Abstract
OBJECTIVE
Prevalence and prognostic impact of cardiovascular disease differ between patients with or without diabetes. We aimed to explore differences in the prevalence and prognosis of myocardial ischemia by automated quantification of total perfusion deficit (TPD) among patients with and without diabetes.
RESEARCH DESIGN AND METHODS
Of 20,418 individuals who underwent single-photon emission computed tomography myocardial perfusion imaging, 2,951 patients with diabetes were matched to 2,951 patients without diabetes based on risk factors using propensity score. TPD was categorized as TPD = 0%, 0% < TPD < 1%, 1% ≤ TPD < 5%, 5% ≤ TPD ≤ 10%, and TPD >10%. Major adverse cardiovascular events (MACE) were defined as a composite of all-cause mortality, myocardial infarction, unstable angina, or late revascularization.
RESULTS
MACE risk was increased in patients with diabetes compared with patients without diabetes at each level of TPD above 0 (P < 0.001 for interaction). In patients with TPD >10%, patients with diabetes had greater than twice the MACE risk compared with patients without diabetes (annualized MACE rate 9.4 [95% CI 6.7–11.6] and 3.9 [95% CI 2.8–5.6], respectively, P < 0.001). Patients with diabetes with even very minimal TPD (0% < TPD < 1%) experienced a higher risk for MACE than those with 0% TPD (hazard ratio 2.05 [95% CI 1.21–3.47], P = 0.007). Patients with diabetes with a TPD of 0.5% had a similar MACE risk as patients without diabetes with a TPD of 8%.
CONCLUSIONS
For every level of TPD >0%, even a very minimal deficit of 0% < TPD < 1%, the MACE risk was higher in the patients with diabetes compared with patients without diabetes. Patients with diabetes with minimal ischemia had comparable MACE risk as patients without diabetes with significant ischemia.
Introduction
Diabetes is an important public health problem, estimated to be present in 25% of individuals over 65 years old in the U.S. in 2017 (1). Numerous previous studies have demonstrated the prognostic value of visually assessed single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) in patients with diabetes (2–4); however, little has been reported regarding quantitative assessment of myocardial perfusion in relation to diabetes. In general populations of patients undergoing SPECT MPI, prior studies have shown that quantitative assessment of the extent and severity of stress perfusion defect using the stress total perfusion deficit (TPD) have provided independent prognostic information (5–7) and that even minimal abnormalities of TPD are associated with increased risk (8,9). However, the prognostic value of quantitative stress perfusion defects in the population with diabetes has not been explored.
Once cardiovascular disease is established, it is associated with a more rapid progression and poorer outcomes in patients with diabetes compared with patients without diabetes (10–12). We hypothesized that the prognostic value of myocardial ischemia might be different between patients with and without diabetes according to myocardial ischemic burden and that the presence of even a minimal perfusion deficit might have greater prognostic significance in patients with diabetes. In a group of propensity-matched patients with and without diabetes undergoing SPECT MPI, we aimed to explore the relationships regarding major adverse cardiac events (MACE) and automated quantification of TPD on SPECT MPI.
Research Design and Methods
Study Population
We used data from subjects enrolled in the REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT). The REFINE SPECT is an international, multicenter, observational cohort study of patients undergoing SPECT MPI with cadmium zinc telluride cameras, designed to evaluate the diagnosis and prognosis using quantitative SPECT MPI. Details of the rationale and design of the REFINE SPECT have been described previously (13). The overall population of REFINE SPECT includes patients with suspected or known coronary artery disease (CAD) who were referred for SPECT imaging at five sites across three countries (Canada, Israel, and the U.S.) from 2009 to 2014. Participating centers enrolled patients, collected data, deidentified clinical and image data, and transfered data to the central core laboratory (Cedars-Sinai Medical Center). All transferred data were checked by experienced nuclear cardiologists at the central core laboratory. Among 20,418 patients, 14,303 patients (3,122 patients with diabetes [22%] and 11,181 patients without diabetes [78%]) were identified after excluding individuals who had a prior history of myocardial infarction, coronary revascularization, cardiac transplantation, and early revascularization (≤90 days of MPI). The institutional review board committees approved the study protocol at each participating center and at the core laboratory.
Image Acquisition
SPECT MPI scanning was performed in all participants using D-SPECT (Spectrum-Dynamics, Haifa, Israel) scanners or GE Discovery NM 530c scanners (GE Healthcare, Haifa, Israel) (14,15). The image acquisition protocols for the registry included rest-stress or stress-rest 1-day, rest-stress 2-day, or stress-only protocols. Stress protocols include symptom-limited Bruce/modified Bruce protocol treadmill exercise testing or pharmacological stress testing combined with low-level exercise whenever possible (16).
Image Process and Quantification of Perfusion Defect
All anonymized Digital Imaging and Communications in Medicine (DICOM) images were transferred from each participating center to the Core laboratory. Experienced core laboratory technologists conducted quality control and were blinded regarding clinical and prognostic information. Quantitative Perfusion SPECT and Quantitative Gated SPECT software programs (Cedars-Sinai Medical Center, Los Angeles, CA) were used to generate myocardial contours, which were manually adjusted to correspond to the myocardium by an experienced core laboratory technologist when necessary. TPD was estimated using stress images by previously described methods (17). The upright position images from the D-SPECT and supine position images from the GE scanner were used to estimate TPD.
Study End Point
The primary end point was MACE. MACE was defined as a composite of all-cause death, myocardial infarction, unstable angina, and late revascularization (>3 months after image acquisition). Patient follow-up was performed locally at each participating center at the time of enrollment. Mortality status was determined using the Social Security Death Index at the centers in the U.S., the Ministry of Health National Death Database at the centers in Israel, and chart review of hospital and medical office records through the OACIS Clinical Information System at the centers in Canada (13). Information regarding revascularization, unstable angina, and myocardial infarction were collected from e-mail questionnaires, telephone contact or medical records (including all clinics, cardiology groups, insurance registries, and hospital visits). For each patient considered to have had MACE, medical records were reviewed and verified by site physicians. After medical record and physician review, all data and events were transferred to the core laboratory. Among 20,489 of initial enrollments, 71 patients were excluded because of lost follow-up, missing clinical information, or nondiagnostic MPI. The first MACE for each patient was recorded.
Statistical Analysis
To reduce the impact of differences in baseline characteristics among patients with and without diabetes, we adjusted for confounding factors using propensity score matching. Propensity score matching is a reliable method to adjust for confounding in observational studies (18). Propensity scores were calculated from the predicted probabilities of a nonparsimonious multiple logistic regression model predicting diabetes status on the basis of the following variables: age, sex, BMI, hypertension, dyslipidemia, current smoking, family history of CAD, type of stress test (pharmacology or exercise), and scanner type. Subsequently, propensity scores were used to match patients with diabetes to patients without diabetes using the Mahalanobis nearest-neighbor matching algorithm based on a caliper of 0.001 (19).
Continuous variables are presented as mean ± SD, and categorical variables are presented as counts (proportions). The normality of the variables was tested by the Shapiro-Wilk test. Continuous variables were compared by unpaired Student t test (normal distribution) or Wilcoxon signed rank test (skewed distributions) and categorical variables by Pearson χ2 test. Paired Student t test (normal distribution) or Wilcoxon matched-pairs signed ranks test (skewed distributions) were performed for paired continuous variables, and the McNemar test was performed for paired categorical variables. The subjects were categorized by the presence of perfusion defect (TPD = 0% or TPD >0%), as well as the extent of perfusion defect (no deficit [0%], very minimal deficit [0% < TPD < 1%], minimal deficit [1 ≤ TPD < 5%], mild deficit [5 ≤ TPD ≤ 10%], and moderate-to-severe deficit [>10%]). The threshold of TPD was derived from previous prognostic analysis (9). Interactions between diabetes and TPD categories on MACE outcomes were assessed in a Cox model containing diabetes and TPD. The interaction term was significant, and the Wald P value for the interaction term was reported. The annualized MACE rate was calculated to determine the risk of MACE across TPD category. An exponential model was used to evaluate the annualized MACE rate according to the continuous TPD level. Cumulative incidences of MACE were estimated for each TPD category using the cumulative incidence function (20,21). A Cox regression analysis was used to estimate the risk of MACE across diabetes groups in each TPD category. The adjusted hazard ratio (HR) was reported with 95% CIs. In addition, competing risks regression was used to perform subdistribution hazards model for MACE risk to adjust for the impact of competing risk on model performance (22). Multivariable models were adjusted for age, sex, BMI, hypertension, dyslipidemia, smoking status, family history of CAD, stress test type, and center location. The predicted probability of undergoing MACE at 1 year and 5 year for each patient was estimated using a Cox model consisting of diabetes status and TPD%. A two-tailed P value of <0.05 was considered statistically significant. All statistical analyses were performed using STATA, version 13 (StataCorp LP, College Station, TX).
Results
Baseline Characteristics
Prior to matching, patients with diabetes were significantly older and had a higher prevalence of most cardiovascular risk factors, but a lower prevalence of family history of CAD compared with patients without diabetes (Table 1). A higher proportion of patients with diabetes underwent pharmacological stress relative to patients without diabetes. After risk adjustment by propensity score matching, all matching parameters did not differ between the patients with and without diabetes. Both groups had a high prevalence of comorbidities, such as hypertension (79%) and dyslipidemia (73%). The proportion of subjects in each TPD category was similar between the patients with and without diabetes; however, the prevalence of TPD >10% was higher in the group with diabetes (Table 1) (TPD >10%, diabetes vs. nondiabetes: 7.6% vs. 5.8%, P = 0.007).
Table 1.
Baseline characteristics in the study population before and after matching
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Patients with diabetes (n = 3,122) | Patients without diabetes (n = 11,181) | P value | Patients with diabetes (n = 2,951) | Patients without diabetes (n = 2,951) | P value | |
| Age, years | 64 ± 11 | 62 ± 12 | <0.001 | 64 ± 11 | 65 ± 12 | 0.077 |
| Sex, male | 1,597 (51) | 5,768 (51) | 0.668 | 1,506 (51) | 1,519 (51) | 0.753 |
| BMI, kg/m2 | 31.4 ± 7.7 | 27.8 ± 5.9 | <0.001 | 31.0 ± 7.3 | 30.8 ± 7.2 | 0.294 |
| Hypertension | 2,462 (79) | 5,743 (51) | <0.001 | 2,309 (78) | 2,323 (79) | 0.585 |
| Dyslipidemia | 2,299 (74) | 5,484 (49) | <0.001 | 2,153 (73) | 2,142 (73) | 0.726 |
| Family history of CAD | 739 (24) | 3,247 (29) | <0.001 | 701 (24) | 694 (24) | 0.853 |
| Smoking | 490 (16) | 2,216 (20) | <0.001 | 470 (16) | 472 (16) | 0.971 |
| Stress type | <0.001 | 0.240 | ||||
| Exercise | 1,239 (40) | 6,086 (54) | 1,198 (40) | 1,155 (39) | ||
| Pharmacology | 1,883 (60) | 5,095 (46) | 1,753 (60) | 1,796 (61) | ||
| Site | <0.001 | 0.004 | ||||
| Assuta | 1,253 (40) | 3,949 (35) | <0.001 | 1,200 (41) | 1,126 (38) | 0.049 |
| BW | 363 (12) | 1,264 (11) | 0.616 | 349 (12) | 327 (11) | 0.369 |
| CSMC | 645 (21) | 2,119 (19) | 0.033 | 597 (20) | 578 (20) | 0.536 |
| Oregon | 530 (17) | 1,666 (15) | 0.004 | 490 (17) | 513 (17) | 0.425 |
| Ottawa | 331 (11) | 2,183 (20) | <0.001 | 315 (11) | 407 (14) | <0.001 |
| TPD categories | <0.001 | 0.019 | ||||
| No deficit (TPD = 0%) | 318 (10) | 1,321 (12) | 0.012 | 303 (10) | 311 (11) | 0.733 |
| Very minimal (0% < TPD < 1%) | 697 (22) | 3,004 (27) | <0.001 | 658 (22) | 719 (24) | 0.060 |
| Minimal (1% ≤ TPD < 5%) | 1,428 (46) | 5,067 (45) | 0.675 | 1,356 (47) | 1,378 (46) | 0.566 |
| Mild (5% ≤ TPD ≤ 10%) | 435 (14) | 1,261 (11) | <0.001 | 409 (14) | 370 (13) | 0.134 |
| Moderate to severe (TPD >10%) | 244 (8) | 528 (5) | <0.001 | 225 (8) | 173 (6) | 0.007 |
Data are presented as mean ± SD or n (%). Assuta, Assuta Medical Center; BW, Brigham and Women’s Hospital; CSMC, Cedars-Sinai Medical Center; Oregon, Oregon Heart and Vascular Institute; Ottawa, Ottawa Heart Institute.
Propensity Matching
Of the 3,122 patients with diabetes, 171 (5%) were excluded by the caliper of 0.001 of matching algorithm. Finally, a total of 2,951 patients with diabetes were propensity matched in a 1-to-1 fashion to 2,951 patients without diabetes. Standard differences of all matching covariates between matched groups were <0.05, and <0.1 indicated excellent covariate balance with a negligible difference in the mean of covariates between groups (18,19) (Supplementary Table 1).
Risk of MACE in Matched Patients With and Without Diabetes
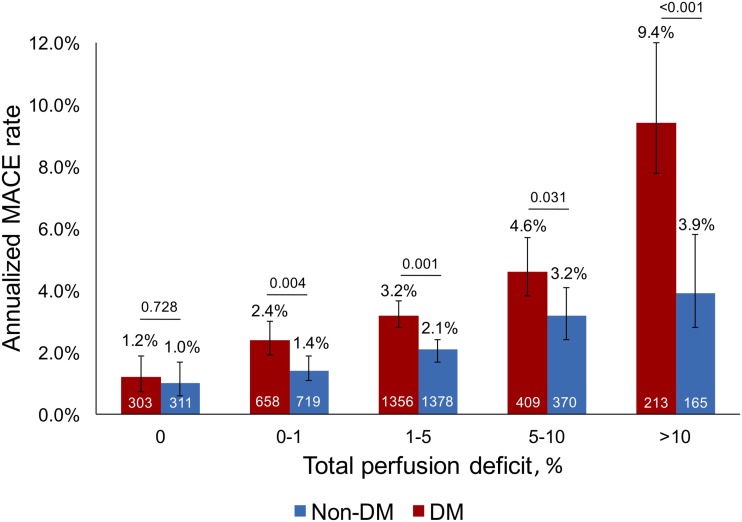
During a median follow-up of 4.6 (IQR 3.6–6.0) years (4.6 [IQR 3.6–5.9] for patients with diabetes and 4.7 [IQR 3.7–6.2] for those without), 468 (16%) and 290 (10%) MACE occurred in patients with and without diabetes, respectively (P < 0.001 for difference). A significant interaction existed between diabetes and risk for MACE across TPD categories (P = 0.024). Among patients with 0% TPD, the annualized MACE rate was 1.2 (95% CI: 0.7–1.9) and 1.0 (95% CI: 0.6–1.7) in patients with and without diabetes, respectively (Fig. 1) (P = 0.728 for difference). In the group with diabetes, MACE increased progressively with each increasing TPD category (P < 0.001). A similar progressive increase in MACE was observed in the group without diabetes, although the MACE rate was consistently lower than that of the group with diabetes in each TPD category (Fig. 1) (P < 0.05 for all and Supplementary Fig. 1). The annualized MACE rate demonstrated the greatest difference between the patients with and without diabetes among those with a TPD >10% (9.4 [95% CI: 7.6–11.6] in diabetes and 3.9 [95% CI: 2.8–5.6] in nondiabetes, P = 0.001 for difference and P < 0.001 for interaction). Notably, a TPD of 0.5% and 3% in patients with diabetes had the same future MACE risk as a TPD of 8% and 11% in patients without diabetes, respectively (Supplementary Table 2).
Figure 1.
Annualized MACE rate in patients with and without diabetes according to TPD categories.
In the Cox regression analysis, age, sex, BMI, family history of CAD, stress test type, and TPD were significantly associated with MACE in both patients with and without diabetes (Table 2). Risk for MACE tended to increase at higher levels of TPD, with a statistically significant correlation in patients with diabetes. The group with diabetes with 0% < TPD < 1% had significantly higher risk compared with those with 0% TPD (HR: 2.08, 95% CI: 1.23–3.53, P = 0.007). In the group without diabetes, patients with 0% < TPD < 1% showed a trend toward higher risk than those with 0% TPD (HR: 1.35, 95% CI: 0.78–2.42, P = 0.307) (Table 2). In the subdistribution hazards model, the prognostic significances of TPD were unchanged in both patients with and without diabetes (Supplementary Table 3).
Table 2.
Cox regression analysis for the prediction of MACE
| Patients with diabetes | Patients without diabetes | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.03 | 1.03–0.04 | <0.001 | 1.06 | 1.04–1.07 | <0.001 |
| Male | 1.43 | 1.19–1.71 | <0.001 | 1.48 | 1.17–1.87 | 0.001 |
| BMI | 0.97 | 0.95–0.98 | <0.001 | 0.97 | 0.95–0.98 | <0.001 |
| Hypertension | 1.29 | 1.02–1.64 | 0.036 | 1.28 | 0.94–1.73 | 0.114 |
| Dyslipidemia | 0.99 | 0.81–1.21 | 0.892 | 1.05 | 0.81–1.37 | 0.697 |
| Smoking | 1.32 | 1.04–1.66 | 0.020 | 1.22 | 0.91–1.66 | 0.178 |
| Family history of CAD | 0.73 | 0.58–0.92 | 0.008 | 0.65 | 0.48–0.89 | 0.006 |
| Pharmacological stress test | 2.27 | 1.84–2.81 | <0.001 | 2.25 | 1.71–2.95 | <0.001 |
| TPD, % increase* | 1.06 | 1.05–1.07 | <0.001 | 1.04 | 1.02–1.06 | <0.001 |
| TPD category* | ||||||
| No deficit (TPD = 0%) | 1 (reference) | — | — | 1 (reference) | ||
| Very minimal (0% < TPD < 1%) | 2.08 | 1.23–3.53 | 0.007 | 1.35 | 0.78–2.42 | 0.307 |
| Minimal (1% ≤ TPD < 5%) | 2.60 | 1.58–4.28 | <0.001 | 1.69 | 0.99–2.90 | 0.056 |
| Mild (5% ≤ TPD ≤ 10%) | 3.43 | 2.02–5.83 | <0.001 | 2.16 | 1.21–3.88 | 0.010 |
| Moderate to severe (TPD >10%) | 6.15 | 3.61–10.48 | <0.001 | 2.59 | 1.37–4.90 | 0.003 |
Multivariate analysis including all matching variables and center location.
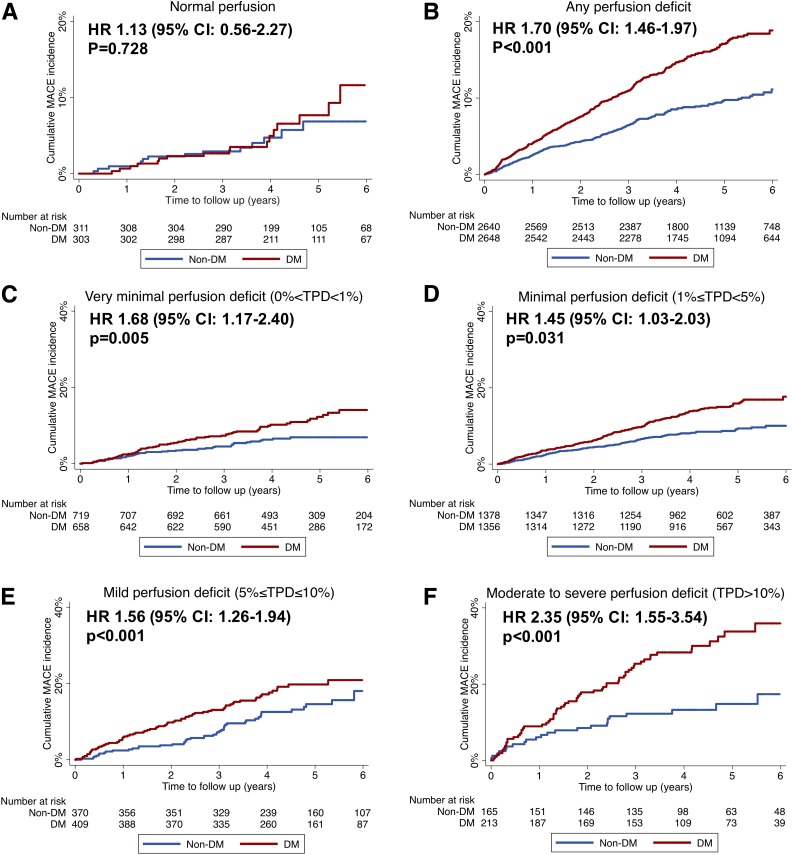
There was no significant difference in MACE risk among patients with and without diabetes with 0% TPD (HR: 1.13, 95% CI: 0.56–2.27, P = 0.728) (Fig. 2A). Among patients with TPD >0%, the risk for MACE was 1.7-fold higher among the group with diabetes relative to patients without diabetes (HR 1.70, 95% CI: 1.46–1.97, P < 0.001) (Fig. 2B). Patients with diabetes had consistently higher risk for MACE compared with patients without diabetes across all TPD categories greater than 0%, including the minimal category of 0% < TPD < 1% (Fig. 2C–F) (all P < 0.05).
Figure 2.
Cumulative Kaplan-Meier survival curve according to diabetes status (diabetes vs. nondiabetes) in patients with (A) normal perfusion or (B–F) perfusion defect. A: No deficit (TPD = 0%). B: Presence of any deficit (TPD >0%). C: Very minimal deficit (0% < TPD < 1%). D: Minimal deficit (1% ≤ TPD < 5%). E: Mild deficit (5% ≤ TPD ≤ 10%). F: Moderate-to-severe deficit (TPD >10%).
Conclusions
In this observational multicenter study, we demonstrated that each level of ischemia as assessed by the quantitative TPD is associated with greater adverse prognosis among patients with diabetes relative to their counterparts without diabetes. The increase in risk associated in the patients with diabetes was seen in all categories of TPD above 0. Importantly, in patients with TPD >10%, the risk of events was more than doubled in patients with diabetes. Even the most minimal presence of TPD (0% < TPD < 1%) was associated with increased risk in the patients with diabetes compared with those with 0% TPD. In addition, patients with diabetes with minimal TPD had comparable MACE risk as patients without diabetes with significant TPD. The group of patients with 0% TPD had a consistent low risk of MACE in both patients with and without diabetes.
Early prognostic studies in patients with diabetes revealed higher cardiovascular event rates compared with patients without diabetes and showed that the annual event rate increased with higher ischemic burden (2–4). More recently, multiple clinical trials confirmed that SPECT MPI improved cardiovascular risk assessment as well as was useful in guiding treatment strategy in patients with diabetes (23,24). However, these studies were based on subjective visual analysis, which depends on observer expertise, and did not address the prognostic differences associated with minimal perfusion defects.
Previous studies have shown that cardiac risk is increased in patients with minimal perfusion defects compared those with normal perfusion defects (6,8). In a large cohort of patients with suspected CAD, Abidov et al. (25) reported that minimal perfusion defects (summed stress score 1–2), resulting in a visual interpretation of “probably normal,” had increased all-cause mortality compared with patients with normal scans. Using quantitative analysis, Nakazato et al. (8) reported that minimal perfusion defects in patients with suspected CAD, defined as 1–4% TPD, were associated with significantly higher all-cause mortality compared with patients with TPD of 0%. More recently, our group demonstrated that the findings of even a very minimal perfusion defect (0% < TPD < 1%) have significant prognostic value in the overall cohort from the REFINE SPECT cohort (9).
The current study has expanded on previous findings by comparing the prognostic value of these very mild defects between groups with and without diabetes. As noted, in this matched subset from the REFINE SPECT registry, the group with diabetes showed an increased risk in patients with 0% < TPD < 1% compared with those without any delectable perfusion defect (TPD = 0%) on SPECT MPI. The reason for this difference is unclear. The 0% < TPD < 1% category may be because of a true mild regional decrease in myocardial perfusion abnormality in which only a very small portion falls below the normal limits that form the basis for quantitation of TPD. The application of deep learning to raw polar maps could provide insight into this possibility (26).
In this study, we compared future MACE risk between patients with and without diabetes for minimal-to-small TPD levels. The large sample size of the current study allowed us to investigate the association of TPD with future MACE risk in this selected population. Interestingly, the prevalence of minimal-to-small TPD were not different between patients with and without diabetes after risk adjustment by propensity score matching. However, the prognostic impact of minimal-to-small TPD between the groups was different. As noted, patients with diabetes with a very minimal TPD level (0.5%) have similar MACE risk as patients without diabetes with a significant TPD level (8%) based on our equation. Therefore, patients with diabetes with even minimal perfusion defect may require medical attention, and those patients could be managed as if they had significant perfusion defects.
Patients with diabetes have been shown to have 2- to 3-fold higher cardiovascular risk compared with patients without diabetes, independent of other cardiovascular risk factors (27,28). However, the impact of diabetes on the MACE risk varies according to patient characteristics, such as age, sex, or the presence or extent of CVD (2–4,11,29,30). In the current study, the annualized MACE rate demonstrated the smallest difference between patients with and without diabetes among those with a TPD of 0%. The differences in MACE risk between patients with and without diabetes increased with greater TPD (P < 0.001 for interaction). This suggests that patients with diabetes are more vulnerable to a greater myocardial ischemic burden than patients without diabetes are, even if they have similar risk factors.
This study has limitations. Although the current study was multicenter and prospective in nature with a large sample size, we cannot discount the follow-up bias as well as the possibility of unmeasured confounding factors that may have influenced the clinical end points. Information regarding baseline and downstream pharmacological management of diabetes was unavailable. Therefore, the effects of specific medical therapy and how it might have influenced the relationship between TPD and MACE cannot be accounted for in this study. Though we used detailed TPD categories to assess the prognostic value of the perfusion defect including the most minimal abnormality (0% < TPD < 1%), the possibility that factors other than the true perfusion abnormality might be causative of this finding, such as increased motion artifact in the sicker patients, could not be excluded. Our findings indicate that there was a high prevalence of mild stress perfusion myocardial abnormalities, with over 50% having TPD that was above 0 but <5%, the usual clinical criterion for an abnormal study. This high prevalence raises concern about how the findings in the minimally abnormal groups would be applied clinically. Additional studies are warranted to validate the prognostic significance of minimal TPD in other cohorts.
In conclusion, patients with diabetes were found to have a higher risk for MACE than patients without diabetes across all categories of quantitative perfusion abnormality. Patients with diabetes were found to have increased MACE risk even with the most minimal quantitative perfusion defects. Patients with diabetes with minimal ischemia had comparable MACE risk as patients without diabetes with significant ischemia.
Appendix
List of Participating Investigators. The participating investigators are as follows: Piotr J. Slomka, Daniel S. Berman, Damini Dey, Balaji K. Tamarappoo, and Guido Germano at Cedars-Sinai Medical Center; Marcelo Di Carli and Sharmila Dorbala at Brigham and Women’s Hospital; Philipp A. Kaufmann at University of Zurich; Tali Sharir at Assuta Medical Center; Terrence D. Ruddy at Ottawa Heart Institute; Matthews B. Fish at Oregon Heart and Vascular Institute; Andrew J. Einstein at Columbia University; Albert J. Sinusas and Edward J. Miller at Yale University; and Timothy M. Bateman at Aspire Foundation.
Supplementary Material
Article Information
Funding. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL089765.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. D.H., A.R., H.G., T.S., M.B.F., P.A.K., A.J.S., T.M.B., J.X.L., L.-H.H., and D.D. have no relevant conflict of interest to disclose. A.J.E. has received research grants from Roche Medical Systems and Toshiba Medical Systems and is a consultant for GE Healthcare. T.D.R. has received research grants from GE Healthcare and Advanced Accelerator Applications. E.J.M. has received grant support from and is a consultant for GE Healthcare. S.D. is a consultant for GE Healthcare and Pfizer. M.D.C. has received institutional grants from Spectrum Dynamics and Gilead and is a consultant for Sanofi and General Electric. G.G., D.S.B., and P.J.S. participate in software royalties for Quantitative Perfusion SPECT software at Cedars-Sinai Medical Center. P.J.S. has received research grant support from Siemens Medical Systems.
Author Contributions. D.H. drafted the manuscript. D.H., A.R., H.G., T.S., A.J.E., M.B.F., T.D.R., P.A.K., A.J.S., E.J.M., T.M.B., S.D., M.D.C., J.X.L., L.-H.H., G.G., D.D., D.S.B., and P.J.S. approved the final article. D.H. and H.G. analyzed and interpreted the data. A.R., T.S., A.J.E., M.B.F., T.D.R., P.A.K., A.J.S., E.J.M., T.M.B., S.D., M.D.C., J.X.L., L.-H.H., G.G., and D.D. contributed to data collection. A.J.E., L.-H.H., D.S.B., and P.J.S. contributed to critically revising the manuscript. P.J.S. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
D.S.B. and P.J.S. are co–senior authors.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1360/-/DC1.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017. Atlanta, GA, Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017 [Google Scholar]
- 2.Berman DS, Kang X, Hayes SW, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol 2003;41:1125–1133 [DOI] [PubMed] [Google Scholar]
- 3.Kang X, Berman DS, Lewin HC, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography in patients with diabetes mellitus. Am Heart J 1999;138:1025–1032 [DOI] [PubMed] [Google Scholar]
- 4.Giri S, Shaw LJ, Murthy DR, et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation 2002;105:32–40 [DOI] [PubMed] [Google Scholar]
- 5.Leslie WD, Tully SA, Yogendran MS, Ward LM, Nour KA, Metge CJ. Prognostic value of automated quantification of 99mTc-sestamibi myocardial perfusion imaging. J Nucl Med 2005;46:204–211 [PubMed] [Google Scholar]
- 6.Xu Y, Nakazato R, Hayes S, et al. Prognostic value of automated vs visual analysis for adenosine stress myocardial perfusion SPECT in patients without prior coronary artery disease: a case-control study. J Nucl Cardiol 2011;18:1003–1009; quiz 1010–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betancur J, Otaki Y, Motwani M, et al. Prognostic value of combined clinical and myocardial perfusion imaging data using machine learning. JACC Cardiovasc Imaging 2018;11:1000–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakazato R, Berman DS, Gransar H, et al. Prognostic value of quantitative high-speed myocardial perfusion imaging. J Nucl Cardiol 2012;19:1113–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otaki Y, Betancur J, Sharir T, et al. 5-year prognostic value of quantitative versus visual MPI in subtle perfusion defects: results from REFINE SPECT. JACC Cardiovasc Imaging. 8 June 2019 [Epub ahead of print]. DOI: 10.1016/j.jcmg.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kip KE, Faxon DP, Detre KM, Yeh W, Kelsey SF, Currier JW. Coronary angioplasty in diabetic patients. The National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. Circulation 1996;94:1818–1825 [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 12.Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008;52:255–262 [DOI] [PubMed] [Google Scholar]
- 13.Slomka PJ, Betancur J, Liang JX, et al. Rationale and design of the REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT). J Nucl Cardiol. 19 June 2018 [Epub ahead of print]. DOI: 10.1007/s12350-018-1326-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambhir SS, Berman DS, Ziffer J, et al. A novel high-sensitivity rapid-acquisition single-photon cardiac imaging camera. J Nucl Med 2009;50:635–643 [DOI] [PubMed] [Google Scholar]
- 15.Herzog BA, Buechel RR, Katz R, et al. Nuclear myocardial perfusion imaging with a cadmium-zinc-telluride detector technique: optimized protocol for scan time reduction. J Nucl Med 2010;51:46–51 [DOI] [PubMed] [Google Scholar]
- 16.Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol 2018;25:1784–1846 [DOI] [PubMed] [Google Scholar]
- 17.Slomka PJ, Nishina H, Berman DS, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol 2005;12:66–77 [DOI] [PubMed] [Google Scholar]
- 18.Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017;69:345–357 [DOI] [PubMed] [Google Scholar]
- 19.Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika 2000;87:706–710 [Google Scholar]
- 20.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695–706 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geskus RB. Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics 2011;67:39–49 [DOI] [PubMed] [Google Scholar]
- 23.Mancini GBJ, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv 2014;7:195–201 [DOI] [PubMed] [Google Scholar]
- 24.Shaw LJ, Cerqueira MD, Brooks MM, et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol 2012;19:658–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abidov A, Hachamovitch R, Hayes SW, et al. Are shades of gray prognostically useful in reporting myocardial perfusion single-photon emission computed tomography? Circ Cardiovasc Imaging 2009;2:290–298 [DOI] [PubMed] [Google Scholar]
- 26.Betancur J, Commandeur F, Motlagh M, et al. Deep learning for prediction of obstructive disease from fast myocardial perfusion SPECT: a multicenter study. JACC Cardiovasc Imaging 2018;11:1654–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preis SR, Pencina MJ, Hwang SJ, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation 2009;120:212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarwar N, Gao P, Seshasai SR, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies [published correction appears in Lancet 2010;376:958]. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana JS, Dunning A, Achenbach S, et al. Differences in prevalence, extent, severity, and prognosis of coronary artery disease among patients with and without diabetes undergoing coronary computed tomography angiography: results from 10,110 individuals from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes): an InteRnational Multicenter Registry. Diabetes Care 2012;35:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004;27:2898–2904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.