Abstract
Enforced egress of hematopoietic stem cells (HSCs) out of the bone marrow (BM) into the peripheral circulation, termed mobilization, has come a long way since its discovery over four decades ago. Mobilization research continues to be driven by the need to optimize the regimen currently available in the clinic with regard to pharmacokinetic and pharmacodynamic profile, costs, and donor convenience. In this review, we describe the most recent findings in the field and how we anticipate them to affect the development of mobilization strategies in the future. Furthermore, the significance of mobilization beyond HSC collection, i.e. for chemosensitization, conditioning, and gene therapy as well as a means to study the interactions between HSCs and their BM microenvironment, is reviewed. Open questions, controversies, and the potential impact of recent technical progress on mobilization research are also highlighted.
Keywords: Mobilization, Hematopoietic stem and progenitor cells, G-CSF, CXCR4, VLA4, CXCR2, BM niche, Chemosensitization, Conditioning, Gene therapy
Introduction
Discovered by pure chance in patients recovering from chemotherapy almost 45 years ago 1, the phenomenon of hematopoietic stem cell (HSC) mobilization has transformed the clinical practice of HSC transplantation 2. It has further extended to indications beyond HSC collection, including mobilization-based chemosensitization, conditioning, and gene therapeutic approaches, which are areas of intensive research. Better understanding of the pathways governing HSC trafficking can provide important insights into how stem cell localization within the bone marrow (BM) is regulated, which explains a continued need for basic research on mobilization to define the underlying molecular and cellular mechanisms.
In mammals, the first definitive HSCs arise in several intra and extraembryonic tissues from which they first migrate into the fetal liver 3, 4. Following expansion in the fetal liver, HSCs continue their journey towards the BM, where the overwhelming majority of adult HSCs are subsequently found in their unique, specialized environments, the BM niches 5, 6. Interestingly, despite the dramatically reduced migratory activity upon BM colonization, a small fraction of adult HSCs can be found in the peripheral circulation at any given time 7– 9. Even though random leakiness of BM retention pathways cannot be excluded as a cause, the regularity of this physiological HSC egress 10– 12 implies a biological function. The number of HSCs in the circulation at steady state can be substantially augmented by a wide variety of endogenous and exogenous stimuli such as growth factors 13– 20, chemotherapy 1, 21– 23, chemokines 24– 27, chemokine and integrin receptor agonists and antagonists 28– 31, bioactive lipids 32, 33, exercise 34, 35, infection, and inflammation 36, 37 ( Figure 1). This enforced egress of HSCs into peripheral blood is called mobilization. While the function of homeostatically circulating HSCs remains enigmatic, pharmacologically induced HSC egress is increasingly used as the preferred strategy to generate grafts for HSC transplantation (HSCT), the only curative therapeutic option for many hematopoietic malignancies as well as non-malignant pathologies. HSCT requires the intravenous infusion of a minimum of 2×10 6 CD34 + stem cells/kg recipient body weight; however, a dose of 5×10 6 CD34 + cells/kg is considered preferable for early, consistent, and long-term multilineage engraftment 116– 118. Each failure or delay to collect sufficient hematopoietic stem/progenitor cells (HSPCs) to proceed to transplantation extends the time of high-dose chemotherapy and increases the risk of disease progression in cancer patients.
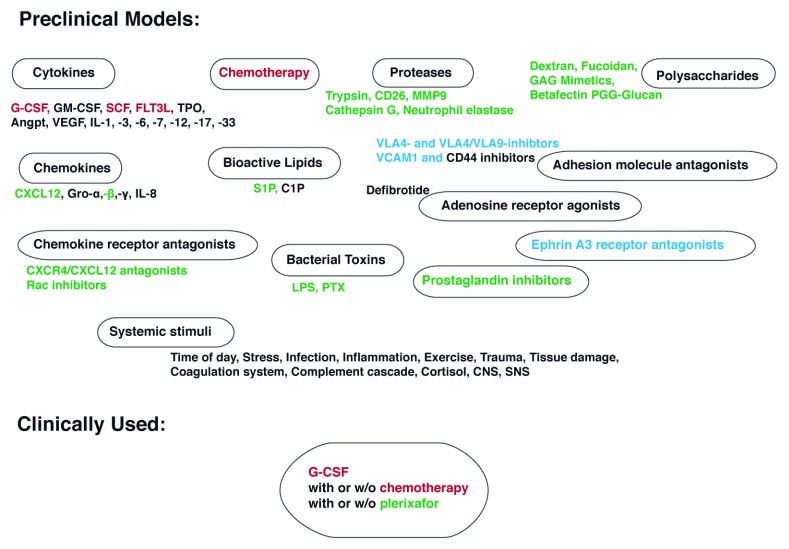
Figure 1. Mobilization stimuli.
A wide variety of stimuli that lead to increased numbers of circulating hematopoietic stem cells (HSCs) have been identified, including but not limited to growth factors (cytokines 17, granulocyte colony-stimulating factor [G-CSF] 19, 38, granulocyte-macrophage colony-stimulating factor [GM-CSF] 20, stem cell factor [SCF] 18, 39, 40, FLT3 ligand [FLT3L] 40– 42, thrombopoietin [TPO] 15, 16, 40, angiopoietin [Angpt] 43, 44, vascular endothelial growth factor [VEGF] 43– 45, and interleukins [ILs] -1 46, -3 47, 48, -6 49, -7 50, -12 51, -17 52, and -33 53, 54), chemotherapy-induced myeloid rebound 1, 23, 55, chemokines (CXCL12 and analogs 27, 56, Gro-α 57, -β 26, 58, 57, 59, and -γ 57, and IL-8 24, 25, 60), chemokine receptor antagonists (CXCR4 antagonists 61, 62, 63 and inhibitors of the intracellular mediator of CXCR4 signaling, the small Rho GTPase Rac1 64, 65), bioactive lipids (sphingosine-1 phosphate [S1P] 66, 67 and ceramide-1 phosphate [C1P] 66, 67) and bacterial toxins 36, 68, 69 (lipopolysaccharide [LPS] 37, 70 and pertussis toxin [PTX] 71– 74), proteases (trypsin 75, matrix metalloprotease 9 [MMP9] 58, 76, CD26 77, 78, cathepsin G 79, and neutrophil elastase 79) and adenosine receptor agonists (defibrotide) 80, 81, inhibitors of adhesive cell interactions 82 (VLA4 28, 29, 83 and VLA4/VLA9 84 inhibitors, VCAM1 85, and CD44 86, 87 blockers) and ephrin A3 receptor antagonists 88, polymeric sugar molecules (dextran 89, fucoidan 89, 90, Betafectin PGG-Glucan 91, and glycosaminoglycan [GAG] mimetics 92), and prostaglandin inhibitors 93. For the majority of the listed stimuli, a direct or indirect targeting of CXCR4 (green) or VLA4 (blue) signaling or both (red) has been documented. On the systemic level, the time of day 10, 11, 94, stress 95, 96 and exercise 97– 100, trauma and tissue damage 101– 103, infection 104 and inflammation 105, 106, coagulation 107– 109 and complement 110– 113 cascade along with cortisol 94, 114 and the central 114 as well as the sympathetic nervous system (SNS) 115 have been shown to affect HSC egress out of the bone marrow (BM) into the peripheral blood. In sharp contrast to the diversity of mobilizing agents discovered and tested in preclinical models, only G-CSF alone (healthy donors) or in conjunction with chemotherapy and plerixafor (patients) is being used in the clinic. CNS, central nervous system.
The need to optimize mobilization regimens with regard to their stem cell yield, side effects and risk profile, cost-effectiveness, and availability for different groups of patients, as well as the need to better understand the communication between HSCs and their niche, continues to drive mobilization research. In this review, we discuss how deciphering the events induced by the most commonly used mobilizing agent, granulocyte colony-stimulating factor (G-CSF), led to the development of new mobilization strategies. We highlight the most recent findings and how we envision the newly discovered mobilization approaches will impact mobilization in the clinic. Alternative applications for mobilization are also reviewed. Lastly, we identify open questions and controversies, prospective directions, and how recent technical advances can be implemented within mobilization research.
Current mobilization regimens
G-CSF-mobilized blood is the preferred graft source for virtually all autologous and an increasing majority of allogeneic HSCTs owing to its generally higher stem cell content, reduced rates of graft failure, and better overall survival as compared to the BM 2, 119, 120. After 4–5 days of treatment with G-CSF, circulating HSPCs increase an average of 50–100-fold 121, 122 as a result of HSPC pool expansion followed by mobilization. The latter is achieved through targeting the two major pathways involved in stem cell retention: chemokine receptor CXCR4- and integrin VLA4-mediated signaling 79, 123, 124. Attenuation of these pathways is achieved on the level of gene expression as well as through proteolytic cleavage 79, 123– 127. While the role of specific proteases involved in the latter, such as neutrophil elastase, cathepsin G, and MMP9, remains controversial 128, 129, the cell surface protease dipeptidyl peptidase 4 (DPP-4, CD26), which cleaves and inactivates the CXCR4 ligand CXCL12, has indeed been shown to be essential for G-CSF-induced mobilization 77, 78.
Shortcomings of G-CSF such as the slow mode of action 17, 130, side effects, and contraindications 131, 132 as well as significant heterogeneity in the mobilization response 121 explain the quest for alternative mobilizing agents 133. Plerixafor (AMD3100), a small molecule bicyclam CXCR4 antagonist, is FDA approved for autologous stem cell mobilization in non-Hodgkin’s lymphoma and multiple myeloma (MM) 134, 135. When combined with G-CSF, plerixafor increases CD34 + concentration 2–3-fold compared to G-CSF alone 134, 135. However, a significant disadvantage of plerixafor is its cost, adding $25,567 per patient compared to G-CSF alone 136. Furthermore, up to 24% of patients undergoing autologous stem cell transplantation for lymphoma receiving plerixafor and G-CSF still fail to collect ≥2×10 6 CD34 + cells/kg in 4 days of apheresis 134, 135. A recent economic analysis determined that reducing apheresis by 1 day can potentially decrease medical costs by $6,600 137. Thus improved/alternative mobilizing agents and strategies are needed.
The long-standing view has been that both HSPC expansion and mobilization are necessary for clinically relevant mobilization. In line with this view, mobilization with CXCR4 or VLA4 antagonists alone fails to achieve numbers that would allow their use without G-CSF, despite promising potential in preclinical models 29, 61, 83, 138– 140. Very recent findings by our group and others challenge this notion and suggest that efficient recruitment of long-term, serially repopulating HSCs can be accomplished within minutes 57, 58. Indeed, CXCR4 or VLA4 blockade, when combined with the stimulation of a different chemokine receptor, CXCR2, results in extremely rapid and potent HSC mobilization in mice with a repopulating capacity similar or even superior to G-CSF-recruited HSCs 57, 58. These observations show that major changes in cellular composition or localization are not required for efficient mobilization. They further highlight the existence of different HSC species that, upon disruption of certain adhesive tethers, can egress from the BM very rapidly with kinetics that appear incompatible with a prior requirement for changes in gene expression.
The key role of the stromal compartment in G-CSF-induced mobilization has long been appreciated 124, 129, 141. Following activation of their G-CSF receptor, BM monocytes/macrophages, the most prominent hematopoietic component of the BM stroma 142– 144, downregulate several retention molecules, including the major CXCR4 ligand, CXCL12, and several VLA4 ligands by non-hematopoietic stroma, resulting in HSPC egress 143, 145– 147. Absence of the G-CSF receptor on monocytes/macrophages abrogates the G-CSF-induced mobilization response 143, whereas the cytokine oncostatin M is thought to mediate communication between monocytes and non-hematopoietic stroma 145, 148. Interestingly, rapid mobilizing agents in general, and chemokines and chemokine receptor antagonists in particular, are assumed to act on hematopoietic cells directly. Our recent findings challenge this view and suggest a critical contribution of stromal (endothelial) CXCR2 targeting upon rapid HSC mobilization by the combination of the CXCR2 ligand truncated Gro-beta (tGro-β) and a VLA4 antagonist 57. Since CXCR2 is absent from the HSPC surface 25, 26, CXCR2-expressing neutrophils have long been assumed to be the responding cell. Upon stimulation with CXCR2 agonists, neutrophils release proteases that cut adhesive interactions between HSPCs and their niche 60, 76, 149, 150. Yet CXCR2 within stroma was sufficient to induce mobilization with tGro-β and a VLA4 antagonist 57, pointing towards changes in endothelial layer permeability 151 as well as crosstalk between endothelia and neutrophils as the “priming” events triggered by CXCR2 activation that boost VLA4 antagonist-induced HSPC egress ( Figure 2).
Figure 2. Mobilization priming effects of CXCR2 stimulation.
Upon activation of the CXCR2 receptor on the surface of neutrophils (NE) and/or endothelial cells (EC), a reciprocal stimulation of the cells occurs that is critical for the subsequent boost of mobilization with a VLA4 or CXCR4 antagonist. Augmented mobilization appears to be a result of increased permeability of the endothelial layer together with other cell contact mediated or soluble factors derived from CXCR2-stimulated cells that reduce hematopoietic stem/progenitor cell (HSPC) retention. Additional inhibition of the VLA4 or CXCR4 receptor results in efficient targeting of the very primitive, serially repopulating HSPCs retained in the bone marrow (BM) primarily via VLA4 or CXCR4 signaling, respectively. The rapid kinetics of CXCR2 agonist + VLA4 or CXCR4 antagonist-induced mobilization preclude major molecular or cellular changes prior to the BM egress and rather suggest a close proximity of the primitive HSPC fraction to the BM sinusoids.
Development of alternative HSPC mobilization regimens and grafts
As we continue to learn about the events and mechanisms regulating HSPC egress, we approach the ultimate goal of developing an “optimal” mobilization strategy to collect sufficient numbers of primitive stem cells with superior properties within a day or two. In contrast to the days when clinical observations determined the applicability of a mobilization approach, educated and targeted designs are becoming the basis for the clinical development of mobilizing agents. In addition to the quantity and fitness of the HSPCs, as reflected in their engraftment capacity, the immunogenic properties of the graft (i.e. the graft-versus-host disease [GvHD] profile) are an important feature requiring optimization, potentially at the cost of stem cell numbers. For example, a substantially reduced incidence of GvHD is observed upon transplantation of CXCR4 antagonist-mobilized grafts, possibly due to co-mobilization of a specific population of dendritic cells (DCs) with immunomodulatory properties, plasmacytoid DCs 140, 152, 153. Along the same lines, grafts mobilized with pegylated G-CSF were superior to standard G-CSF in that they were associated with less GvHD 154, 155 while graft-versus-leukemia (GvL) effects were improved through mobilization of invariant natural killer T (iNKT) cells 155. Susceptibility of the mobilized HSPCs to further molecular manipulation, e.g. using gene therapy, is another important criterion when defining the “optimal” mobilization strategy. Lastly, if proven to be suitable for mobilized HSPCs, recently described methods for ex vivo expansion of HSCs 156 are expected to shift the emphasis on HSPC quality over quantity even further.
Studies with CXCR4 and VLA4 antagonists, tested in VLA4 and CXCR4 knockout mice, respectively, implied an independence between the two axes 139, 157, 158. This suggests that subsets of HSPCs are being retained in the BM by either CXCR4 or VLA4. Combined with the knowledge of the complexity and multiplicity of events induced in the course of G-CSF mobilization 129, 133, co-existence of these (and possibly other) functionally distinct HSPC populations suggests combinatorial mobilization approaches as the best alternatives to G-CSF. Thus, the small molecule Me6TREN reportedly inhibits CXCR4 and VLA4 signaling simultaneously, possibly through upregulation of the protease MMP9 159. However, given the controversy regarding the role of MMP9 for mobilization 128, other approaches should be explored. In addition to cell-intrinsic HSPC retention pathways, disruption of endothelial layer integrity, along with the endothelial cell activation and subsequent crosstalk between endothelial and mature hematopoietic cells, should be included in designing “optimal” mobilization. Recent data suggest that Viagra (sildenafil citrate), a phosphodiesterase type 5 (PDE5) inhibitor which blocks the degradation of cyclic GMP in the smooth muscle cells lining blood vessels, resulting in vasodilation, can synergize with plerixafor to rapidly mobilize stem cells in mice 160.
Various techniques for ex vivo graft manipulation (e.g. T cell depletion and CD34 enrichment 161– 164) have been developed that entail extended periods during which the HSPCs stay outside of their natural environment and therefore, unsurprisingly, exhibit reduced stem cell capacity 165, 166. From further in-depth analyses of differentially mobilized blood (see below), we expect to learn not only how to target specific HSPC populations but also how to mobilize HSPCs without a concurrent mobilization of mature cells, T-cells in particular. In general, a priori cell type-specific targeting remains challenging because of the high conservation of migratory and retention pathways between different hematopoietic cell types. Nevertheless, selective HSPC mobilization represents an intriguing goal that would help reduce additional graft manipulation.
Mobilization beyond stem cell collection
Chemosensitization
In addition to supplying HSPCs with the factors required for their normal development, the BM microenvironment is also a refuge for malignant cells, allowing them to escape cytotoxic therapies and cause disease relapse 167, 168. This provides a rationale for targeting the interactions between tumor cells and the BM, with the goal of sensitizing them to therapy. Pathways responsible for the anchorage and survival of malignant cells and resistance to chemotherapy largely overlap with those of normal HSPCs 168, 169. Accordingly, blockade of CXCR4 and VLA4 signaling and/or G-CSF was tested in conjunction with chemotherapy in pre-clinical models of acute myeloid leukemia (AML 170– 173), acute 174, 175 and chronic 176 lymphoid leukemia, and MM 177. Moreover, the FDA-approved CXCR4 antagonist plerixafor has been tested as a chemosensitizing agent alone and in combination with G-CSF in patients with relapsed AML 178, 179. While the mobilizing capacity varied substantially, an overall benefit from adding mobilizing agent(s) to chemotherapy has been reported, prolonging survival and decreasing tumor burden 170, 172, 177, 180 or even eradicating disease 175. The benefits of this approach in AML and other hematologic malignancies, in spite of these preclinical as well as early clinical studies, remain both unclear and controversial.
Conditioning
As HSPCs are pharmacologically driven from the BM into circulation, the temporarily unoccupied spaces (niches) in theory become available to new cells, e.g. the HSPCs introduced into a mobilized recipient during transplantation. The utility of mobilization for non-cytotoxic and on-target conditioning prior to HSCT is supported by the fact that mobilized cells return to the BM after spending some time in peripheral circulation, as shown in studies of parabiotic mice 181. Yet virtually all attempts at mobilization alone for conditioning of an adult host before HSCT have been unsuccessful (Karpova and Rettig, unpublished data). It is unclear whether the reason is that the cells introduced exogenously are inherently disadvantaged (less fit?) compared with endogenously circulating HSPCs or whether the mobilizing agent interferes with the repopulating capacity of the transplanted cells. An intriguing alternative explanation is that owing to targeting/recruitment of a specific population during the mobilization process, and by extension because of emptying of very specific niches, only HSPCs mobilized with the same mobilizing regimen are able to engraft BM niches that become available. Interestingly, since BM- or fetal liver-derived HSPCs have been used to engraft mobilized recipients (Karpova and Rettig, unpublished data), the possibility that a qualitative rather than quantitative approach might lead to successful, persistent engraftment is untested. Given recent reports of successful conditioning using antibody–drug conjugates targeting the pan leukocyte marker CD45 182 and the CD117-targeting antibodies 183– 185, or a cocktail of monoclonal antibodies depleting CD47-expressing cells along with T cells, NK cells, and HSPCs 186, mobilization-based conditioning may not be a promising approach in postnatal recipients. However, fetal HSPC mobilization with a VLA4 antagonist followed by in utero HSCT results in increased donor HSC homing to the fetal liver and enhanced long-term allogeneic engraftment in mice 187. Therefore, mobilization-based conditioning regimens might be applicable for in utero HSCT.
Gene therapy
Manipulating HSCs to correct mutations that cause inherited diseases of the hematopoietic system such as sickle cell anemia and beta-thalassemia represents a potential cure, with recent advances in gene therapeutic approaches (CRISPR-Cas9, TALEN, and ZFN) 188– 193 allowing sustainable correction of the genetic defects. Autologous HSCT is the method of choice in this setting, whereby instead of extracting HSCs for subsequent ex vivo manipulation, stem cells are mobilized into the circulation and subjected to gene therapy in situ 194– 196. As discussed above, HSC collection and ex vivo editing inevitably leads to a diminished stem cell capacity, which can be avoided by editing the cells in the peripheral blood. Proof of principle for mobilization-based gene therapy was reported following mobilization with G-CSF plus a CXCR4 antagonist, with sustained expression of the introduced transgene over a period of 5 months 196. We believe this approach should be developed further, e.g. by using it in combination with mobilization strategies to preferentially recruit stem cells with superior repopulating capacity into the circulation. In addition, these cells may be more susceptible to therapeutic gene editing. Apart from its obvious therapeutic benefit, this approach might become useful for studying functional differences between HSCs that have been mobilized into the circulation and returned to the BM and HSCs that remain in the BM niche.
Biology of the hematopoietic niche
The discovery of compounds and pathways that enable HSPC displacement from the niche has provided important insights into the regulation of HSPC trafficking and maintenance. For example, detailed analysis of the mechanisms underlying G-CSF-induced mobilization was indispensable for establishing monocytes/macrophages as crucial components of the BM niche and understanding their crosstalk with the non-hematopoietic stroma 143, 145– 147. More recent studies demonstrated that bone marrow dendritic cells regulate endothelial cell function in part through CXCR2 signaling, resulting in HSPC mobilization and loss of bone marrow macrophages 151. Similarly, studies with the CXCR2 ligand tGro-β disclosed the role of another mature leukocyte population, neutrophils, in HSPC trafficking 57, 58, 76. Together with the observation that the circadian release of HSPCs into the circulation is synchronized by daily return of aged neutrophils from the circulation into the BM 197, these findings implicate neutrophils as critical mediators of HSPC localization at steady state and upon enforced egress. On the molecular level, the recognition that all physiological, pathological, and pharmacological mobilization stimuli described to date interfere with VLA4 or CXCR4 signaling or both ( Figure 1) highlights the key roles of these two pathways in HSPC trafficking.
Open questions and future directions
Homeostatic HSPC trafficking
The physiological function and regulation of daily HSPC egress remain elusive. We know that, similar to their mature counterparts, the release of HSPCs from the BM follows a circadian rhythm 11, 94, 198, with sympathetic nervous system-derived adrenergic signals acting through beta(3)-adrenergic receptors on BM stroma to downregulate CXCL12 signaling (and therefore retention 10, 11). While we understand the purpose of migration of more differentiated hematopoietic cells out of the BM to undergo maturation, encounter antigens, proliferate, etc., the role(s) of HSPCs found in blood and other peripheral tissues is speculative. Exchange between different parts of the hematopoietic system has been suggested to be mediated by homeostatic HSPC migration 7, 133 and is supported by observations from parabiotic 9, 199, 200 as well as partially irradiated 201, 202 mice. An alternative explanation implies a possible immune surveillance function of HSPCs that have also been found in the lymphoid system 203 and non-hematopoietic tissues with crucial immune functions such as the intestine 204. Furthermore, expression of MHC class II molecules, otherwise restricted to professional antigen-presenting cells, has been detected in HSPCs 205, 206.
Prediction of HSPC mobilization success and failure
With regard to inadequate HSPC mobilization, one must distinguish between disease- and/or treatment-associated failure and failure of G-CSF mobilization in healthy donors. Of note, less than 1% of “healthy allogeneic donors” fail to collect an optimal (5×10 6 CD34/kg) or minimal (2×10 6 CD34/kg) amount of CD34 + cells after a standard 4–5-day regimen of G-CSF mobilization 133. This relatively uncommon event may represent the extreme heterogeneity of HSPC mobilization seen among the general population with no known medical conditions or prior exposure to chemotherapy or radiation. Thus, CD34 counts between 5 and 500 cells per µl blood have been reported 121. The general consensus, derived from studies of poor- and well-mobilizing mouse strains 207, 208, as well as repetitive mobilization of healthy donors 121, 209, is that genetic factors determine the mobilization response in healthy individuals. However, single nucleotide polymorphisms in any of the obvious candidate genes (including CXCL12 210, VCAM1 211, 212, and CD44 212, 213) do not correlate with mobilization efficacy in response to G-CSF or plerixafor in larger population studies 133, 214, 215. Knowledge of high or low HSPC mobilization potential could be translated into donor screening prior to mobilization to help predict response and potentially to guide the best mobilization strategy.
The probability of mobilization failure in patients directly correlates with the amount and extent of prior cytotoxic exposure 216– 218. Other clinical and demographic features of normal donors and patients undergoing autologous stem cell mobilization that predict poor mobilization include age 217, 219, resting platelet counts 217, 220, and a history of diabetes mellitus 221. Failure rates have decreased since the CXCR4 antagonist plerixafor was approved for the mobilization of HSPCs for MM and non-Hodgkin lymphoma patients when given in conjunction with G-CSF 133– 135. However, given that the diminished mobilization response results from the substantially reduced HSPC pool in these patients 222, approaches that both potently expand HSPCs and induce their egress into the circulation are needed. We recently reported that the administration of CXCR4 antagonists to mice by subcutaneous continuous infusion for 1–4 weeks induces a robust mobilization response that significantly exceeds the mobilization achieved with bolus drug injections and was 25–50-fold greater than a 5-day course of G-CSF 158. Moreover, continuous infusion of CXCR4 inhibitors leads to a two- to four-fold expansion of the HSPC pool in the bone marrow that exhibits a distinct repopulating advantage when tested in serial competitive transplantation experiments. Similarly, others have shown that the FMS-like tyrosine kinase 3 ligand (Flt3L) stimulates the expansion and mobilization of HSPCs in animals and humans 223– 225.
Profiling of differentially mobilized blood
Gene expression profiling of the HSPC populations mobilized with different agents has been performed, mostly using microarrays 57, 226. Not unexpectedly, increased relative expression of genes involved in lymphoid development were detected in grafts mobilized with a CXCR4 antagonist alone and in conjunction with G-CSF as compared to G-CSF alone 226. A closer similarity between BM-resident and CXCR4 antagonist-mobilized HPSCs as compared to BM-resident versus G-CSF-mobilized HSPCs had been proposed to be due to the fast kinetics of CXCR4 antagonist-induced mobilization. However, the opposite was detected when comparing the three, with BM-resident HSPCs showing a profile much closer to that of G-CSF-mobilized HSPCs 57. It would appear from these studies that CXCR4 disruption recruits a specific rather than representative fraction of the BM HSPCs. By contrast, VLA4 antagonist + tGro-β-mobilized HSPCs have a profile very similar to that of BM-resident as well as G-CSF-mobilized HSPCs, indicating that rapid kinetics of mobilization can indeed be associated with an HSPC profile closely related to BM-resident HSPCs 57.
Single cell RNA sequencing (scRNA-seq) is currently revolutionizing the field of hematopoietic cancer research by defining the heterogeneity of malignant cells and the supporting network of non-malignant cells 227– 230. Naturally, scRNA-seq analysis and comparison of differentially mobilized HSPCs will provide key insights and may strengthen the notion that they are comprised of different HSPC subsets rather than representing a homogenous population with an overall altered expression profile depending on the agent used. Moreover, we anticipate that concurrent analysis of peripheral blood and BM HSPC compartments will shed new light on the unique identity and specific origin of mobilized cells that respond to specific mobilizing agents. In general, single cell characterization of mobilized blood/HSPCs is expected to be particularly informative with rapid-mobilizing agents, where the kinetics of the cell recruitment would not allow for major changes of the cell identity or localization prior to BM egress.
Interestingly, despite the more elaborate isolation process for non-hematopoietic as opposed to hematopoietic cells, a detailed characterization of the stromal populations using single cell approaches has been published already 231– 233. However, the contribution of the newly identified populations to HSPC retention remains unexplored. Ultimately, simultaneous analysis of stroma and HSPCs based on their proximity using spatial transcriptomics promises to reveal potentially unique relationships between certain stromal and hematopoietic cell types and thereby define the biological roles of the long-argued diversity within the hematopoietic niche.
Summary
Pharmacologically induced egress of HSPCs from the BM has become an indispensable tool in HSCT with all autologous and over 80% of allogeneic transplants performed with mobilized blood. Mechanistic insights gained from studying the complex chain of events induced during mobilization with G-CSF have paved the way for the rational design of alternative mobilization approaches without the inherent shortcomings of G-CSF such as slow mode of action and side effects. Similar to the recruitment of healthy HSPCs into the circulation, targeting of various BM retention pathways has been explored as a means to sensitize leukemic cells and thereby improve the efficacy of chemotherapy. Moreover, mobilization of HSPCs as a non-cytotoxic conditioning strategy as well as for gene therapy represents two other applications of mobilization beyond mere collection of a stem cell graft. Understanding the physiological function of homeostatic HSPC trafficking and identifying the genetic determinants of mobilization efficiency, along with characterizing the differentially mobilized HSPC populations on a single cell level, represent some of the directions of future mobilization research.
Acknowledgements
We kindly thank Joel Eissenberg (St Louis University School of Medicine) and Elisa Donato (German Cancer Research Center [DKFZ] and DKFZ-ZMBH Alliance) for critical revision of the manuscript, helpful comments, and discussion. DK is a past scholar of the German Academic Exchange Service (postdoctoral fellowship ID: 57054578, 2014–2016).
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Jean-Pierre Levesque, Faculty of Medicine, University of Queensland, Herston, Queensland, Australia
Mariusz Ratajczak, Stem Cell Program, Department of Medicine, Division of Hematology and Oncology, University of Louisville, Louisville, Kentucky, USA
Funding Statement
MPR acknowledges funding from the National Institutes of Health, National Cancer Institute (R50 CA211466), and the Alvin J. Siteman Cancer Center, Siteman Investment Program award, supported by The Foundation for Barnes-Jewish Hospital Cancer Frontier Fund, the Barnard Trust, and a National Cancer Institute Cancer Center support grant (P30 CA091842). JFD is supported by grants from the National Institutes of Health (P50 CA171963, Project 4 Leader) and NIH/National Cancer Institute (R35 CA210084).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Richman CM, Weiner RS, Yankee RA: Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47(6):1031–9. [PubMed] [Google Scholar]
- 2. Henig I, Zuckerman T: Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med J. 2014;5(4):e0028. 10.5041/RMMJ.10162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burns CE, Zon LI: Homing sweet homing: odyssey of hematopoietic stem cells. Immunity. 2006;25(6):859–62. 10.1016/j.immuni.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 4. Medvinsky A, Rybtsov S, Taoudi S: Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138(6):1017–31. 10.1242/dev.040998 [DOI] [PubMed] [Google Scholar]
- 5. Lo Celso C, Scadden DT: The haematopoietic stem cell niche at a glance. J Cell Sci. 2011;124(Pt 21):3529–35. 10.1242/jcs.074112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schofield R: The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 7. Wright DE, Wagers AJ, Gulati AP, et al. : Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933–6. 10.1126/science.1064081 [DOI] [PubMed] [Google Scholar]
- 8. Goodman JW, Hodgson GS: Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19(6):702–14. 10.1182/blood.V19.6.702.702 [DOI] [PubMed] [Google Scholar]
- 9. Abkowitz JL, Robinson AE, Kale S, et al. : Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102(4):1249–53. 10.1182/blood-2003-01-0318 [DOI] [PubMed] [Google Scholar]
- 10. Méndez-Ferrer S, Chow A, Merad M, et al. : Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16(4):235–42. 10.1097/MOH.0b013e32832bd0f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Méndez-Ferrer S, Lucas D, Battista M, et al. : Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–7. 10.1038/nature06685 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Scheiermann C, Kunisaki Y, Lucas D, et al. : Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37(2):290–301. 10.1016/j.immuni.2012.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Briddell RA, Hartley CA, Smith KA, et al. : Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential. Blood. 1993;82(6):1720–3. [PubMed] [Google Scholar]
- 14. McNiece IK, Briddell RA, Yan XQ, et al. : The role of stem cell factor in mobilization of peripheral blood progenitor cells. Leuk Lymphoma. 1994;15(5–6):405–9. 10.3109/10428199409049743 [DOI] [PubMed] [Google Scholar]
- 15. Vadhan-Raj S, Murray LJ, Bueso-Ramos C, et al. : Stimulation of megakaryocyte and platelet production by a single dose of recombinant human thrombopoietin in patients with cancer. Ann Intern Med. 1997;126(9):673–81. 10.7326/0003-4819-126-9-199705010-00001 [DOI] [PubMed] [Google Scholar]
- 16. Murray LJ, Luens KM, Estrada MF, et al. : Thrombopoietin mobilizes CD34+ cell subsets into peripheral blood and expands multilineage progenitors in bone marrow of cancer patients with normal hematopoiesis. Exp Hematol. 1998;26(3):207–16. [PubMed] [Google Scholar]
- 17. Nervi B, Link DC, Dipersio JF: Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99(3):690–705. 10.1002/jcb.21043 [DOI] [PubMed] [Google Scholar]
- 18. Morstyn G, Brown S, Gordon M, et al. : Stem cell factor is a potent synergistic factor in hematopoiesis. Oncology. 1994;51(2):205–14. 10.1159/000227335 [DOI] [PubMed] [Google Scholar]
- 19. Matsunaga T, Sakamaki S, Kohgo Y, et al. : Recombinant human granulocyte colony-stimulating factor can mobilize sufficient amounts of peripheral blood stem cells in healthy volunteers for allogeneic transplantation. Bone Marrow Transplant. 1993;11(2):103–8. 10.11501/3101317 [DOI] [PubMed] [Google Scholar]
- 20. Mayer P, Lam C, Obenaus H, et al. : Recombinant human GM-CSF induces leukocytosis and activates peripheral blood polymorphonuclear neutrophils in nonhuman primates. Blood. 1987;70(1):206–13. 10.1182/blood.V70.1.206.206 [DOI] [PubMed] [Google Scholar]
- 21. To LB, Haylock DN, Kimber RJ, et al. : High levels of circulating haemopoietic stem cells in very early remission from acute non-lymphoblastic leukaemia and their collection and cryopreservation. Br J Haematol. 1984;58(3):399–410. 10.1111/j.1365-2141.1984.tb03987.x [DOI] [PubMed] [Google Scholar]
- 22. Stiff PJ, Murgo AJ, Wittes RE, et al. : Quantification of the peripheral blood colony forming unit-culture rise following chemotherapy. Could leukocytaphereses replace bone marrow for autologous transplantation? Transfusion. 1983;23(6):500–3. 10.1046/j.1537-2995.1983.23684074271.x [DOI] [PubMed] [Google Scholar]
- 23. To LB, Davy ML, Haylock DN, et al. : Autotransplantation using peripheral blood stem cells mobilized by cyclophosphamide. Bone Marrow Transplant. 1989;4(5):595–6. [PubMed] [Google Scholar]
- 24. Laterveer L, Lindley IJ, Heemskerk DP, et al. : Rapid mobilization of hematopoietic progenitor cells in rhesus monkeys by a single intravenous injection of interleukin-8. Blood. 1996;87(2):781–8. 10.1182/blood.V87.2.781.bloodjournal872781 [DOI] [PubMed] [Google Scholar]
- 25. Laterveer L, Lindley IJ, Hamilton MS, et al. : Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85(8):2269–75. 10.1182/blood.V85.8.2269.bloodjournal8582269 [DOI] [PubMed] [Google Scholar]
- 26. Fukuda S, Bian H, King AG, et al. : The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood. 2007;110(3):860–9. 10.1182/blood-2006-06-031401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pelus LM, Bian H, Fukuda S, et al. : The CXCR4 agonist peptide, CTCE-0021, rapidly mobilizes polymorphonuclear neutrophils and hematopoietic progenitor cells into peripheral blood and synergizes with granulocyte colony-stimulating factor. Exp Hematol. 2005;33(3):295–307. 10.1016/j.exphem.2004.11.008 [DOI] [PubMed] [Google Scholar]
- 28. Bonig H, Watts KL, Chang KH, et al. : Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells. 2009;27(4):836–7. 10.1002/stem.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramirez P, Rettig MP, Uy GL, et al. : BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114(7):1340–3. 10.1182/blood-2008-10-184721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papayannopoulou T, Nakamoto B: Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci U S A. 1993;90(20):9374–8. 10.1073/pnas.90.20.9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craddock CF, Nakamoto B, Andrews RG, et al. : Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90(12):4779–88. [PubMed] [Google Scholar]
- 32. Juarez JG, Harun N, Thien M, et al. : Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119(3):707–16. 10.1182/blood-2011-04-348904 [DOI] [PubMed] [Google Scholar]
- 33. Ogle ME, Olingy CE, Awojoodu AO, et al. : Sphingosine-1-Phosphate Receptor-3 Supports Hematopoietic Stem and Progenitor Cell Residence Within the Bone Marrow Niche. Stem Cells. 2017;35(4):1040–52. 10.1002/stem.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Zaldivar F, Eliakim A, Radom-Aizik S, et al. : The effect of brief exercise on circulating CD34 + stem cells in early and late pubertal boys. Pediatr Res. 2007;61(4):491–5. 10.1203/pdr.0b013e3180332d36 [DOI] [PubMed] [Google Scholar]
- 35. Barrett AJ, Longhurst P, Sneath P, et al. : Mobilization of CFU-C by exercise and ACTH induced stress in man. Exp Hematol. 1978;6(7):590–4. [PubMed] [Google Scholar]
- 36. Cline MJ, Golde DW: Mobilization of hematopoietic stem cells (CFU-C) into the peripheral blood of man by endotoxin. Exp Hematol. 1977;5(3):186–90. [PubMed] [Google Scholar]
- 37. Benner R, Rijnbeek AM, Molendijk W, et al. : Genetic control of lipopolysaccharide-induced mobilization of CFUs. Dissociation between early and delayed mobilization of CFUs in complement C5-deficient mice and LPS non-responder mice. Cell Tissue Kinet. 1981;14(2):143–51. 10.1111/j.1365-2184.1981.tb00519.x [DOI] [PubMed] [Google Scholar]
- 38. Welte K, Bonilla MA, Gillio AP, et al. : Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987;165(4):941–8. 10.1084/jem.165.4.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakamura Y, Tajima F, Ishiga K, et al. : Soluble c-kit receptor mobilizes hematopoietic stem cells to peripheral blood in mice. Exp Hematol. 2004;32(4):390–6. 10.1016/j.exphem.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 40. Luens KM, Travis MA, Chen BP, et al. : Thrombopoietin, kit ligand, and flk2/flt3 ligand together induce increased numbers of primitive hematopoietic progenitors from human CD34 +Thy-1 +Lin - cells with preserved ability to engraft SCID-hu bone. Blood. 1998;91(4):1206–15. 10.1182/blood.V91.4.1206 [DOI] [PubMed] [Google Scholar]
- 41. He S, Chu J, Vasu S, et al. : FLT3L and plerixafor combination increases hematopoietic stem cell mobilization and leads to improved transplantation outcome. Biol Blood Marrow Transplant. 2014;20(3):309–13. 10.1016/j.bbmt.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Urdziková L, Likavčanová-Mašínová K, Vaněček V, et al. : Flt3 ligand synergizes with granulocyte-colony-stimulating factor in bone marrow mobilization to improve functional outcome after spinal cord injury in the rat. Cytotherapy. 2011;13(9):1090–104. 10.3109/14653249.2011.575355 [DOI] [PubMed] [Google Scholar]
- 43. Hattori K, Dias S, Heissig B, et al. : Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193(9):1005–14. 10.1084/jem.193.9.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moore MA, Hattori K, Heissig B, et al. : Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. 10.1111/j.1749-6632.2001.tb03572.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Rafii S, Heissig B, Hattori K: Efficient mobilization and recruitment of marrow-derived endothelial and hematopoietic stem cells by adenoviral vectors expressing angiogenic factors. Gene Ther. 2002;9(10):631–41. 10.1038/sj.gt.3301723 [DOI] [PubMed] [Google Scholar]
- 46. Pruijt JF, van Kooyk Y, Figdor CG, et al. : Anti-LFA-1 blocking antibodies prevent mobilization of hematopoietic progenitor cells induced by interleukin-8. Blood. 1998;91(11):4099–105. [PubMed] [Google Scholar]
- 47. Down JD, Awwad M, Kurilla-Mahon B, et al. : Increases in autologous hematopoietic progenitors in the blood of baboons following irradiation and treatment with porcine stem cell factor and interleukin-3. Transplant Proc. 2000;32(5):1045–6. 10.1016/s0041-1345(00)01111-8 [DOI] [PubMed] [Google Scholar]
- 48. MacVittie TJ, Farese AM, Davis TA, et al. : Myelopoietin, a chimeric agonist of human interleukin 3 and granulocyte colony-stimulating factor receptors, mobilizes CD34 + cells that rapidly engraft lethally x-irradiated nonhuman primates. Exp Hematol. 1999;27(10):1557–68. 10.1016/s0301-472x(99)00092-2 [DOI] [PubMed] [Google Scholar]
- 49. Pettengell R, Luft T, de Wynter E, et al. : Effects of interleukin-6 on mobilization of primitive haemopoietic cells into the circulation. Br J Haematol. 1995;89(2):237–42. 10.1111/j.1365-2141.1995.tb03295.x [DOI] [PubMed] [Google Scholar]
- 50. Grzegorzewski KJ, Komschlies KL, Jacobsen SE, et al. : Mobilization of long-term reconstituting hematopoietic stem cells in mice by recombinant human interleukin 7. J Exp Med. 1995;181(1):369–74. 10.1084/jem.181.1.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jackson JD, Yan Y, Brunda MJ, et al. : Interleukin-12 enhances peripheral hematopoiesis in vivo. Blood. 1995;85(9):2371–6. 10.1182/blood.V85.9.2371.bloodjournal8592371 [DOI] [PubMed] [Google Scholar]
- 52. Schwarzenberger P, Huang W, Oliver P, et al. : Il-17 mobilizes peripheral blood stem cells with short- and long-term repopulating ability in mice. J Immunol. 2001;167:2081–6. 10.4049/jimmunol.167.4.2081 [DOI] [PubMed] [Google Scholar]
- 53. Kim J, Kim W, Le HT, et al. : IL-33-induced hematopoietic stem and progenitor cell mobilization depends upon CCR2. J Immunol. 2014;193(7):3792–802. 10.4049/jimmunol.1400176 [DOI] [PubMed] [Google Scholar]
- 54. Alt C, Yuan S, Wu F, et al. : Long-Acting IL-33 Mobilizes High-Quality Hematopoietic Stem and Progenitor Cells More Efficiently Than Granulocyte Colony-Stimulating Factor or AMD3100. Biol Blood Marrow Transplant. 2019;25(8):1475–85. 10.1016/j.bbmt.2019.05.030 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Green DJ, Bensinger WI, Holmberg LA, et al. : Bendamustine, etoposide and dexamethasone to mobilize peripheral blood hematopoietic stem cells for autologous transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2016;51(10):1330–6. 10.1038/bmt.2016.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hattori K, Heissig B, Tashiro K, et al. : Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97(11):3354–60. 10.1182/blood.v97.11.3354 [DOI] [PubMed] [Google Scholar]
- 57. Karpova D, Rettig MP, Ritchey J, et al. : Targeting VLA4 integrin and CXCR2 mobilizes serially repopulating hematopoietic stem cells. J Clin Invest. 2019;129(7):2745–59. 10.1172/JCI124738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoggatt J, Singh P, Tate TA, et al. : Rapid Mobilization Reveals a Highly Engraftable Hematopoietic Stem Cell. Cell. 2018;172(1–2):191–204.e10. 10.1016/j.cell.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Pelus LM, Fukuda S: Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34(8):1010–20. 10.1016/j.exphem.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 60. Pruijt JF, Fibbe WE, Laterveer L, et al. : Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9). Proc Natl Acad Sci U S A. 1999;96(19):10863–8. 10.1073/pnas.96.19.10863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Broxmeyer HE, Orschell CM, Clapp DW, et al. : Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–18. 10.1084/jem.20041385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bonig H, Chudziak D, Priestley G, et al. : Insights into the biology of mobilized hematopoietic stem/progenitor cells through innovative treatment schedules of the CXCR4 antagonist AMD3100. Exp Hematol. 2009;37(3):402–15.e1. 10.1016/j.exphem.2008.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rettig MP, Ansstas G, DiPersio JF: Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26(1):34–53. 10.1038/leu.2011.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cancelas JA, Lee AW, Prabhakar R, et al. : Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11(8):886–91. 10.1038/nm1274 [DOI] [PubMed] [Google Scholar]
- 65. Ghiaur G, Lee A, Bailey J, et al. : Inhibition of RhoA GTPase activity enhances hematopoietic stem and progenitor cell proliferation and engraftment. Blood. 2006;108(6):2087–94. 10.1182/blood-2006-02-001560 [DOI] [PubMed] [Google Scholar]
- 66. Karapetyan AV, Klyachkin YM, Selim S, et al. : Bioactive lipids and cationic antimicrobial peptides as new potential regulators for trafficking of bone marrow-derived stem cells in patients with acute myocardial infarction. Stem Cells Dev. 2013;22(11):1645–56. 10.1089/scd.2012.0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ratajczak MZ, Suszynska M, Borkowska S, et al. : The role of sphingosine-1 phosphate and ceramide-1 phosphate in trafficking of normal stem cells and cancer cells. Expert Opin Ther Targets. 2013;18(1):95–107. 10.1517/14728222.2014.851671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Velders GA, Verzaal P, Van Os RP, et al. : Endotoxins serve as cofactors in cytokine induced mobilization of peripheral blood stem cells. Blood. 2000;96 Reference Source [Google Scholar]
- 69. Velders GA, van Os R, Hagoort H, et al. : Reduced stem cell mobilization in mice receiving antibiotic modulation of the intestinal flora: Involvement of endotoxins as cofactors in mobilization. Blood. 2004;103(1):340–6. 10.1182/blood-2002-07-2270 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Molendijk WJ, Oudenaren A, Dijk H, et al. : Complement split product C5a mediates the lipopolysaccharide-induced mobilization of CFU-s and haemopoietic progenitor cells, but not the mobilization induced by proteolytic enzymes. Cell Tissue Kinet. 1986;19(4):407–17. 10.1111/j.1365-2184.1986.tb00738.x [DOI] [PubMed] [Google Scholar]
- 71. Schneider OD, Weiss AA, Miller WE: Pertussis toxin signals through the TCR to initiate cross-desensitization of the chemokine receptor CXCR4. J Immunol. 2009;182(9):5730–9. 10.4049/jimmunol.0803114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Papayannopoulou T, Priestley GV, Bonig H, et al. : The role of G-protein signaling in hematopoietic stem/progenitor cell mobilization. Blood. 2003;101(12):4739–47. 10.1182/blood-2002-09-2741 [DOI] [PubMed] [Google Scholar]
- 73. Bonig H, Priestley GV, Papayannopoulou T: Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood. 2006;107(1):79–86. 10.1182/blood-2005-05-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bonig H, Priestley GV, Nilsson LM, et al. : PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood. 2004;104(8):2299–306. 10.1182/blood-2004-04-1605 [DOI] [PubMed] [Google Scholar]
- 75. Fehér I, Gidáli J: Mobilizable stem cells: characteristics and replacement of the pool after exhaustion. Exp Hematol. 1982;10(8):661–7. [PubMed] [Google Scholar]
- 76. Pelus LM, Bian H, King AG, et al. : Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103(1):110–9. 10.1182/blood-2003-04-1115 [DOI] [PubMed] [Google Scholar]
- 77. Christopherson K, Cooper S, Hangoc G, et al. : CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26 -/- mice. Exp Hematol. 2003;31(11):1126–34. 10.1016/j.exphem.2003.07.002 [DOI] [PubMed] [Google Scholar]
- 78. Christopherson KW, 2nd, Cooper S, Broxmeyer HE: Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101(12):4680–6. 10.1182/blood-2002-12-3893 [DOI] [PubMed] [Google Scholar]
- 79. Lévesque JP, Hendy J, Takamatsu Y, et al. : Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111(2):187–96. 10.1172/JCI15994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Scalia R, Kochilas L, Campbell B, et al. : Effects of defibrotide on leukocyte-endothelial cell interaction in the rat mesenteric vascular bed: role of P-selectin. Methods Find Exp Clin Pharmacol. 1996;18(10):669–76. [PubMed] [Google Scholar]
- 81. Carlo-Stella C, Di Nicola M, Magni M, et al. : Defibrotide in combination with granulocyte colony-stimulating factor significantly enhances the mobilization of primitive and committed peripheral blood progenitor cells in mice. Cancer Res. 2002;62(21):6152–7. [PubMed] [Google Scholar]
- 82. Vermeulen M, Le Pesteur F, Gagnerault MC, et al. : Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92(3):894–900. 10.1182/blood.V92.3.894 [DOI] [PubMed] [Google Scholar]
- 83. Bonig H, Wundes A, Chang KH, et al. : Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111(7):3439–41. 10.1182/blood-2007-09-112052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cao B, Zhang Z, Grassinger J, et al. : Therapeutic targeting and rapid mobilization of endosteal HSC using a small molecule integrin antagonist. Nat Commun. 2016;7:11007. 10.1038/ncomms11007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Kikuta T, Shimazaki C, Ashihara E, et al. : Mobilization of hematopoietic primitive and committed progenitor cells into blood in mice by anti-vascular adhesion molecule-1 antibody alone or in combination with granulocyte colony-stimulating factor. Exp Hematol. 2000;28(3):311–7. 10.1016/s0301-472x(99)00151-4 [DOI] [PubMed] [Google Scholar]
- 86. Zoeller M: CD44v10 in hematopoiesis and stem cell mobilization. Leuk Lymphoma. 2000;38(5–6):463–80. 10.3109/10428190009059265 [DOI] [PubMed] [Google Scholar]
- 87. Rösel M, Khaldoyanidi S, Zawadzki V, et al. : Involvement of CD44 variant isoform v10 in progenitor cell adhesion and maturation. Exp Hematol. 1999;27(4):698–711. 10.1016/s0301-472x(98)00082-4 [DOI] [PubMed] [Google Scholar]
- 88. Ting MJ, Day BW, Spanevello MD, et al. : Activation of ephrin A proteins influences hematopoietic stem cell adhesion and trafficking patterns. Exp Hematol. 2010;38(11):1087–98. 10.1016/j.exphem.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 89. Sweeney EA, Priestley GV, Nakamoto B, et al. : Mobilization of stem/progenitor cells by sulfated polysaccharides does not require selectin presence. Proc Natl Acad Sci U S A. 2000;97(12):6544–9. 10.1073/pnas.97.12.6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sweeney EA, Lortat-Jacob H, Priestley GV, et al. : Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 2002;99(1):44–51. 10.1182/blood.v99.1.44 [DOI] [PubMed] [Google Scholar]
- 91. Patchen ML, Liang J, Vaudrain T, et al. : Mobilization of peripheral blood progenitor cells by Betafectin PGG-Glucan alone and in combination with granulocyte colony-stimulating factor. Stem Cells. 1998;16(13):208–17. 10.1002/stem.160208 [DOI] [PubMed] [Google Scholar]
- 92. Albanese P, Caruelle D, Frescaline G, et al. : Glycosaminoglycan mimetics-induced mobilization of hematopoietic progenitors and stem cells into mouse peripheral blood: structure/function insights. Exp Hematol. 2009;37(9):1072–83. 10.1016/j.exphem.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 93. Hoggatt J, Mohammad KS, Singh P, et al. : Differential stem- and progenitor-cell trafficking by prostaglandin E 2. Nature. 2013;495(7441):365–9. 10.1038/nature11929 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Stenzinger M, Karpova D, Unterrainer C, et al. : Hematopoietic-Extrinsic Cues Dictate Circadian Redistribution of Mature and Immature Hematopoietic Cells in Blood and Spleen. Cells. 2019;8(9):pii: E1033. 10.3390/cells8091033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McKim DB, Yin W, Wang Y, et al. : Social Stress Mobilizes Hematopoietic Stem Cells to Establish Persistent Splenic Myelopoiesis. Cell Rep. 2018;25(9):2552–2562.e3. 10.1016/j.celrep.2018.10.102 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Machaliński B, Paczkowska E, Koziarska D, et al. : Mobilization of human hematopoietic stem/progenitor-enriched CD34+ cells into peripheral blood during stress related to ischemic stroke. Folia Histochem Cytobiol. 2006;44(2):97–101. [PubMed] [Google Scholar]
- 97. Morici G, Zangla D, Santoro A, et al. : Supramaximal exercise mobilizes hematopoietic progenitors and reticulocytes in athletes. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1496–503. 10.1152/ajpregu.00338.2005 [DOI] [PubMed] [Google Scholar]
- 98. Sandri M, Adams V, Gielen S, et al. : Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111(25):3391–9. 10.1161/CIRCULATIONAHA.104.527135 [DOI] [PubMed] [Google Scholar]
- 99. Emmons R, Niemiro GM, Owolabi O, et al. : Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J Appl Physiol (1985). 2016;120(6):624–32. 10.1152/japplphysiol.00925.2015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Kroepfl JM, Pekovits K, Stelzer I, et al. : Exercise increases the frequency of circulating hematopoietic progenitor cells, but reduces hematopoietic colony-forming capacity. Stem Cells Dev. 2012;21(16):2915–25. 10.1089/scd.2012.0017 [DOI] [PubMed] [Google Scholar]
- 101. Grzelak I, Olszewski WL, Zaleska M, et al. : Surgical trauma evokes a rise in the frequency of hematopoietic progenitor cells and cytokine levels in blood circulation. Eur Surg Res. 1998;30(3):198–204. 10.1159/000008577 [DOI] [PubMed] [Google Scholar]
- 102. Kollet O, Dar A, Shivtiel S, et al. : Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–64. 10.1038/nm1417 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 103. Hennemann B, Ickenstein G, Sauerbruch S, et al. : Mobilization of CD34 + hematopoietic cells, colony-forming cells and long-term culture-initiating cells into the peripheral blood of patients with an acute cerebral ischemic insult. Cytotherapy. 2008;10(3):303–11. 10.1080/14653240801949994 [DOI] [PubMed] [Google Scholar]
- 104. Burberry A, Zeng MY, Ding L, et al. : Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe. 2014;15(6):779–91. 10.1016/j.chom.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ratajczak MZ, Adamiak M, Plonka M, et al. : Mobilization of hematopoietic stem cells as a result of innate immunity-mediated sterile inflammation in the bone marrow microenvironment-the involvement of extracellular nucleotides and purinergic signaling. Leukemia. 2018;32(5):1116–23. 10.1038/s41375-018-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Adamiak M, Bujko K, Cymer M, et al. : Novel evidence that extracellular nucleotides and purinergic signaling induce innate immunity-mediated mobilization of hematopoietic stem/progenitor cells. Leukemia. 2018;32(9):1920–31. 10.1038/s41375-018-0122-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Tjwa M, Sidenius N, Moura R, et al. : Membrane-anchored uPAR regulates the proliferation, marrow pool size, engraftment, and mobilization of mouse hematopoietic stem/progenitor cells. J Clin Invest. 2009;119(4):1008–18. 10.1172/JCI36010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tjwa et al. Plasmin therapy enhances mobilization of HPCs after G-CSF. Blood. 2008;112:4048–4050. Blood. 2009;113(21):5368 10.1182/blood-2009-02-206284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tjwa M, Janssens S, Carmeliet P: Plasmin therapy enhances mobilization of HPCs after G-CSF. Blood. 2008;112(10):4048–50. 10.1182/blood-2008-07-166587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Janowska-Wieczorek A, Marquez-Curtis LA, Shirvaikar N, et al. : The role of complement in the trafficking of hematopoietic stem/progenitor cells. Transfusion. 2012;52(12):2706–16. 10.1111/j.1537-2995.2012.03636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Borkowska S, Suszynska M, Mierzejewska K, et al. : Novel evidence that crosstalk between the complement, coagulation and fibrinolysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs). Leukemia. 2014;28(11):2148–54. 10.1038/leu.2014.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Borkowska S, Suszynska M, Wysoczynski M, et al. : Mobilization studies in C3-deficient mice unravel the involvement of a novel crosstalk between the coagulation and complement cascades in mobilization of hematopoietic stem/progenitor cells. Leukemia. 2013;27(9):1928–30. 10.1038/leu.2013.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Adamiak M, Abdelbaset-Ismail A, Suszynska M, et al. : Novel evidence that the mannan-binding lectin pathway of complement activation plays a pivotal role in triggering mobilization of hematopoietic stem/progenitor cells by activation of both the complement and coagulation cascades. Leukemia. 2017;31(1):262–5. 10.1038/leu.2016.278 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 114. Pierce H, Zhang D, Magnon C, et al. : Cholinergic Signals from the CNS Regulate G-CSF-Mediated HSC Mobilization from Bone Marrow via a Glucocorticoid Signaling Relay. Cell Stem Cell. 2017;20(5):648–658.e4. 10.1016/j.stem.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 115. Katayama Y, Battista M, Kao WM, et al. : Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–21. 10.1016/j.cell.2005.10.041 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 116. Weaver CH, Hazelton B, Birch R, et al. : An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86(10):3961–9. 10.1182/blood.V86.10.3961.bloodjournal86103961 [DOI] [PubMed] [Google Scholar]
- 117. Bensinger W, Appelbaum F, Rowley S, et al. : Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13(10):2547–55. 10.1200/JCO.1995.13.10.2547 [DOI] [PubMed] [Google Scholar]
- 118. Benedetti G, Patoia L, Giglietti A, et al. : Very large amounts of peripheral blood progenitor cells eliminate severe thrombocytopenia after high-dose melphalan in advanced breast cancer patients. Bone Marrow Transplant. 1999;24(9):971–9. 10.1038/sj.bmt.1702008 [DOI] [PubMed] [Google Scholar]
- 119. Russell NH, Hunter A, Rogers S, et al. : Peripheral blood stem cells as an alternative to marrow for allogeneic transplantation. Lancet. 1993;341(8858):1482. 10.1016/0140-6736(93)90929-b [DOI] [PubMed] [Google Scholar]
- 120. van Besien K, Shore T, Cushing M: Peripheral-blood versus bone marrow stem cells. N Engl J Med. 2013;368(3):287–8. 10.1056/NEJMc1214025 [DOI] [PubMed] [Google Scholar]
- 121. Mueller MM, Bialleck H, Bomke B, et al. : Safety and efficacy of healthy volunteer stem cell mobilization with filgrastim G-CSF and mobilized stem cell apheresis: results of a prospective longitudinal 5-year follow-up study. Vox Sang. 2013;104(1):46–54. 10.1111/j.1423-0410.2012.01632.x [DOI] [PubMed] [Google Scholar]
- 122. Hölig K, Kramer M, Kroschinsky F, et al. : Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers: 12 years of single-center experience in 3928 donors. Blood. 2009;114(18):3757–63. 10.1182/blood-2009-04-218651 [DOI] [PubMed] [Google Scholar]
- 123. Salvucci O, Jiang K, Gasperini P, et al. : MicroRNA126 contributes to granulocyte colony-stimulating factor-induced hematopoietic progenitor cell mobilization by reducing the expression of vascular cell adhesion molecule 1. Haematologica. 2012;97(6):818–26. 10.3324/haematol.2011.056945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Semerad CL, Christopher MJ, Liu F, et al. : G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–7. 10.1182/blood-2004-01-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Winkler IG, Hendy J, Coughlin P, et al. : Serine protease inhibitors serpina1 and serpina3 are down-regulated in bone marrow during hematopoietic progenitor mobilization. J Exp Med. 2005;201(7):1077–88. 10.1084/jem.20042299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lévesque JP, Hendy J, Takamatsu Y, et al. : Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30(5):440–9. 10.1016/s0301-472x(02)00788-9 [DOI] [PubMed] [Google Scholar]
- 127. Lévesque JP, Hendy J, Winkler IG, et al. : Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31(2):109–17. 10.1016/s0301-472x(02)01028-7 [DOI] [PubMed] [Google Scholar]
- 128. Levesque JP, Liu F, Simmons PJ, et al. : Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104(1):65–72. 10.1182/blood-2003-05-1589 [DOI] [PubMed] [Google Scholar]
- 129. Greenbaum AM, Link DC: Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25(2):211–7. 10.1038/leu.2010.248 [DOI] [PubMed] [Google Scholar]
- 130. Anderlini P, Przepiorka D, Huh Y, et al. : Duration of filgrastim mobilization and apheresis yield of CD34 + progenitor cells and lymphoid subsets in normal donors for allogeneic transplantation. Br J Haematol. 1996;93(4):940–2. 10.1046/j.1365-2141.1996.d01-1747.x [DOI] [PubMed] [Google Scholar]
- 131. Anderlini P, Przepiorka D, Champlin R, et al. : Biologic and clinical effects of granulocyte colony-stimulating factor in normal individuals. Blood. 1996;88(8):2819–25. 10.1182/blood.V88.8.2819.bloodjournal8882819 [DOI] [PubMed] [Google Scholar]
- 132. Anderlini P, Champlin RE: Biologic and molecular effects of granulocyte colony-stimulating factor in healthy individuals: recent findings and current challenges. Blood. 2008;111(4):1767–72. 10.1182/blood-2007-07-097543 [DOI] [PubMed] [Google Scholar]
- 133. Karpova D, Wiercinska E, Bönig H: Mobilisierung hämatopoietischer Stammzellen. Transfusionsmedizin. 2013;3(3):127–39. 10.1055/s-0032-1325071 [DOI] [Google Scholar]
- 134. DiPersio JF, Micallef IN, Stiff PJ, et al. : Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767–73. 10.1200/JCO.2008.20.7209 [DOI] [PubMed] [Google Scholar]
- 135. DiPersio JF, Stadtmauer EA, Nademanee A, et al. : Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–6. 10.1182/blood-2008-08-174946 [DOI] [PubMed] [Google Scholar]
- 136. Kymes SM, Pusic I, Lambert DL, et al. : Economic evaluation of plerixafor for stem cell mobilization. Am J Manag Care. 2012;18(1):33–41. [PMC free article] [PubMed] [Google Scholar]
- 137. Hosing C, Smith V, Rhodes B, et al. : Assessing the charges associated with hematopoietic stem cell mobilization and remobilization in patients with lymphoma and multiple myeloma undergoing autologous hematopoietic peripheral blood stem cell transplantation. Transfusion. 2011;51(6):1300–13. 10.1111/j.1537-2995.2011.03176.x [DOI] [PubMed] [Google Scholar]
- 138. Devine SM, Vij R, Rettig M, et al. : Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990–8. 10.1182/blood-2007-12-130179 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 139. Karpova D, Dauber K, Spohn G, et al. : The novel CXCR4 antagonist POL5551 mobilizes hematopoietic stem and progenitor cells with greater efficiency than Plerixafor. Leukemia. 2013;27(12):2322–31. 10.1038/leu.2013.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Karpova D, Bräuninger S, Wiercinska E, et al. : Mobilization of hematopoietic stem cells with the novel CXCR4 antagonist POL6326 (balixafortide) in healthy volunteers-results of a dose escalation trial. J Transl Med. 2017;15(1):2. 10.1186/s12967-016-1107-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Winkler IG, Pettit AR, Raggatt LJ, et al. : Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26(7):1594–601. 10.1038/leu.2012.17 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 142. Chow A, Lucas D, Hidalgo A, et al. : Bone marrow CD169 + macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–71. 10.1084/jem.20101688 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 143. Christopher MJ, Rao M, Liu F, et al. : Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251–60. 10.1084/jem.20101700 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 144. Liu F, Poursine-Laurent J, Link DC: Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000;95(10):3025–31. 10.1182/blood.V95.10.3025 [DOI] [PubMed] [Google Scholar]
- 145. Albiero M, Poncina N, Ciciliot S, et al. : Bone Marrow Macrophages Contribute to Diabetic Stem Cell Mobilopathy by Producing Oncostatin M. Diabetes. 2015;64(8):2957–68. 10.2337/db14-1473 [DOI] [PubMed] [Google Scholar]
- 146. Winkler IG, Sims NA, Pettit AR, et al. : Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23):4815–28. 10.1182/blood-2009-11-253534 [DOI] [PubMed] [Google Scholar]
- 147. Barbier V, Winkler IG, Lévesque JP: Mobilization of hematopoietic stem cells by depleting bone marrow macrophages. Methods Mol Biol. 2012;904:117–38. 10.1007/978-1-61779-943-3_11 [DOI] [PubMed] [Google Scholar]
- 148. Minehata K, Takeuchi M, Hirabayashi Y, et al. : Oncostatin m maintains the hematopoietic microenvironment and retains hematopoietic progenitors in the bone marrow. Int J Hematol. 2006;84(4):319–27. 10.1532/IJH97.06090 [DOI] [PubMed] [Google Scholar]
- 149. Pruijt JF, Verzaal P, van Os R, et al. : Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci U S A. 2002;99(9):6228–33. 10.1073/pnas.092112999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fibbe WE, Pruijt JF, Velders GA, et al. : Biology of IL-8-induced stem cell mobilization. Ann N Y Acad Sci. 1999;872:71–82. 10.1111/j.1749-6632.1999.tb08454.x [DOI] [PubMed] [Google Scholar]
- 151. Zhang J, Supakorndej T, Krambs JR, et al. : Bone marrow dendritic cells regulate hematopoietic stem/progenitor cell trafficking. J Clin Invest. 2019;129(7):2920–31. 10.1172/JCI124829 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 152. Rettig MP, McFarland K, Ritchey J, et al. : Preferential Mobilization of CD34+ Plasmacytoid Dendritic Cell Precursors by Plerixafor. Blood. 2009;114(22):32 10.1182/blood.V114.22.32.32 [DOI] [Google Scholar]
- 153. Schroeder MA, Rettig MP, Lopez S, et al. : Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood. 2017;129(19):2680–92. 10.1182/blood-2016-09-739722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Morris ES, MacDonald KP, Rowe V, et al. : Donor treatment with pegylated G-CSF augments the generation of IL-10-producing regulatory T cells and promotes transplantation tolerance. Blood. 2004;103(9):3573–81. 10.1182/blood-2003-08-2864 [DOI] [PubMed] [Google Scholar]
- 155. Banovic T, MacDonald KP, Markey KA, et al. : Donor treatment with a multipegylated G-CSF maximizes graft-versus-leukemia effects. Biol Blood Marrow Transplant. 2009;15(1):126–30. 10.1016/j.bbmt.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 156. Wilkinson AC, Ishida R, Kikuchi M, et al. : Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature. 2019;571(7763):117–21. 10.1038/s41586-019-1244-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 157. Christopher MJ, Liu F, Hilton MJ, et al. : Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114(7):1331–9. 10.1182/blood-2008-10-184754 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 158. Karpova D, Ritchey JK, Holt MS, et al. : Continuous blockade of CXCR4 results in dramatic mobilization and expansion of hematopoietic stem and progenitor cells. Blood. 2017;129(21):2939–49. 10.1182/blood-2016-10-746909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhang J, Ren X, Shi W, et al. : Small molecule Me6TREN mobilizes hematopoietic stem/progenitor cells by activating MMP-9 expression and disrupting SDF-1/CXCR4 axis. Blood. 2014;123(3):428–41. 10.1182/blood-2013-04-498535 [DOI] [PubMed] [Google Scholar]
- 160. Smith-Berdan S, Bercasio A, Rajendiran S, et al. : Viagra Enables Efficient, Single-Day Hematopoietic Stem Cell Mobilization. Stem Cell Reports. 2019;13(5):787–92. 10.1016/j.stemcr.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 161. Handgretinger R: New approaches to graft engineering for haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):664–73. 10.1053/j.seminoncol.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 162. Handgretinger R: Negative depletion of CD3(+) and TcRαβ(+) T cells. Curr Opin Hematol. 2012;19(6):434–9. 10.1097/MOH.0b013e3283582340 [DOI] [PubMed] [Google Scholar]
- 163. Bethge WA, Haegele M, Faul C, et al. : Haploidentical allogeneic hematopoietic cell transplantation in adults with reduced-intensity conditioning and CD3/CD19 depletion: fast engraftment and low toxicity. Exp Hematol. 2006;34(12):1746–52. 10.1016/j.exphem.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 164. Spohn G, Wiercinska E, Karpova D, et al. : Automated CD34+ cell isolation of peripheral blood stem cell apheresis product. Cytotherapy. 2015;17(10):1465–71. 10.1016/j.jcyt.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 165. Kumar S, Geiger H: HSC Niche Biology and HSC Expansion Ex Vivo. Trends Mol Med. 2017;23(9):799–819. 10.1016/j.molmed.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 166. Eaves CJ: Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125(17):2605–13. 10.1182/blood-2014-12-570200 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 167. Lane SW, Scadden DT, Gilliland DG: The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114(6):1150–7. 10.1182/blood-2009-01-202606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Schroeder MA, DiPersio JF: Mobilization of hematopoietic stem and leukemia cells. J Leukoc Biol. 2012;91(1):47–57. 10.1189/jlb.0210085 [DOI] [PubMed] [Google Scholar]
- 169. Konopleva M, Tabe Y, Zeng Z, et al. : Therapeutic targeting of microenvironmental interactions in leukemia: mechanisms and approaches. Drug Resist Updat. 2009;12(4–5):103–13. 10.1016/j.drup.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Nervi B, Ramirez P, Rettig MP, et al. : Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113(24):6206–14. 10.1182/blood-2008-06-162123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Nervi B, Holt M, Rettig MP, et al. : AMD3100 Mobilizes Acute Promyelocytic Leukemia Cells from the Bone Marrow into the Peripheral Blood and Sensitizes Leukemia Cells to Chemotherapy.ASH Annu Meet Abstr.2005. Reference Source [Google Scholar]
- 172. Zeng Z, Shi YX, Samudio IJ, et al. : Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113(24):6215–24. 10.1182/blood-2008-05-158311 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 173. Matsunaga T, Takemoto N, Sato T, et al. : Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9(9):1158–65. 10.1038/nm909 [DOI] [PubMed] [Google Scholar]
- 174. Parameswaran R, Yu M, Lim M, et al. : Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia. 2011;25(8):1314–23. 10.1038/leu.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 175. Hsieh YT, Gang EJ, Geng H, et al. : Integrin alpha4 blockade sensitizes drug resistant pre-B acute lymphoblastic leukemia to chemotherapy. Blood. 2013;121(10):1814–8. 10.1182/blood-2012-01-406272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Burger M, Hartmann T, Krome M, et al. : Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106(5):1824–30. 10.1182/blood-2004-12-4918 [DOI] [PubMed] [Google Scholar]
- 177. Azab AK, Runnels JM, Pitsillides C, et al. : CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–51. 10.1182/blood-2008-10-186668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Uy GL, Rettig MP, Motabi IH, et al. : A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119(17):3917–24. 10.1182/blood-2011-10-383406 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 179. Uy GL, Rettig MP, Stone RM, et al. : A phase 1/2 study of chemosensitization with plerixafor plus G-CSF in relapsed or refractory acute myeloid leukemia. Blood Cancer J. 2017;7(3):e542. 10.1038/bcj.2017.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Beider K, Begin M, Abraham M, et al. : CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp Hematol. 2011;39(3):282–92. 10.1016/j.exphem.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 181. Chen J, Larochelle A, Fricker S, et al. : Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood. 2006;107(9):3764–71. 10.1182/blood-2005-09-3593 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 182. Palchaudhuri R, Saez B, Hoggatt J, et al. : Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat Biotechnol. 2016;34(7):738–45. 10.1038/nbt.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 183. Pang WW, Czechowicz A, Poyser J, et al. : Anti-Human CD117 Antibodies Mediate Clearance of Myelodysplastic Syndrome Hematopoietic Stem Cells and Facilitate Establishment of Normal Hematopoiesis in Transplantation. Biol Blood Marrow Transplant. 2018;24(3):S230–S231. 10.1016/j.bbmt.2017.12.211 [DOI] [Google Scholar]
- 184. Czechowicz A, Kraft D, Weissman IL, et al. : Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318(5854):1296–9. 10.1126/science.1149726 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 185. Czechowicz A, Palchaudhuri R, Scheck A, et al. : Selective hematopoietic stem cell ablation using CD117-antibody-drug-conjugates enables safe and effective transplantation with immunity preservation. Nat Commun. 2019;10(1):617. 10.1038/s41467-018-08201-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 186. George BM, Kao KS, Kwon HS, et al. : Antibody Conditioning Enables MHC-Mismatched Hematopoietic Stem Cell Transplants and Organ Graft Tolerance. Cell Stem Cell. 2019;25(2):185–192.e3. 10.1016/j.stem.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 187. Kim AG, Vrecenak JD, Boelig MM, et al. : Enhanced in utero allogeneic engraftment in mice after mobilizing fetal HSCs by α4β1/7 inhibition. Blood. 2016;128(20):2457–61. 10.1182/blood-2016-06-723981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Byrne SM, Mali P, Church GM: Genome editing in human stem cells. Meth Enzymol. 2014;546:119–38. 10.1016/B978-0-12-801185-0.00006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yu KR, Natanson H, Dunbar CE: Gene Editing of Human Hematopoietic Stem and Progenitor Cells: Promise and Potential Hurdles. Hum Gene Ther. 2016;27(10):729–40. 10.1089/hum.2016.107 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 190. Bak RO, Dever DP, Porteus MH: CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat Protoc. 2018;13(2):358–76. 10.1038/nprot.2017.143 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 191. Kuo CY, Long JD, Campo-Fernandez B, et al. : Site-Specific Gene Editing of Human Hematopoietic Stem Cells for X-Linked Hyper-IgM Syndrome. Cell Rep. 2018;23(9):2606–16. 10.1016/j.celrep.2018.04.103 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 192. Cavazzana M, Bushman FD, Miccio A, et al. : Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat Rev Drug Discov. 2019;18(6):447–62. 10.1038/s41573-019-0020-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 193. Naldini L: Genetic engineering of hematopoiesis: current stage of clinical translation and future perspectives. EMBO Mol Med. 2019;11(3):pii: e9958. 10.15252/emmm.201809958 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 194. Yannaki E, Karponi G, Zervou F, et al. : Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with β-thalassemia major. Hum Gene Ther. 2013;24(10):852–60. 10.1089/hum.2013.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Yannaki E, Papayannopoulou T, Jonlin E, et al. : Hematopoietic stem cell mobilization for gene therapy of adult patients with severe β-thalassemia: results of clinical trials using G-CSF or plerixafor in splenectomized and nonsplenectomized subjects. Mol Ther. 2012;20(1):230–8. 10.1038/mt.2011.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Richter M, Saydaminova K, Yumul R, et al. : In vivo transduction of primitive mobilized hematopoietic stem cells after intravenous injection of integrating adenovirus vectors. Blood. 2016;128(18):2206–17. 10.1182/blood-2016-04-711580 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 197. Casanova-Acebes M, Pitaval C, Weiss LA, et al. : Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–35. 10.1016/j.cell.2013.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Lucas D, Battista M, Shi PA, et al. : Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3(4):364–6. 10.1016/j.stem.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 199. Harris JE, Ford CE, Barnes DW, et al. : Evidence from Parabiosis for an Afferent Stream of Cells. Nature. 1964;201:886–7. 10.1038/201886a0 [DOI] [PubMed] [Google Scholar]
- 200. McBride RA, Simonsen M: Cellular and Humoral Phenomena during the Inductive Phase of Parabiosis Tolerance. Transplantation. 1965;3:140–54. 10.1097/00007890-196503000-00002 [DOI] [PubMed] [Google Scholar]
- 201. Croizat H, Frindel E, Tubiana M: The effect of partial body irradiation on haemopoietic stem cell migration. Cell Tissue Kinet. 1980;13(3):319–25. 10.1111/j.1365-2184.1980.tb00470.x [DOI] [PubMed] [Google Scholar]
- 202. Murate T, Utsumi M, Hotta T, et al. : Hematopoietic stem cell migration and proliferation after partial body irradiation: Significant role of the spleen in hematopoietic recovery. Nippon Ketsueki Gakkai Zasshi. 1983;46(4):867–75. [PubMed] [Google Scholar]
- 203. Massberg S, Schaerli P, Knezevic-Maramica I, et al. : Immunosurveillance by Hematopoietic Progenitor Cells Trafficking through Blood, Lymph, and Peripheral Tissues. Cell. 2007;131(5):994–1008. 10.1016/j.cell.2007.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 204. Fu J, Zuber J, Martinez M, et al. : Human Intestinal Allografts Contain Functional Hematopoietic Stem and Progenitor Cells that Are Maintained by a Circulating Pool. Cell Stem Cell. 2019;24(2):227–239.e8. 10.1016/j.stem.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 205. Bettinotti MP, Lucas DP, Ghiaur G, et al. : HLA-Class I and II Antigens are Expressed in CD34+ Cells: Implications for HLA Mismatched Bone Marrow Transplantation (BMT). Biol Blood Marrow Transplant. 2018;24(3):S420–S421. 10.1016/j.bbmt.2017.12.431 [DOI] [Google Scholar]
- 206. Greinix HT, Storb R, Bartelmez SH: Specific growth inhibition of primitive hematopoietic progenitor cells mediated through monoclonal antibody binding to major histocompatibility class II molecules. Blood. 1992;80(8):1950–6. [PubMed] [Google Scholar]
- 207. Roberts AW, Foote S, Alexander WS, et al. : Genetic Influences Determining Progenitor Cell Mobilization and Leukocytosis Induced by Granulocyte Colony-Stimulating Factor. Blood. 1997;89(8):2736–44. 10.1182/blood.V89.8.2736 [DOI] [PubMed] [Google Scholar]
- 208. Ryan MA, Nattamai KJ, Xing E, et al. : Pharmacological inhibition of EGFR signaling enhances G-CSF-induced hematopoietic stem cell mobilization. Nat Med. 2010;16(10):1141–6. 10.1038/nm.2217 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 209. Cohen KS, Cheng S, Larson MG, et al. : Circulating CD34 + progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121(8):e50–e56. 10.1182/blood-2012-05-424846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Bogunia-Kubik K, Gieryng A, Dlubek D, et al. : The CXCL12-3'A allele is associated with a higher mobilization yield of CD34 progenitors to the peripheral blood of healthy donors for allogeneic transplantation. Bone Marrow Transplant. 2009;44(5):273–8. 10.1038/bmt.2009.30 [DOI] [PubMed] [Google Scholar]
- 211. B MA, M C, A B, et al. : VCAM1 RS1041163 polymorphism influences G-CSF mobilization of CD34+ cells. Haematologica. 2010. [Google Scholar]
- 212. Martin-Antonio B, Carmona M, Falantes J, et al. : Impact of constitutional polymorphisms in VCAM1 and CD44 on CD34 + cell collection yield after administration of granulocyte colony-stimulating factor to healthy donors. Haematologica. 2011;96(1):102–9. 10.3324/haematol.2010.026401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Szmigielska-Kaplon A, Szemraj J, Hamara K, et al. : Polymorphism of CD44 influences the efficacy of CD34 + cells mobilization in patients with hematological malignancies. Biol Blood Marrow Transplant. 2014;20(7):986–91. 10.1016/j.bbmt.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 214. Schulz M, Karpova D, Spohn G, et al. : Variant rs1801157 in the 3’UTR of SDF-1ß Does Not Explain Variability of Healthy-Donor G-CSF Responsiveness. PLoS One. 2015;10(3):e0121859. 10.1371/journal.pone.0121859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Schroeder MA, Rettig MP, Uy GL, et al. : Cxcl12 G801A Polymorphisms Do Not Predict Response to Mobilization by Plerixafor in Normal Allogeneic Stem Cell Donors. Biol Blood Marrow Transplant. 2012;18(2):S261 10.1016/j.bbmt.2011.12.169 [DOI] [Google Scholar]
- 216. To LB, Levesque JP, Herbert KE: How I treat patients who mobilize hematopoietic stem cells poorly. Blood. 2011;118(17):4530–40. 10.1182/blood-2011-06-318220 [DOI] [PubMed] [Google Scholar]
- 217. Olivieri J, Attolico I, Nuccorini R, et al. : Predicting failure of hematopoietic stem cell mobilization before it starts: The predicted poor mobilizer (pPM) score. Bone Marrow Transplant. 2018;53(4):461–73. 10.1038/s41409-017-0051-y [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 218. Bhamidipati P, Wang S, Sturgill T, et al. : Predicting autologous stem cell mobilization failure in hematologic malignancies. Blood. 2013;122(21):2034 10.1182/blood.V122.21.2034.2034 [DOI] [Google Scholar]
- 219. Duong HK, Bolwell BJ, Rybicki L, et al. : Predicting hematopoietic stem cell mobilization failure in patients with multiple myeloma: a simple method using day 1 CD34+ cell yield. J Clin Apher. 2011;26(3):111–5. 10.1002/jca.20278 [DOI] [PubMed] [Google Scholar]
- 220. Kuittinen T, Nousiainen T, Halonen P, et al. : Prediction of mobilisation failure in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant. 2004;33(9):907–12. 10.1038/sj.bmt.1704466 [DOI] [PubMed] [Google Scholar]
- 221. Giralt S, Costa L, Schriber J, et al. : Optimizing Autologous Stem Cell Mobilization Strategies to Improve Patient Outcomes: Consensus Guidelines and Recommendations. Biol Blood Marrow Transplant. 2014;20(3):295–308. 10.1016/j.bbmt.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 222. Jiang L, Malik S, Litzow M, et al. : Hematopoietic stem cells from poor and good mobilizers are qualitatively equivalent. Transfusion. 2012;52(3):542–8. 10.1111/j.1537-2995.2011.03286.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Papayannopoulou T, Nakamoto B, Andrews RG, et al. : In vivo effects of Flt3/Flk2 ligand on mobilization of hematopoietic progenitors in primates and potent synergistic enhancement with granulocyte colony-stimulating factor. Blood. 1997;90(2):620–9. 10.1182/blood.V90.2.620 [DOI] [PubMed] [Google Scholar]
- 224. Anandasabapathy N, Breton G, Hurley A, et al. : Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant. 2015;50(7):924–30. 10.1038/bmt.2015.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Brasel K, McKenna HJ, Morrissey PJ, et al. : Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88(6):2004–12. 10.1182/blood.V88.6.2004.bloodjournal8862004 [DOI] [PubMed] [Google Scholar]
- 226. Donahue RE, Jin P, Bonifacino AC, et al. : Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34 + cell populations based on global gene and microRNA expression signatures. Blood. 2009;114(12):2530–41. 10.1182/blood-2009-04-214403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Ziegenhain C, Vieth B, Parekh S, et al. : Quantitative single-cell transcriptomics. Brief Funct Genomics. 2018;17(4):220–32. 10.1093/bfgp/ely009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 228. Rodriguez-Meira A, Buck G, Clark SA, et al. : Unravelling Intratumoral Heterogeneity through High-Sensitivity Single-Cell Mutational Analysis and Parallel RNA Sequencing. Mol Cell. 2019;73(6):1292–1305.e8. 10.1016/j.molcel.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 229. Petti AA, Williams SR, Miller CA, et al. : Mutation detection in thousands of acute myeloid leukemia cells using single cell RNA-sequencing. bioRxiv 2018. 10.1101/434746 [DOI] [Google Scholar]
- 230. Nam AS, Kim KT, Chaligne R, et al. : High throughput droplet single-cell Genotyping of Transcriptomes (GoT) reveals the cell identity dependency of the impact of somatic mutations. bioRxiv 2018. 10.1101/444687 [DOI] [Google Scholar]
- 231. Baryawno N, Przybylski D, Kowalczyk MS, et al. : A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell. 2019;177(7):1915–1932.e16. 10.1016/j.cell.2019.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 232. Severe N, Karabacak NM, Gustafsson K, et al. : Stress-Induced Changes in Bone Marrow Stromal Cell Populations Revealed through Single-Cell Protein Expression Mapping. Cell Stem Cell. 2019;25(4):570–583.e7. 10.1016/j.stem.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 233. Tikhonova AN, Dolgalev I, Hu H, et al. : The bone marrow microenvironment at single-cell resolution. Nature. 2019;569(7755):222–8. 10.1038/s41586-019-1104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation