SUMMARY
Glioblastoma multiforme (GBM) is the most common and most aggressive malignant primary brain tumor in humans. Clinically useful molecular markers that help predict response to therapy and prognosis are still rare. The research was conducted in 55 patients with GBM, 26 (47.3%) women and 29 (52.7%) men, mean age 62.58 years. On immunohistochemical analysis, primary antibody to CD44 (dilution 1:50) and primary antibody to endoglin (CD105) (dilution 1:250) were used to evaluate neovascularization. Statistical analysis showed negative correlation between CD44 and survival (p=0.023) (higher expression of CD44 was correlated with shorter survival), but there was no correlation between neovascularization determined by CD105 in GBM and patient survival. Thus, significant individual predictors of longer survival were lower expression of CD44 (p=0.004), higher Karnofsky score (p=0.045), and female gender (p=0.017). The results obtained suggested the possible role of CD44 in the progression and tumor neovascularization of GBM.
Key words: Glioblastoma; Brain neoplasms; Prognosis; Immunohistochemistry; CD44 protein, human; Endoglin
Introduction
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor in adults. The mean progression-free survival is just over 6 months. It is widely acknowledged that the incurable nature of GBM is primarily attributable to the infiltrative growth of the cancer. Treatment with surgical resection, chemotherapy and radiation is invariably followed by tumor recurrence (1).
The prognosis of GBM patients is based on determining the prognostic value of various factors, including tumor markers. CD44 is a transmembrane glycoprotein that regulates cell growth, survival, differentiation and migration, and is thereby widely considered to be involved in carcinogenesis. CD44 is expressed in multiple forms, the most common being CD44 standard (CD44). As this protein has been examined in many cancer types, we employed immunohistochemical analysis of CD44 in GBM and correlated the expression of CD44 with neovascularization and survival of GBM patients. The identification of tumor markers such as CD44 could be of prognostic value in GBM patients and could help set up an individual treatment plan for these patients, with the goal of prolonging survival (2-4).
Also significant in GBM is angiogenesis, whereby endoglin (CD105) (accessory receptor for the transforming growth factor beta, TGF-β) is used as a marker which can determine density of the newly formed tumor blood vessels and can be used to quantify angiogenesis. These factors are especially significant for research of future therapeutic options based on angiogenesis, which have been proven useful in GBM patients so far (3).
Materials and Methods
Patients
Archival materials of 55 patients having undergone surgery for primary glioblastoma at the Department of Neurosurgery between January 2003 and December 2009 were used in the study. Data on patient survival were obtained from Cancer Registry of the Croatian Public Health Institute. Karnofsky score and survival time (number of days from day of operation to day of death) were established for every patient. Only patients with all clinical data available and with adequate histopathological findings were included in the study; patients treated with radiotherapy or chemotherapy, as well as patients with recurrent GBM were excluded from the analysis.
Immunohistochemistry
Paraffin blocks with GBM from every patient who matched the mentioned criteria were separated out of the archive. The material was processed by a standard histological method which includes fixation of tissue in 10% neutral buffered formalin and embedding into paraffin blocks, cutting into 5-µm thickness, deparaffinizing and staining with the standard hematoxylin and eosin method (HE).
Primary antibody for CD44 (dilution 1:50, Dako, Denmark) and primary antibody for endoglin (CD105) (dilution 1:250, Dako, Denmark) for determining microvascular density (MVD) were used in the study. Neovascularization determined by CD105 was marked as MVD/105. Immunohistochemical analysis was performed by the labeled streptavidin biotin (LSAB) method as a visualization system on a DAKO TechMate TM (Dako, Denmark) automated machine for immunohistochemical staining with the use of the microwave streptavidin immunoperoxidase (MSIP) protocol. Results of immunohistochemical analysis for CD44 marker were analyzed by light microscope and shown semiquantitatively by determining the percentage of immunoreactive tumor cells in the total selected cross section of tumor tissue. The endoglin (CD105) marker served to assess density of the newly formed tumor blood vessels. MVD was measured in the most active area of tumor neovascularization, which was determined by viewing histologic tumor cross sections under small magnification (X40) using Olympus CX 41 microscope in three non-overlapping large fields of view (X400). Every separated lumen surrounded by endoglin positive cells or, in the absence of lumen, every endoglin positive cell was considered to be a newly formed blood vessel. The mean value of three measurements was used on data processing.
Statistical analysis
Statistical analysis was performed by using the STATISTICA ver. 6.0 (StatSoft Inc., Tulsa, OK, USA) statistical package with the use of descriptive statistics. Regression analysis (parametric and nonparametric, depending on the type of distribution) was used to determine correlation of single variables, while the χ2-test or Fisher exact test was used to compare qualitative variables among subgroups. Outcomes related to survival were analyzed using survival life tables and Cox models of proportional hazards. Kruskal-Wallis test was also performed. The level of statistical significance was set at p<0.05.
Results
Patient characteristics
Among 55 GBM patients there were 26 (47.3%) women and 29 (52.7%) men. According to age, they were divided into those aged ≤65 (n=29; 52.7%) and >65 (n=26; 47.3%). The mean patient Karnofsky score was 75.45 and mean survival 183.82 days.
CD44 and MVD/CD105 expression
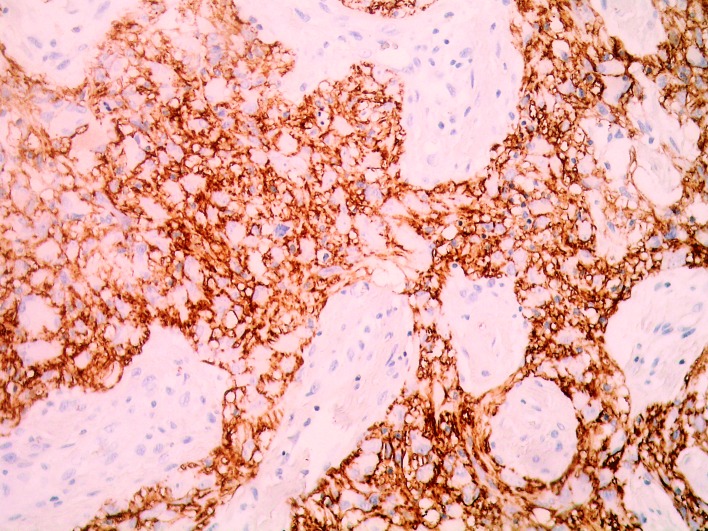
The mean CD44 expression in glioblastoma tumors was 64.91 (range 40.00-90.00, SD 17.23) (Fig. 1). The mean expression of endoglin and mean density of newly formed blood vessels in glioblastomas (MVD/CD105) was 15.64 (range 3.67-52.33, SD 8.91).
Fig. 1.
Strong CD44 activity in the majority of glioblastoma cells (magnification X400).
Descriptive statistical analysis of patients according to age, survival, CD44, MVD/CD105 and Karnofsky score is illustrated in Table 1. Statistical analysis of parameters yielded a number of statistically significant correlations (Table 2). There was negative correlation between CD44 and survival (greater expression of CD44 correlated with shorter survival; p=0.023) and positive correlation between Karnofsky score and survival (higher Karnofsky score correlated with longer survival; p=0.018). A relation was also demonstrated between greater expression of CD44 and female sex (p=0.039).
Table 1. Characteristics of glioblastoma multiforme patients: age, length of survival, CD44, MVD/CD105 and Karnofsky score (N=55).
| Arithmetic mean | SD | Min | Max | Percentile | |||
|---|---|---|---|---|---|---|---|
| 25th | Median | 75th | |||||
| Age (yrs) | 62.58 | 9.24 | 47.00 | 77.00 | 55.00 | 63.00 | 71.00 |
| Survival (days) | 183.82 | 133.16 | 7.00 | 512.00 | 87.00 | 151.00 | 263.00 |
| MVD/CD105 | 15.64 | 8.91 | 3.67 | 52.33 | 9.33 | 13.00 | 21.00 |
| CD44 | 64.91 | 17.23 | 40.00 | 90.00 | 50.00 | 65.00 | 80.00 |
| Karnofsky score | 75.45 | 20.89 | 40.00 | 100.00 | 60.00 | 80.00 | 90.00 |
MVD = microvascular density
Table 2. Correlation coefficients among age, length of survival (days) and particular clinical parameters.
| MVD/CD105 | CD44 | Karnofsky score | Age | Survival (days) | Sex | ||
|---|---|---|---|---|---|---|---|
| MVD/CD105 | Correlation coefficient | 1.000 | 0.234 | 0.120 | -0.036 | 0.072 | 0.022 |
| p | 0.085 | 0.383 | 0.793 | 0.602 | 0.846 | ||
| n | 55 | 55 | 55 | 55 | 55 | 55 | |
| CD44 | Correlation coefficient | 0.234 | 1.000 | -0.091 | 0.022 | -0.306 | 0.241 |
| p | 0.085 | 0.508 | 0.874 | 0.023 | 0.039 | ||
| n | 55 | 55 | 55 | 55 | 55 | 55 | |
| Karnofsky score | Correlation coefficient | 0.120 | -0.091 | 1.000 | -0.242 | 0.318 | -0.093 |
| p | 0.383 | 0.508 | 0.075 | 0.018 | 0.437 | ||
| n | 55 | 55 | 55 | 55 | 55 | 55 | |
| Age | Correlation coefficient | -0.036 | 0.022 | -0.242 | 1.000 | -0.189 | 0.142 |
| p | 0.793 | 0.874 | 0.075 | 0.167 | 0.212 | ||
| n | 55 | 55 | 55 | 55 | 55 | 55 | |
| Survival (days) | Correlation coefficient | 0.072 | -0.306 | 0.318 | -0.189 | 1.000 | 0.087 |
| p | 0.602 | 0.023 | 0.018 | 0.167 | 0.438 | ||
| n | 55 | 55 | 55 | 55 | 55 | 55 | |
| Sex* | Correlation coefficient | 0.022 | 0.241 | -0.093 | 0.142 | 0.087 | 1.000 |
| p | 0.846 | 0.039 | 0.437 | 0.212 | 0.438 | . | |
| n | 55 | 55 | 55 | 55 | 55 | 55 | |
*Kendall’s tau_b; MVD = microvascular density; figures in bold are statistically significant
Patients were grouped according to the level of CD44 expression (0-45, 46-75 and 76-100), whereby no statistically significant difference was found between their results (p=0.066 was the borderline result). Analysis according to sex and age did not show significant differences among the groups of CD44 expression (sex differences were at borderline level, p=0.066).
Different CD44 groups were also analyzed according to particular parameters, i.e. age, MVD/CD105, Karnofsky score and survival (Table 3). There was no statistically significant difference, except for the relation of CD44 and survival (p=0.0117), i.e. the mean survival in the CD44 group 0-45 was 185.67 days, in CD44 group 46-75 it was 234.42 days, and in the CD44 group 76-100 it was 118.74 days. Significant differences were only recorded in survival, which was longest in the 46-75 group (mean, 234.42 days) and significantly shorter in the 76-100 group (mean, 118.74 days).
Table 3. Age, MVD/CD105, Karnofsky score, survival in individual CD44 groups (Kruskal-Wallis test).
| CD44 group | n | Arithmetic mean | SD | Min | Max | Percentile | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25th | Median | 75th | |||||||||||
| Age | 0-45 | 12 | 65.17 | 8.89 | 47.00 | 77.00 | 57.25 | 67.50 | 70.75 | ||||
| 46-75 | 24 | 60.50 | 9.51 | 48.00 | 77.00 | 51.75 | 60.00 | 68.75 | |||||
| 76-100 | 19 | 63.58 | 9.00 | 49.00 | 75.00 | 55.00 | 65.00 | 72.00 | |||||
| MVD/CD105 | 0-45 | 12 | 14.56 | 8.10 | 6.00 | 32.00 | 8.42 | 11.50 | 21.58 | ||||
| 46-75 | 24 | 14.19 | 5.96 | 3.67 | 24.67 | 10.00 | 12.50 | 20.08 | |||||
| 76-100 | 19 | 18.16 | 11.94 | 6.67 | 52.33 | 8.78 | 13.00 | 22.67 | |||||
| Karnofsky score | 0-45 | 12 | 74.17 | 22.75 | 40.00 | 100.00 | 52.50 | 70.00 | 100.00 | ||||
| 46-75 | 24 | 78.75 | 19.85 | 40.00 | 100.00 | 62.50 | 85.00 | 97.50 | |||||
| 76-100 | 19 | 72.11 | 21.49 | 40.00 | 100.00 | 50.00 | 80.00 | 90.00 | |||||
| Survival (days)* | 0-45 | 12 | 185.67 | 142.00 | 7.00 | 477.00 | 74.00 | 173.50 | 271.25 | ||||
| 46-75 | 24 | 234.42 | 142.93 | 23.00 | 512.00 | 126.00 | 208.50 | 370.50 | |||||
| 76-100 | 19 | 118.74 | 82.98 | 13.00 | 352.00 | 59.00 | 121.00 | 151.00 | |||||
| Kruskal Wallis test | df | p | |||||||||||
| Age | 2.00 | 2 | 0.367 | ||||||||||
| MVD/CD105 | 0.89 | 2 | 0.641 | ||||||||||
| Karnofsky score | 1.10 | 2 | 0.577 | ||||||||||
| Survival (days) | 8.19 | 2 | 0.017 | ||||||||||
*Kendall’s tau_b; MVD = microvascular density; figures in bold are statistically significant
Accordingly, it should be noted that significant individual predictors of longer survival were lower CD44 expression (beta=-0.39; p=0.004), higher Karnofsky score (beta=0.26; p=0.045) and female sex (beta=0.31; p=0.017), while the strongest individual predictor was CD44 expression. Correlation between CD44 expression and density of newly formed blood vessels (MVD/CD105) was not demonstrated; CD44 expression correlated statistically significantly with patient survival, but there was no correlation between density of newly formed blood vessels (MVD/CD105) in GBM and patient survival (Table 4).
Table 4. Linear regression model of survival prognosis.
| Nonstandardized coefficient | Standardized coefficient | t | p | 95% CI | |||
|---|---|---|---|---|---|---|---|
| B | Standard error | Beta | Lower | Upper | |||
| Age | -2.34 | 1.81 | -0.16 | -1.30 | 0.201 | -5.97 | 1.29 |
| Female sex* | 83.03 | 33.56 | 0.31 | 2.47 | 0.017 | 15.60 | 150.47 |
| MVD/CD105 | 1.60 | 1.87 | 0.11 | 0.86 | 0.396 | -2.15 | 5.36 |
| CD44* | -3.03 | 1.00 | -0.39 | -3.03 | 0.004 | -5.05 | -1.02 |
| Karnofsky score* | 1.65 | 0.80 | 0.26 | 2.05 | 0.045 | 0.04 | 3.26 |
95% CI = 95% confidence interval; *Kendall’s tau_b; MVD = microvascular density; figures in bold are statistically significant
Discussion
There are numerous studies of the prognostic value of CD44, which have different results. CD44 is known to be a transmembrane glycoprotein, the various isoforms of which are encoded based on alternative RNA cutting, and the most numerous isoform variant is the standard type CD44. CD44 expression in tissues has primarily been detected by immunohistochemistry (IHC) and reverse transcription polymerase chain reaction (RT-PCR) (5-12). It is important to understand the complexities of this molecule given its ability to function at the center of multiple signaling highways and to act as a tumor microenvironment sensory tool (2, 5, 6). However, CD44-mediated biology goes beyond the complexity of a molecule that either promotes or inhibits cancer because CD44 regulates cellular processes that can do both. Decades of research have shown that CD44 participates in major oncogenic signaling networks and complexes with oncogens that promote every aspect of tumor progression. Conversely, CD44 signaling also mediates contact inhibition and inhibits cell invasion and angiogenesis. CD44 is extremely sensitive to changes in the microenvironment, and although much is known about its biology, its reaction to changing extra- and intracellular conditions is still the subject of active research (7).
Aside from the participation of CD44 in matrix adhesion, activation and lymphocyte navigation, wound healing, growth promotion, cellular survival and migration, it also participates in tumor growth and metastasizing (3-5, 8, 13). The importance of CD44 is shown in tumorigenesis, since CD44 mediates the interaction of extracellular matrix and intracellular cytoskeleton, and shows interaction with growth factors and matrix metalloproteinases, thus activating signaling pathways which may stimulate tumor growth and suppression of apoptosis (5, 6). Changes in CD44 molecule expression have been noticed in various types of tumor, such as breast tumor, soft tissue sarcomas, as well as neuroblastomas (6-8).
Research into the role of CD44 in the development of neoplasms has shown contradicting results. Thus, colorectal carcinoma showed increased CD44 expression, whereas in prostate carcinoma, there was a correlation between lower CD44 expression and metastatic progression (9, 10, 14). Similar to that, some studies noted that overexpression of CD44 was related to better prognosis, whereas others showed that lower CD44 expression contributed to tumor invasion (14-16).
We found negative correlation between CD44 and survival in GBM patients by statistical analysis, i.e. increased CD44 expression was related to decreased survival, supporting the potential prognostic significance of that marker in this tumor. Otherwise, there was positive correlation between Karnofsky score and survival, i.e. higher Karnofsky score correlated with longer survival in GBM patients (1, 2, 17).
The potential influence of CD44 on tumor growth and expansion could be exerted in many ways. CD44 probably stimulates proliferation, motility and/or invasiveness of tumor cells (it probably recruites and activates matrix metalloproteinases attached to cell surface) (1, 2, 18). It is also possible that CD44 promotes tumor angiogenesis by regulating endothelial cells and/or recruiting, i.e. activating inflammatory cells. Besides that, CD44 has been shown to be involved in other mechanisms of carcinogenesis as well, i.e. noticeable is the role of brain tumor stem cells that are involved in tumor progression, whereby CD44 could be a contributing factor (19, 20).
On the other hand, several studies showed that lower CD44 expression correlated with lower survival in patients with different types of cancer, and some authors demonstrated it in GBM patients as well (21, 22). Therefore, although higher CD44 expression has been frequently found to correlate with worse tumor prognosis, lower CD44 values may also indicate that malignant cells are more resistant to chemotherapeutic agents.
Special importance of the role of angiogenesis in tumors should be borne in mind, thus also in GBM which is highly vascularized (15, 20, 23-29). Angiogenesis has an important role in the progression of this tumor. Tumor angiogenesis is regulated by proangiogenic and antiangiogenic factors produced by tumor, as well as by the surrounding and infiltrating host cells (24, 25). Tumor blood vessels and normal blood vessels are distinguished by their organization, vascular patterns and density of newly formed small blood vessels. The advantage of endoglin lies in its expression that is only evident in endothelial cells which are proliferating, but not in normal blood vessels or tumor cells (26, 27). Sica et al. (28) studied neovascularization in peritumoral areas, as well as CD105 and nestin expression besides density of small blood vessels, in order to assess their possible prognostic value. They found tumor neoangiogenesis to develop in peritumoral GBM tissue, whereby pericytes contributed closely and CD105-MVD had a prognostic value in the areas at longer distance from tumor edge (28). Our results of determining blood vessel density by analyzing CD105/MVD showed no correlation between CD105/MVD expression and patient survival.
Since angiogenesis plays a major role in GBM growth, there are treatment options based on angiogenesis which have shown clinical efficacy in GBM patients (24). The administration of the potentially therapeutic antiangiogenic agents [such as anti-vascular endothelial growth factor VEGF/VEGF receptor (VEGFR) agents] has shown clinical benefits in GBM patients (30-33).The possibility of inhibiting signaling to the angiogenic factors VEGF and hepatocyte growth factor (HGF) by targeting CD44v6 variant is a very important approach to antitumor treatment (20, 26, 30-33).
In conclusion, the results of our study showed negative correlation between CD44 and survival. Monitoring and research of CD44 is related to the possible treatment modalities in GBM patients, since CD44 can be the target of antiangiogenic therapy.
References
- 1.Reavey-Cantwell JF, Haroun RI, Zahurak M, Clatterbuck RE, Parker RJ, Mehta R, et al. The prognostic value of tumor markers in patients with glioblastoma multiforme: analysis of 32 patients and review of the literature. J Neurooncol. 2001;55:195–204. 10.1023/A:1013845004294 [DOI] [PubMed] [Google Scholar]
- 2.Wei KC, Huang CY, Chen PY, Feng LY, Wu TW, Chen SM, et al. Evaluation of the prognostic value of CD44 in glioblastoma multiforme. Anticancer Res. 2010;30:253–9. [PubMed] [Google Scholar]
- 3.Marhaba R, Zöller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–31. 10.1023/B:HIJO.0000032354.94213.69 [DOI] [PubMed] [Google Scholar]
- 4.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. 10.1038/nrm1004 [DOI] [PubMed] [Google Scholar]
- 5.Peiper M, Sato T, Zurakowski D, Eisenberger C, Heinecke A, Hosch S, et al. CD44s expression is associated with improved survival in soft tissue sarcoma. Anticancer Res. 2004;24:1053–6. [PubMed] [Google Scholar]
- 6.Terpe HJ, Christiansen H, Gonzalez M, Berthold F, Lampert F. Differentiation and prognosis of neuroblastoma in correlation to the expression of CD44s. Eur J Cancer. 1995;31A:549–52. 10.1016/0959-8049(95)00061-M [DOI] [PubMed] [Google Scholar]
- 7.Woodman AC, Sugiyama M, Yoshida K, Sugino T, Borgya A, Goodson S, et al. Analysis of anomalous CD44 gene expression in human breast, bladder, and colon cancer and correlation of observed mRNA and protein isoforms. Am J Pathol. 1996;149:1519–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Jothy S. CD44 and its partners in metastasis. Clin Exp Metastasis. 2003;20(3):195–201. 10.1023/A:1022931016285 [DOI] [PubMed] [Google Scholar]
- 9.Iczkowski KA, Omara-Opyene AL, Shah GV. The predominant CD44 splice variant in prostate cancer binds fibronectin, and calcitonin stimulates its expression. Anticancer Res. 2006;26:2863–72. [PubMed] [Google Scholar]
- 10.Okada H, Yoshida J, Sokabe M, Wakabayashi T, Hagiwara M. Suppression of CD44 expression decreases migration and invasion of human glioma cells. Int J Cancer. 1996;66:255–60. [DOI] [PubMed] [Google Scholar]
- 11.Novak-Bilić G, Vučić M, Japundžić I, Meštrović-Štefekov J, Stanić-Duktaj S, Lugović-Mihić L. Irritant and allergic contact dermatitis – skin lesion characteristics. Acta Clin Croat. 2018;57(4):713–20. 10.20471/acc.2018.57.04.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesar M, Stanec M, Lesar N, Vrdoljak DV, Zore Z, Banović M, et al. Immunohistochemical differentiation of triple negative breast cancer. Acta Clin Croat. 2016;55(1):3–8. 10.20471/acc.2016.55.01.1 [DOI] [PubMed] [Google Scholar]
- 13.Mihić J, Rotim K, Vučić M, Novak-Bilić G, Lugović-Mihić L. Značenje angiogeneze i ekspresije CD44 u glioblastomu. Acta Med Croatica. 2017;71:201–7. [in Croatian] [Google Scholar]
- 14.Huh JW, Kim HR, Kim YJ, Lee JH, Park YS, Cho SH, et al. Expression of standard CD44 in human colorectal carcinoma: association with prognosis. Pathol Int. 2009;59:241–6. 10.1111/j.1440-1827.2009.02357.x [DOI] [PubMed] [Google Scholar]
- 15.DeLisser HM. CD44: target for antiangiogenesis therapy. Blood. 2009;114:5114–5. 10.1182/blood-2009-10-246397 [DOI] [PubMed] [Google Scholar]
- 16.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–9. 10.1080/10408360290795574 [DOI] [PubMed] [Google Scholar]
- 17.Stojsavljević M, Tasić G, Nikolić I, Repac N, Janićijević A, Sćepanović V, et al. Glioblastoma multiforme brain tumors located in the motor cortex – specific findings in comparison with low grade gliomas of the same localization: analysis of a sixty patient series. Acta Clin Croat. 2015;54(4):402–8. [PubMed] [Google Scholar]
- 18.Harmanci D, Erbayraktar Z, Sayin O, Guner GA. In vitro effects of selenium on human glioblastoma multiforme cell lines: a preliminary study. Acta Clin Croat. 2017;56(1):48–57. doi: . 10.20471/acc.2017.56.01.08 [DOI] [PubMed] [Google Scholar]
- 19.Napier SL, Healy ZR, Schnaar RL, Konstantopoulos K. Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44v and alternative sialofucosylated selectin ligands. J Biol Chem. 2007;282:3433–41. 10.1074/jbc.M607219200 [DOI] [PubMed] [Google Scholar]
- 20.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delliser H, et al. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. 10.1016/j.matbio.2006.08.261 [DOI] [PubMed] [Google Scholar]
- 21.Sillanpää S, Anttila MA, Voutilainen K, Tammi RH, Saarikoski SV, Kosma VM. CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clin Cancer Res. 2003;9:5318–24. [PubMed] [Google Scholar]
- 22.Esteban F, Bravo JJ, González-Moles MA, Bravo M, Ruiz-Avila I, Gil-Montoya JA. Adhesion molecule CD44 as a prognostic factor in laryngeal cancer. Anticancer Res. 2005;25:1115–21. [PubMed] [Google Scholar]
- 23.Božić B, Rotim K, Kogler A, Broz R, Krpina H, Čupić H, et al. Cerebellar glioblastoma in the elderly – case report. Acta Clin Croat. 2009;48(2):175–8. [PubMed] [Google Scholar]
- 24.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7:1152–60. 10.1016/S1474-4422(08)70260-6 [DOI] [PubMed] [Google Scholar]
- 25.Brunckhorst MK, Wang H, Lu R, Yu Q. Angiopoietin-4 promotes glioblastoma progression by enhancing tumor cell viability and angiogenesis. Cancer Res. 2010;70:7283–93. Epub 2010 Sep 7. 10.1158/0008-5472.CAN-09-4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–90. [PubMed] [Google Scholar]
- 27.Smith SJ, Tilly H, Ward JH, Macarthur DC, Lowe J, Coyle B, et al. CD105 (Endoglin) exerts prognostic effects via its role in the microvascular niche of paediatric high grade glioma. Acta Neuropathol. 2012;124:99–110. 10.1007/s00401-012-0952-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sica G, Lama G, Anile C, Geloso MC, La Torre G, De Bonis P, et al. Assessment of angiogenesis by CD105 and nestin expression in peritumor tissue of glioblastoma. Int J Oncol. 2011;38:41–9. [PubMed] [Google Scholar]
- 29.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. 10.1038/nrc2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh S, Goi T, Naruse T, Ueda Y, Kurebayashi H, Nakazawa T, et al. Cancer stem cell marker in circulating tumor cells: expression of CD44 variant exon 9 is strongly correlated to treatment refractoriness, recurrence and prognosis of human colorectal cancer. Anticancer Res. 2015;35:239–44. [PubMed] [Google Scholar]
- 31.Wang N, Jain RK, Batchelor TT. New directions in anti-angiogenic therapy for glioblastoma. Neurotherapeutics. 2017;14(2):321–32. 10.1007/s13311-016-0510-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalpathy-Cramer J, Chandra V, Da X, Ou Y, Emblem KE, Muzikansky A, et al. Phase II study of tivozanib, an oral VEGFR inhibitor, in patients with recurrent glioblastoma. J Neurooncol. 2017;131(3):603–10. 10.1007/s11060-016-2332-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas AA, Fisher JL, Hampton TH, Christensen BC, Tsongalis GJ, Rahme GJ, et al. Immune modulation associated with vascular endothelial growth factor (VEGF) blockade in patients with glioblastoma. Cancer Immunol Immunother. 2017;66(3):379–89. 10.1007/s00262-016-1941-3 [DOI] [PMC free article] [PubMed] [Google Scholar]