Abstract
Background
This study aimed to summarize the previously published literature on the role of platelet-to-lymphocyte ratio (PLR) on overall survival (OS) in patients with gastric cancer.
Methods
We systematically searched PubMed, EmBase, and the Cochrane library to identify eligible studies to review. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the random-effects model. Sensitivity and subgroup analyses were performed, and publication bias was assessed.
Results
A total of 28 studies comprising 15,617 patients with gastric cancer were included in this meta-analysis. The pooled results indicated that elevated PLR was associated with poor OS (HR: 1.37; 95% CI: 1.24–1.51; P < 0.001). A significant publication bias was observed (Egger test, P = 0.036; Begg test, P = 0.017). After adjusting for publication bias using the trim and fill method, an adjusted pooled HR of 1.19 (95% CI: 1.08–1.33; P = 0.001) was observed. Subgroup analyses indicated an elevated PLR in retrospective studies. Studies conducted in Turkey, the UK, the USA, and Costa Rica; studies with a sample size of < 1000, with < 70% male patients, and with patients treated with chemotherapy; studies with PLR cutoff value of ≥200; and studies with lower quality as determined by the Newcastle-Ottawa Scale all showed greater harmful effects on OS than their corresponding subsets (P < 0.05).
Conclusions
An elevated PLR was associated with poor OS in patients with gastric cancer. These results might differ between studies due to differences in design, country of origin, sample size, sex proportion, treatment strategy, PLR cutoff value, and study quality.
Keywords: Prognosis, Blood platelets, Lymphocyte count, Meta-analysis, Stomach neoplasms
Background
Gastric cancer is the second most common cancer in China. Nearly 679,100 new gastric cancer cases are diagnosed, and 498,000 patients die from gastric cancer annually [1]. Patients are usually diagnosed at advanced or metastatic stages due to the lack of clinical symptoms specific to gastric cancer, making it an extremely deadly disease with unfavorable prognosis despite the development of new surgical techniques, chemotherapy, and radiotherapy [2]. Currently, the standard treatment strategy for metastatic gastric cancer includes chemotherapy and targeted therapy. The response rate to first-line treatment ranges from 27 to 54% [3–5]. Therefore, simple, low-cost methods to evaluate the prognosis of gastric cancer should be explored.
Several studies have indicated that the immune system can affect tumor growth, with neutrophils, lymphocytes, monocytes, and platelets possibly playing an important role in the tumor-induced systemic inflammatory response [6, 7]. This response may accelerate tumor development and metastasis through the following mechanisms: promoting secretion of inflammatory mediators and cytokines, inhibiting apoptosis, and damaging the tumor cell DNA [8]. Previous meta-analyses have already demonstrated the prognostic role of platelet-to-lymphocyte ratio (PLR) in gastrointestinal cancers [9–11], and whether this association differs according to patients’ characteristics remains controversial. Therefore, this study was conducted to update the magnitude for the role of PLR on overall survival (OS) of patients with gastric cancer. Moreover, differences in this association based on patients’ characteristics were also investigated.
Methods
Data sources, search strategy, and selection criteria
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2009 [12]. Studies that investigated the role of PLR on OS in patients with gastric cancer were eligible for inclusion in this meta-analysis, with no restriction on language of publication. Utilizing the Boolean logic, the core search template in PubMed, EmBase, and the Cochrane library was [(“PLR” OR “platelet lymphocyte ratio”) AND (“gastric cancer” OR “stomach cancer”) AND (“prognosis” OR “survival”)]. Each database was searched from its date of inception through November 2018. Manual searches of the reference lists of eligible studies and relevant reviews obtained in the database search were also carried out to identify any relevant new studies or studies otherwise missed.
Two independent reviewers conducted the literature search and study selection process following a standardized flowchart. The inclusion criteria for this meta-analysis were as follows: 1) patients: all studies including patients diagnosed with gastric cancer, irrespective of stage; 2) comparison: all studies comparing high PLR with low PLR; and 3) outcome: all studies reporting OS. Furthermore, studies designed as either prospective or retrospective were included, whereas those including patients with secondary cancers in addition to gastric cancer were excluded.
Data collection and quality assessment
Data collection and quality assessment were performed by two independent reviewers, and any inconsistencies or disputes were settled by a third independent reviewer. Collected data from each study included the first author’s name, year of publication, study design, country of origin, sample size, sex proportion and mean age of the study cohort, treatment strategy, disease status, cutoff value of PLR used to define elevated level, and OS. The quality of each study was evaluated using the Newcastle-Ottawa Scale (NOS) which consists of the following 3 subscales: selection (4 items), comparability (1 item), and outcome (3 items). The NOS is quite comprehensive and has been partially validated for evaluating the quality of observational studies in meta-analysis [13]. The “star system” of NOS ranges from 0 to 9; studies with 7–9 stars are considered high quality, whereas those with ≤6 stars are considered low quality.
Statistical analysis
The prognostic role of PLR on OS for patients with gastric cancer was analyzed by abstracting the hazard ratios (HRs) and 95% confidence intervals (CIs) reported in each individual study. The pooled results were then calculated using the random-effects model, which considers that the true underlying effect varies across included studies [14, 15]. Heterogeneity among included studies was calculated using the I-square and Q statistics, with I-square of > 50.0% or P < 0.10 indicating significant heterogeneity [16, 17]. Then, sensitivity analysis then performed to assess the stability of pooled results [18]. Subgroup analyses were also conducted to evaluate the relationship between PLR and OS according to the study design, country of origin, sample size, sex proportion and mean age of cohort, treatment strategy, disease status, PLR cutoff value, and NOS score. P-values between subgroups were also calculated using the interaction t-test [19]. Publication bias was investigated with qualitative and quantitative methods, including funnel plot, Egger test [20], and Begg test [21]. P-values for pooled results were two-sided, and the inspection level was 0.05. All statistical analyses were computed with STATA software (version 10.0; Stata Corporation, College Station, TX, USA).
Results
Literature search
The initial search in the 3 electronic databases identified 143 studies, of which 106 were excluded due to duplication and irrelevance to this meta-analysis. Thirty-seven potentially eligible studies were selected for further evaluation; 9 were excluded due to the following reasons: same study population (n = 2), OS not reported as outcome (n = 4), and secondary cancers were included (n = 3). Manual searches of the reference lists of these studies identified 17 articles, and all of them were already included in the initial electronic searches. Finally, 28 studies were selected for meta-analysis [22–49]. The study selection process is presented as PRISMA flowchart in Fig. 1, and the baseline characteristics of the included studies are shown in Table 1.
Fig. 1.
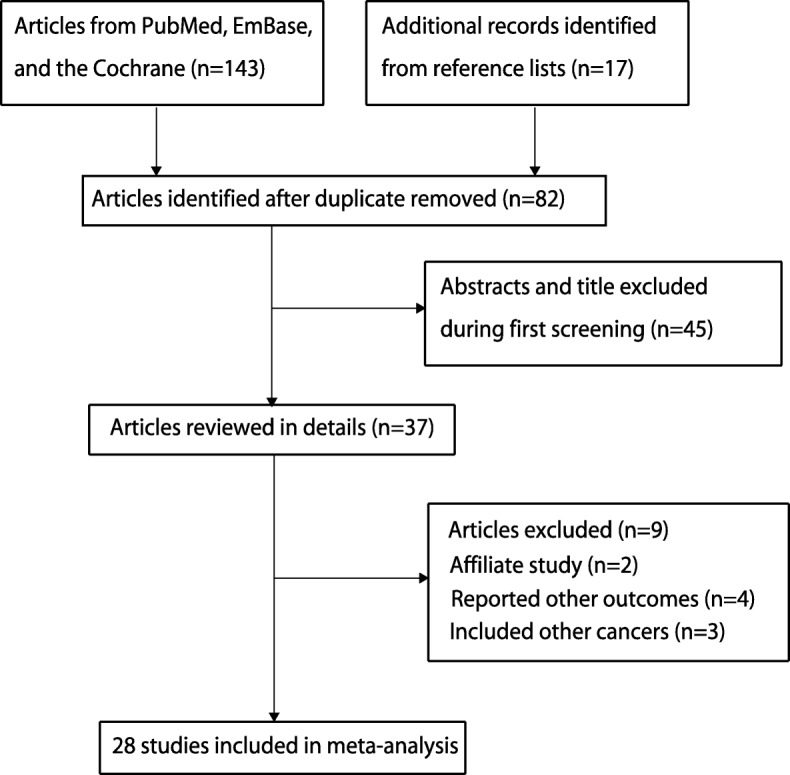
A flow diagram of the literature search and trials selection process
Table 1.
Baseline characteristics of the selected studies
| Study | Publication year | Study design | Country | Sample size | Percent of male (%) | Mean age (years) | Treatment strategy | Disease status | Cutoff value of PLR | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Aliustaoglu [23] | 2010 | Retrospective | Turkey | 168 | 67.8 | 60.1 | Chemotherapy | Advanced | 160 | 6 |
| Lee [32] | 2013 | Retrospective | Korea | 174 | 65.5 | 55.0 | Chemotherapy | Advanced | 160 | 8 |
| Jiang [30] | 2014 | Prospective | China | 377 | 67.1 | 64.0 | Surgery | Early | 184 | 7 |
| Wang [45] | 2014 | Retrospective | China | 439 | 72.7 | NA | Mixed | Advanced | 160 | 7 |
| Lian [33] | 2015 | Retrospective | China | 162 | 69.8 | 56.3 | Surgery | All | 208 | 8 |
| Aldemir [22] | 2015 | Retrospective | Turkey | 103 | 56.3 | 58.0 | Mixed | Early and Advanced | 170 | 7 |
| Deng [24] | 2015 | Retrospective | China | 389 | 72.5 | 65.0 | Surgery | All | 132 | 8 |
| Gunaldi [28] | 2015 | Retrospective | Turkey | 245 | 72.2 | 59.6 | Mixed | All | 160 | 7 |
| Hsu [29] | 2015 | Retrospective | China | 1030 | 64.5 | NA | Surgery | All | 132 | 7 |
| Kim [31] | 2015 | Prospective | Korea | 1986 | 66.3 | 58.2 | Surgery | Early | 126 | 7 |
| Liu [34] | 2015 | Retrospective | China | 455 | 69.0 | 59.0 | Surgery | Early | 188 | 6 |
| Sun [39] | 2015 | Retrospective | China | 632 | 65.3 | 57.0 | Surgery | All | 140 | 7 |
| Wang [42] | 2015 | Retrospective | China | 120 | 62.5 | 68.0 | Chemotherapy | Advanced | 235 | 8 |
| Feng [25] | 2016 | Retrospective | China | 3243 | 78.3 | 57.3 | Mixed | Advanced | 130 | 7 |
| Sun [40] | 2016 | Retrospective | China | 305 | 66.2 | 57.0 | Surgery | Early | 120 | 8 |
| Zhou [49] | 2016 | Retrospective | China | 451 | 71.8 | NA | Surgery | Early | 255 | 7 |
| Wen [46] | 2017 | Retrospective | UK | 253 | 66.1 | 75.5 | Surgery | All | 150 | 6 |
| Fuentes [26] | 2017 | Retrospective | USA | 112 | 66.1 | 58.0 | Mixed | Advanced | 260 | 6 |
| Song [38] | 2017 | Retrospective | China | 1990 | 73.7 | 62.0 | Surgery | Advanced | 139 | 7 |
| Wang [43] | 2017 | Retrospective | China | 273 | 68.1 | 56.7 | Chemotherapy | Advanced | 202 | 6 |
| Wang [44] | 2017 | Retrospective | China | 444 | 63.3 | 56.0 | Surgery | All | 120 | 7 |
| Ramos-Esquivel [36] | 2018 | Retrospective | Costa Rica | 381 | 57.2 | 61.2 | Mixed | All | 350 | 7 |
| Petrillo [35] | 2018 | Retrospective | Italy | 151 | 64.2 | 62.0 | Chemotherapy | Advanced | 157 | 8 |
| Saito [37] | 2018 | Retrospective | Japan | 453 | 73.1 | 67.7 | Surgery | All | 173 | 7 |
| Gong [27] | 2018 | Retrospective | China | 91 | 75.8 | 55.0 | Mixed | Advanced | 108 | 7 |
| Zhang [48] | 2018 | Retrospective | China | 182 | 67.0 | 65.0 | Mixed | All | 172 | 7 |
| Tang [41] | 2018 | Retrospective | China | 104 | 71.2 | NA | Chemotherapy | Advanced | 131 | 6 |
| Zhang [47] | 2018 | Retrospective | China | 904 | 74.4 | NA | Surgery | All | 160 | 7 |
Study characteristics
Two prospective and 26 retrospective studies reporting a total of 15,617 patients with gastric cancer were included in this meta-analysis. The sample size ranged from 91 to 3243, and the proportion of male patients ranged from 56.3 to 78.3%. Eighteen studies were conducted in China, 3 in Turkey, 2 in Korea, 1 in Japan, 1 in the UK, 1 in the USA, 1 in Costa Rica, and 1 in Italy. Fourteen studies included patients treated with surgery, 6 with chemotherapy, and the remaining 8 included patients who received combined treatment strategies. Five studies included patients in early stages, 11 with advanced stages, and the remaining 12 with all stages. The mean patient age in the included studies ranged from 55.0 to 75.5 years, and the PLR cutoff value used to define elevated level ranged from 108 to 305. Study quality was evaluated using the NOS: 6 studies had 8 stars, 16 had 7 stars, and the remaining 6 had 6 stars.
Meta-analysis and sensitivity analysis
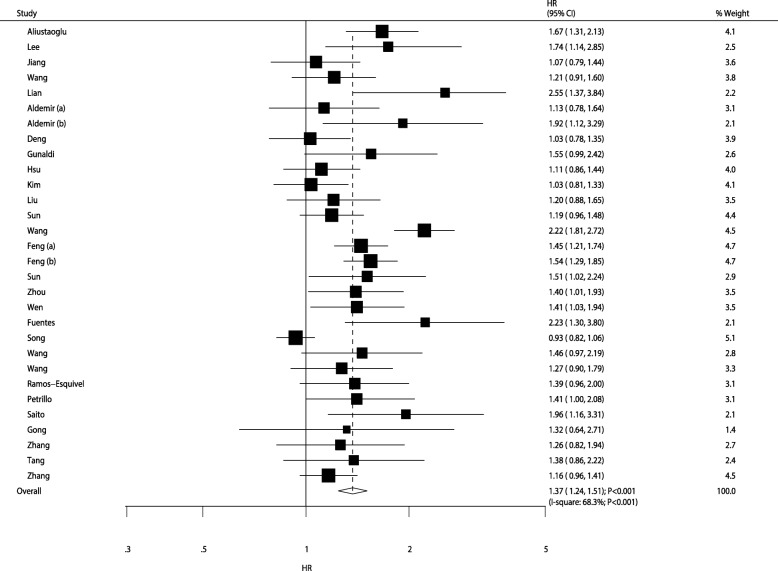
After pooling all included studies, patients with gastric cancer with an elevated PLR were noted to have lower OS than those with lower PLR level (HR: 1.37; 95% CI: 1.24–1.51; P < 0.001; Fig. 2). Significant heterogeneity among the included studies was observed (I-square: 68.3%; P < 0.001). The results of sensitivity analyses are presented in Table 2; we noted that higher PLR was associated with lower OS in the pooled conclusion. Moreover, studies conducted by Wang et al. [42] and Song et al. [38] were noted to be responsible for most of the heterogeneity in the summary results.
Fig. 2.
The prognostic role of PLR on OS in gastric cancer patients
Table 2.
Sensitivity analysis for overall survival
| Excluding study | Including studies | HR and 95% CI | P value | Heterogeneity (%) | P value for heterogeneity |
|---|---|---|---|---|---|
| Aliustaoglu [23] | 22,24–49 | 1.36 (1.23–1.50) | < 0.001 | 67.9 | < 0.001 |
| Lee [32] | 22–31,33–49 | 1.36 (1.23–1.50) | < 0.001 | 68.8 | < 0.001 |
| Jiang [30] | 22–29,31–49 | 1.38 (1.25–1.53) | < 0.001 | 68.8 | < 0.001 |
| Wang [45] | 22–44,46–49 | 1.38 (1.25–1.52) | < 0.001 | 69.3 | < 0.001 |
| Lian [33] | 22–32,34–49 | 1.35 (1.23–1.49) | < 0.001 | 67.0 | < 0.001 |
| Aldemir [22] (a) | 23–49 | 1.38 (1.25–1.52) | < 0.001 | 69.2 | < 0.001 |
| Aldemir [22] (b) | 23–49 | 1.36 (1.23–1.50) | < 0.001 | 68.7 | < 0.001 |
| Deng [24] | 22,23,25–49 | 1.39 (1.25–1.53) | < 0.001 | 68.3 | < 0.001 |
| Gunaldi [28] | 22–27,29–49 | 1.37 (1.24–1.51) | < 0.001 | 69.2 | < 0.001 |
| Hsu [29] | 22–28,30–49 | 1.38 (1.25–1.53) | < 0.001 | 68.8 | < 0.001 |
| Kim [31] | 22–30,32–49 | 1.39 (1.25–1.53) | < 0.001 | 68.2 | < 0.001 |
| Liu [34] | 22–33,35–49 | 1.38 (1.25–1.52) | < 0.001 | 69.3 | < 0.001 |
| Sun [39] | 22–38,40–49 | 1.38 (1.25–1.53) | < 0.001 | 69.1 | < 0.001 |
| Wang [42] | 22–41,43–49 | 1.33 (1.22–1.45) | < 0.001 | 55.8 | < 0.001 |
| Feng [25] (a) | 22–24,26–49 | 1.37 (1.23–1.52) | < 0.001 | 68.9 | < 0.001 |
| Feng [25] (b) | 22–24,26–49 | 1.36 (1.23–1.51) | < 0.001 | 68.1 | < 0.001 |
| Sun [40] | 22–39,41–49 | 1.37 (1.24–1.51) | < 0.001 | 69.2 | < 0.001 |
| Zhou [49] | 22–48 | 1.37 (1.24–1.52) | < 0.001 | 69.3 | < 0.001 |
| Wen [46] | 22–45,47–49 | 1.37 (1.24–1.51) | < 0.001 | 69.3 | < 0.001 |
| Fuentes [26] | 22–25,27–49 | 1.36 (1.23–1.49) | < 0.001 | 68.0 | < 0.001 |
| Song [38] | 22–37,39–49 | 1.39 (1.28–1.52) | < 0.001 | 54.1 | < 0.001 |
| Wang [43] | 22–42,44–49 | 1.37 (1.24–1.51) | < 0.001 | 69.3 | < 0.001 |
| Wang [44] | 22–43,45–49 | 1.37 (1.24–1.52) | < 0.001 | 69.3 | < 0.001 |
| Ramos-Esquivel [36] | 22–35,37–49 | 1.37 (1.24–1.51) | < 0.001 | 69.3 | < 0.001 |
| Petrillo [35] | 22–34,36–49 | 1.37 (1.24–1.51) | < 0.001 | 69.3 | < 0.001 |
| Saito [37] | 22–36,38–49 | 1.36 (1.23–1.50) | < 0.001 | 68.5 | < 0.001 |
| Gong [27] | 22–26,28–49 | 1.37 (1.24–1.51) | < 0.001 | 69.4 | < 0.001 |
| Zhang [48] | 22–47,49 | 1.37 (1.24–1.52) | < 0.001 | 69.3 | < 0.001 |
| Tang [41] | 22–40,42–49 | 1.37 (1.24–1.51) | < 0.001 | 69.3 | < 0.001 |
| Zhang [47] | 22–46,48,49 | 1.38 (1.25–1.53) | < 0.001 | 68.9 | < 0.001 |
Subgroup analysis
Subgroup analyses for the prognostic role of PLR on OS in gastric cancer are presented in Table 3 and Additional file 1: Figures S1, S2, S3, S4, S5, S6, S7, S8 and S9. Increased PLR was found to be associated with lower OS in gastric cancer in most subsets. However, PLR was not significantly associated with OS in prospectively designed studies, nor in studies conducted in Japan and Korea. When comparing relative ratios between subgroups, PLR was found to be higher in the pooled results from retrospective studies. Studies conducted in Turkey, the UK, the USA, and Costa Rica; studies with sample size of < 1000; studies including < 70% male patients; studies with patients treated with chemotherapy; studies with PLR cutoff value ≥200; and studies of lower quality as determined by the NOS score all showed greater harmful effects on OS as compared to their corresponding subgroups (Table 2).
Table 3.
Subgroup analysis for overall survival
| Factor | Groups | Number of cohorts | HR and 95% CI | P value | Heterogeneity (%) | P value for heterogeneity | P value between subgroups |
|---|---|---|---|---|---|---|---|
| Study design | Prospective | 2 | 1.05 (0.87–1.27) | 0.625 | 0.0 | 0.868 | 0.022 |
| Retrospective | 28 | 1.40 (1.26–1.55) | < 0.001 | 68.6 | < 0.001 | ||
| Country | China | 19 | 1.32 (1.16–1.49) | < 0.001 | 75.0 | < 0.001 | 0.045 |
| Japan or Korea | 3 | 1.45 (0.94–2.25) | 0.092 | 71.4 | 0.030 | ||
| Other | 8 | 1.51 (1.33–1.72) | < 0.001 | 0.0 | 0.503 | ||
| Sample size | ≥ 1000 | 11 | 1.25 (1.09–1.45) | 0.002 | 69.2 | < 0.001 | < 0.001 |
| < 1000 | 19 | 1.44 (1.28–1.63) | < 0.001 | 60.4 | < 0.001 | ||
| Percent male | ≥ 70.0 | 10 | 1.31 (1.10–1.55) | 0.002 | 72.9 | < 0.001 | 0.014 |
| < 70.0 | 20 | 1.40 (1.25–1.58) | < 0.001 | 63.5 | < 0.001 | ||
| Mean age (years) | ≥ 60.0 | 13 | 1.41 (1.16–1.71) | 0.001 | 82.2 | < 0.001 | 0.168 |
| < 60.0 | 12 | 1.39 (1.23–1.57) | < 0.001 | 41.6 | 0.064 | ||
| Treatment strategy | Surgery | 14 | 1.21 (1.08–1.35) | 0.001 | 56.5 | 0.005 | < 0.001 |
| Chemotherapy | 6 | 1.70 (1.43–2.03) | < 0.001 | 40.4 | 0.136 | ||
| Mixed | 10 | 1.44 (1.31–1.59) | < 0.001 | 0.0 | 0.545 | ||
| Disease status | Early | 6 | 1.18 (1.04–1.34) | 0.012 | 0.0 | 0.533 | 0.076 |
| Advanced | 13 | 1.51 (1.26–1.82) | < 0.001 | 82.0 | < 0.001 | ||
| All | 11 | 1.29 (1.14–1.45) | < 0.001 | 35.1 | 0.118 | ||
| Cutoff value | ≥ 200 | 6 | 1.79 (1.43–2.24) | < 0.001 | 56.8 | 0.041 | < 0.001 |
| < 200 | 24 | 1.28 (1.17–1.40) | < 0.001 | 53.9 | 0.001 | ||
| NOS scale | High | 24 | 1.34 (1.20–1.50) | < 0.001 | 72.0 | < 0.001 | 0.039 |
| Low | 6 | 1.49 (1.30–1.72) | < 0.001 | 0.0 | 0.416 |
Publication Bias
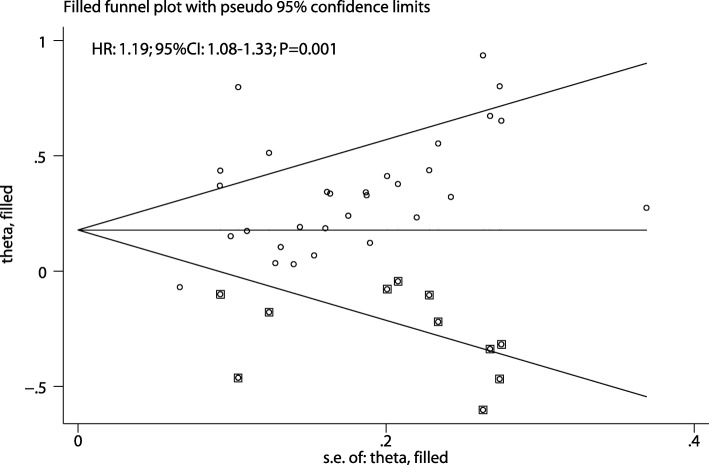
Publication bias for the prognostic role of PLR on OS in gastric cancer was assessed and is presented in Fig. 3. Results of the Egger and Begg tests showed significant publication bias (P = 0.036 and P = 0.017, respectively). Our finding that elevated PLR is associated with lower OS did not change after the adjustment for publication bias using the trim and fill method [50]. The adjusted pooled HR was 1.19 (95% CI: 1.08–1.33; P = 0.001; Fig. 4).
Fig. 3.
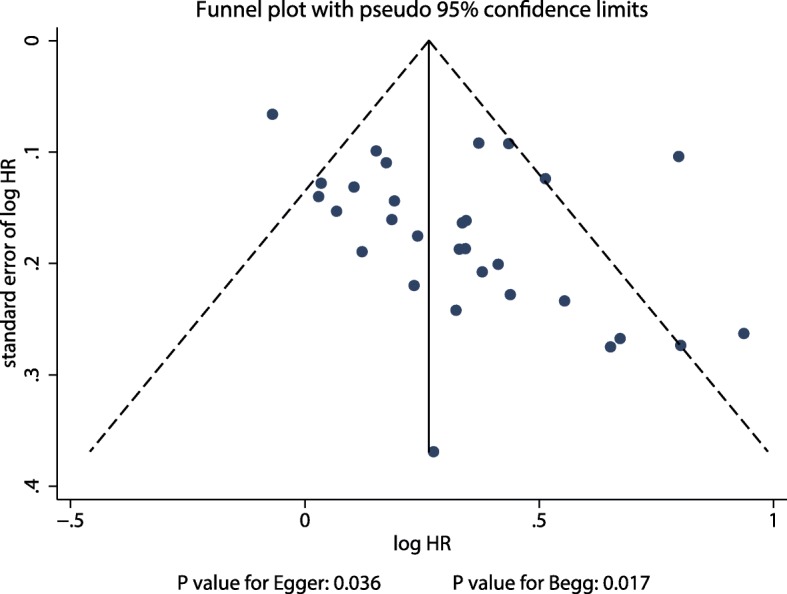
Publication bias for the prognostic role of PLR on OS in gastric cancer patients
Fig. 4.
The pooled result adjusted by the trim and fill method
Discussion
The current meta-analysis was based on all published observational studies that explored the prognostic role of PLR on OS in gastric cancer, and the prognostic ability of elevated PLR on OS was compared between subgroups based on pre-defined factors. This comprehensive quantitative meta-analysis comprised a total of 15,617 patients with gastric cancer from 2 prospective and 26 retrospective studies with a wide range of study and patient characteristics. The pooled results indicated that elevated PLR was significantly associated with lower OS in gastric cancer. This result is stable and not altered by excluding any specific study from the analysis. The results of subgroup analyses indicated that elevated PLR predicted poor OS in most subsets. In the pooled retrospective studies, elevated PLR was noted to cause greater harmful effects on OS than their corresponding subgroups in studies conducted in Turkey, the UK, the USA, and Costa Rica; studies with sample size of < 1000; studies with < 70% male patients; studies with patients treated with chemotherapy; studies with PLR cutoff value of ≥200; and studies of lesser quality.
A previous meta-analysis based on 13 studies found that elevated PLR was associated with poor OS, but without significant effect on disease-free survival [51]. Subgroup analyses indicated that the prognostic roles of PLR on OS differed based on race, treatment strategy, disease status, and cutoff value of PLR. However, data from these included studies were assessed and revealed that some data were not consistent with that of the original study. The study conducted by Zhou et al. indicated an elevated PLR was not significantly associated with OS in patients with gastric cancer according to 3 studies [52]. Moreover, Xu et al. conducted a meta-analysis of 8 studies and concluded an elevated PLR was not associated with OS in patients with gastric cancer, but was significantly correlated with greater risk of lymph node metastasis, serosal invasion, and advanced stage risk [53]. However, stratified analyses according to some characteristics, including the mean age of patients, sex proportion, and study quality, were not addressed. Furthermore, numerous relevant studies were published in 2017 and 2018, but were not yet included in any pooled results. Therefore, this meta-analysis was conducted to thoroughly evaluate the prognostic role of PLR on OS in gastric cancer and include newer updated studies.
The pooled results indicated that elevated PLR was significantly associated with poor OS in gastric cancer. However, several studies included in the meta-analysis did not observe this. Jiang et al. showed that neutrophil–lymphocyte ratio (NLR) and PLR are prognostic factors for operable gastric cancer, whereas PLR was not a prognostic factor for OS [30]. Wang et al. found the median survival time in patients with PLR of > 160 and PLR of < 160 as 8.5 months and 10 months, respectively; this small difference was not statistically significant [45]. Aldemir et al. found that PLR could not significantly predict OS in patients with early-stage gastric cancer but could in those with advanced gastric cancer [22]. Deng et al. suggested that preoperative PLR was significantly correlated with tumor progression and poor prognosis in patients with gastric cancer after a surgical resection [24]. Gunaldi et al. found no significant association between PLR and OS in gastric cancer of all stages [28]. Hsu et al. used PLR of 132 as the cutoff value and found that elevated PLR was not associated with OS in gastric cancer at all stages [29]. Similarly, the study conducted by Kim et al. suggested that PLR and NLR were associated with gastric cancer prognosis and indicated that NLR was more predictive of OS than PLR [31]. Several other studies also did not find elevated PLR to be associated with OS in patients with gastric cancer [27, 34, 36, 38, 39, 41, 43, 44, 47, 48]. These results might vary due to the study design, disease stage, and cutoff values of PLR. Differences between studies in median survival rates might be biased due to the relationship of PLR with tumor size and disease stage.
Subgroup analyses indicated that the prognostic role of PLR on OS in gastric cancer might be affected by the study design, country of origin, sample size, sex proportion, treatment strategy, cutoff values of PLR, and study quality. This condition potentially occurs due to the following reasons: 1) the number of included studies was not balanced between subgroups, which might affect the pooled results; 2) weighted pooled results could affect the prognostic role of PLR on OS in patients with specific characteristics; 3) background therapies and tumor stage are associated with the prognosis of patients with gastric cancer; and 4) study quality was correlated with evidence level and reliability of pooled results.
Although this study provided a comprehensive meta-analysis for the prognostic role of PLR on OS in gastric cancer, several limitations should be acknowledged: 1) most studies included were retrospective in design, which might introduce confounding variables, thus overestimating the pooled result; 2) different adjusted models, treatment strategies, and tumor stages among included patients might introduce a substantial heterogeneity among the included studies; 3) a significant publication bias among the included studies was observed, although the adjusted result was calculated; and 4) individual data were not available and more detailed analyses not conducted.
Conclusion
In conclusion, the pooled result indicated that elevated PLR was associated with poor OS in patients with gastric cancer. Moreover, the adjusted HR indicated decreased harmful effects after adjusting for potential publication bias. Furthermore, the prognostic role of PLR on OS might be affected by the study design, country of origin, sample size, treatment strategy, cutoff values of PLR, and study quality. Further large-scale prospective studies should be conducted to verify the findings in this study and evaluate the role of PLR on the prognosis (progression-free survival and disease-free survival) of gastric cancer.
Supplementary information
Additional file 1. Subgroup analyses for overall survival. Subgroup analyses for overall survival based on study design, country, sample size, percent male, mean age, treatment strategy, disease status, cutoff value and study quality.
Acknowledgements
Not applicable.
Abbreviations
- CIs
Confidence intervals
- HRs
Hazard ratios
- NLR
Neutrophil–lymphocyte ratio
- NOS
Newcastle-Ottawa Scale
- OS
Overall survival
- PLR
Platelet-to-lymphocyte ratio
Authors’ contributions
WJC, LYX contributed to the conception or design of the study. XMY, DWC, YJZ and NWZ contributed to acquisition, analysis or interpretation of this study. WJC, XMY and LYX drafted the manuscript. WJC and LYX critically revised the manuscript. All authors gave final approval and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weijuan Cao, Email: 770180309@qq.com.
Xiaomin Yao, Email: 630088408@qq.com.
Danwei Cen, Email: cendanwei0901@sina.com.
Yajun Zhi, Email: zyj0532@qq.com.
Ningwei Zhu, Email: 1058207756@qq.com.
Liyong Xu, Email: 1529228295@qq.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12876-020-1167-x.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Ma J, Yao S, Li XS, Kang HR, Yao FF, Du N. Neoadjuvant therapy of DOF regimen plus Bevacizumab can increase surgical resection Ratein locally advanced gastric Cancer: a randomized, controlled study. Medicine (Baltimore) 2015;94:e1489. doi: 10.1097/MD.0000000000001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START) J Cancer Res Clin Oncol. 2014;140:319–328. doi: 10.1007/s00432-013-1563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 6.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 9.Huang XZ, Chen WJ, Zhang X, Wu CC, Zhang CY, Sun SS, Wu J. An elevated platelet-to-lymphocyte ratio predicts poor prognosis and Clinicopathological characteristics in patients with colorectal Cancer: a meta-analysis. Dis Markers. 2017;2017:1053125. doi: 10.1155/2017/1053125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai Q, Melandro F, Larghi Laureiro Z, Giovanardi F, Ginanni Corradini S, Ferri F, Hassan R, Rossi M, Mennini G. Platelet-to-lymphocyte ratio in the setting of liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. World J Gastroenterol. 2018;24:1658–1665. doi: 10.3748/wjg.v24.i15.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Tao L, Lu M, Xiu D. Prognostic role of platelet to lymphocyte ratio in pancreatic cancers: a meta-analysis including 3028 patients. Medicine (Baltimore) 2018;97:e9616. doi: 10.1097/MD.0000000000009616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells GSB, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2009. [Google Scholar]
- 14.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Mak. 2005;25:646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Deeks JJ, Higgins JP, Altman DG. Analysing Data and Undertaking Meta-Analyses. In: GS HJ, editor. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: The Cochrane Collaboration; 2008. [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- 19.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. Syst Rev Health Care. 2001;2:285–312. doi: 10.1002/9780470693926.ch15. [DOI] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 22.Aldemir MN, Turkeli M, Simsek M, Yildirim N, Bilen Y, Yetimoglu H, Bilici M, Tekin SB. Prognostic value of baseline neutrophil-lymphocyte and platelet-lymphocyte ratios in local and advanced gastric Cancer patients. Asian Pac J Cancer Prev. 2015;16:5933–5937. doi: 10.7314/APJCP.2015.16.14.5933. [DOI] [PubMed] [Google Scholar]
- 23.Aliustaoglu M, Bilici A, Ustaalioglu BB, Konya V, Gucun M, Seker M, Gumus M. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol. 2010;27:1060–1065. doi: 10.1007/s12032-009-9335-4. [DOI] [PubMed] [Google Scholar]
- 24.Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F, Gao T, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. doi: 10.1186/s12967-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng F, Sun L, Zheng G, Liu S, Liu Z, Xu G, Guo M, Lian X, Fan D, Zhang H. Low lymphocyte-to-white blood cell ratio and high monocyte-to-white blood cell ratio predict poor prognosis in gastric cancer. Oncotarget. 2017;8:5281–5291. doi: 10.18632/oncotarget.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuentes HE, Oramas DM, Paz LH, Wang Y, Andrade XA, Tafur AJ. Venous thromboembolism is an independent predictor of mortality among patients with gastric Cancer. J Gastrointest Cancer. 2018;49:415–421. doi: 10.1007/s12029-017-9981-2. [DOI] [PubMed] [Google Scholar]
- 27.Gong W, Zhao L, Dong Z, Dou Y, Liu Y, Ma C, Qu X. After neoadjuvant chemotherapy platelet/lymphocyte ratios negatively correlate with prognosis in gastric cancer patients. J Clin Lab Anal. 2018;32:e22364. doi: 10.1002/jcla.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunaldi M, Goksu S, Erdem D, Gunduz S, Okuturlar Y, Tiken E, Kahraman S, Inan YO, Genc TB, Yildirim M. Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte ratios in patients with gastric cancer: a multicenter study. Int J Clin Exp Med. 2015;8:5937–5942. [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu JT, Liao CK, Le PH, Chen TH, Lin CJ, Chen JS, Chiang KC, Yeh TS. Prognostic value of the preoperative neutrophil to lymphocyte ratio in Resectable gastric Cancer. Medicine (Baltimore) 2015;94:e1589. doi: 10.1097/MD.0000000000001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang N, Deng JY, Liu Y, Ke B, Liu HG, Liang H. The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers. 2014;19:444–451. doi: 10.3109/1354750X.2014.926567. [DOI] [PubMed] [Google Scholar]
- 31.Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric Cancer? Ann Surg Oncol. 2015;22:4363–4370. doi: 10.1245/s10434-015-4518-z. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, Kim HJ. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian L, Xia YY, Zhou C, Shen XM, Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR, Wu MY, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. 2015;15:899–907. doi: 10.3233/CBM-150534. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Sun X, Liu J, Kong P, Chen S, Zhan Y, Xu D. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric Cancer. Transl Oncol. 2015;8:339–345. doi: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrillo A, Laterza MM, Tirino G, Pompella L, Ventriglia J, Pappalardo A, Famiglietti V, Martinelli E, Ciardiello F, Orditura M, et al. Systemic-inflammation-based score can predict prognosis in metastatic gastric cancer patients before first-line chemotherapy. Future Oncol. 2018;14:2493–2505. doi: 10.2217/fon-2018-0167. [DOI] [PubMed] [Google Scholar]
- 36.Ramos-Esquivel A, Cordero-Garcia E, Brenes-Redondo D, Alpizar-Alpizar W. The neutrophil-lymphocyte ratio is an independent prognostic factor for overall survival in Hispanic patients with gastric adenocarcinoma. J Gastrointest Cancer. 2018. PMID: 30003495. [DOI] [PubMed]
- 37.Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ashida K, Fujiwara Y. Prognostic significance of platelet-based inflammatory indicators in patients with gastric Cancer. World J Surg. 2018;42:2542–2550. doi: 10.1007/s00268-018-4527-8. [DOI] [PubMed] [Google Scholar]
- 38.Song S, Li C, Li S, Gao H, Lan X, Xue Y. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther. 2017;10:3145–3154. doi: 10.2147/OTT.S138039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun KY, Xu JB, Chen SL, Yuan YJ, Wu H, Peng JJ, Chen CQ, Guo P, Hao YT, He YL. Novel immunological and nutritional-based prognostic index for gastric cancer. World J Gastroenterol. 2015;21:5961–5971. doi: 10.3748/wjg.v21.i19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun X, Liu X, Liu J, Chen S, Xu D, Li W, Zhan Y, Li Y, Chen Y, Zhou Z. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio in predicting survival for patients with stage I-II gastric cancer. Chin J Cancer. 2016;35:57. doi: 10.1186/s40880-016-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang C, Cheng X, Yu S, Wang Y, Hou J, Li Q, Shen Z, Liu T, Cui Y. Platelet-to-lymphocyte ratio and lymphocyte-to-white blood cell ratio predict the efficacy of neoadjuvant chemotherapy and the prognosis of locally advanced gastric cancer patients treated with the oxaliplatin and capecitabine regimen. Onco Targets Ther. 2018;11:7061–7075. doi: 10.2147/OTT.S176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Liu ZY, Xia YY, Zhou C, Shen XM, Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR, et al. Changes in neutrophil/lymphocyte and platelet/lymphocyte ratios after chemotherapy correlate with chemotherapy response and prediction of prognosis in patients with unresectable gastric cancer. Oncol Lett. 2015;10:3411–3418. doi: 10.3892/ol.2015.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Jin, Qu Jinglei, Li Zhi, Che Xiaofang, Liu Jing, Teng Yuee, Jin Bo, Zhao Mingfang, Liu Yunpeng, Qu Xiujuan. Pretreatment platelet-to-lymphocyte ratio is associated with the response to first-line chemotherapy and survival in patients with metastatic gastric cancer. Journal of Clinical Laboratory Analysis. 2017;32(1):e22185. doi: 10.1002/jcla.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang K, Diao F, Ye Z, Zhang X, Zhai E, Ren H, Li T, Wu H, He Y, Cai S, Chen J. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer. 2017;36:75. doi: 10.1186/s40880-017-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Yang Y, Zhang YP, Zou Z, Qian X, Liu B, Wei J. Prognostic value of carbohydrate tumor markers and inflammation-based markers in metastatic or recurrent gastric cancer. Med Oncol. 2014;31:289. doi: 10.1007/s12032-014-0289-9. [DOI] [PubMed] [Google Scholar]
- 46.Wen J, Bedford M, Begum R, Mitchell H, Hodson J, Whiting J, Griffiths E. The value of inflammation based prognostic scores in patients undergoing surgical resection for oesophageal and gastric carcinoma. J Surg Oncol. 2018;117:1697–1707. doi: 10.1002/jso.25057. [DOI] [PubMed] [Google Scholar]
- 47.Zhang LX, Wei ZJ, Xu AM, Zang JH. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg. 2018;56:320–327. doi: 10.1016/j.ijsu.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine (Baltimore) 2018;97:e0144. doi: 10.1097/MD.0000000000010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X, Xu L, Huang Z, Zhang L, Zhang H, Zhu W, Liu P. The hematologic markers as prognostic factors in patients with resectable gastric cancer. Cancer Biomark. 2016;17:359–367. doi: 10.3233/CBM-160648. [DOI] [PubMed] [Google Scholar]
- 50.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 51.Gu X, Gao XS, Cui M, Xie M, Peng C, Bai Y, Guo W, Han L, Gu X, Xiong W. Clinicopathological and prognostic significance of platelet to lymphocyte ratio in patients with gastric cancer. Oncotarget. 2016;7:49878–49887. doi: 10.18632/oncotarget.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, Wang T, Zhu W, Liu P. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Z, Xu W, Cheng H, Shen W, Ying J, Cheng F, Xu W. The prognostic role of the platelet-lymphocytes ratio in gastric Cancer: a meta-analysis. PLoS One. 2016;11:e0163719. doi: 10.1371/journal.pone.0163719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Subgroup analyses for overall survival. Subgroup analyses for overall survival based on study design, country, sample size, percent male, mean age, treatment strategy, disease status, cutoff value and study quality.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.