Abstract
Introduction:
Although diabetes through several possible mechanisms such as increased microvascular pathology and inefficiency of glucose utilization during cognitive tasks can be associated with cognitive impairment, there is inconclusive evidence that shows elderly diabetic patients under therapy have higher cognitive function compared to their non-diabetics counterparts. The present study was conducted to elucidate the association between diabetes and cognitive function in later life.
Methods:
Data for this study, consisting of 2202 older adults aged 60 years and above, were taken from a population-based survey entitled “Identifying Psychosocial and Identifying Economic Risk Factor of Cognitive Impairment among Elderly. Data analysis was conducted using the IBM SPSS Version 23.0.
Results:
The mean of MMSE was found to be 22.67 (SD = 4.93). The overall prevalence of self-reported diabetes was found to be 23.6% (CI95%: 21.8% - 25.4%). The result of independent t-test showed diabetic subjects had a higher mean score of MMSE (M = 23.05, SD =4 .55) than their counterparts without diabetes (M = 22.55, SD = 5.04) (t = -2.13 p<.05). The results of multiple linear regression analysis showed that diabetes was not significantly associated with cognitive function, after controlling the possible confounding factors.
Conclusions:
The findings from the current study revealed that diabetes is not associated with cognitive decline. This study supports the findings that long-term treatment of diabetes may reduce the risk of cognitive decline. This finding may provide new opportunities for the prevention and management of cognitive decline.
Keywords: Aged, diabetes, cognitive function, microvascular pathology, cognitive impairment, MMSE
1. INTRODUCTION
Diabetes and dementia are two most common medical conditions among older adults [1]. Due to effective prevention and treatment strategies for the macro and microvascular complications of diabetes, people with diabetes are living longer, which might result in the emerging new complications such as dementia [2]. Several studies have assessed the association between diabetes and dementia and most studies identified diabetes as a risk factor for dementia in old age [3-5]. There are several potential mechanisms that explain the association between diabetes and dementia [2, 6-13]. One of the potential underlying mechanisms is insulin resistance and deficiency that may interact with amyloid-β protein and tau protein phosphorylation that might lead to the onset and development of dementia [2, 13].
In addition, diabetes as a complex metabolic disorder can be associated with some identified risk factors, such as vascular disease, impaired glucose metabolism, chronic inflammation, hypoglycemia and hyperglycemia, APOE-ε4 allele, and oxidative stress which appears to play a role in the onset and development of dementia [6-12].
Although, as mentioned above, there is a growing body of evidence suggesting that diabetes may increase the risk of dementia [14, 15], there are some other studies that show diabetes treatment may reduce the risk of cognitive decline [16-18]. In light of this evidence, diabetes treatment that may reduce the risk of dementia has gained the interest for scientists and resulted in conflicting results. For example, the findings from an epidemiological trial showed that those diabetic patients who were long-term users of metformin, had a slightly higher risk of dementia than those who did not receive the drug [19].
The contrasting findings from the recent studies [16-19] imply that more research is needed for a consistent understanding of the impact of dementia treatment on cognitive function. Therefore, the present cross-sectional study was conducted on 2202 older adults to elucidate the association between diabetes and cognitive function in later life.
2. METHODS
The data for this study were drawn from the cross-sectional national survey entitled “Identifying Psychosocial and Identifying Economic Risk Factor of Cognitive Impairment among Elderly”. The survey employed a multi-stage proportional cluster random sampling technique to obtain a sample of community-dwelling older adults in Malaysia. The detailed methodology of the survey has been previously reported elsewhere [20, 21].
3. ETHICAL CONSIDERATIONS
The study was approved by the Medical Research Ethics Committee of Faculty of Medicine and Health Science of Universiti Putra Malaysia, in accordance with the Helsinki Declaration, World Medical Association (WMA). After explaining the purpose of the study, informed consent was orally obtained from all participants.
4. MEASURES
4.1. Cognitive Function
The cognitive function was measured by a validated Malay version of the Mini-Mental State Examination (MMSE) [22]. The total raw score was used in the analyses.
4.2. Diabetes
The self-reported method was used to assess previously diagnosed diabetes by asking respondents, “Have you ever been told by a doctor that you had diabetes?. The self-reported diagnosis of diabetes has been found as a valid method to assess diabetes in epidemiological studies [23-25].
4.3. Confounding Factors
Socio-demographic confounding factors contributing to cognitive function [26, 27], such as age, sex, marital status, years of education, household income, and employment status were assessed.
4.4. Data Analysis
To fulfill the study purposes both descriptive and inferential statistics were employed using IBM SPSS version 23. First, the univariate was conducted to assess variables distribution. Second, a multiple linear regression was used to assess the effect of diabetes on cognitive function. The assumption of the normal distribution for numeric variables was assessed by skewness and kurtosis indices. The results showed that skewness and kurtosis values were within the acceptable range of -2 to +2 [28]. A two-tailed α value of 0.05 was considered as the level of statistical significance.
5. RESULTS
The sample for the current study consisted of 2,202 community-dwelling Malaysian older adults, with an average age of 69.05 (SD= 6.24) years. Table 1 presents socio-demographic characteristics of the respondents. As can be seen from Table 1, 47.9% of the respondents participated in the survey were men, and 31.3% were unmarried. Most respondents had a primary education (57.8%), 21.4% obtained a secondary and tertiary education, and around one-fifth of the respondents reported having no formal education. The mean of MMSE was found to be 22.67 (SD=4.93). The overall prevalence of self-reported diabetes was found to be 23.6% (CI95%: 21.8% - 25.4%) among older adults aged 60 years and above.
Table 1. Socio-demographic characteristics of the respondents.
| - | Frequency | Percent | |
|---|---|---|---|
| Sex | Female | 1148 | 52.1 |
| Male | 1054 | 47.9 | |
| Employment Status | Not working | 1709 | 77.6 |
| Working | 493 | 22.4 | |
| Marital Status | Unmarried | 690 | 31.3 |
| Married | 1512 | 68.7 | |
| Diabetes | No | 1682 | 76.4 |
| Yes | 520 | 23.6 | |
| Educational Level | No formal | 458 | 20.8 |
| Primary | 1273 | 57.8 | |
| Secondary and tertiary | 471 | 21.4 | |
| Age | 60-69 | 1260 | 57.2 |
| 70-79 | 810 | 36.8 | |
| 80+ | 132 | 6.0 | |
| Household Income | Less than RM 500 | 1163 | 52.8 |
| RM 500+ | 1039 | 47.2 | |
RM: Ringgit Malaysia.
As depicted in Fig. (1), the result of an independent t-test showed a significant difference between respondents with diabetes and those without diabetes in mean score of MMSE (t=-2.13 p<.05), wherein diabetic subjects had a higher mean score of MMSE (M=23.05, SD=4.55) than their counterparts without diabetes (M=22.55, SD=5.04).
Fig. (1).
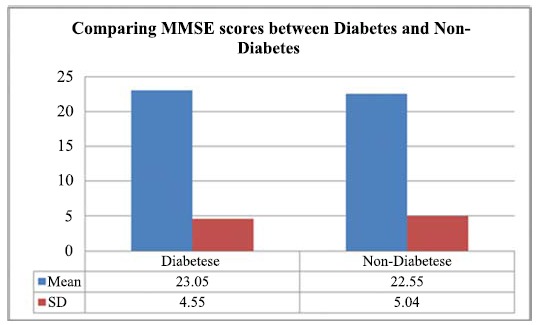
Comparing MMSE scores between diabetes and non-diabetes participants.
The assumptions for multiple linear regression analysis including linearity, homoscedasticity and collinearity were tested and the findings showed that the assumptions were met. The results of multiple linear regression analysis emerged as a significant model that was able to explain 14% of the variance in cognitive function, (F (9, 2176) =125.68, R2 =.34, p<.001). As can be found from Table 2, diabetes was not significantly associated with cognitive function, after adjusting for age, sex, years of education, marital status, employment status, and household income.
Table 2. Results of multiple linear regression analysis.
| Variable | B | SE | Beta | t | P-value | 95%CI |
|---|---|---|---|---|---|---|
| Age | -0.07 | 0.02 | -0.08 | -3.76 | P<.001 | .01 .09 |
| Sex | 0.35 | 0.23 | 0.04 | 1.51 | .130 | -.41 .49 |
| Marital Status | 0.45 | 0.24 | 0.04 | 1.84 | .065 | -.43 .51 |
| Employment status | 0.23 | 0.25 | 0.02 | 0.94 | .345 | -.47 .51 |
| Years of education | 0.34 | 0.03 | 0.27 | 11.74 | P<.001 | .21 .33 |
| Household Income | 0.76 | 0.21 | 0.08 | 3.56 | P<.001 | .33 .49 |
| Diabetes | 0.29 | 0.23 | 0.03 | 1.26 | .209 | -.42 .48 |
F(7, 2190)=51.23, P<.001, R2=.14.
6. DISCUSSION
The main purpose of the present study was to elucidate the association between diabetes and cognitive function in later life.
According to the present study, about one-fourth (23.6%) of the Malaysian older adults had diabetes. The prevalence of diabetes observed in older Malaysians aged 60 years and over is lower than the finding reported by the study in Australia. The prevalence of self-reported diabetes in a sample of Indigenous Australians aged 40-92 years was 37.11% [24]. However, the prevalence of self-reported diabetes in our study was higher than what has been documented by a Brazilin study, wherein 15.4% of the sample presented self-reported diabetes [29].
Our finding is closely consistent with the results of a recent study in Malaysia which showed that the prevalence of diabetes was 22.7% among older adults 60 years and above [30].
The result of the bivariate analysis revealed that diabetic subjects had a higher mean score of cognitive function than their counterparts without diabetes. Although, there is a growing body of scientific investigations indicating that diabetes is associated with an increased risk of developing cognitive impairment and dementia [31-33], the findings from the current study revealed that diabetic respondents had a higher score of cognitive function. As the first line oral diabetes medication in Malaysia is metformin [34], it can be said that metformin may be associated with the lowest risk of cognitive impairment. This finding is consistent with previous studies [23-25, 35, 36] that have demonstrated using metformin for diabetes management is contributed to lower possibility of cognitive impairment.
Metformin as an orally active biguanide clearly reduces insulin levels, inflammation and thrombosis, and the risks of metabolic syndrome [2]. Additionally, there is evidence indicating that metformin acts as an antiaging factor that promotes health [37, 38].
CONCLUSION
The findings from the current study revealed that diabetes is not associated with cognitive decline. This study supports the findings that long-term treatment of diabetes may reduce the risk of cognitive decline [13]. These findings imply that metformin could play a role in preventing dementia [39]. This finding may provide new opportunities for the prevention and management of cognitive decline.
LIMITATIONS
Although the current study benefits from its large sample size, there are a few limitations that should be acknowledged. Firstly, the study employed a cross-sectional design which impedes causal inferences. Although there is a substantial agreement between self-reported and physician assessment for diabetes [40], the second possible limitation of this study is that the assessment of diabetes was based on the self-report measure.
ACKNOWLEDGEMENTS
We would like to express our gratitude to all co-researchers, fieldworkers, staffs, local authorities and subjects for their willingness to cooperate with us to make our study a success.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Medical Research Ethics Committee of Faculty of Medicine and Health Science of Universiti Putra, Malaysia.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. The reported experiments on humans were in accordance with the ethical standards of the committee responsible for human experimentation (institutional national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/).
CONSENT FOR PUBLICATION
Informed consent was orally obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
The data that support the finding of this study are available from the corresponding author Dr. Tengku A. Hamid (tengkuaizan06@gmail.com), upon reasonable request.
FUNDING
The authors would like to acknowledge the financial support under the Long Term Research Grant Scheme (LRGS) provided by the Ministry of Education Malaysia (LRGS/BU/ 2012/UKM-UKM/K/01).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Luchsinger J.A. Diabetes, related conditions, and dementia. J. Neurol. Sci. 2010;299(1):35–38. doi: 10.1016/j.jns.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natan M.B., Danino S., Freundlich N., Barda A., Yosef R.M. Intention of nursing students to work in geriatrics. Res. Gerontol. Nurs. 2015;8(3):140–147. doi: 10.3928/19404921-20150219-03. [DOI] [PubMed] [Google Scholar]
- 3.Peila R., Rodriguez B.L., Launer L.J. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies. Honolulu-Asia Aging Study Diabetes. 2002;51(4):1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 4.Akomolafe A., Beiser A., Meigs J.B., Au R., Green R.C., Farrer L.A., et al. Diabetes mellitus and risk of developing Alzheimer disease: Results from the framingham study. Arch. Neurol. 2006;63(11):1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 5.Cheng D., Noble J., Tang M.X., Schupf N., Mayeux R., Luchsinger J.A. Type 2 diabetes and late-onset alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2011;31(6):424–430. doi: 10.1159/000324134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrone C., Eriksson O., Lindholm D. Diabetes drugs and neurological disorders: New views and therapeutic possibilities. Lancet Diabetes Endocrinol. 2014;2(3):256–262. doi: 10.1016/S2213-8587(13)70125-6. [DOI] [PubMed] [Google Scholar]
- 7.Markowicz-Piasecka M., Sikora J., Szydłowska A., Skupień A., Mikiciuk-Olasik E., Huttunen K.M. Metformin- A future therapy for neurodegenerative diseases. Pharm. Res. 2017;34(12):2614–2627. doi: 10.1007/s11095-017-2199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ascher-Svanum H., Chen Y.F., Hake A., Kahle-Wrobleski K., Schuster D., Kendall D., et al. Cognitive and functional decline in patients with mild alzheimer dementia with or without comorbid diabetes. Clin Therapeut. 2015;37(6):1195–1205. doi: 10.1016/j.clinthera.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Banks W.A., Owen J.B., Erickson M.A. Insulin in the brain: There and back again. Pharmacol. Ther. 2012;136(1):82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Cesari M., Liu F., Dong B., Vellas B. Effects of diabetes mellitus on cognitive decline in patients with Alzheimer disease: A systematic review. Can. J. Diabetes. 2017;41(1):114–119. doi: 10.1016/j.jcjd.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Strachan M.W., Reynolds R.M., Frier B.M., Mitchell R.J., Price J.F. The relationship between type 2 diabetes and dementia. Br. Med. Bull. 2008;88(1):131–146. doi: 10.1093/bmb/ldn042. [DOI] [PubMed] [Google Scholar]
- 12.González-Reyes E.R., Aliev G., Ávila-Rodrigues M., Barreto E.G. Alterations in glucose metabolism on cognition: A possible link between diabetes and dementia. Curr. Pharm. Des. 2016;22(7):812–818. doi: 10.2174/1381612822666151209152013. [DOI] [PubMed] [Google Scholar]
- 13.Bowling A. Research methods in health: Investigating health and health services. 4th ed. Milton Keynes: Open University Press; 2014. [Google Scholar]
- 14.Hsu C.C., Wahlqvist M.L., Lee M.S., Tsai H.N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimers Dis. 2011;24(3):485–493. doi: 10.3233/JAD-2011-101524. [DOI] [PubMed] [Google Scholar]
- 15.Bruce D.G., Davis W.A., Casey G.P., Starkstein S.E., Clarnette R.M., Foster J., et al. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia. 2008;51(2):241–248. doi: 10.1007/s00125-007-0894-7. [DOI] [PubMed] [Google Scholar]
- 16.Herath P.M., Cherbuin N., Eramudugolla R., Anstey K.J. The effect of diabetes medication on cognitive function: evidence from the PATH Through Life study. BioMed Res. Int. 2016;2016:7208429. doi: 10.1155/2016/7208429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asadbegi M., Yaghmaei P., Salehi I., Ebrahim-Habibi A., Komaki A. Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res. Bull. 2016;121:178–185. doi: 10.1016/j.brainresbull.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A., Bisht B., Dey C.S. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology. 2011;60(6):910–920. doi: 10.1016/j.neuropharm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Imfeld P., Bodmer M., Jick S.S., Meier C.R. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: A population-based case-control study. J. Am. Geriatr. Soc. 2012;60(5):916–921. doi: 10.1111/j.1532-5415.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 20.Momtaz Y.A., Hamid T.A., Haron S.A., Bagat M.F. Flourishing in later life. Arch. Gerontol. Geriatr. 2016;63:85–91. doi: 10.1016/j.archger.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Shahar S., Omar A., Vanoh D., Hamid T.A., Mukari S.Z.M.S., Din N.C., et al. Approaches in methodology for population-based longitudinal study on neuroprotective model for healthy longevity (TUA) among Malaysian Older Adults. Aging Clin. Exp. Res. 2016;28(6):1089–1104. doi: 10.1007/s40520-015-0511-4. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim N.M., Shohaimi S., Chong H.T., Rahman A.H., Razali R., Esther E., et al. Validation study of the mini-mental state examination in a Malay-speaking elderly population in Malaysia. Dement. Geriatr. Cogn. Disord. 2009;27(3):247–253. doi: 10.1159/000203888. [DOI] [PubMed] [Google Scholar]
- 23.Yuan X., Liu T., Wu L., Zou Z.Y., Li C. Validity of self-reported diabetes among middle-aged and older Chinese adults: The China health and retirement longitudinal study. BMJ Open. 2015;5(4):e006633. doi: 10.1136/bmjopen-2014-006633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keel S., Foreman J., Xie J., van Wijngaarden P., Taylor H.R., Dirani M. The prevalence of self-reported diabetes in the Australian national eye health survey. PLoS One. 2017;12(1):e0169211. doi: 10.1371/journal.pone.0169211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xaverius P.K., Salas J., Kiel D. Differences in pregnancy planning between women aged 18-44, with and without diabetes: Behavioral risk factor surveillance system analysis. Diabetes Res. Clin. Pract. 2013;99(1):63–68. doi: 10.1016/j.diabres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Hamid T.A., Krishnaswamy S., Abdullah S.S., Momtaz Y.A. Sociodemographic risk factors and correlates of dementia in older Malaysians. Dement. Geriatr. Cogn. Disord. 2010;30(6):533–539. doi: 10.1159/000321672. [DOI] [PubMed] [Google Scholar]
- 27.Momtaz Y.A., Hamid T.A., Yusoff S., Ibrahim R. Do depression and educational attainment mediate the association between ethnicity and dementia? Gerontology. 2013;59(3):206–212. doi: 10.1159/000342254. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.Y. Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod. 2013;38(1):52–54. doi: 10.5395/rde.2013.38.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francisco P.M., Belon A.P., Barros M.B., Carandina L., Alves M.C., Goldbaum M., et al. Self-reported diabetes in the elderly: Prevalence, associated factors, and control practices. Public Health Notebooks. 2010;26(1):175–184. doi: 10.1590/s0102-311x2010000100018. [DOI] [PubMed] [Google Scholar]
- 30.Rampal S., Rampal L., Rahmat R., Zain A.M., Yap Y.G., Mohamed M., et al. Variation in the prevalence, awareness, and control of diabetes in a multiethnic population: A nationwide population study in Malaysia. Asia Pac. J. Public Health. 2010;22(2):194–202. doi: 10.1177/1010539509334816. [DOI] [PubMed] [Google Scholar]
- 31.Cukierman T., Gerstein H.C., Williamson J.D. Cognitive decline and dementia in diabetes- Systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 32.Stewart R., Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet. Med. 1999;16(2):93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- 33.Cheng G., Huang C., Deng H., Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta‐analysis of longitudinal studies. Intern. Med. J. 2012;42(5):484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 34.Hasan S.S. A comparative drug utilisation study of the treatment of diabetes in Malaysia and Australia. Australas. Med. J. 2015;8(6):179–188. doi: 10.4066/AMJ.2015.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell J.M., Stephenson M.D. Courten Bd, Chapman I, Bellman SM, Aromataris E. Metformin use associated with reduced risk of dementia in patients with diabetes: A systematic review and meta-analysis. J. Alzheimers Dis. 2018;65(4):1225–1236. doi: 10.3233/JAD-180263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng T.P., Feng L., Yap K.B., Lee T.S., Tan C.H., Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J. Alzheimers Dis. 2014;41(1):61–68. doi: 10.3233/JAD-131901. [DOI] [PubMed] [Google Scholar]
- 37.Podhorecka M., Ibanez B., Dmoszyńska A. Metformin- Its potential anti-cancer and anti-aging effects. Postepy Hig. Med. Dosw. 2017;71:170–175. doi: 10.5604/01.3001.0010.3801. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y., Zhou K., Wang R., Liu Y., Kwak Y.D., Ma T., et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc. Natl. Acad. Sci. USA. 2009;106(10):3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell J.M., Stephenson M.D., de Courten B., Chapman I., Bellman S.M., Aromataris E. Metformin and Alzheimer’s disease, dementia and cognitive impairment: A systematic review protocol. JBI Database Syst. Rev. Implement. Reports. 2017;15(8):2055–2059. doi: 10.11124/JBISRIR-2017-003380. [DOI] [PubMed] [Google Scholar]
- 40.Okura Y., Urban L.H., Mahoney D.W., Jacobsen S.J., Rodeheffer R.J. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J. Clin. Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the finding of this study are available from the corresponding author Dr. Tengku A. Hamid (tengkuaizan06@gmail.com), upon reasonable request.


