37 ± 0.00a | 666.5 | 6 | 0.0001 |
| M: 0.19 ± 0.00b | ||||
| I: 0.19 ± 0.00b | ||||
| Male calf | O: 0.30 ± 0.07a | 14.87 | 5 | 0.0277 |
| M: 0.05 ± 0.00b | ||||
| I: 0.13 ± 0.03 | ||||
| Female calf | O: 0.33 ± 0.02a | 125.7 | 5 | 0.0013 |
| M: 0.05 ± 0.00bc | ||||
| I: 0.22 ± 0.02bd | ||||
| Adult male | O: 0.33 ± 0.04a | 55.98 | 5 | 0.0042 |
| M: 0.05 ± 0.02b | ||||
| I: 0.10 ± 0.00b | ||||
| Adult female | O: 0.26 ± 0.00a | 180.5 | 5 | 0.0007 |
| M: 0.07 ± 0.00bc | ||||
| I: 0.17 ± 0.01bd | ||||
Note: Cellular measurement across the blubber layers followed by the same letter was not significantly different at p = 0.05. O*= outer, M*= middle, I*= inner.
Table 3.
Cellular measurements across the layers in reproductive states
| Groups | Mean ± SD | F | d.f. | p |
|
Adipocyte Size (µm2) | ||||
| Adult female | O*: 54.26 ± 8.75a | 152.4 | 5 | 0.0010 |
| M*:149.2 ± 1.88bc | ||||
| I*: 87.78 ± 3.32bd | ||||
| Pregnant | O: 34.90 ± 5.73 | 0.98 | 5 | 0.4693 |
| M: 225.1 ± 216.2 | ||||
| I: 164.1 ± 103.8 | ||||
| Lactating | O: 58.23 ± 21.4 | 3.79 | 5 | 0.1510 |
| M: 426.0 ± 258.2 | ||||
| I: 80.50 ± 13.99 | ||||
|
Area Ratio of Structural Fibers (%) | ||||
| Adult female | O: 0.26 ± 0.00a | 180.5 | 5 | 0.0007 |
| M: 0.07 ± 0.00bc | ||||
| I: 0.17 ± 0.01bd | ||||
| Pregnant | O: 0.29 ± 0.04 | 6.39 | 5 | 0.0828 |
| M: 0.10 ± 0.09 | ||||
| I: 0.10 ± 0.02 | ||||
| Lactating | O: 0.31 ± 0.02a | 17.47 | 5 | 0.0222 |
| M: 0.04 ± 0.01b | ||||
| I: 0.13 ± 0.07 | ||||
Note: Cellular measurement across the blubber layers followed by the same letter was not significantly different at p = 0.05. O*= outer, M*= middle, I*= inner.
Differences in blubber morphology with sex and age
As expected, adipocyte cell size in the fetus group was significantly smallest across the ontogenetic groups in all three layers. Both male and female calves then start a robust increase in adipocyte size, peaking at the adult stage. In the middle and inner layers, adipocyte cell size was significantly greater in the adult male vs. adult female, but no difference was reported between the male calves vs. female calves (Table 4).
Table 4.
Cellular measurements across the ontogenetic groups
| Layers | Fetus (mean ± SD) | Male calf (mean ± SD) | Female calf (mean ± SD) | Adult male (mean ± SD) | Adult female (mean ± SD) | F | d.f. | p |
|
Adipocyte Size (µm2) | ||||||||
| Outer | 9.57 ± 1.06a | 28.65 ± 10.95 | 17.49 ± 1.47 c | 44.34 ± 4.03bd | 54.26 ± 8.75bd | 15.81 | 9 | 0.0048 |
| Middle | 17.33 ± 1.76a | 96.62 ± 38.56c | 69.41 ± 2.06e | 247.6 ± 32.94bd.f.g | 149.2 ± 1.88bh | 29.71 | 9 | 0.0011 |
| Inner | 18.17 ± 1.87a | 72.51 ± 40.29c | 47.68 ± 4.51e | 181.7 ± 11.38bd.f.g | 87.78 ± 3.32h | 21.42 | 9 | 0.0024 |
|
Area Ratio of Structural Fibers (%) | ||||||||
| Outer | 0.37 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.02 | 0.07 ± 0.00 | 2.30 | 9 | 0.1924 |
| Middle | 0.19 ± 0.00a | 0.05 ± 0.19b | 0.05 ± 0.00b | 0.05 ± 0.02b | 0.06 ± 0.01b | 57.58 | 9 | 0.0002 |
| Inner | 0.19 ± 0.00a | 0.13 ± 0.03c | 0.22 ± 0.02de | 0.10 ± 0.00bf | 0.17 ± 0.01 | 11.28 | 9 | 0.0102 |
Note: Cellular measurement across the ontogenetic groups followed by the same letter was not significantly different at p = 0.05.
The area ratio of structural fiber in fetus group was significantly higher across the ontogenetic groups in the middle and inner layers. Only in the inner layer, the area ratio of structural fiber was significantly higher in the FC vs MC and AM. However, no significant difference was observed in the outer layer (Table 4).
Blubber morphological changes across reproductive states
The adipocyte cell size and area ratio of structural fiber in all the three layers were not significantly different across the reproductive states (Table 5).
Table 5.
Cellular measurements across the reproductive states
| Layers | Adult female (mean ± SD) | Pregnant (mean ± SD) | Lactating (mean ± SD) | F | d.f. | p |
|
Adipocyte Size (µm2) | ||||||
| Outer | 54.26 ± 8.75 | 34.90 ± 5.73 | 58.23 ± 21.40 | 1.64 | 5 | 0.3291 |
| Middle | 149.2 ± 1.88 | 225.1 ± 216.2 | 426 ± 258.2 | 1.082 | 5 | 0.4427 |
| Inner | 87.78 ± 3.32 | 164.1 ± 103.8 | 80.50 ± 13.99 | 1.17 | 5 | 0.4210 |
|
Area Ratio of Structural Fibers (%) | ||||||
| Outer | 0.26 ± 0.00 | 0.29 ± 0.04 | 0.31 ± 0.02 | 1.63 | 5 | 0.3317 |
| Middle | 0.07 ± 0.00 | 0.10 ± 0.09 | 0.04 ± 0.01 | 0.72 | 5 | 0.5517 |
| Inner | 0.17 ± 0.01 | 0.10 ± 0.02 | 0.13 ± 0.07 | 0.94 | 5 | 0.4797 |
Note: Cellular measurement across the reproductive states followed by the same letter was not significantly different at p = 0.05.
DISCUSSION
Blubber depths
In our study, we found an increasing overall mean blubber depth across the ontogenetic stages of the EAFP from the fetus to adult stage. This ontogenetic blubber developmental pattern was similar to that of the bottlenose dolphin reported by Struntz et al. (2004). Similar to our study, they found a significantly thinner blubber depth in the fetal group compared to adult groups. Relative to total body length and weight, we found a significant positive correlation with blubber depths, as was also observed in the bottlenose dolphin (Struntz et al. 2004), gray whales (Eschrichtius robustus) (Rice and Wolman 1971), and blue and fin whales (Nishiwaki and Hayashi 1950; Nishiwaki and Oye 1951). Like EAFP, the blubber thickness of Eubalaena australis calves increases during the first months of suckling while the calves of E. glacialis in the last months of suckling, were as thick as juvenile whales (Miller et al. 2011). Thicker blubber in the EAFP calves might be due to the provision of positive energy balance via the mother’s milk (Lockyer 1995; Dearolf et al. 2000; McLellan et al. 2002; Miller et al. 2011) in order to provide energy reserves, positive buoyancy, and insulation for the young animal (Dearolf et al. 2000; McLellan et al. 2002).
Across the reproductive states, we found a significantly thicker blubber depth both in pregnant and lactating groups vs. adult female. However, no significant difference was observed between pregnant and lactating groups. The reproductive stage changes with age in cetacean and therefore, like fin and blue whales, we observed a similar extra metabolic demand during pregnancy (Lockyer 1981; Anabella et al. 2017). They recorded about 25% thicker blubber and high lipid contents in pregnant fin and blue whales compared to non-breeding females (Aguilar and Borrell 1990). The high lipid content during pregnancy might be very crucial for normal fetal development. The lack of difference in the blubber thickness between pregnant and lactating groups indicates that EAFP, like North Atlantic harbor porpoises (Phocoena phocoena L.) (McLellan et al. 2002), invest equally in blubber as a percentage of total body mass. However, Gómez-Campos et al. (2011) noticed no significant differences in the blubber thickness of striped dolphin (Stenella coeruleoalba) among the pregnant, lactating, resting females or calves and immature groups. Thus, in Stenella coeruleoalba maximum blubber thickness did not give any benefit to female reproductive status discrimination. In contrast to our results, Mohl-Hansen (1954), Lockyer (1995), and Koopman (1998) reported an overall decline in the blubber thickness from calves to adult harbor porpoise, as well as thicker blubber in calves and thinner blubber in simultaneously lactating and pregnant females in different waters. The enantiometric thickness of blubber in harbour porpoises indicates that calves and adults may have different energetic and thermoregulatory strategies, resulting in different adaptation of blubber morphology (Huxley and Teissier 1936).
Indices such as different girths, blubber depths, body axis, and other morphometric measurements have been used to investigate nutritional status and are largely accepted and presently used for pinnipeds and baleen whales (Konishi 2006; Lockyer et al. 1985; Pitcher and Calkins 2000). However, in odontocetes, these parameters have not always been effective (Caon et al. 2007; Evans et al. 2003), except in the harbor porpoise (Phocoena phocoena), where there is a relationship between reproductive state and blubber thickness (Koopman 1998). Presently, there are uncertainties concerning their accuracy to exactly indicate the nutritional status of each individual. Evidence suggests that these indices are insensitive, biased, described by intrinsic errors of measurement, and lack a correlation with alteration in total body fat reserves (Aguilar et al. 2007). Furthermore, the sample size in our study was too small and the blubber depths could be affected by several other parameters, including the nutritional status of the animals (Nabi et al. 2017). Therefore, further studies are needed with a large sample size to uncover this physiological phenomenon in the EAFP.
Blubber Stratification
Histological analysis showed a three-layer stratified blubber in the EAFP. A similar three-layer blubber was also observed in the bottlenose dolphin (Struntz et al. 2004) and also previously reported by Zeng et al. (2015) in the same species on the basis of an ultrasound examination. An increasing overall mean blubber depth by one-way ANOVA across the ontogenetic stages of the EAFP from fetuses to adult stages also observed in the mean adipocyte size, which significantly increased throughout all vigorous life history categories. Across the depth of blubber layers, this increase in cell size was not uniform and only the largest increase was observed in the middle and inner layers. A similar pattern was also noticed by Struntz et al. (2004) in bottlenose dolphins. Blubber development in the fetal stage is due to propagation of adipocytes rather than an increase in adipocyte size. Adipocyte proliferation in humans and pigs initiates close to the muscle/integument boundary and propagate superficially. During the third trimester, expansion of adipocyte dimensions accelerates due to abrupt lipid accumulation, resulting from the de novo synthesis of glucose and uptake of maternal free fatty acids (Hudson and Hull 1975; Labbe et al. 1989). In our study, a postnatal increase in blubber thickness was independent of the adipocyte cell count, and the resulting increase in cell size might be due to lipid accumulation, as concluded by Struntz et al. (2004) in bottlenose dolphins. The significance of smaller adipocyte size in the early ontogenetic stages might only provide insulation to the body, as highly nutritious energy is delivered by the mother via milk. Thinner blubber in fetuses might also be an adaptation for allowing an easy birth.
Lactation and pregnancy are states that may affect the blubber morphology and dynamics of blubber lipids. Further, lactation in mammalian reproduction is energetically more costly than gestation (Scantlebury et al. 2000; Kastelein et al. 2002). In our study, we did not observe significant variations in the adipocyte cell size across the reproductive states. However, we found a non-significant increasing trend in the inner layer across the reproductive states that could support the hypothesis that the smaller adipocyte size in these lactating females might be due to lipid mobilization (i.e., shrinkage of adipocyte and lower lipid content) and vice versa in pregnant females, as observed in the bottlenose dolphins (Struntz et al. 2004). The shrinkage of the adipocyte in the inner layer due to energy mobilization was also observed by Koopman et al. (2002) in starved harbor porpoises. In lactating females, there might also be an association between adipocyte size and lactating period, i.e., the greater the lactating duration, the smaller the adipocyte size. Therefore, a female dolphin with a bigger calf might have lost more lipids than one captured with smaller calves due to high energetic demands (Struntz et al. 2004).
For the average area ratio of structural fiber, no specific pattern was noted across the ontogenetic groups. However, each group showed a higher average area ratio of structural fiber in the outer layer. Montie et al. (2008) reported significantly higher densities of structural fibers in the outer layer, which probably anchor the dermal papillae, providing structural aid to the skin (Aguilar and Borrell 1990; Struntz et al. 2004) throughout all life stages. Variation in the structural fibers of other layers indicates that the outer layer is static while other layers are dynamic and have a major role in lipid mobilization.
CONCLUSIONS
In summary, factors such as ontogeny and reproductive are correlated to differences in the blubber morphology of EAFP. During birth, thinner blubber due to smaller adipocyte size might be an adaptation to allow for easy birth. This thinner blubber might be enough to provide thermoregulation and bouncy. Later, an increase in the blubber thickness is the result of an increase in cell size for energy storage and mobilization, which is most evident in pregnant and lactating groups. To further understand the morphology and physiology of blubber, research on a large sample size is needed to focus on the lipid composition of the blubber layers and molecular and hormonal control of blubber dynamics.
Acknowledgments
Acknowledgment: We are thankful to the National Natural Science Foundation of China (No. 31430080) for financial support.
List of abbreviations
- EAFP
East Asian Finless Porpoise.
- NBF
Neutral Buffered Formalin
- H&E
Hematoxylin and Eosin.
Footnotes
Authors’ contributions: GN conceived the study, analyzed the data, and drafted the manuscript. JJ and XZ collected and analyzed the samples. YH and DW supervised the study.
Competing interest: JJ, GN, XZ, YH, and DW declare that they have no conflict of interest.
Availability of data and materials: Additional data and materials will be provided by the corresponding author on request.
Consent for publication: Not applicable.
Ethics approval consent to participate: The whole procedure strictly adhered to Chinese law and ethical guidelines for wild animals.
References
- Aguilar A, Borrell A. 1990. Patterns of lipid content and stratification in the blubber of fin whales (Balaenoptera physalus). J Mammal 71:544–554. doi:10.2307/1381793.
- Aguilar A, Borrell A, Gómez-Campos E. 2007. The reliability of blubber thickness as a measure of body condition in large whales. 59th Annual Meeting of the Scientific Committee of the International Whaling Commission (SC/59/O17).
- Akin PA, Peltier KM, Miller RB. 1993. Techniques for the preparation and examination of reproductive samples collected from dolphins in the eastern tropical Pacific. U.S. Dep. Commer. NOAA Tech. Memo NMFS-SWFSC-192.
- American Society of Mammalogists. 1961. Standardized methods for measuring and recording data on the smaller cetaceans. J Mammal 42:471–476. doi:10.2307/1377364.
- Anabella SF, Florencia GM, Aníbal GN, Alberto CE, Laura DS. 2017. Reproductive parameters of female long-finned pilot whales (Globicephala melas edwardii) from the Southwestern Atlantic. Zool Stud 56:39. doi:10.6620/ZS.2017.56-39. [DOI] [PMC free article] [PubMed]
- Caon G, Fialho CB, Danilewicz D. 2007. Body fat condition in Franciscanas (Pontoporia blainvillei) in Rio Grande do Sul, Southern Brazil. J Mammal 88:1335–1341. doi:10.1644/06-MAMM-A-364R.1.
- Chen HC, Farese RV. 2002. Determination of adipocyte size by computer image analysis. Lipid Res 43:986–989. [PubMed]
- Chen R, Li W, Jiang W, Zheng B, Li J. 2018. The development of mother-calf interactions during the first year in Yangtze finless porpoises (Neophocaena asiaeorientalis asiaeorientalis). Zool Stud 57:23. doi:10.6620/ZS.2018.57-23. [DOI] [PMC free article] [PubMed]
- Dearolf JL, McLellan WA, Dillaman RM, Frierson D Jr, Pabst DA. 2000. Precocial development of axial locomotor muscle in bottlenose dolphins (Tursiops Truncatus). J Morphol 244:203– 215. doi:10.1002/(SICI)1097-4687(200006)244:3<203::AID-JMOR5>3.0.CO;2-V. [DOI] [PubMed]
- Dunkin RC, McLellan WA, Blum JE, Pabst D. 2005. The ontogenetic changes in the thermal properties of blubber from Atlantic bottlenose dolphin Tursiops truncatus. J Exp Biol 208:1469– 1480. doi:10.1242/jeb.01559. [DOI] [PubMed]
- Evans K, Hindell MA, Thiele D. 2003. Body fat condition in sperm whales, Physeter macrocephalus, from southern Australian waters. Comp Biochem Physiol 134:847–862. doi:10.1016/s1095-6433(03)00045-x. [DOI] [PubMed]
- Gao A, Zhou K. 1993. Growth and reproduction of three populations of finless porpoise, Neophocaena phocaenoides, in Chinese waters. Aquat Mamm 19:3-12.
- Gómez-Campos E, Borrell A, Aguilar A. 2011. Assessment of nutritional condition indices across reproductive states in the striped dolphin (Stenella coeruleoalba). J Exp Mar Biol Ecol 405:18–24. doi:10.1016/j.jembe.2011.05.013.
- Gómez-Campos EA, Borrell A, Correas J, Aguilar A. 2015. Topographical variation in lipid content and morphological structure of the blubber in the striped dolphin. Scientia Marina 79:189–197. doi:10.3989/scimar.04093.25A.
- Hamilton JL, McLellan WA, Pabst DA. 2004. Functional morphology of tailstock blubber of the harbor porpoise (Phocoena phocoena). J Morphol 26:105–117. [DOI] [PubMed]
- Hudson DG, Hull D. 1975. Growth of adipose tissue in the fetal rabbit. Biol Neonate 27:71–79. doi:10.1159/000240761. [DOI] [PubMed]
- Huxley JS, Teissier G. 1936. Terminology of relative growth. Nature 137:780–781.
- Jefferson TA, Myrick Jr AC, Chivers SJ. 1994. Small cetacean dissection and sampling: a field guide. NOAA Tech. Mem., NMFS-SWFSC-198.
- Kastelein RA, Vaughan N, Walton S, Wiepkema PR. 2002. Food intake and body measurements of Atlantic bottlenose dolphins (Tursiops truncatus) in captivity. Mar Environ Res 53:199–218. doi:10.1016/S0141-1136(01)00123-4. [DOI] [PubMed]
- Kipps EK, McLellan WA, Rommel SA, Pabst DA. 2002. Skin density and its influence on buoyancy in the manatee (Trichechus manatus latirostris), harbor porpoise (Phocoena phocoena), and bottlenose dolphin (Tursiops truncatus). Mar Mamm Sci 18:765–778. doi:10.1111/j.1748-7692.2002.tb01072.x.
- Konishi K. 2006. Characteristics of blubber distribution and body condition indicators for Antarctic minke whales (Balaenoptera bonaerensis). Mamm Study 31:15–22. doi:10.3106/1348-6160(2006)31[15:COBDAB]2.0.CO;2.
- Koopman HN. 1998. Topographical distribution of the blubber of harbor porpoises (Phocoena phocoena). J Mammal 79:260–270. doi:10.2307/1382862.
- Koopman H, Pabst D, McLellan W, Dillaman R, Read A. 2002. Changes in blubber distribution and morphology associated with starvation in the harbor porpoise (Phocoena phocoena): evidence for regional differences in blubber structure and function. Physiol Biochem Zool 75:498–512. doi:10.1086/342799. [DOI] [PubMed]
- Labbe S, Barbet JP, Copin H, Maillet M, Schramm B. 1989. Development of adipose tissue in the human fetus. Prog Clin Biol Res 295:281–286. [PubMed]
- Lockyer C. 1981. Growth and energy budgets of large baleen whales from the Southern Hemisphere. In: Mammals of the Sea, FAO Fisheries Series (ed) Food and Agriculture Association of the United Nations, Rome.
- Lockyer C. 1995. Aspects of the biology of the harbor porpoise. Phocoena phocoena. from British waters. In: Blix AS, Walloe L, Ulltang O (ed) Whales, seals, fish and man, Elsevier Science, Amsterdam, Netherlands.
- Lockyer CH, McConell LC, Waters TD. 1985. Body condition in terms of anatomical and biochemical assessment of body fat in North Atlantic fin and sei whales. Can J Zool 63:2328–2338. doi:10.1139/z85-345.
- McLellan WA, Koopman HN, Rommel SA, Read AJ, Potter CW, Nicolas JR, Westgate AJ, Pabst DA. 2002. Ontogenetic allometry and body composition of harbour porpoises (Phocoena phocoena, L.) from the western North Atlantic. J Zool (London) 257:457–471. doi:10.1017/S0952836902001061.
- Miller CA, Reeb D, Best PB, Knowlton AR, Brown MW, Moore MJ. 2011. Blubber thickness in right whales Eubalaena glacialis and Eubalaena australis related with reproduction, life history status and prey abundance. Mar Ecol Prog Ser 438:267–83. doi:10.3354/meps09174.
- Mohl-Hansen U. 1954. Investigations on reproduction and growth of the porpoise (Phocaena phocaena (L.) from the Baltic. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening 116:369–400.
- Montie EW, Garvin SR, Fair PA, Bossart GD, Mitchum GB, McFee WE, Speakman T, Starczak VR, Hahn ME. 2008. Blubber morphology in wild bottlenose dolphins (Tursiops truncatus) from the Southeastern United States: influence of geographic location, age class, and reproductive state. J Morphol 269:496– 511. doi:10.1002/jmor.10602. [DOI] [PubMed]
- Nabi G, Hao Y, Zeng X, Wang D. 2017. Assessment of Yangtze finless porpoises (Neophocaena asiaorientalis) through biochemical and hematological parameters. Zool Stud 56:31. doi:10.6620/ZS.2017.56-31. [DOI] [PMC free article] [PubMed]
- Nishiwaki M, Oye T. 1951. Biological investigation on blue whales (Balaenoptera musculus) and fin whales (Bala - enoptera physalus) caught by the Japanese Antarctic whaling fleets. Sci Rep Whales Res Inst Tokyo 5:91–167.
- Nishiwaki M, Hayashi K. 1950. Biological survey of fin and blue whales taken in the Antarctic season 1947–48 by the Japanese fleet. Sci Rep Whales Res Inst Tokyo 3:132–190.
- Pabst DA. 2000. To bend a dolphin: Convergence of force transmission designs in cetaceans and scombrid fishes. Amer Zool 40:146–155. doi:10.1093/icb/40.1.146.
- Parry DA. 1949. The structure of whale blubber and a discussion of its thermal properties. Q J Microsc Sci 90:13–25. [PubMed]
- Pitcher KW, Calkins DG. 2000. Steller sea lion body condition indices. Mar Mam Sci 16:427–436. doi:10.1111/j.1748-7692.2000.tb00934.x.
- Rice DW, Wolman AA. 1971. The life history and ecology of the gray whale (Eschrichtius robustus), Spec Publ 3. American Society of Mammalogists, Stillwater, OK.
- Ryg M, Smith TG, Øritsland NA. 1988. Thermal significance of the topographical distribution of blubber in ringed seals (Phoca hispida). Can B Fish Aquat Sci 45:985–992. doi:10.1139/f88-121.
- Samuel AM, Worthy GA. 2004. Variability in fatty acid composition of bottlenose dolphin (Tursiops truncatus) blubber as a function of body site, season, and reproductive state. Can J Zool 82:1933–1942. doi:10.1139/z05-001.
- Scantlebury M, Butterwick R, Speakman JR. 2000. Energetics of lactation in domestic dog (Canis familiaris) breeds of two sizes. Comp Biochem Physiol A, Mol Integr Physiol 125:197–210. doi:10.1016/s1095-6433(99)00175-0. [DOI] [PubMed]
- Struntz DJ, McLellan WA, Disllaman R, Blum J, Kucklick J, Pabst DA. 2004. Blubber development in bottlenose dolphins (Tursiops truncatus). J Morphol 259:7–20. doi:10.1002/jmor.10154. [DOI] [PubMed]
- Wang JY, Yang SC, Wang BJ, Wang LS. 2010. Distinguishing between two species of finless porpoises (Neophocaena phocaenoides and N. asiaeorientalis) in areas of sympatry. Mammalia 74:305–310. doi:10.1515/mamm.2010.029.
- Wang J, Frasier T, Yang S, White B. 2008. Detecting recent speciation events: The case of the finless porpoise (genus Neophocaena). Heredity 101:145–155. doi:10.1038/hdy.2008.40. [DOI] [PubMed]
- Xiao Y, Nabi G, Yang J, Hao Y, Wang D. 2018. Hormonal regulation of testicular development in the finless Porpoise Neophocaena asiaeorientalis sunameri: preliminary evidence from testicular histology and immunohistochemistry. Zool Stud 57:41. doi:10.6620/ZS.2018.57-41. [DOI] [PMC free article] [PubMed]
- Zeng X, Junhua J, Yujiang H, Ding W. 2015. Topographical distribution of blubber in finless porpoises (Neophocaena asiaeorientalis sunameri): a result from adapting to living in coastal waters. Zool Stud 54:32. doi:10.1186/s40555-015-0111-1. [DOI] [PMC free article] [PubMed]