Abstract
OBJECTIVE
Many patients with hyperglycemic crises present with combined features of diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS). The implications of concomitant acidosis and hyperosmolality are not well known. We investigated hospital outcomes in patients with isolated or combined hyperglycemic crises.
RESEARCH DESIGN AND METHODS
We analyzed admissions data listing DKA or HHS at two academic hospitals. We determined 1) the frequency distributions of HHS, DKA, and combined DKA-HHS (DKA criteria plus elevated effective osmolality); 2) the relationship of markers of severity of illness and clinical comorbidities with 30-day all-cause mortality; and 3) the relationship of hospital complications associated with insulin therapy (hypoglycemia and hypokalemia) with mortality.
RESULTS
There were 1,211 patients who had a first admission with confirmed hyperglycemic crises criteria, 465 (38%) who had isolated DKA, 421 (35%) who had isolated HHS, and 325 (27%) who had combined features of DKA-HHS. After adjustment for age, sex, BMI, race, and Charlson Comorbidity Index score, subjects with combined DKA-HHS had higher in-hospital mortality compared with subjects with isolated hyperglycemic crises (adjusted odds ratio [aOR] 2.7; 95% CI 1.4, 4.9; P = 0.0019). In all groups, hypoglycemia (<40 mg/dL) during treatment was associated with a 4.8-fold increase in mortality (aOR 4.8; 95% CI 1.4, 16.8). Hypokalemia ≤3.5 mEq/L was frequent (55%). Severe hypokalemia (≤2.5 mEq/L) was associated with increased inpatient mortality (aOR 4.9; 95% CI 1.3, 18.8; P = 0.02).
CONCLUSIONS
Combined DKA-HHS is associated with higher mortality compared with isolated DKA or HHS. Severe hypokalemia and severe hypoglycemia are associated with higher hospital mortality in patients with hyperglycemic crises.
Introduction
Hyperglycemic crises are severe, acute, metabolic complications of diabetes that include the hyperosmolar hyperglycemic state (HHS) and diabetic ketoacidosis (DKA). A large proportion of studies investigating these hyperglycemic complications have focused on DKA, the most common hyperglycemic crisis. HHS, a syndrome characterized by severe hyperglycemia, hyperosmolality, and dehydration in the absence of ketoacidosis, is associated with a higher mortality compared with DKA (1).
Even though DKA mortality is <2%, the number of DKA-related hospitalizations across all age-groups continues to increase in the U.S. and the U.K (2,3). According to the Centers for Disease Control and Prevention, hospitalizations for DKA in the U.S. increased by 6.3% per year between 2000 and 2014 (2). In the U.K, the incidence of hospitalizations for DKA in patients with type 1 diabetes rose by ∼4% every year between 1998 and 2013 (3). Most reported cases of HHS are in older patients, but recent reports suggest an increase in the incidence of HHS cases in children and young adults (4). The overall proportion of patients presenting with HHS, however, is not well defined. Given the limited data available regarding the frequency, clinical characteristics, and prognosis of patients presenting with combined features of DKA and HHS, there is no accepted definition to characterize this population. Some small studies have reported that up to 30% of patients with DKA have combined features of HHS and DKA (5,6). However, the hospital outcomes of patients with combined DKA-HHS are not well known (1).
Recommendations for the management of hyperglycemic crises are similar for patients with DKA and HHS (1,7). Since the mortality rate in patients with HHS is considered much higher than in patients with DKA (5,8), it would be helpful to identify the clinical characteristics of patients with different subtypes of isolated hyperglycemic crises and in subjects presenting with combined DKA-HHS. This will provide estimates on rates of complications associated with severity of disease and help identify factors associated with worse outcomes.
Based on our clinical experience, we hypothesized that patients with combined features of HHS and DKA have worse outcomes compared with patients with isolated HHS or DKA alone. To test our hypothesis, we used individual-level data obtained from the Clinical Data Warehouse at Emory University and conducted three separate analyses: 1) we determined the frequency distributions of HHS, DKA, and combined DKA-HHS; 2) we examined predictors of all-cause hospital mortality and clinical outcomes; and 3) we examined the relationship of early hospital complications commonly related to insulin therapy (hypoglycemia and hypokalemia) with all-cause hospital mortality.
Research Design and Methods
Data Source
We conducted an observational study analyzing individual-level admission and hospitalization data of patients admitted with an index admission for hyperglycemic crises at Emory Hospitals (Emory University Hospital and Emory University Hospital Midtown) between 1 June 2005 and 1 June 2015. Data were obtained through the Clinical Data Warehouse infrastructure program. We first identified patient encounters according to ICD-9 codes for DKA and HHS. In addition, we included patients with admission blood glucose (BG) levels >600 mg/dL (33.3 mmol/L) and hyperosmolality. We then confirmed the diagnosis according to admission laboratory values with the presence of metabolic acidosis and/or hyperosmolality (we used estimations of osmolality as commonly used in clinical practice) (9). Patients with a diagnosis of DKA or HHS but without laboratory results on admission consistent with our case definition were excluded. Subsequent encounters were also excluded.
Hyperosmolality was defined as an effective plasma osmolality ≥300 mOsm/kg. Case definition of hyperglycemic crises on admission was:
HHS: ICD-9 code for HHS + effective osmolality [2(Na) + glucose/18)] ≥300 mOsm/kg, + bicarbonate >18 mEq/L, or plasma glucose level >600 mg/dL (33.3 mmol/L) + plasma effective osmolality ≥300 mOsm/kg + bicarbonate >18 mEq/L;
DKA: ICD-9 code for DKA with confirmed bicarbonate ≤18 mEq/L on admission;
Combined DKA-HHS: ICD-9 code for DKA with confirmed bicarbonate ≤18 mEq/L on presentation + effective osmolality ≥300 mOsm/kg.
Data were extracted for each patient during the index hospitalization, including demographics and anthropometrics (age, BMI, sex, race), admission and inpatient laboratory values (hemoglobin A1c, plasma glucose, pH, ketones, total serum osmolality [2(Na) + glucose/18 + blood urea nitrogen/2], effective serum osmolality [2(Na) + glucose/18)], anion gap, serum sodium, serum creatinine, estimated glomerular filtration rate, and lactic acid. To extract data on comorbidities and hospital complications, we used ICD-9 codes to include cardiac complications, deep vein thrombosis/venous thromboembolism (DVT/VTE), cerebral edema, and rhabdomyolysis. Acute kidney injury (AKI) was defined as a 0.5 mg/dL increase in creatinine level among subjects admitted with creatinine <5 mg/dL. Data on length of hospital stay and all-cause in-hospital mortality were obtained. Fatal events were defined as deaths occurring within 30 days of admission. To limit potential misclassification, we excluded patients labeled on admission as DKA or HHS who did not meet admission criteria. The Emory University Institutional Review Board approved the study protocol.
Statistical Analysis
Descriptive statistics were used to determine the proportion of hyperglycemic crises (DKA, HHS, and combined DKA-HHS) and clinical characteristics of hospitalized adult patients with diabetes. A total of 1,907 patients with 2,950 episodes of hyperglycemic crises were identified. Of them, 1,211 subjects had a first event with confirmed admission criteria. We compared demographic and clinical outcome measures between subtypes of hyperglycemic crises. On the basis of the normality of the data, continuous variables were compared using ANOVA or Kruskal-Wallis test for three-group comparisons. Categorical data were compared using χ2 or the Fisher exact test. Multivariate regression analysis was performed to assess the influence of demographic and clinical characteristics on hospital outcomes. The main outcome of interest was hospital mortality. Additional outcomes included hypoglycemia (<70 mg/dL), severe hypoglycemia (<40 mg/dL), hypokalemia (<3.5 mEq/L), severe hypokalemia (<2.5 mEq/L), and length of stay according to DKA, HHS, and DKA-HHS categories. Metabolic parameters (BG, anion gap, bicarbonate, effective osmolality) were evaluated according to quartiles. Models evaluating the role of metabolic parameters on clinical outcomes were adjusted for demographic variables. Metabolic parameters that are correlated or determined by each other (e.g., anion gap and bicarbonate or effective osmolality and glucose) were not included simultaneously in the adjusted models. We also explored the association of higher osmolality levels with complications. Receiver operating characteristic curves were used to relate admission-effective osmolality cut points and age and the rate of hospital outcomes. The area under the curve, or C statistic, was used as a measure of the predictive accuracy. All comparisons were two-sided, with statistical significance defined as P < 0.05. The log-rank test was used to test the difference among the Kaplan-Meier survival curves. Cox proportional hazards regression analysis adjusted for age, sex, and BMI was used to compare rates of patient in-hospital 30-day survival among hyperglycemic crises categories groups. Interaction terms were dropped from the final models due to nonsignificant P values. Results are expressed as the hazard ratio (HR) with 95% CI. All analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Among 1,211 patients with confirmed criteria for DKA and HHS, 465 (38%) had isolated DKA, 421 (35%) had isolated HHS, and 325 (27%) had combined features of DKA-HHS. Compared with isolated DKA and combined DKA-HHS patients, patients with isolated HHS were older and more likely to be African American (83%) and to have a higher BMI (28.9 kg/m2) (Table 1). Despite no differences in admission HbA1c among groups, the patients with combined DKA-HHS had higher admission BG (828 ± 296 mg/dL, 46.0 ± 16.4 mmol/L) compared with patients with isolated HHS (797 ± 287 mg/dL, 44.3 ± 15.9 mmol/L) and DKA (490 ± 197 mg/dL, 27.2 ± 10.9 mmol/L) (P < 0.001). Patients identified by ICD-9 code for HHS had an average BG of 790 ± 288 mg/dL (25th percentile: 578; 75th percentile: 1,000 mg/dL) that was not significantly different compared with the average of patients identified by admission glucose levels (no ICD-9 code) with a mean BG 883 ± 262 (25th percentile: 676; 75th percentile: 1,056 mg/dL) (P = 0.074).
Table 1.
Clinical characteristics according to subtypes of hyperglycemic crises
| DKA (n = 465) | HHS (n = 421) | Combined DKA-HHS (n = 325) | P value | |
|---|---|---|---|---|
| Age, years | 43 ± 17 | 57 ± 4 | 49 ± 18 | <0.001 |
| Female sex, n (%) | 245 (53) | 211 (50) | 169 (52) | 0.74 |
| BMI, kg/m2 | 27.3 ± 7.5 | 28.9 ± 8.3 | 26.5 ± 6.7 | <0.001 |
| African American race, n (%) | 312 (67) | 349 (83) | 256 (79) | <0.001 |
| HbA1c, % | 11.7 ± 2.9 | 12.1 ± 2.8 | 11.6 ± 2.7 | 0.12 |
| Admit BG, mg/dL | 490 ± 197 | 797 ± 287 | 828 ± 296 | <0.001 |
| Bicarbonate, mEq/L | 12.2 ± 3.7 | 23.7 ± 3.9 | 11.8 ± 4.5 | <0.001 |
| Anion gap | 20.1 ± 5.4 | 14.3 ± 4.8 | 25.4 ± 6.9 | <0.001 |
| pH | 7.23 ± 0.2 | 7.35 ± 0.1 | 7.17 ± 0.2 | <0.001 |
| Effective osmolality, mOsm/kg | 288.1 ± 8.3 | 316 ± 18.9 | 316 ± 15 | <0.001 |
| Total osmolality, mOsm/kg | 311.1 ± 16.0 | 352.8 ± 29.6 | 355.3 ± 27.6 | <0.001 |
| Sodium, mg/dL | 130.5 ± 5.7 | 136.1 ± 10.0 | 134.8 ± 7.5 | <0.001 |
| Potassium, mEq/L | 4.9 ± 1.1 | 4.8 ± 1.1 | 5.5 ± 1.2 | <0.001 |
| Potassium minimum on day 2, mEq/L | 3.6 ± 0.6 | 3.6 ± 0.5 | 3.4 ± 0.5 | <0.001 |
| β-Hydroxybutyrate, mmol/L | 5.8 ± 2.6 | 2.2 ± 2.2 | 6.0 ± 2.9 | <0.001 |
| Heart failure, n (%) | 37 (8) | 46 (11) | 25 (8) | 0.20 |
| Stroke, n (%) | 1 (0.2) | 7 (1.7) | 2 (0.6) | 0.05 |
| Acute myocardial infarction, n (%) | 10 (2) | 15 (4) | 10 (3) | 0.45 |
| DVT, n (%) | 10 (2) | 4 (1) | 10 (3) | 0.09 |
| Hypokalemia <3.5 mEq/L within 48 h, n (%) | 252 (54) | 208 (51) | 196 (62) | 0.01 |
| Severe hypokalemia <2.5 mEq/L within 48 h, n (%) | 16 (3) | 9 (2) | 9 (3) | 0.56 |
| Hypoglycemia <70 mg/dL, n (%) | 74 (16) | 52 (12) | 49 (15) | 0.30 |
| Hypoglycemia <40 mg/dL, n (%) | 7 (2) | 9 (2) | 8 (2) | 0.63 |
| Cerebral edema, n (%) | 4 (0.9) | 1 (0.2) | 2 (0.6) | 0.50 |
| AKIa, n (%) | 34 (7) | 32 (8) | 18 (6) | 0.50 |
| Vasopressor use, n (%) | 42 (9) | 29 (7) | 43 (13) | 0.01 |
| Rhabdomyolysis, n (%) | 4 (0.9) | 14 (3.3) | 4 (1.2) | 0.02 |
| Length of stay, days | 3.3 (2.1, 6.2) | 3.8 (2.4,7.5) | 3.8 (2.4, 6.2) | 0.02 |
| In-hospital death, n (%) | 14 (3) | 20 (5) | 27 (8) | 0.003 |
Data are presented as mean ± SD or median (interquartile range) unless otherwise indicated. P values represent results of ANOVA or Kruskal-Wallis tests for continuous variables or the χ2 or Fisher test for categorical variables. aAKI is defined as a 0.5 mg/dL increase in the creatinine level among subjects admitted with creatinine <5 mg/dL.
Predictors of Mortality
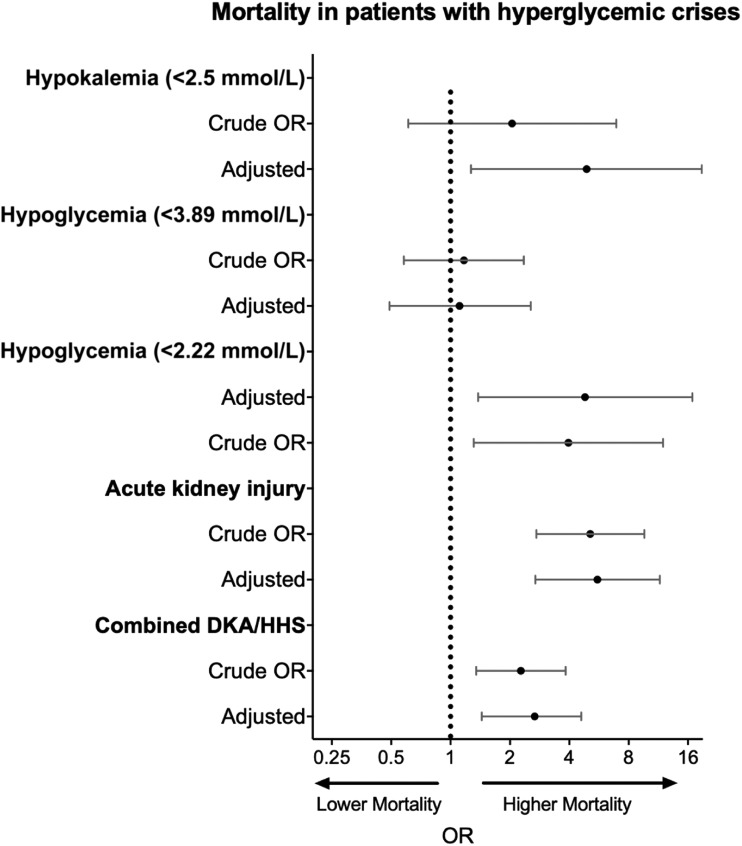
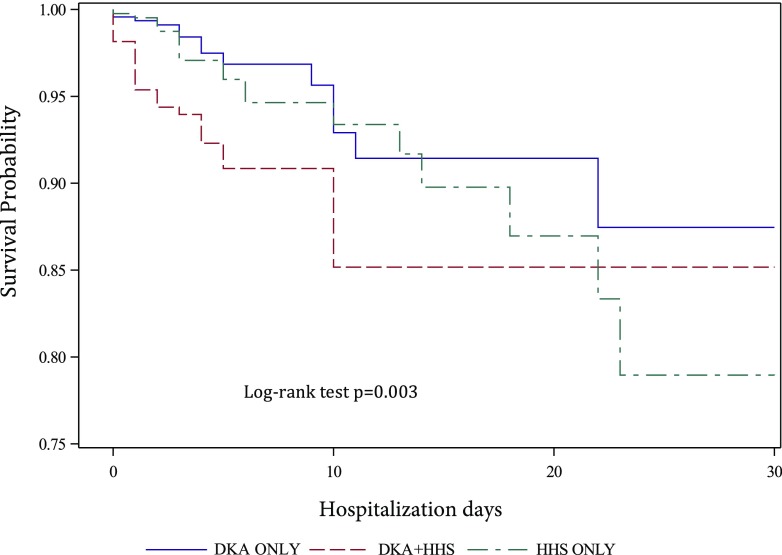
After adjustment for age, sex, and BMI, patients with combined DKA-HHS had higher hospital mortality compared with those with isolated DKA or HHS. Patients presenting with a combination of DKA and HHS had the highest mortality (8%) compared with patients with isolated HHS (5%) or DKA (3%) (P = 0.003) (Figs 1 and 2). The HRs for DKA-HHS were 2.7 (95% CI 1.4, 5.3) vs. isolated HHS and 1.8 (95% CI 0.9, 3.6) vs. isolated DKA. After adjusting for hyperglycemic crises, sex, BMI, and race, mortality was associated with increasing age (adjusted OR [aOR] 1.05; 95% CI 1.03, 1.07; P < 0.0001) and Charlson Comorbidity Index (aOR 1.47; 95% CI 1.29, 1.69; P < 0.0001). Admission plasma glucose level was not associated with mortality (P = 0.44). In contrast, a lower bicarbonate level on admission was associated with increased mortality after adjusting for covariates (P = 0.04) (Supplementary Table 1).
Figure 1.
Odds of mortality according hospital complications and hyperglycemic crises. AKI defined as an elevation of creatinine of 0.5 mg/dL from admission; hypoglycemia, <40 mg/dL; hypokalemia, <2.5 mEq/L; mortality, death occurring during admission. Model adjusted for age, sex, BMI, race, and Charlson Comorbidity Index.
Figure 2.
Adjusted survival analysis comparing the probability of 30-day inpatient mortality according to hyperglycemic crisis category. Higher mortality risk was observed for those with combined DKA-HHS vs. isolated HHS (HR 2.7; 95% CI 1.4, 5.3), DKA-HHS vs. isolated DKA (HR 1.8; 95% CI 0.9, 3.6); log-rank test P = 0.003.
Effective and total osmolality were highly correlated (r = 0.9, P < 0.001). Effective osmolality values of 300, 320, and 350 mOsm/kg corresponded to a total osmolality of 328, 362, and 394 mOsm/kg, respectively. Both effective and total osmolality were associated with DVT/VTE (effective osmolality: OR 1.02 [95% CI 1, 1.04], P = 0.03; total osmolality: OR 1.01 [1, 1.02], P = 0.01) and rhabdomyolysis (effective osmolality: OR 1.03 [1.01, 1.04], P < 0.0001; total osmolality: OR 1.02 [1.01, 1.03], P = 0.0002). Both effective and total osmolality were associated with increased mortality (OR 1.02 [1.01, 1.03] and 1.01 [1.01, 1.02], both P < 0.01), but the association was not significant after adjusting for age, sex, BMI, race, and Charlson Comorbidity Index (P = 0.97 and P = 0.49, respectively). Effective osmolality >320 mOsm/kg had good specificity (82%) but poor sensitivity (31%) to discriminate fatalities (C statistic 0.57). A combination of age (>50 years) and effective osmolality (≥300 mOsm/kg) increased the predictive accuracy (sensitivity to 82% and specificity to 52%; C statistic 0.70) to discriminate case fatalities (Supplementary Fig. 2). Patients with combined DKA-HHS had higher odds of DVT/VTE compared with those with isolated crises; however, the association did not reach statistical significance (aOR 2.07; 95% CI 0.85, 5.03). Sepsis was associated with increased mortality (crude OR 2.94 [1.39, 6.23], P = 0.0049); however, the association did not reach statistical significance in the adjusted model (aOR 2.03 [0.88, 4.67], P = 0.09).
Hospital Complications
The development of AKI, hypokalemia, and hypoglycemia during the hospital stay was associated with poor hospital outcome. AKI was associated with a 5.5-fold increased mortality in adjusted and unadjusted analysis (aOR 5.56; 95% CI 2.69, 11.50) (Table 2); however, it was not different according to hyperglycemic crises subtypes. Patients with combined DKA-HHS had the highest potassium levels on admission and the lowest potassium concentration during the first 48 h after admission (P < 0.01 for both). Within 48 h of admission, potassium levels declined on average from 4.9 ± 1.1 to 3.6 ± 0.6 mEq/L, 4.8 ± 1.1 to 3.5 ± 0.5 mEq/L, and 5.5 ± 1.2 to 3.4 ± 0.5 mEq/L among patients with DKA alone, HHS alone, and combined DKA-HHS, respectively. Patients with DKA-HHS had the highest rate of hypokalemia within 48 h of admission (62%). Severe hypokalemia (≤2.5 mEq/L) occurred in 75 patients (6.3%) and was associated with increased mortality (OR 3.17; 95% CI 1.49, 6.76). The association between hypokalemia (within 48 h, n = 34) and mortality remained significant after adjusting for hyperglycemic crises categories, demographic variables, and metabolic parameters on admission (aOR 4.90; 95% CI 1.27, 18.84; P = 0.02) (Table 2). Similarly, the development of hypoglycemia <40 mg/dL (2.22 mmol/L) within 48 h of admission was associated with a 4.8-fold increase in mortality after adjusting for demographic variables and hyperglycemic crises categories (aOR 4.81; 95% CI 1.38, 16.83). Concomitant hypokalemia (<3.5 mEq/L) and hypoglycemia (<70 mg/dL) occurred in 102 patients (8%). No episodes of severe hypokalemia with severe hypoglycemia were recorded. No difference in mortality was observed when comparing the later study period (after year 2010) vs. the earlier period (OR 1.082; 95% CI 0.571, 2.049).
Table 2.
Mortality according to acute metabolic complications among patients presenting with hyperglycemic crises
| OR | aORa | aORb | aORc | |
|---|---|---|---|---|
| Hypokalemia | ||||
| Potassium ≥2.5 mEq/L | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Potassium < 2.5 mEq/L | 2.05 (0.61, 6.92)* | 3.8 (1.04, 13.92)† | 4.64 (1.24, 17.35)† | 4.90 (1.27, 18.84)† |
| Hypoglycemia | ||||
| BG ≥40 mg/dL | 1.00 (Reference) | 1.00 (Reference) | 1.0 (Reference) | 1.00 (Reference) |
| BG <40 mg/dL | 3.96 (1.31, 11.96)† | 4.45 (1.36, 14.58)† | 4.73 (1.38, 16.27)† | 4.81 (1.38, 16.83)† |
| AKI | ||||
| No AKI | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| AKI | 5.11 (2.72, 9.61)‡ | 5.19 (2.58, 10.45)‡ | 5.30 (2.6, 10.82)‡ | 5.56 (2.69, 11.5)‡ |
Model adjusted for age, sex, BMI, and race.
Model adjusted for age, sex, BMI, race, and Charlson Comorbidity Index.
Model adjusted for age, sex, BMI, race, Charlson Comorbidity Index, and hyperglycemic crisis category (isolated DKA, isolated HHS, or combined DKA-HHS).
P < 0.05;
P < 0.001;
P = NS.
Conclusions
Our study is, to our knowledge, the largest study aiming to determine the distribution and hospital outcomes of individuals presenting with isolated DKA and HHS or with combined DKA-HHS based on confirmed diagnosis of hyperglycemic crises on admission. We found that one in four patients have combined features of DKA and HHS and that this phenotype is associated with an approximately twofold increase in mortality compared with isolated hyperglycemic crises. We also observed that the development of hypoglycemia and hypokalemia, two common complications related to insulin therapy, is associated with increased mortality independently of hyperglycemic crisis category.
Current diagnostic criteria of hyperglycemic crises recommended by the American Diabetes Association (ADA) and international guidelines include 1) HHS: plasma glucose >600 mg/dL (33.3 mmol/L), plasma effective osmolality >320 mOsm/kg, and an absence of significant ketoacidosis; and 2) DKA: plasma glucose >250 mg/dL (13.9 mmol/L), arterial pH <7.3, serum bicarbonate <18 mEq/L, positive urine or serum ketones, and anion gap >10, with or without altered cognitive status (9–12). Commentaries and small observational studies have suggested that these conditions coexist frequently, but the characteristics and clinical course of patients with combined DKA-HHS remain epidemiologically poorly characterized. Management strategies are also not clear for individuals with HHS or combined DKA-HHS. The Joint British Diabetes Societies Guideline, for example, recommend starting insulin at the time of initial fluid resuscitation only if there is ketonemia along with hyperosmolality. The HHS guideline also suggests that insulin should be avoided until the glucose concentration stops dropping (12). However, this is not clearly delineated in the ADA guidelines.
No systematic analysis has been conducted in a large cohort of patients presenting with different subtypes of hyperglycemic crises; however, small case series have suggested that patients with HHS have worse outcomes compared with patients with isolated DKA, but data adjusting for covariates and severity of disease or evaluating the overlap of DKA and HHS have not been rigorously reported. Our results provide estimates that highlight the importance of identifying patients with concomitant DKA and HHS, a distinctive high-risk category. Our results also indicate that among subjects presenting with hyperglycemic crises, a combination of HHS with DKA was associated with the highest mortality (8%) compared with isolated crises (5% for isolated HHS and 3% for isolated DKA).
On the basis of mostly small retrospective case series, the estimated mortality is close to 20% among patients presenting with HHS (8). This likely reflects the use of higher osmolality cutoffs or the use of severe cognitive status impairment as criteria to identify patients with HHS in older cohorts. Osmolality criteria are derived from case series of HHS described in the 1970s when interest increased about the role of hyperosmolality in the acute cognitive changes associated with severe hyperglycemia (13–16). Furthermore, the cutoff of BG >600 mg/dL for the diagnosis of HHS was based on the observation that these levels were usually accompanied by total serum osmolality >350 mOsm/kg according to the experience of the investigators describing the initial case series (13). Before 2001, total serum osmolality was commonly used (>330 mOsm/kg); however, it was later changed to “effective osmolality” >320 mOsm/kg because it correlates with severe cognitive status changes (6). This calculation excludes urea (an ineffective osmole, as it is permeable and freely flows into cells) (5,6). Nevertheless, effective and total osmolality are highly correlated and have a similar association with outcome (both were associated similarly with DVT/VTE, rhabdomyolysis, and mortality). One of the limited number of prospective studies including patients with HHS was conducted at our institution and used a total osmolality cutoff of 320 mOsm/L (5). With this criterion, 74% of patients were categorized as having impaired cognitive status, and 23% were categorized as comatose. Mortality was reported in 4% of patients (5). Studies using higher cutoffs not surprisingly have shown very high mortality rates up to 20% (8). In this study, we used an effective osmolality cutoff of 300 mOsm/L (normal osmolality values range from 275 to 295 mOsm/kg), which is abnormal and correlates to a total osmolality of ∼328 mOsm/L. We think it may not be appropriate to wait for very high osmolality cutoffs to label patients as having hyperosmolality to intervene with a standardized protocol. Using an effective osmolality of >300 mOsm/kg helps classify patients with DKA as a higher-risk population when concomitant hyperosmolality is present. The Joint British Diabetes Societies for Inpatient Care recommend reducing the osmolality by 3–8 mOsm/kg/h in patients with HHS, while ADA recommends early insulin therapy targeting a higher BG value (compared with DKA) and close monitoring of cognitive status. These discrepancies based on limited data clearly suggest a need for a joint effort to find the best management strategies for patients with HHS with and without DKA.
In addition, low bicarbonate concentration was independently associated with increased mortality, while admission glucose was not. This highlights the importance of acidosis as a predictive mortality factor in patients with hyperglycemic crises and the known role of fluid resuscitation and insulin therapy protocols to target acidosis as a main goal of management, rather than reduction of BG levels alone.
Approximately 95% of patients admitted with hyperglycemic crises present with high-normal or elevated serum potassium levels (9,17,18), despite having a total body potassium deficiency ranging from 3 to 6 mmol/kg due to osmotic diuresis, emesis, and hyperaldosteronism (9). The absolute or relative insulin deficit, along with hyperglycemia and ketoacidosis, lead to an extracellular potassium shift (19,20). During insulin therapy; however, many patients develop a significant lowering of the serum potassium concentration due to intracellular shifting of potassium, activation of Na/K ATPase, correction of acidosis, and increase in kaliuresis (21,22). To prevent hypokalemia, clinical guidelines recommend that potassium be administered even in patients presenting with normal or high-normal potassium levels <5.3 mEq/L and that starting insulin infusion be held in patients with potassium levels <3.3 mEq/L (9). In this study, hypokalemia <3.5 mEq/L was observed in 55% of patients during insulin therapy. The average decline in serum potassium during the first 48 h was 1.37 ± 1.13 mEq/L in patients with isolated DKA, 1.18 ± 1.16 in patients with HHS, and 2.12 ± 1.26 mEq/L in patients with combined DKA-HHS. Severe hypokalemia ≤2.5 mEq/L was independently associated with almost fivefold-higher odds for death. Severe hypokalemia (<2.5 mEq/L) has been associated with a mortality rate >30% in the hospital (23).
Studies using protocols with lower doses of insulin (now standard of care) in patients with DKA (24–26) have demonstrated a lower risk of hypokalemia in a controlled setting. The implementation of standard protocols that include potassium administration should be encouraged in all facilities caring for patients with severe hyperglycemia (9).
A common complication of intravenous insulin therapy is hypoglycemia. Prior studies have reported an incidence of DKA treatment-related hypoglycemia ranging from 13 to 30% (5,27,28). These data from observational studies in real-world settings contrast with the data from the several randomized controlled studies using low-dose insulin protocols showing lower rates (<5%) of hypoglycemia under controlled conditions (24–26,29). More recently, with the use of a computerized system in patients with DKA requiring intravenous insulin therapy, we have observed a significantly lower incidence of hypoglycemia (30). The incidence of hypoglycemia (<70 mg/dL) may vary according to the BG target and the multiplier (0.01–0.03) used in the computerized algorithm, but in general, the risk of severe hypoglycemia is minimal (30). Of interest, severe hypoglycemia was associated with a 4.8-fold increase in mortality after adjusting for multiple variables (aOR 4.8; 95% CI 1.4, 16.8; P = 0.014).
Insulin-induced hypoglycemia and hypokalemia can lead to fatal cardiac arrhythmias. Experimental data have shown that hypoglycemia can lead to an acquired long QT syndrome, which could precipitate ventricular tachycardia and sudden death (31). It has also been shown that QT dispersion can be prevented by potassium replacement. During insulin infusion, these cardiac conduction abnormalities appear to be a consequence of sympathoadrenal stimulation acting both directly on the myocardium and through decreases in potassium levels (31,32). Our results highlight the strong association between hypoglycemia and hypokalemia with mortality and raise awareness for the need to implement effective protocols to closely monitor both glucose and potassium levels during insulin therapy.
About one in every five patients with DKA is readmitted within 30 days (33). In this study, we included only first admissions, as recurrent events would have significant impact on the interpretation of our results (lack of survival data outside the Emory Healthcare System). Nevertheless, it is important to recognize that DKA recurrence has been shown to be significantly associated with increased mortality. Zhong et al. (3) reported that, compared with patients with single admissions, those with three or more DKA-related admissions had a twofold increase in all-cause mortality (3). Similarly, a retrospective cohort from the U.K. noted that those with two to five DKA-related admissions had a threefold increase of mortality, while those with more than five admissions had a sixfold mortality increase (34). A study similarly evaluating recurrent DKA in the Chicago area noted that for each DKA readmission, the odds of mortality increased 1.28 times (95% CI 1.04, 1.58) (35). Further research is needed to understand the underlying factors that lead to readmission and interventions to prevent DKA recurrence.
AKI is associated with increased mortality in the hospital and is a frequent complication of DKA (36,37). Multiple potential mechanisms, including volume overload, hyperkalemia, acid-base derangements, increased risk of infections, or a proinflammatory response leading to lung injury, have been suggested. It is also possible that AKI is colinear with unmeasured elements of comorbidity (36). With its limitations we adjusted for a comorbidity score to account for underlying conditions. In patients with hyperglycemic crises, we could speculate that additional factors could increase the risk of AKI and poor outcomes, including the severity of ketosis, hyperglycemia, dehydration, or rhabdomyolysis (37–39). Not surprisingly, our cohort showed increased mortality among those with worsening kidney function. Further research is needed to understand the causal relationships between risk factors and AKI and the consequences of AKI in patients with hyperglycemic crises.
The findings from our study are subject to several limitations. Misclassification based on ICD-9 codes is common in the hospital setting and could have led to an over- or underestimation of the frequency of combined DKA-HHS. Since pH and β-hydroxybutyrate levels are not consistently measured in clinical practice, there is a potential for additional misclassification of hyperglycemic crises. To reduce misclassification bias, we only included patients with a confirmed diagnosis based on admission biochemical parameters commonly used in clinical practice. Recently, VanderWeele et al. (40) confirmed the limitations of extracting data from electronic medical records using ICD codes alone. A review of records showed that a large proportion of subjects (47%) with an ICD diagnosis of DKA did not have confirmatory biochemical data on admission (40). We believe our results provide good estimates from a large population of patients with confirmed admission data, although this may represent a sicker population than commonly reported in clinical practice or derived from national estimates using only ICD data. Other patient characteristics that may affect the patients' risk and response to therapy, such as duration of diabetes, were not available. The rate of complications or comorbidities is also subject to information bias. With its known limitations, we used a Charlson Comorbidity Index to adjust for comorbidities (41). Assessing clinical risk in patients who are along the continuum of DKA-HHS is complex, and there is a need for clinical prediction tools for hospital morbidity and mortality (42). Our results highlight the predictive role of age, osmolality, bicarbonate, and treatment-related severe hypokalemia and hypoglycemia in hospital mortality. Patients with other metabolic derangements associated with low bicarbonate levels (e.g., lactic acidosis) could have been included. Selection bias is possible, as patients with DKA diagnosis but bicarbonate levels >18 mEq/L were excluded. Therefore, our findings might not be applicable to patients with very mild derangements in metabolic parameters. In addition, subjects with mixed metabolic disorders could also have been excluded. We do not know whether there was a role for the use of sodium–glucose cotransporter 2 inhibitors as a precipitating factor in this cohort; such patients may present with BG levels <250 mg/dL (“euglycemia”) and may have a prolonged duration of DKA (43). This is an uncommon presentation, and this drug class was not commonly used during the study period. To obtain complete data, we limited our study to a single academic institution. Despite these limitations, we identified a large number of patients fitting common criteria of hyperglycemic crises in this analysis.
We do not know whether patients with combined features of DKA and HHS need to be treated differently compared with patients with isolated DKA or HHS. We recommend that providers taking care of patients with hyperglycemic crises use insulin infusion algorithms with a low risk of hypoglycemia and monitor and replete potassium levels closely, particularly among patients with DKA-HHS (>60% developed hypokalemia, with an average 2 mEq/L drop in potassium levels within 48 h of admission). Lack of an appropriate implementation of guidelines may lead to poor management and increased risk of life-threatening changes in glucose in glucose and potassium. Dhatariya et al. (28) reported that despite potassium replacement (77%), in accordance with protocol in the U.K., a large proportion of patients (67%) had a potassium level <4 mEq/L within 24 h of presentation. Similar findings were reported by Galm et al. (44) in Canada, where 47% of patients developed hypokalemia (<3.5 mEq/L) despite 91% of them receiving potassium replacement. The severity of acidosis and dehydration (hyperosmolality) suggests also that patients need a higher level of care compared with patients with mild to moderate DKA, who can occasionally be treated in the emergency department only, and can be even considered for subcutaneous therapy following previously published protocols (29).
Conclusion
No prospective studies have determined best treatment strategies for the management of patients with HHS or combined DKA-HHS (1). From this large cohort including >1,200 patients presenting with hyperglycemic crises, the following conclusions and recommendations can be drawn: 1) a combination of HHS with DKA is common and is associated with increased mortality, and 2) hypokalemia and hypoglycemia within 48 h of admission are common complications among all subtypes of hyperglycemic crises and are associated with increased mortality. With the growing diabetes epidemic and rise in the incidence of hyperglycemic crises, there is an urgent need to develop effective programs that identify subjects at the highest risk for poor outcomes and that ensure the avoidance of hypokalemia and hypoglycemia, as well as initiatives to conduct prospective studies to identify better treatment strategies for patients with high mortality risk such as those with HHS with and without DKA.
Supplementary Material
Article Information
Acknowledgments. The authors would like to thank Shailesh Nair from the Research and Woodruff Health Sciences Information Technology (R-WIT) Department at Emory University and Dr. Darin Olson from the Department of Medicine/Endocrinology at Emory University for helpful comments on an earlier draft of the manuscript.
Funding. This study was supported by a clinical research grant from the Jacobs Research Funds to Emory University. F.J.P., P.V., K.M.V.N., and G.E.U. are partially supported by National Institutes of Health grants from the National Institute of General Medical Sciences (1K23-GM-128221-01A1 [F.J.P.]), the Eunice Kennedy ShriverNational Institute of Child Health and Human Development (3K12-HD-085850-03S1 [P.V.]), the National Institute of Diabetes and Digestive and Kidney Diseases (1P30-DK-111024-01 [K.M.V.N. and G.E.U.]), and the National Center for Advancing Translational Sciences (UL1-TR-002378 [G.E.U.]).
The supporters of the study were not involved in the study design, data collection, analysis or interpretation of the results, or preparation of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. F.J.P. designed the study and wrote the first draft of the manuscript. K.T. and S.C. extracted and organized the data and reviewed the manuscript. H.W. conducted the statistical analysis. R.J.G., M.F., G.D., P.V., A.M., U.G., and K.M.V.N. critically reviewed the manuscript. G.E.U. critically reviewed the study design and the manuscript. F.J.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1168/-/DC1.
References
- 1.Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 2014;37:3124–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2018;67:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998-2013: a retrospective cohort study. Diabetes Care 2018;41:1870–1877 [DOI] [PubMed] [Google Scholar]
- 4.Rosenbloom AL. Hyperglycemic hyperosmolar state: an emerging pediatric problem. J Pediatr 2010;156:180–184 [DOI] [PubMed] [Google Scholar]
- 5.Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med 1997;157:669–675 [PubMed] [Google Scholar]
- 6.Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care 2001;24:131–153 [DOI] [PubMed] [Google Scholar]
- 7.Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am 2017;101:587–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadini GP, de Kreutzenberg SV, Rigato M, et al. Characteristics and outcomes of the hyperglycemic hyperosmolar non-ketotic syndrome in a cohort of 51 consecutive cases at a single center. Diabetes Res Clin Pract 2011;94:172–179 [DOI] [PubMed] [Google Scholar]
- 9.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park BE, Meacham WF, Netsky MG. Nonketotic hyperglycemic hyperosmolar coma. Report of neurosurgical cases with a review of mechanisms and treatment. J Neurosurg 1976;44:409–417 [DOI] [PubMed] [Google Scholar]
- 11.Zeitler P, Haqq A, Rosenbloom A, Glaser N; Drugs and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society . Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr 2011;158:9–14, 14.e1–2 [DOI] [PubMed] [Google Scholar]
- 12.Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS hyperosmolar hyperglycaemic guidelines group . Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015;32:714–724 [DOI] [PubMed] [Google Scholar]
- 13.Arieff AI, Carroll HJ. Hyperosmolar nonketotic coma with hyperglycemia: abnormalities of lipid and carbohydrate metabolism. Metabolism 1971;20:529–538 [DOI] [PubMed] [Google Scholar]
- 14.Arieff AI, Carroll HJ. Nonketotic hyperosmolar coma with hyperglycemia: clinical features, pathophysiology, renal function, acid-base balance, plasma-cerebrospinal fluid equilibria and the effects of therapy in 37 cases. Medicine (Baltimore) 1972;51:73–94 [DOI] [PubMed] [Google Scholar]
- 15.Fulop M, Tannenbaum H, Dreyer N. Ketotic hyperosmolar coma. Lancet 1973;2:635–639 [DOI] [PubMed] [Google Scholar]
- 16.Gerich JE, Martin MM, Recant L. Clinical and metabolic characteristics of hyperosmolar nonketotic coma. Diabetes 1971;20:228–238 [DOI] [PubMed] [Google Scholar]
- 17.Murthy K, Harrington JT, Siegel RD. Profound hypokalemia in diabetic ketoacidosis: a ther apeutic challenge. Endocr Pract 2005;11:331–334 [DOI] [PubMed] [Google Scholar]
- 18.Arora S, Cheng D, Wyler B, Menchine M. Prevalence of hypokalemia in ED patients with diabetic ketoacidosis. Am J Emerg Med 2012;30:481–484 [DOI] [PubMed] [Google Scholar]
- 19.Adrogué HJ, Lederer ED, Suki WN, Eknoyan G. Determinants of plasma potassium levels in diabetic ketoacidosis. Medicine (Baltimore) 1986;65:163–172 [DOI] [PubMed] [Google Scholar]
- 20.Harris AN, Grimm PR, Lee HW, et al. Mechanism of hyperkalemia-induced metabolic acidosis. J Am Soc Nephrol 2018;29:1411–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlotti AP, St George-Hyslop C, Bohn D, Halperin ML. Hypokalemia during treatment of diabetic ketoacidosis: clinical evidence for an aldosterone-like action of insulin. J Pediatr 2013;163:207–212.e1 [DOI] [PubMed] [Google Scholar]
- 22.West ML, Magner PO, Richardson RM, Halperin ML. A renal mechanism limiting the degree of potassium loss in severely hyperglycemic patients. Am J Nephrol 1988;8:373–378 [DOI] [PubMed] [Google Scholar]
- 23.Paltiel O, Salakhov E, Ronen I, Berg D, Israeli A. Management of severe hypokalemia in hospitalized patients: a study of quality of care based on computerized databases. Arch Intern Med 2001;161:1089–1095 [DOI] [PubMed] [Google Scholar]
- 24.Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med 1976;84:633–638 [DOI] [PubMed] [Google Scholar]
- 25.Heber D, Molitch ME, Sperling MA. Low-dose continuous insulin therapy for diabetic ketoacidosis. Prospective comparison with “conventional” insulin therapy. Arch Intern Med 1977;137:1377–1380 [PubMed] [Google Scholar]
- 26.Burghen GA, Etteldorf JN, Fisher JN, Kitabchi AQ. Comparison of high-dose and low-dose insulin by continuous intravenous infusion in the treatment of diabetic ketoacidosis in children. Diabetes Care 1980;3:15–20 [DOI] [PubMed] [Google Scholar]
- 27.Malone ML, Klos SE, Gennis VM, Goodwin JS. Frequent hypoglycemic episodes in the treatment of patients with diabetic ketoacidosis. Arch Intern Med 1992;152:2472–2477 [PubMed] [Google Scholar]
- 28.Dhatariya KK, Nunney I, Higgins K, Sampson MJ, Iceton G. National survey of the management of Diabetic Ketoacidosis (DKA) in the UK in 2014. Diabet Med 2016;33:252–260 [DOI] [PubMed] [Google Scholar]
- 29.Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care 2004;27:1873–1878 [DOI] [PubMed] [Google Scholar]
- 30.Ullal J, Aloi JA, Reyes-Umpierrez D, et al. Comparison of computer-guided versus standard insulin infusion regimens in patients with diabetic ketoacidosis. J Diabetes Sci Technol 2018;12:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes 2003;52:1469–1474 [DOI] [PubMed] [Google Scholar]
- 32.Kacheva S, Karges B, Göller K, Marx N, Mischke K, Karges W. QT prolongation caused by insulin-induced hypoglycaemia - an interventional study in 119 individuals. Diabetes Res Clin Pract 2017;123:165–172 [DOI] [PubMed] [Google Scholar]
- 33.Everett E, Mathioudakis NN. Association of socioeconomic status and DKA readmission in adults with type 1 diabetes: analysis of the US National Readmission Database. BMJ Open Diabetes Res Care 2019;7:e000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia 2016;59:2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mays JA, Jackson KL, Derby TA, et al. An evaluation of recurrent diabetic ketoacidosis, fragmentation of care, and mortality across Chicago, Illinois. Diabetes Care 2016;39:1671–1676 [DOI] [PubMed] [Google Scholar]
- 36.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006;10:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orban JC, Maizière EM, Ghaddab A, Van Obberghen E, Ichai C. Incidence and characteristics of acute kidney injury in severe diabetic ketoacidosis. PLoS One 2014;9:e110925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amin A, Gandhi B, Torre S, et al. Rhabdomyolysis-induced acute kidney injury in diabetic emergency: underdiagnosed and an important association to be aware of. Case Rep Med 2018;2018:4132738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hohenegger M, Rudas B. Kidney function in experimental diabetic ketosis. Diabetologia 1971;7:334–338 [DOI] [PubMed] [Google Scholar]
- 40.VanderWeele J, Pollack T, Oakes DJ, et al. Validation of data from electronic data warehouse in diabetic ketoacidosis: caution is needed. J Diabetes Complications 2018;32:650–654 [DOI] [PubMed] [Google Scholar]
- 41.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 42.Rimailho A, Riou B, Dadez E, Richard C, Auzépy P. Prognostic factors in hyperglycemic hyperosmolar nonketotic syndrome. Crit Care Med 1986;14:552–554 [DOI] [PubMed] [Google Scholar]
- 43.Pujara S, Ioachimescu A. Prolonged ketosis in a patient with euglycemic diabetic ketoacidosis secondary to dapagliflozin. J Investig Med High Impact Case Rep 2017;5:2324709617710040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galm BP, Bagshaw SM, Senior PA. Acute management of diabetic ketoacidosis in adults at 3 teaching hospitals in Canada: a multicentre, retrospective cohort study. Can J Diabetes 2019;43:309–315.e2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.