Abstract
OBJECTIVE
To study plasma and dietary linoleic acid (LA) in relation to type 2 diabetes risk in post–myocardial infarction (MI) patients.
RESEARCH DESIGN AND METHODS
We included 3,257 patients aged 60–80 years (80% male) with a median time since MI of 3.5 years from the Alpha Omega Cohort and who were initially free of type 2 diabetes. At baseline (2002–2006), plasma LA was measured in cholesteryl esters, and dietary LA was estimated with a 203-item food-frequency questionnaire. Incident type 2 diabetes was ascertained through self-reported physician diagnosis and medication use. Hazard ratios (with 95% CIs) were calculated by Cox regressions, in which dietary LA isocalorically replaced the sum of saturated (SFA) and trans fatty acids (TFA).
RESULTS
Mean ± SD circulating and dietary LA was 50.1 ± 4.9% and 5.9 ± 2.1% energy, respectively. Plasma and dietary LA were weakly correlated (Spearman r = 0.13, P < 0.001). During a median follow-up of 41 months, 171 patients developed type 2 diabetes. Plasma LA was inversely associated with type 2 diabetes risk (quintile [Q]5 vs. Q1: 0.44 [0.26, 0.75]; per 5%: 0.73 [0.62, 0.86]). Substitution of dietary LA for SFA+TFA showed no association with type 2 diabetes risk (Q5 vs. Q1: 0.78 [0.36, 1.72]; per 5% energy: 1.18 [0.59, 2.35]). Adjustment for markers of de novo lipogenesis attenuated plasma LA associations.
CONCLUSIONS
In our cohort of post-MI patients, plasma LA was inversely related to type 2 diabetes risk, whereas dietary LA was not related. Further research is needed to assess whether plasma LA indicates metabolic state rather than dietary LA in these patients.
Introduction
Dietary guidelines for the prevention of coronary heart disease promote the replacement of trans fatty acids (TFAs) and saturated fatty acids (SFAs) by cis unsaturated fat, especially polyunsaturated fatty acids (PUFAs) (1). Linoleic acid (LA; 18:2n-6) is the predominant type of dietary PUFA, with a contribution of 4–6% to total energy intake (% energy) in Western diets (2).
The importance of PUFAs for the prevention of type 2 diabetes is not yet clear. Results from population-based studies showed inconsistent associations between type 2 diabetes risk and dietary LA (3–8), and also when studied as total n-6 fatty acid (FA) (9,10) or as total PUFA (11,12). Inverse associations were found when SFA was theoretically replaced by PUFA (11,12) and not when PUFA replaced total carbohydrates (4,9). However, a recent analysis using data from three cohorts in the U.S. observed that isocaloric replacement by LA for SFA, TFA, or carbohydrates was associated with lower type 2 diabetes risk (8). Inconsistencies might also be due to incomplete adjustment for relevant confounders such as total energy intake (3,5) or dietary fiber (7,10).
Plasma LA has been investigated as a biomarker of dietary LA (13). Moderate correlations (0.2–0.3) between dietary, assessed by food-frequency questionnaire, and plasma LA have been reported in nonpatient populations, but correlations up to 0.7 were also reported when dietary history or multiple-day food records were used (13). Plasma LA has been used to investigate associations of LA with type 2 diabetes risk in mostly general populations (14,15). A pooling study of 8 European population-based cohorts with ∼27,000 individuals showed a relative risk (RR) of 0.54 for incident type 2 diabetes in the upper versus lower quintile of plasma phospholipids LA (14). This was confirmed in a larger pooling study including 20 prospective cohort studies of ∼40,000 individuals, which showed an RR of 0.57 when comparing extreme quintiles of circulating LA in various lipid compartments (15).
Plasma LA is influenced not only by dietary LA but also by endogenous metabolic processes. Liver fat accumulation has been associated with lower plasma LA and higher plasma palmitic acid in an elderly population without diabetes (16). Higher δ-6-desaturase (D6D) activity, estimated by the ratio of 18:3n-6 to LA in blood fractions, predicted worsening of glycemia (17).
Patients with diabetes who have had a myocardial infarction (MI) had a higher risk for recurrent cardiovascular events and mortality compared with patients who did not have diabetes (18,19). In the Alpha Omega Cohort, we showed that a better dietary fat quality, studied by using theoretical replacement of SFA plus TFA by PUFA (predominantly LA), was related to a 34% lower risk of cardiovascular disease mortality, on top of advanced drug treatment (20). Whether having better dietary fat quality or plasma FA composition could also lower type 2 diabetes risk in these patients is still unknown. We therefore examined the associations of both plasma LA, measured in cholesteryl esters, and dietary LA (replacing SFA plus TFA) and the incidence of type 2 diabetes during ∼3 years of follow-up in our cohort of post-MI patients.
Research Design and Methods
Patients and Study Design
The Alpha Omega Cohort is a prospective observational study of 4,837 drug-treated Dutch patients aged 60–80 years with a verified clinically diagnosed MI up to 10 years before study enrollment. The Alpha Omega Cohort is registered with ClinicalTrials.gov, NCT03192410. Baseline measurements took place between 2002 and 2006, as described in detail elsewhere (21,22). These patients were free living, mostly men (78%), and some also reported a history of stroke (7%) or type 2 diabetes (17%). Most of the patients were treated with statins (86%) and antihypertensive medications (89%) (22). During the first 40 months of follow-up, patients took part in an intervention study (Alpha Omega Trial) with low doses of n-3 PUFA (treatment groups: α-linolenic acid, eicosapentaenoic acid + docosahexaenoic acid, α-linolenic acid+ eicosapentaenoic acid + docosahexaenoic acid, and placebo), which had no effect on major cardiovascular events (21). Patients were monitored for type 2 diabetes incidence (outcome for the current study) for the duration of the intervention study.
The current analysis excluded patients with prevalent type 2 diabetes at baseline (n = 1,014). Prevalent type 2 diabetes at baseline was defined when a patient reported having received the diagnosis from a physician, was taking antidiabetic drugs, or had an elevated plasma glucose level (≥7.0 mmol/L if fasted for ≥4 h or ≥11.1 mmol/L if not fasted) (21). We further excluded patients with missing data on plasma cholesteryl esters or patients with >5% of unknown FA (n = 61) and missing dietary data or extreme energy intake (<800 or >8,000 kcal/day for men, <600 or >6,000 kcal/day for women; n = 334). Additionally, we excluded 171 patients with extreme unsaturated FA intakes (<2.5th or >97.5th percentile) to obtain reliable risk estimates when analyzing FA intake on a continuous scale, as reported previously (20). A total of 3,257 patients remained for analysis (Supplementary Fig. 1).
Dietary Data Collection
Dietary intake data were collected at baseline by a 203-item food-frequency questionnaire (FFQ). The FFQ was an extended version of a reproducible, validated 104-item questionnaire that was specifically designed for estimating FAs and cholesterol intake (23,24). The original FFQ showed good relative validity for LA intake compared with dietary history interviews (Pearson r = 0.65) (23). Patients were instructed to report their usual food intake over the past month, including the type of food, frequency, amount, and preparation methods, if applicable. For example, when patients indicated that they consumed baked potatoes, they were also asked to indicate which type and brand of fat was used for preparation. A trained dietitian checked the returned FFQs and obtained additional information by telephone for items that were unclear or missing. Double data entry was performed, and inconsistencies were solved. Total energy and nutrient intakes were calculated from food consumption data using the 2006 Dutch Food Consumption Database (Nederlands Voedingsstoffenbestand [NEVO]) (25). Dietary intake of LA and other macronutrients was expressed as percentages of energy (% energy), excluding calories from alcohol.
Assessment of Plasma FAs
Samples of 30 mL venous blood, either fasting or nonfasting, were drawn at the patients’ home or at the hospital. Blood was collected in EDTA-containing Vacutainers, packed in a sealed envelope, and sent by postal mail to a central laboratory (26). FAs were measured in plasma cholesteryl esters. Detailed procedures of FA measurement have been described previously (27). In short, lipids from EDTA plasma were separated by solid-phase extraction silica columns to obtain the cholesteryl esters fraction (Chrompack, Middelburg, the Netherlands). Cholesteryl esters FAs were identified by gas chromatography through comparison with known standards (Nu-Chek Prep, Elysian, MN). Individual FAs were expressed as the percentage of total FAs. For LA, the within-run and between-run coefficient of variation was ≤0.6% and ≤1.2%, respectively.
Ascertainment of Incident Type 2 Diabetes
Type 2 diabetes was ascertained based on a self-reported physician’s diagnosis and/or the initiation of antidiabetic medication. Type 2 diabetes was assessed after 12 months, 24 months, at midterm examination (20 months), and at the end of the Alpha Omega Trial phase, with a median (interquartile range [IQR]) follow-up of 40.7 (36.8–41.5) months, through telephone calls and questionnaires. The date of diagnosis or start of medication use was reported by the patients. If this information was missing, the midpoint between two interview dates was used (10% of cases). Diagnosis of incident type 2 diabetes was not based on plasma glucose concentrations because blood samples were only collected for part of the cohort during the follow-up.
Other Measurements
BMI was calculated as measured weight (kg) divided by the squared height (m2). Educational level was assessed in four categories as primary education, lower secondary education, higher secondary or lower tertiary education, and higher tertiary education. Smoking status was reported in three categories as never, former, or current. Alcohol intake, estimated from the FFQ, was categorized as no (ethanol intake of 0 g/day), low (>0–10 g/day), moderate (>10–20 g/day for women or >10–30 g/day for men), or high (>20 g/day for women or >30 g/day for men). Physical activity was assessed by using the validated Physical Activity Scale for the Elderly (28) and categorized as low (no activity or only light activity, ≤3 METs), intermediate (>0 to <5 days/week of moderate or vigorous activity, >3 METs), or high (≥5 days/week of moderate or vigorous activity, >3 METs). Medication use was coded according to the Anatomical Therapeutic Chemical Classification System. Codes were A10 for antidiabetic drugs, C02, C03, C07, C08, and C09 for antihypertensive drugs, C10AA and C10B for statins, and B01 for antithrombotic drugs (29). Blood lipids and glucose were analyzed by standard kits using a Hitachi 912 Autoanalyzer (Roche Diagnostics, Basel, Switzerland) (22). Blood was collected in fasting state in 44.5% of the cohort (4 to <12 h for 414 patients and ≥12 h for 1,035 patients). Prediabetes was defined as plasma glucose between 6.1 and 6.9 mmol/L after ≥4 h of fasting. Family history of type 2 diabetes was considered present when patients reported at least one parent with type 2 diabetes. To describe overall diet quality of these patients, we computed a healthy nutrient and food score using information from the FFQ, as reported previously (30).
Statistical Analysis
Baseline characteristics are presented as mean and SD for normally distributed variables, median and IQR for skewed variables, or percentage for categorical variables. Spearman correlation was computed between dietary and plasma LA. Survival time in years was computed from the date of study enrollment until 1) onset of type 2 diabetes, 2) death, or 3) end of follow-up. Associations of dietary and plasma LA with incident type 2 diabetes were analyzed using Cox proportional hazard analysis. Proportional hazards assumption was tested by log-log plots and by including time-dependent covariates in the Cox models; all assumptions were met. Hazard ratios (HRs) and 95% CIs were calculated in quintiles of dietary or plasma LA, and the lowest quintile was set as the reference category. The P value for trend across quintiles was obtained by assigning each patient median dietary or plasma LA values for the category, and we modeled this value as continuous in the Cox models. Analyses were repeated with LA on a continuous scale (per 5% energy increase in dietary LA; per 5% increase in plasma LA). Per 5% increase for continuous analysis was chosen mainly because it approximated the range between the extreme quintiles of dietary or plasma LA in the present analysis or was also used by other studies (6,12). Missing values for covariables (n = 1 for smoking, n = 2 for BMI, n = 13 for education level, and n = 16 for physical activity) were imputed with sex-specific median (continuous variable) or mode (categorical variable) to retain all patients in the analyses.
The following models were used: model 1 adjusted for age (years), sex and Alpha Omega Trial treatment code (four categories); model 2 additionally adjusted for physical activity (three categories), smoking status (three categories), educational level (four categories), BMI (kg/m2), family history of type 2 diabetes (yes/no), total energy intake excluding calories from alcohol (kcal/day), alcohol intake (four categories), dietary fiber (g/day), and dietary cholesterol (mg/day). For dietary LA, we additionally investigated a replacement of sum of SFA and TFA by LA in an isocaloric substitution model (model 3). In model 3, we simultaneously included the percentages of energy from LA, carbohydrates, protein, and the remaining FA types: n-3 PUFA and cis-MUFA in the model, and left SFA and TFA out of the model. Coefficients for dietary LA from this Cox model may be interpreted as the estimated effect of replacing a certain percentage of energy contributed by SFA+TFA for equivalent energy from LA, holding constant the total energy intake and other macronutrients. Restricted cubic spline analyses were performed to evaluate the linearity of the associations in the fully adjusted models. Knots were positioned at the 5th, 50th, and 95th percentile, and the value at the 5th percentile of dietary or plasma LA was considered as the reference value (31).
Subgroup analyses were performed by sex, age (<65 or ≥65 years), and obesity (BMI <30 or ≥30 kg/m2), as previously examined in other studies (4,6,12,15), and time since MI (≤5 or >5 years). Additionally, we investigated alcohol intake (no, low, moderate, or high) and statin use (yes or no) since in this cohort we observed a lower plasma LA level in statin users and patients with high alcohol intake compared with patients without these characteristics (27). Potential effect modification by these variables was statistically tested by including interaction terms with plasma LA in model 2. Sensitivity analyses for dietary and plasma LA were performed by excluding patients with prediabetes at baseline.
In order to explore additional confounding or mediating effects of metabolic factors on the association between plasma LA and type 2 diabetes incidence, we added the following covariables to the fully adjusted model (one at a time) in post hoc analyses: triacylglycerols (mmol/L); palmitic acid (16:0, %) and the palmitoleic acid–to–palmitic acid ratio (16:1n-7/16:0) as markers for de novo lipogenesis; and oleic acid (18:1n-9) percentage, as the other major FA present in cholesteryl esters and may be derived from de novo lipogenesis.
Finally, to explore associations between FA metabolism and incident type 2 diabetes, we examined associations of selected markers of FA metabolism with incident type 2 diabetes by using these markers as an exposure variable in model 2. We selected plasma 16:0, 18:1n-9, and the ratio of palmitic acid to LA (16:0/18:2n-6) as markers for liver fat content (16,32), the ratio of arachidonic acid to dihomo-γ-linolenic acid (20:4n-6/20:3n-6) as an estimation of delta-5 desaturase activity, and the ratio γ-linolenic acid to LA (18:3n-6/18:2n-6) as estimated delta-6 desaturase (D6D) activity.
All analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC). Two-sided P values <0.05 were considered statistically significant.
Results
Patients were on average 68.9 ± 5.5 years old, had a BMI of 27.4 ± 3.5 kg/m2 (75% overweight or obese), and 80.5% were men. Median (IQR) time since MI for these patients was 3.5 (1.6–6.3) years. Most of the patients used antithrombotic drugs (97.7%), antihypertensive drugs (89.0%), or statins (86.4%). Dietary and plasma LA were weakly correlated (r = 0.13, P < 0.001). Median dietary LA increased from 3.5 to 8.8% energy across dietary quintiles, with a smaller range (5.6–6.3% energy) across plasma quintiles (Table 1). Median proportions of plasma LA increased from 49.3 to 51.2% of total FA across quintiles of dietary LA, whereas this range was larger (43.9%–56.3% of total FA) across quintiles of plasma LA. During a median follow-up of 41 months (10,277 person-years), 171 cases of type 2 diabetes occurred. No patients were lost during follow-up.
Table 1.
Baseline characteristics of 3,257 post-MI patients from the Alpha Omega Cohort by quintiles of dietary and plasma LA
| Quintiles of dietary LA (% energy) | Quintiles of plasma LA (%) | |||||
|---|---|---|---|---|---|---|
| Q1 (n = 651) | Q3 (n = 651) | Q5 (n = 651) | Q1 (n = 652) | Q3 (n = 651) | Q5 (n = 651) | |
| Median (range) | 3.5 (1.2–4.1) | 5.6 (5.1–6.2) | 8.8 (7.6–13.9) | 43.9 (28.5–46.1) | 50.1 (48.9–51.4) | 56.3 (54.3–67.6) |
| Age, years | 69.4 ± 5.7 | 68.8 ± 5.5 | 68.6 ± 5.4 | 68.2 ± 5.7 | 68.7 ± 5.4 | 69.8 ± 5.5 |
| Men | 471 (72.4) | 536 (82.3) | 569 (87.4) | 504 (77.3) | 511 (78.5) | 553 (85.0) |
| BMI, kg/m2 | 27.6 ± 3.7 | 27.4 ± 3.5 | 27.1 ± 3.2 | 27.6 ± 3.8 | 27.4 ± 3.2 | 26.7 ± 3.3 |
| Time since MI, years | 3.6 (1.5–6.3) | 3.6 (1.7–6.1) | 3.1 (1.4–6.3) | 3.1 (1.4–5.9) | 3.4 (1.5–6.2) | 4.0 (1.8–6.6) |
| Smoking | ||||||
| Never | 136 (20.9) | 102 (15.7) | 86 (13.2) | 92 (14.1) | 109 (16.7) | 112 (17.2) |
| Former | 414 (63.6) | 452 (69.4) | 432 (66.4) | 417 (64.0) | 440 (67.6) | 435 (66.9) |
| Current | 101 (15.5) | 97 (14.9) | 133 (20.4) | 143 (21.9) | 102 (15.7) | 103 (15.8) |
| Physical activity* | ||||||
| Low | 260 (40.2) | 256 (39.4) | 259 (40.0) | 257 (39.5) | 263 (40.4) | 269 (41.3) |
| Middle | 228 (35.3) | 247 (38.0) | 262 (40.5) | 266 (40.9) | 250 (38.4) | 243 (37.3) |
| High | 158 (24.5) | 147 (22.6) | 126 (19.5) | 127 (19.5) | 138 (21.2) | 139 (21.4) |
| Highest level of education | ||||||
| Primary | 124 (19.2) | 133 (20.5) | 128 (19.8) | 120 (18.5) | 125 (19.2) | 137 (21.1) |
| Lower secondary | 233 (36.0) | 228 (35.1) | 268 (41.4) | 223 (34.4) | 249 (38.2) | 229 (35.2) |
| Higher secondary or lower tertiary | 209 (32.3) | 203 (31.2) | 189 (29.2) | 208 (32.0) | 202 (31.0) | 203 (31.2) |
| Higher tertiary | 81 (12.5) | 86 (13.2) | 62 (9.6) | 98 (15.1) | 75 (11.5) | 81 (12.5) |
| Alcohol intake* | ||||||
| No | 32 (4.9) | 18 (2.8) | 35 (5.4) | 27 (4.1) | 17 (2.6) | 36 (5.5) |
| Low | 337 (51.8) | 344 (52.8) | 306 (47.0) | 262 (40.2) | 347 (53.3) | 378 (58.1) |
| Moderate | 172 (26.4) | 179 (27.5) | 202 (31.0) | 170 (26.1) | 182 (28.0) | 183 (28.1) |
| High | 110 (16.9) | 110 (16.9) | 108 (16.6) | 193 (29.6) | 105 (16.1) | 54 (8.3) |
| Medication use | ||||||
| Statins | 560 (86.0) | 564 (86.6) | 563 (86.5) | 602 (92.3) | 587 (90.2) | 464 (71.3) |
| Antithrombotic drugs | 633 (97.2) | 640 (98.3) | 641 (98.5) | 637 (97.7) | 631 (96.9) | 637 (97.8) |
| Antihypertensive drugs | 581 (89.2) | 580 (89.1) | 590 (90.6) | 586 (89.9) | 576 (88.5) | 572 (87.9) |
| Serum lipids, mmol/L†‡§ | ||||||
| Total cholesterol | 4.81 ± 0.95 | 4.73 ± 0.93 | 4.70 ± 0.98 | 4.71 ± 0.98 | 4.66 ± 0.88 | 4.83 ± 0.95 |
| LDL cholesterol | 2.67 ± 0.81 | 2.60 ± 0.76 | 2.59 ± 0.85 | 2.49 ± 0.81 | 2.54 ± 0.75 | 2.80 ± 0.84 |
| HDL cholesterol | 1.34 ± 0.36 | 1.30 ± 0.32 | 1.30 ± 0.35 | 1.32 ± 0.37 | 1.31 ± 0.35 | 1.29 ± 0.33 |
| Triacylglycerols | 1.58 (1.18–2.11) | 1.58 (1.18–2.21) | 1.59 (1.16–2.19) | 1.76 (1.28–2.48) | 1.56 (1.17–2.17) | 1.46 (1.11–1.97) |
| Plasma glucose, mmol/L‡§ | 5.40 (4.96–5.98) | 5.44 (4.96–6.10) | 5.38 (4.90–6.00) | 5.57 (5.04–6.28) | 5.45 (5.00–6.00) | 5.33 (4.90–5.84) |
| Prediabetes* | 39 (6.1) | 39 (6.1) | 36 (5.6) | 53 (8.2) | 34 (5.3) | 36 (5.6) |
| Family history of type 2 diabetes | 104 (16.0) | 104 (16.0) | 104 (16.0) | 108 (16.6) | 101 (15.5) | 108 (16.6) |
| Dietary factors | ||||||
| Energy, kcal/day | 1,750 ± 485 | 1,856 ± 502 | 1,938 ± 502 | 1,775 ± 501 | 1,858 ± 516 | 1,938 ± 490 |
| Protein, % energy | 16.4 ± 3.0 | 15.6 ± 2.7 | 14.6 ± 2.5 | 15.8 ± 2.8 | 15.5 ± 3.0 | 15.2 ± 2.6 |
| Total fat, % energy | 31.5 ± 5.9 | 32.6 ± 4.6 | 38.6 ± 4.9 | 35.0 ± 6.2 | 35.0 ± 5.9 | 36.0 ± 6.2 |
| SFA, % energy | 12.7 ± 3.3 | 13.1 ± 2.8 | 13.5 ± 3.2 | 13.2 ± 3.2 | 12.9 ± 2.9 | 13.0 ± 3.0 |
| cis-MUFA, % energy | 8.4 ± 2.0 | 9.9 ± 1.9 | 11.4 ± 1.8 | 10.1 ± 2.4 | 9.9 ± 2.1 | 9.9 ± 2.2 |
| PUFA, % energy | 5.6 ± 1.9 | 7.1 ± 0.9 | 10.8 ± 1.6 | 7.1 ± 2.2 | 7.6 ± 2.2 | 8.2 ± 2.3 |
| TFA, % energy | 0.73 ± 0.22 | 0.76 ± 0.18 | 0.78 ± 0.16 | 0.76 ± 0.20 | 0.75 ± 0.18 | 0.75 ± 0.18 |
| Carbohydrates, % energy | 52.1 ± 6.2 | 49.7 ± 5.6 | 44.6 ± 5.4 | 49.2 ± 6.4 | 49.4 ± 6.1 | 48.8 ± 6.4 |
| Fiber, g/day | 21.3 ± 7.0 | 21.7 ± 6.7 | 21.1 ± 6.2 | 20.4 ± 6.7 | 21.6 ± 6.6 | 23.2 ± 6.9 |
| Cholesterol, mg/day | 181 ± 68 | 189 ± 70 | 181 ± 68 | 187 ± 72 | 185 ± 66 | 183 ± 68 |
| Healthy nutrient and food score | 22.1 ± 6.2 | 22.7 ± 6.1 | 22.6 ± 6.1 | 21.6 ± 6.3 | 22.6 ± 5.9 | 23.6 ± 5.8 |
| Plasma FA composition | ||||||
| SFA, % total FA | 13.2 ± 1.2 | 13.1 ± 1.0 | 12.9 ± 1.0 | 14.1 ± 1.1 | 13.1 ± 0.7 | 12.1 ± 0.7 |
| MUFA, % total FA | 22.9 ± 3.2 | 22.4 ± 3.1 | 21.6 ± 3.1 | 26.3 ± 3.0 | 22.2 ± 1.7 | 19.1 ± 1.8 |
| PUFA, % total FA | 62.4 ± 4.0 | 63.1 ± 3.9 | 64.1 ± 3.7 | 58.0 ± 3.3 | 63.4 ± 1.8 | 67.6 ± 2.1 |
| n-3 PUFA, % total FA | 2.4 (2.0–3.2) | 2.4 (2.0–3.0) | 2.3 (1.9–2.8) | 2.8 (2.2–3.6) | 2.4 (2.0–3.0) | 2.0 (1.6–2.4) |
| n-6 PUFA, % total FA | 59.5 ± 4.3 | 60.3 ± 4.2 | 61.5 ± 4.1 | 54.7 ± 3.4 | 60.6 ± 1.9 | 65.4 ± 2.2 |
| Arachidonic acid (20:4n-6), % total FA | 8.2 ± 2.0 | 8.3 ± 2.1 | 8.4 ± 2.1 | 9.4 ± 2.2 | 8.6 ± 1.8 | 6.9 ± 1.5 |
| Palmitic acid (16:0), % total FA | 11.4 ± 0.8 | 11.3 ± 0.8 | 11.2 ± 0.8 | 12.1 ± 0.8 | 11.3 ± 0.6 | 10.5 ± 0.6 |
| Palmitoleic acid (16:1n-7), % total FA | 2.9 ± 1.2 | 2.6 ± 1.1 | 2.4 ± 1.0 | 3.9 ± 1.4 | 2.5 ± 0.7 | 1.9 ± 0.6 |
| Oleic acid (18:1n-9), % total FA | 18.0 ± 2.2 | 17.8 ± 2.2 | 17.3 ± 2.2 | 20.3 ± 2.0 | 17.7 ± 1.4 | 15.6 ± 1.5 |
| Liver fat proxy (16:0/18:2n-6) | 0.23 ± 0.04 | 0.23 ± 0.04 | 0.22 ± 0.03 | 0.28 ± 0.03 | 0.22 ± 0.01 | 0.18 ± 0.01 |
| Estimated D6D activity (18:3n-6/18:2n-6) | 0.021 ± 0.008 | 0.020 ± 0.008 | 0.020 ± 0.008 | 0.029 ± 0.009 | 0.020 ± 0.006 | 0.013 ± 0.004 |
Values are shown as mean ± SD, median (IQR), or n (%) unless otherwise indicated. % energy, percentage of total energy intake, excluding energy from alcohol.
Classification described in text (research design and methods).
To convert to mg/dL, divide by 0.02586 for total, LDL, and HDL cholesterol and by 0.01129 for triacylglycerols.
Fasting <4 h (n = 1,676), fasting 4 to <8 h (n = 314), fasting 8 to <12 h (n = 100), fasting ≥12 h (n = 1,035), fasting status unknown (n = 132).
Missing values for 61 patients for total cholesterol, HDL cholesterol, and triacylglycerols, 253 patients for LDL cholesterol, 31 patients for plasma glucose, and 1 patient for healthy nutrient and food score.
Dietary LA
Patients with higher dietary LA had a lower BMI and were more likely to be men and to be current smokers. The proportion of patients with high alcohol intake, using statins, and the concentrations of plasma glucose and serum triacylglycerols did not vary across quintiles of dietary LA. Those in the higher quintiles of dietary LA had higher intake of total energy, proportion of energy from total fat, SFA, and cis-MUFA, and lower protein and carbohydrates. Fiber intake was similar across quintiles (Table 1).
Dietary LA was not associated with type 2 diabetes risk after adjusting for demographic, lifestyle characteristics, dietary fiber, and dietary cholesterol (model 2) across quintiles (Q) (HRQ5vs.Q1 0.88; 95% CI 0.54, 1.44; P-trend = 0.97) (Table 2). When SFA and TFA were theoretically replaced by LA, dietary LA was not associated with type 2 diabetes risk across quintiles (HRQ5vs.Q1 0.78; 95% CI 0.36, 1.72; P-trend = 0.84) and continuously (HRper 5% 1.18; 95% CI 0.59, 2.35; P = 0.64). Exclusion of patients with prediabetes yielded similar results as in the total sample.
Table 2.
HRs and 95% CI for dietary LA in quintiles and per 5% energy and incident type 2 diabetes in 3,257 post-MI patients from the Alpha Omega Cohort
| Quintiles of dietary LA | Per 5% energy | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Q1 (n = 651) | Q2 (n = 652) | Q3 (n = 651) | Q4 (n = 652) | Q5 (n = 651) | P-trend* | |||
| Median dietary LA, % energy | 3.5 | 4.6 | 5.6 | 6.8 | 8.8 | |||
| Cases | 37 | 30 | 34 | 39 | 31 | |||
| Model 1† | 1.00 | 0.79 (0.49, 1.28) | 0.90 (0.56, 1.43) | 1.04 (0.66, 1.64) | 0.81 (0.50, 1.31) | 0.72 | 1.01 (0.70, 1.44) | 0.96 |
| Model 2‡ | 1.00 | 0.80 (0.49, 1.29) | 0.95 (0.59, 1.51) | 1.12 (0.71, 1.76) | 0.88 (0.54, 1.44) | 0.97 | 1.09 (0.76, 1.58) | 0.64 |
| Model 3§ | 1.00 | 0.72 (0.44, 1.19) | 0.86 (0.51, 1.45) | 1.01 (0.56, 1.81) | 0.78 (0.36, 1.72) | 0.84 | 1.18 (0.59, 2.35) | 0.64 |
% energy, percentage of total energy intake, excluding energy from alcohol.
P values for trend were obtained by assigning each patient median dietary LA for the category and this value was modeled as continuous.
Model 1 was adjusted for age (continuous; years), sex (men/women), and Alpha Omega Trial treatment code (four categories).
Model 2: model 1 plus physical activity (three categories), smoking status (three categories), educational level (four categories), BMI (continuous; kg/m2), family history of type 2 diabetes (yes/no), total energy intake (excluding calories from alcohol, continuous; kcal/day), alcohol consumption (four categories), dietary fiber (g/day; continuous), and dietary cholesterol (mg/day; continuous).
Model 3 (substitution of SFA and TFA for LA): model 2 plus dietary protein (% energy; continuous), carbohydrates (% energy; continuous), n-3 PUFAs (% energy; continuous), and cis-MUFAs (% energy; continuous).
Plasma LA
Patients with higher plasma LA had lower BMI, plasma glucose, and serum triacylglycerol concentrations. They were less likely to be current smokers, to have high alcohol intake, and to be statin users compared with those in the lower quintiles. Patients with higher plasma LA also had higher total energy intake, percentage of energy from PUFA, and dietary fiber intake (Table 1).
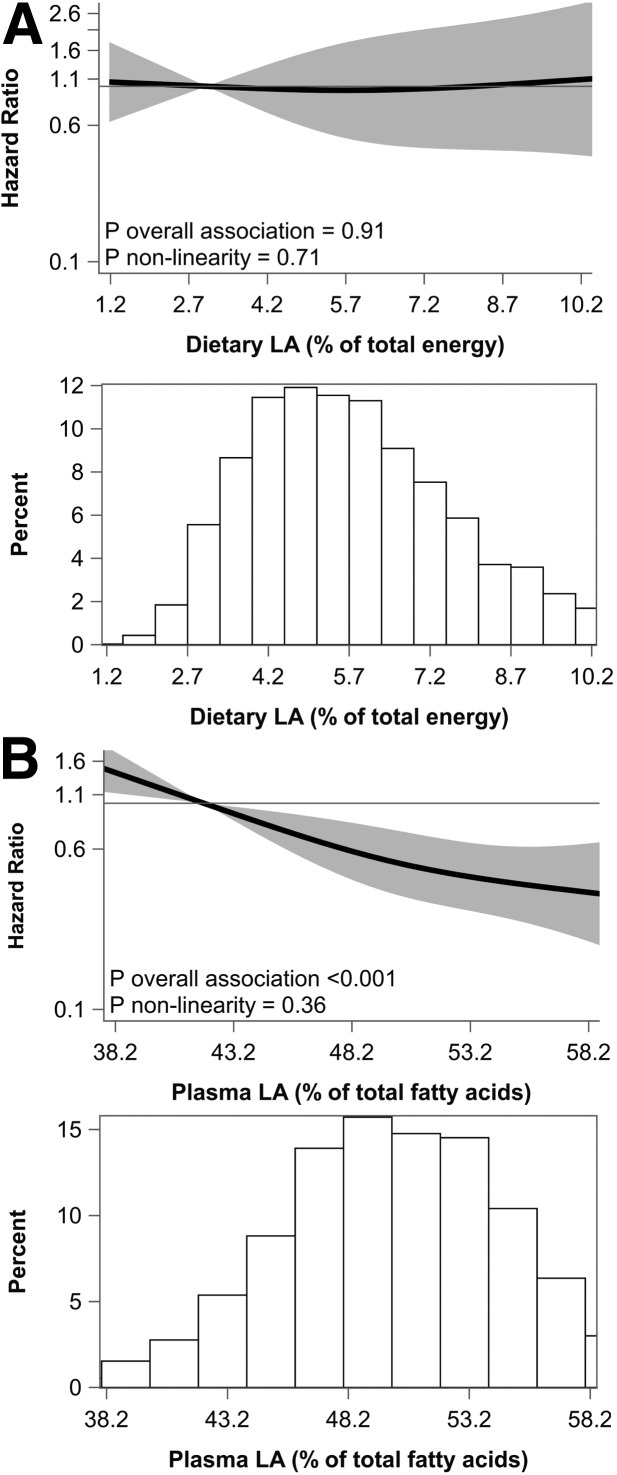
After multivariable adjustments, there was a strong inverse association between plasma LA across quintiles (HRQ5vs.Q1 0.44; 95% CI 0.26, 0.75; P-trend = 0.001) (Table 3). In continuous analyses, each 5% higher plasma LA was associated with 27% lower risk of type 2 diabetes (HR 0.73; 95% CI 0.62, 0.86; P < 0.001). Results from restricted cubic splines analysis indicated a possible linear relation (P nonlinearity = 0.36) (Fig. 1). Association between plasma LA and incident type 2 diabetes was modified by sex (P interaction = 0.009), with an inverse association being more pronounced in women (Supplementary Table 1). In this cohort, women had a lower median (IQR) alcohol intake (2.7 [0.0–9.6] g/day) than men (9.8 [3.0–22.1] g/day). Age, BMI, alcohol intake, and statin use did not modify the associations.
Table 3.
HRs and 95% CI for plasma LA in quintiles and per 5% and incident type 2 diabetes in 3,257 post-MI patients from the Alpha Omega Cohort
| Quintiles of plasma LA | Per 5% | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Q1 (n = 652) | Q2 (n = 650) | Q3 (n = 651) | Q4 (n = 653) | Q5 (n = 651) | P-trend* | |||
| Median plasma LA, % | 43.9 | 47.6 | 50.1 | 52.8 | 56.3 | |||
| Cases | 49 | 38 | 31 | 31 | 22 | |||
| Model 1† | 1.00 | 0.76 (0.50, 1.16) | 0.63 (0.40, 0.98) | 0.61 (0.39, 0.96) | 0.42 (0.26, 0.70) | <0.001 | 0.73 (0.63, 0.85) | <0.001 |
| Model 2‡ | 1.00 | 0.74 (0.48, 1.14) | 0.64 (0.40, 1.00) | 0.60 (0.38, 0.94) | 0.44 (0.26, 0.75) | 0.001 | 0.73 (0.62, 0.86) | <0.001 |
P values for trend were obtained by assigning each patient median plasma LA for the category and this value was modeled as continuous.
Model 1 was adjusted for age (continuous; years), sex (men/women), and Alpha Omega Trial treatment code (four categories).
Model 2: model 1 plus physical activity (three categories), smoking status (three categories), educational level (four categories), BMI (continuous; kg/m2), family history of type 2 diabetes (yes/no), total energy intake (excluding calories from alcohol, continuous; kcal/day), alcohol intake (four categories), dietary fiber (g/day; continuous), and dietary cholesterol (mg/day; continuous).
Figure 1.
Associations of dietary LA (replacing SFA + TFA) (A) and plasma LA (B) with incident type 2 diabetes in 3,257 post-MI patients. Solid lines are risk estimates evaluated by restricted cubic splines from Cox models showing the shape of associations on a continuous scale with three knots located at the 5th, 50th, and 95th percentiles. The y-axis shows the multivariable HRs for type 2 diabetes incidence for any dietary or plasma LA value compared with the reference values set at the 5th percentile of dietary LA (3.0% energy) or 5th percentile of plasma LA (41.9%). Gray areas indicate 95% CIs. Results are presented for the fully adjusted models for dietary LA (model 3; adjusted for variables in model 2 [age, sex, Alpha Omega Trial treatment code, physical activity, smoking status, educational level, BMI, family history of type 2 diabetes, total energy intake excluding alcohol, alcohol consumption, dietary fiber, dietary cholesterol] plus dietary protein, carbohydrates, n-3 PUFA, and cis-MUFA) and plasma LA (model 2). Distribution of values of dietary or plasma LA are displayed in a histogram under each spline; % energy, percentage of total energy intake, excluding energy from alcohol.
In sensitivity analyses including only patients without prediabetes at baseline (n = 3,073), the associations were similar to the main analysis, as shown in results across quintiles (HRQ5vs.Q1 0.50; 95% CI 0.28, 0.88; P for trend = 0.014) and on a continuous scale (HRper 5% 0.76; 95% CI 0.63, 0.91; P = 0.003) (Supplementary Table 2).
Post Hoc Analyses for Plasma LA
The associations between plasma LA and incident type 2 diabetes were attenuated when the model included plasma 18:1n-9, 16:0, or 16:1n-7/16:0. Inclusion of triacylglycerols did not change the observed associations (Supplementary Table 3). Further examination of the markers of FA metabolism showed that most of these markers were associated with higher type 2 diabetes risk, with HRs ranging from 1.74 for estimated D6D activity to 2.65 for 18:1n-9, when comparing extreme quintiles within each FA marker (Supplementary Table 4). Estimated delta-6 desaturase activity was not associated with type 2 diabetes risk.
Conclusions
In the current study, a 5% increase in plasma LA in cholesteryl esters was linearly associated with a 27% lower risk of type 2 diabetes in a high-risk population of post-MI patients, independent of age, sex, BMI, family history, and lifestyle characteristics. Dietary LA was not associated with type 2 diabetes risk.
Our findings for dietary LA are not in line with previous observational studies replacing SFA with PUFA in a theoretical substitution that found inverse associations with incident type 2 diabetes in cohorts of women (11,12). A recent analysis using data from three U.S. cohorts showed that substitution of 5% energy from dietary LA for SFA and 2% energy for TFA was associated with 14% and 17% lower risk of type 2 diabetes, respectively, and that these associations were consistently observed in men and in women (8). A meta-analysis of 13 randomized controlled feeding trials showed that isocaloric replacement of PUFA for SFA or carbohydrates, where primarily n-6 FA was used as the intervention arm, decreased insulin resistance but did not affect glucose concentrations (33). Total PUFA also includes n-3 FA, the influence of which may be limited but cannot be excluded (34).
Our findings for plasma LA are in line with previous studies in mostly general populations. Two recent large prospective studies, including a comparative meta-analysis (14) and a pooled analysis (15), consistently showed that increasing proportions of LA in different blood fractions, including cholesteryl esters, are associated with a lower risk of type 2 diabetes. A possible explanation for the association between higher plasma LA and lower type 2 diabetes risk is through the increase of the unsaturation degree of the membrane lipids. A higher degree of unsaturation may increase membrane fluidity and the number of insulin receptors and decrease receptor affinity, which could result in higher insulin sensitivity (35,36). We also observed that the inverse association between plasma LA and type 2 diabetes risk was more pronounced in women. However, this effect measure modification by sex was not observed in other studies (4,14,15). We suspected that the stronger inverse association in women could be because the alcohol intake of male patients in this cohort was higher than the intake of the female patients. Furthermore, when the association between plasma LA and type 2 diabetes risk was examined in patients with different alcohol intake categories, patients with no alcohol intake were shown to have the lowest type 2 diabetes risk, although the interaction term did not reach significance. The possible influence of other factors related to metabolic condition in these patients on the association of LA and type 2 diabetes risk will be further discussed below.
In earlier population-based studies that examined both plasma and dietary LA within the same cohort, generally inverse associations with type 2 diabetes were found for plasma LA, while the associations for dietary LA varied (3–5). To explain the inconsistent observations between plasma and dietary LA, the authors of the earlier studies attributed the null association for dietary LA to the subjective nature of the dietary assessment method, which is suggested to provide a lower ability to detect true associations than objective LA biomarkers (4,5). Another possible explanation was the existence of metabolic differences between individuals who later did and did not develop type 2 diabetes (3,4).
In relation to the dietary assessment method, dietary intake data used in the present analyses were collected by a validated FFQ specifically designed for the assessment of FA intake. We also recently showed that replacing dietary SFA and TFA by unsaturated FA was associated with a lower risk of mortality from coronary heart disease in the same cohort (20). This further supported the qualitative validity of our FFQ to rank patients adequately according to their FAs intake. Explanation for the inconsistent observations for plasma and dietary LA in the current study may therefore be related not solely to the dietary assessment method but also to other factors, such as metabolic conditions influencing the plasma composition of FA.
Populations at higher risk for type 2 diabetes may already have altered plasma FA composition, including a decrease in the plasma LA proportion. In our population of post-MI patients, those with low plasma LA had a more adverse metabolic risk profile, with higher BMI, plasma glucose, and serum triacylglycerol concentrations. They also consumed more alcohol and often were statin users. Previously, we reported a generally weak correlation between dietary and circulating LA (Spearman correlation <0.20) and that this weak correlation might be partly due to alcohol and/or statins use (27). The more adverse metabolic profile observed in patients with low plasma LA was not observed for dietary LA.
It is possible that the patients with a more adverse metabolic risk profile had an impaired liver function, such as in nonalcoholic fatty liver disease, and this may explain why we observed an increased risk of type 2 diabetes in patients with a low plasma LA. Nonalcoholic fatty liver disease has been strongly associated with an increased risk of type 2 diabetes (37). In nonalcoholic fatty liver disease, de novo lipogenesis is upregulated and/or FA oxidation is downregulated (38). These processes may promote a larger proportion of plasma SFA and MUFA, leading to a smaller plasma LA proportion (13). In addition, insulin resistance has been associated with high D6D activity and low plasma LA proportion (4). Indeed, in the post hoc analyses of the present cohort, we found that higher proportion of the FA in the de novo lipogenesis pathway, oleic acid and palmitic acid, and higher estimated D6D activity were associated with increased type 2 diabetes risk. Furthermore, adjustments for markers of de novo lipogenesis (palmitic acid, oleic acid, and ratio of palmitoleic acid to palmitic acid) also attenuated associations of plasma LA. These changes in FA composition due to the impaired metabolism may additionally explain the weak correlation between plasma and dietary LA in our population.
This study has some limitations. The Alpha Omega Cohort is a rather homogenous population of predominantly male post-MI patients; therefore, the range of dietary LA and plasma LA proportion might not be comparable to other studies in healthy populations. Nevertheless, the range of dietary and plasma LA in this patient population was quite similar to studies in generally healthy populations (3–6,8,15). Other limitations are the relatively short follow-up time (median of 41 months) and the self-reported diagnosis of incident type 2 diabetes, which might have led to an underestimation of the number of cases and attenuation of associations.
To conclude, in this cohort of post-MI patients, plasma LA, but not dietary LA, was associated with lower type 2 diabetes risk. Our results suggest that metabolic conditions affecting plasma LA, rather than dietary LA intake, may be responsible for this association. Further research on the factors affecting plasma LA and its utility as a biomarker of LA intake in patient populations is warranted.
Supplementary Material
Article Information
Acknowledgments. The authors thank Eveline Waterham (Division of Human Nutrition and Health, Wageningen University) for logistic support and data management and Dr. Paul Hulshof, Robert Hovenier, and Marlies Diepeveen-de Bruin (Division of Human Nutrition and Health, Wageningen University) for analysis of plasma FA.
Funding. The Alpha Omega Cohort emerged from the Alpha Omega Trial (ClinicalTrials.gov identifier NCT00127452), which was funded by the Dutch Heart Foundation (Hartstichting) (grant 200T401), National Institutes of Health National Heart, Lung, and Blood Institute (grant R01HL076200), and Office of Dietary Supplements. This research was supported in part by the Netherlands Organisation for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek) through its Graduate Programme on Food Structure, Digestion and Health.
Duality of Interest. This research was supported in part by Upfield. Unilever R&D Vlaardingen provided financial support for plasma FA determinations in the Alpha Omega Cohort. A.J.W. and P.L.Z. are employed by Unilever, Vlaardingen, the Netherlands. J.M.G. received funding from Unilever R&D for epidemiological studies of dietary and circulating FAs and cardiovascular disease. Unilever is a producer of food consumer products. It divested its spreads business, which has operated since July 2018 under the name Upfield. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.P., A.J.W., and M.C.H. wrote the manuscript. K.P., A.J.W., M.C.H., L.K.K., S.S.S.-M., J.d.G., P.L.Z., and J.M.G. interpreted the results and critically revised the manuscript for intellectual content. K.P. and M.C.H. analyzed data. S.S.S.-M. and J.M.G. designed research. J.M.G. had primary responsibility for the final content and handled funding and supervision. All of the authors read and approved the final manuscript. J.M.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the EPI|Lifestyle 2018 Scientific Sessions of the American Heart Association, New Orleans, LA, 20–23 March 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1483/-/DC1.
References
- 1.FAO/WHO (Ed.) Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation. Geneva, Switzerland, World Health Organization, 2010. (publ. no. FAO Food and Nutrition paper 91) [PubMed] [Google Scholar]
- 2.Harika RK, Eilander A, Alssema M, Osendarp SJM, Zock PL. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: a systematic review of data from 40 countries. Ann Nutr Metab 2013;63:229–238 [DOI] [PubMed] [Google Scholar]
- 3.Hodge AM, English DR, O’Dea K, et al. . Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–197 [DOI] [PubMed] [Google Scholar]
- 4.Kröger J, Zietemann V, Enzenbach C, et al. . Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2011;93:127–142 [DOI] [PubMed] [Google Scholar]
- 5.Patel PS, Sharp SJ, Jansen E, et al. . Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010;92:1214–1222 [DOI] [PubMed] [Google Scholar]
- 6.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–424 [DOI] [PubMed] [Google Scholar]
- 7.Dow C, Mangin M, Balkau B, et al. . Fatty acid consumption and incident type 2 diabetes: an 18-year follow-up in the female E3N (Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale) prospective cohort study. Br J Nutr 2016;116:1–9 [DOI] [PubMed] [Google Scholar]
- 8.Zong G, Liu G, Willett WC, et al. . Associations between linoleic acid intake and incident type 2 diabetes among U.S. men and women. Diabetes Care 2019;42:1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brostow DP, Odegaard AO, Koh W-P, et al. . Omega-3 fatty acids and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr 2011;94:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ericson U, Hellstrand S, Brunkwall L, et al. . Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr 2015;101:1065–1080 [DOI] [PubMed] [Google Scholar]
- 11.Meyer KA, Kushi LH, Jacobs DR Jr., Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 2001;24:1528–1535 [DOI] [PubMed] [Google Scholar]
- 12.Salmerón J, Hu FB, Manson JE, et al. . Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–1026 [DOI] [PubMed] [Google Scholar]
- 13.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–380 [DOI] [PubMed] [Google Scholar]
- 14.Forouhi NG, Imamura F, Sharp SJ, et al. . Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med 2016;13:e1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JHY, Marklund M, Imamura F, et al.; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) . Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol 2017;5:965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosqvist F, Bjermo H, Kullberg J, et al. . Fatty acid composition in serum cholesterol esters and phospholipids is linked to visceral and subcutaneous adipose tissue content in elderly individuals: a cross-sectional study. Lipids Health Dis 2017;16:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lankinen MA, Stančáková A, Uusitupa M, et al. . Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia 2015;58:2533–2544 [DOI] [PubMed] [Google Scholar]
- 18.Zengin E, Bickel C, Schnabel RB, et al.; Christoph Sinning for the AtheroGene–Study Investigators . Risk factors of coronary artery disease in secondary prevention--results from the AtheroGene--study. PLoS One 2015;10:e0131434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36:1163–1170 [DOI] [PubMed] [Google Scholar]
- 20.Mölenberg FJM, de Goede J, Wanders AJ, Zock PL, Kromhout D, Geleijnse JM. Dietary fatty acid intake after myocardial infarction: a theoretical substitution analysis of the Alpha Omega Cohort. Am J Clin Nutr 2017;106:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group . n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–2026 [DOI] [PubMed] [Google Scholar]
- 22.Geleijnse JM, Giltay EJ, Schouten EG, et al.; Alpha Omega Trial Group . Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. Am Heart J 2010;159:539–546.e2 [DOI] [PubMed] [Google Scholar]
- 23.Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr 1993;58:489–496 [DOI] [PubMed] [Google Scholar]
- 24.Feunekes IJ, Van Staveren WA, Graveland F, De Vos J, Burema J. Reproducibility of a semiquantitative food frequency questionnaire to assess the intake of fats and cholesterol in The Netherlands. Int J Food Sci Nutr 1995;46:117–123 [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Public Health and the Environment (RIVM) (Ed.) Nederlands Voedingsstoffenbestand (NEVO) (Dutch Food Composition Database). Bilthoven, the Netherlands, National Institute for Public Health and the Environment (RIVM), 2006 [Google Scholar]
- 26.Giltay EJ, Geleijnse JM, Schouten EG, Katan MB, Kromhout D. High stability of markers of cardiovascular risk in blood samples. Clin Chem 2003;49:652–655 [DOI] [PubMed] [Google Scholar]
- 27.Pertiwi K, Kok DE, Wanders AJ, de Goede J, Zock PL, Geleijnse JM. Circulating n-3 fatty acids and linoleic acid as indicators of dietary fatty acid intake in post-myocardial infarction patients. Nutr Metab Cardiovasc Dis 2019;29:343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuit AJ, Schouten EG, Westerterp KR, Saris WHM. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol 1997;50:541–546 [DOI] [PubMed] [Google Scholar]
- 29.WHO Collaborating Centre for Drug Statistics Methodology (Ed.) Anatomical Therapeutic Chemical Classification System (ATC). Oslo, Norway, World Health Organization, 2009 [Google Scholar]
- 30.Sijtsma FPC, Soedamah-Muthu SS, de Goede J, et al. . Healthy eating and lower mortality risk in a large cohort of cardiac patients who received state-of-the-art drug treatment. Am J Clin Nutr 2015;102:1527–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–1057 [DOI] [PubMed] [Google Scholar]
- 32.Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest 1996;97:2081–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanders AJ, Blom WAM, Zock PL, Geleijnse JM, Brouwer IA, Alssema M. Plant-derived polyunsaturated fatty acids and markers of glucose metabolism and insulin resistance: a meta-analysis of randomized controlled feeding trials. BMJ Open Diabetes Res Care 2019;7:e000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JHY, Micha R, Imamura F, et al. . Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 2012;107(Suppl. 2):S214–S227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginsberg BH, Brown TJ, Simon I, Spector AA. Effect of the membrane lipid environment on the properties of insulin receptors. Diabetes 1981;30:773–780 [DOI] [PubMed] [Google Scholar]
- 36.Pilon M. Revisiting the membrane-centric view of diabetes. Lipids Health Dis 2016;15:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballestri S, Zona S, Targher G, et al. . Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:936–944 [DOI] [PubMed] [Google Scholar]
- 38.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.