Abstract
Background:
Aging is a complex irreversible process that is not only related to an individual’s genetic make-up but also to lifestyle choices and environmental exposures. Like every other structure in human body, the Neuromuscular Junction (NMJ) is not averse to aging.
Objectives:
The prime objective is to analyse the microscopic and macroscopic changes at the NMJs with aging.
Methods:
For the purpose of review we evaluated data from resources like Pubmed, Ovid, UCLA libraries and USC libraries.
Results:
We review various morphological, physiological, immunological, and biochemical changes in NMJs with aging and their management.
Conclusion:
The alterations in NMJs secondary to aging are inevitable. It is vital that neurologists clearly understand the pathophysiology of NMJ aging and differentiate between physiological and pathological effects of aging. With the current knowledge of science, the changes in NMJ aging can be better prevented rather than cured.
Keywords: Neuromuscular junction, aging, atrophy, neurotransmitter, synapse, oxidative stress, sarcopenia
1. INTRODUCTION
Aging in peripheral nerves has been reported to resemble a “dying back” neuropathy in that the most severe and earliest age-related changes occur at the most distal levels of the nerve fibers. Although various intrinsic and extrinsic causes for aging have been identified, the exact molecular mechanisms remain elusive. Neuromuscular junction (NMJ) is a specialized unit in the peripheral nervous system between a motor nerve terminal and skeletal muscle fiber that includes three major components- the presynaptic terminal, the synaptic cleft and the post synaptic membrane. The presynaptic motor nerve terminal is specialized for neurotransmitter release. It is responsible for the synthesis, storage and release of neurotransmitter Acetylcholine (ACh) which is packed in vesicles. The synaptic cleft not only helps in chemical and electrical transmission of stimuli but also contains an enzyme Acetyl Cholinesterase (AChE) to terminate synaptic transmission. Lastly, the post synaptic membrane contains a high concentration of Acetylcholine Receptors (AChR) to transmit the signal via muscle contraction and relaxation. Aging of NMJ leads to muscle aging described as sarcopenia, the etio-pathogenesis and therapeutic options of which have been discussed in our previous review [1].
2. MATERIAL AND METHODS
For the purpose of review various database including Pubmed, Ovid, UCLA libraries and USC libraries was searched for relevant articles. The initial list of selected papers was enriched by individual suggestions of the authors of the present review.
3. HISTORY
The history of NMJ dates back centuries when “Curare”, popularly known as “Flying Death”, was used by South American Indians to hunt. It was only in 1846 that a French physiologist Claude Bernard concluded that curare acted peripherally at the NMJ and established the cellular basis of synaptic transmission. In 1897, Sherrington proposed the term ‘synapse’ in the Foster's textbook of physiology to designate a particular neuronal connection in the spinal reflex arc without any morphological evidence or being able to determine whether the connection was continuous or not. The notion of synapse later contributed to the concept of chemical transmission with adrenalin and acetylcholine (Ach). It was René Couteaux who gave vital morphological basis to the concept of synapse. More recently, Sir John Eccles and Sir Bernard Katz were honored with the Nobel Award of 1963 and 1970, respectively for their immense contribution to the field of synaptology. Finally, in 1997, Rosenberg was the first one to use the term Sarcopenia as an aging-related loss of muscle mass [2].
4. DEVELOPMENT AND GENETICS
The synaptic development is composed of three cells: The Motor neuron, the myofiber, and the Schwann cell, each of which arises from three different regions of the growing embryo. The myofibers are derived from individual myoblasts which originate in the mesoderm and fuse to form a multi-nucleated myotube. Motor neurons arise from the neural tube and initiate preliminary contact with the myotube. Schwann cells arise from the neural crest and are led by the axons to their destination where they form a covering over the innervating axons. The movement of these axons is guided by the growth cone, a filamentous projection of the axon that actively searches for neurotrophins released by the myotube [3]. Meanwhile, the microscopic development of NMJ is a rather complex phenomenon which involves a few presynaptic factors like Ach and agrin; few postsynaptic receptors and proteins like AChR, Muscle Specific tyrosine Kinase (MuSK), rapsyn, Downstream of kinase 7 (Dok7) and low-density Lipoprotein receptor related protein 4 (Lrp4). A genetic or acquired abnormality of any of these factors may lead to NMJ disorders. When the motor-nerve growth cone comes in contact with the muscle it induces a narrow, distinct endplate zone in the mid-muscle that is marked by a high density of AChR clustering. MuSK plays a vital role in clustering of AChR by a process called pre-patterning during development of the postsynaptic membranes. In this process, the presynaptic agrin binds with Lrp4 to activate MuSK [4, 5]. Upon activation by its ligand agrin, MuSK signals via two proteins called Dok-7 and rapsyn, to induce clustering of AChRs. The release of agrin from presynaptic nerve terminal not only stimulates AChRs clustering but also aids in selecting only those AChRs that are opposite to the nerve terminal on the postsynaptic membrane. The AChRs that are not stabilized by agrin are later dispersed [6]. Based on the differences in AChR cluster formation and opposition of nerve terminals and Schwann cells, there are two different patterns of NMJ formation. In Fast synapsing muscles (e.g. tibialis anterior or extensor digitorum longus), a mature NMJ is formed within one day whereas in Delayed synapsing muscles (e.g. diaphragm or soleus), this process requires up to five days. A genetic mutation of MuSK, AChR can cause Congenital Myasthenic Syndrome (CMS) and an acquired deficiency can cause Myasthenia Gravis (MG) and Lambert-Eaton Myasthenic Syndrome (LEMS). Butikofer et al. reported that removal of neural agrin from synaptic basal lamina by neurotrypsin results in the early fragmentation of NMJ [7]. NMJ fragmentation and subsequent denervation seem to substantially contribute to sarcopenia and hence, proteins involved in NMJ signaling, such as Lrp4, MuSK or Dok7 are interesting targets. Thus, compounds that locally enhance NMJ signaling might be able to stabilize NMJs and prevent their fragmentation as seen in Fig. (1) [8].
Fig. (1).
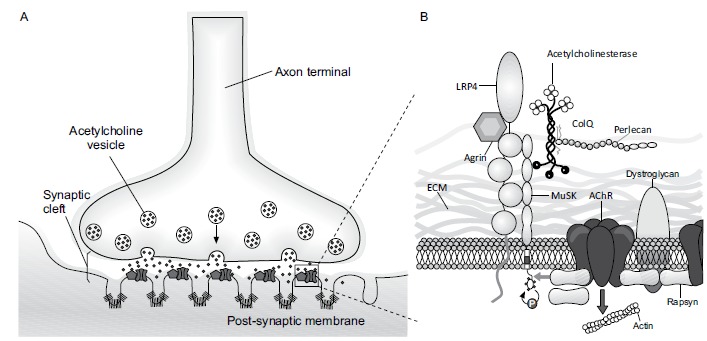
Neuromuscular Junction with associated proteins.
5. MORPHOLOGY
The age associated morphological changes in NMJ occur at both pre and postsynaptic levels. Furthermore, there are several theories about morphological changes with aging. Some studies suggest that morphological changes are confined to nerve endings with little or no degeneration or loss of primary axons, and that aging is associated with a functional denervation. On the other hand, there are studies suggesting that age-related changes in NMJ morphology vary among different muscle types and could potentially be related to muscle activity levels [9]. In rat models, it was demonstrated that NMJs become smaller and less abundant with aging [10]. At presynaptic levels, the nerve terminals exhibit abnormal thinning, distension and sprouting. Although the motor axons compensate for the loss of synaptic sites by sprouting and adding new junctions, this compensatory effect diminishes dramatically with aging. At the postsynaptic level, the end plates decrease in size and reduce in number, length and density of postsynaptic folds. There is a gradual decrease in the number of ACh receptors per junction and postsynaptic endplates are severely fragmented [11]. A few other cell types such as terminal Schwann cells which play an important role in axonal guidance and synaptic repair following denervation, may also contribute to a functional decline of the neuromuscular system in aging. There are several possible etiologies for NMJ fragmentation with aging. First theory is related to the degeneration of muscle fiber segments underlying the synapse. The other possible etiology of NMJ fragmentation with aging can be explained by the appearance of neuronal lesions or complete motor neuron death leading to muscle fiber denervation followed by re-innervation of the same post-synaptic apparatus by a new neuronal sprout from neighboring neurons. Finally, the rearrangement of molecular events could lead to a slow and constant decay of synaptic regions with age progression [12, 13].
6. ELECTRON MICROSCOPY
Electron microscopy has helped to provide detailed insight into the morphological transformations of NMJs. The morphology of Subneural Apparatuses (SNAs) at neuromuscular junctions was first examined in 1999 by scanning electron microscopy in aged rats. It was reported that most of the aged SNAs were mainly characterized by a large number of cup-like depressions with numerous slit-like junctional folds. Additionally, numerous slits were often found outside the depressions [14]. Ultrastructural study of NMJ of Drosophila melanogaster showed a qualitative and quantitative increase in mean synaptic bouton size during adult life with the largest boutons present in the aged fly. The subsynaptic reticulum became progressively thinner, and naked boutons were found in aged flies. These traits indicate an age-associated increment in autophagy, larger synaptic vesicles, and impaired endocytosis [15]. Ultrastructural findings of the presynaptic terminal show age-related decline in synaptic vesicles, mitochondrial content, and nerve terminal area in mice models. On the postsynaptic membrane, there is an increase in the number of branched sub-synaptic folds, an increased number of sub-sarcolemmal vesicles, as well as increased lipofuscin deposits.
On the other hand, there is a prominent change in mitochondrial structure with NMJ aging in axon terminals, including cristae disruption, swelling and appearance of mega-mitochondria due to multiple fusion between adjacent mitochondria [16]. The large number of mitochondria in the nerve ending play an important role in providing energy and buffering of calcium ions. Both are necessary factors for excitation-contraction coupling, and thus a decrease in the number and function of mitochondria with aging may disrupt both neurotransmission and vesicular recycling.
Based on the evidence from light microscopy, there was an increase in nerve terminal length and number of intra-synaptic branches, with no change in muscle fiber diameter or numbers of axons entering the junction in aged mice. When visualized by scanning electron microscopy, motor endplates appear slightly elevated, and the elliptical plateaux ('raised areas') with smooth surfaces into which the synaptic clefts are etched. The primary clefts are often interrupted by narrow short out pouchings approximately perpendicular to the long axis of the primary cleft. The oval primary cleft islets are more frequent and there is increased randomness and branching of secondary clefts. Additionally, both light and scanning microscopy give concordant quantitative evidence that nerve terminals and the underlying postsynaptic cleft are longer and more branched in aged mice. The observed expansion of the synaptic area in the aged neuromuscular junction may be compensatory, trying to preserve neuromuscular function. The cumulative result of these mentioned factors points toward the plasticity of adult neuromuscular synaptic structure [17].
7. HUMAN STUDIES
Based on the morphometric analysis the postsynaptic area and postsynaptic membrane length is significantly greater and the number and/or depth of the secondary synaptic clefts of adults is greater than those of infants. In the aged subjects however, presynaptic membrane length and postsynaptic membrane density showed a significant decline which could be due to the regressive changes of pre-and postsynaptic structure with aging. A large variation is found in the postsynaptic area, postsynaptic membrane length, and membrane length ratio of adults and aged group [18]. A light microscopic study of end plates and related structures in external intercostal muscles of humans aged between 4 and 77 years showed that although the end plates maintained the same size they did not increase in number and showed no sprouting of terminal axons. On electron microscopy the end plates became more complex mainly at the postsynaptic side with increased length and branching of the postsynaptic membrane and enlargement of the postsynaptic area, and degeneration of junctional folds. NMJs in the aged showed irregularly shaped presynaptic nerve terminals with little branching of the postsynaptic membrane. The Schwann cell processes were seen intruding into the primary synaptic cleft. It was found that degeneration of postsynaptic membrane with consequent focal denervation of NMJs is a primary event in the age-related changes of end plates. The muscle fibers showed a minor degree of type grouping in old age owing to the loss of motor neurons with age [19].
8. PHYSIOLOGY
There are several studies targeted towards understanding the physiological changes in NMJ with aging. It was reported that aged rats have a marked synaptic depression at the neuromuscular junction as compared to the young. This is commonly associated with smaller quantities of ACh released per action potential and onset of presynaptic conduction block at lower frequencies of nerve stimulation. As the neuromuscular function declines during aging it has been suggested that the muscle tone may be preserved by increasing the release of Ach. Interestingly, some recent trials using mice models have also demonstrated that increasing cholinergic transmission at the NMJs accelerates the degeneration of the NMJs. Also, in the aged rats the membrane potentials are lower, and the rate of spontaneous transmitter release is faster. One or more of these differences between aged and young rats could be explained by age-related differences in the extent of K+ accumulation during repetitive nerve stimulation. It was found that diffusion of K+ away from the end plate is probably slower in aged than in young rats [20]. Smith et al. tried to study the conduction velocity and synaptic delay of the motoneurons using electrophysiologic techniques. They observed that the speed of conduction did not change with age, but the average synaptic delay increased. They also observed that the total extracellular space (synaptic cleft widths) was about 32% larger in the aged rats [21]. Neuromuscular electrical stimulation was found to reduce age-related changes in NMJ morphology by restoring the relationship between pre- and post- synaptic volumes.
9. BIOCHEMICAL
NMJ is known to undergo several biochemical alterations during aging. Postsynaptic activation of calcium/calmodulin-dependent protein kinase ll was found to promote coordinated pre- and postsynaptic maturation of drosophila neuromuscular junctions [22]. A muscle-specific intermediate filament protein, desmin is found to be essential for maintaining the complex folded structure of the postsynaptic apparatus of the neuromuscular junctions. Studies suggest the destabilization of the NMJ through proteolytic cleavage of agrin at the onset of a pathogenic pathway ending in sarcopenia [7]. Acute blockade of signaling through the Tyrosine kinase receptor B (TrkB) attenuates neuromuscular transmission and fragments postsynaptic AChRs in adult mice, suggesting that TrkB signaling may impact the aging process of sarcopenia [23]. Studies have identified APP/APLP2 as key regulators of structure and function of developing neuromuscular synapses by ensuring proper Ca channel function.
The role of oxidative stress in NMJ aging is not well known. The decrease of the life span in studied mice mostly related to the pathologies the mice developed, thus revealing that oxidative stress may not affect aging directly, but rather effect the progression of disease as an environmental factor [24]. For instance, homozygous deletion of Cu, Zn superoxide dismutase causes a prominent NMJ degeneration similar to that observed in old wild type sarcopenic animals.
When Graus et al. investigated the influence of age on the induction of Experimental Autoimmune Myasthenia Gravis (EAMG) they discovered that the postsynaptic membrane in aged rats is resistant to autoantibody attack and that the lack of AChR degradation by antigenic modulation in aged rats is due to altered AChR density and distribution or rigidity of the postsynaptic membrane and not macrophage related [25]. Later, based on the immunological and electrophysiological data, it was postulated that the age-related susceptibility to EAMG is influenced by anti-rat AChR antibody titers. The possible role of CD44 in ALS-induced NMJ pathology was established using high resolution morphological methods, tissue fractionation and RT-PCR [26].
On the other hand, at the molecular level, cytokines, Neutrophin-3 (NT-3), Neutrophin-4 (NT-4), Brain Derived Neurotrophic Factor (BDNF), Glia-cell Derived Neurotrophic Factor (GDNF) and other growth factors can modulate NMJ aging [11]. For instance, BDNF improves synaptic function by increasing pre-synaptic depolarization at the NMJ. GDNF as a potent trophic factor for motor neuron survival might play a role in remodeling of the NMJ since a recent study shows increased GDNF protein levels at the end plate of soleus and extensor digitorum longus muscles following exercise [27]. Also, Insulin-like Growth Factor 1 (IGF-1) promotes motor neuron survival and protects NMJ from oxidative stress so decrease in IGF-1 level with aging may lead to NMJ degeneration and motor unit denervation [28].
Agrin-MuSK signaling pathway has an important role in clustering of Acetylcholine Receptors on the post-synaptic membrane and differentiation of NMJ and based on a recent study, proteolysis of agrin in transgenic mice leads to precocious aging of NMJ. In fact, increase in cleavage of agrin at NMJ to 90 and 22 kDa N- and C- terminal fragments by overexpression of neuronal neurotrypsin in transgenic mice can result in the same changes in NMJ structure seen with aging [29].
Laminins are a group of signaling and adhesin proteins found in the NMJ that help in aligning the pre and post synaptic specializations for effective neurotransmission across the NMJ. Numerous studies have highlighted the role played by Laminin-α4 in maintaining the morphology of NMJ [30]. Mice deficient in Laminin-α4 show higher percentage of misaligned NMJs with signs of premature aging such as fragmentation of Acetylcholine Receptors (AChRs), partial innervation and sprouting of axons. Similar changes were also seen in older Wild-type mice who were then found to have decreased laminin-α4 expression at the NMJ. These findings were supported by Lee et al. who recently reported altered Laminin-α4 expression prior to decline of neurotransmission in aging wild-type mice [31].
Wnt signaling pathway as a large group of secreted glycoproteins regulates the development and function of synapses and down regulation of this pathway with aging leads to the reduction in muscle regeneration and repair capacity [32].
Peroxisome Proliferator-activated receptor Gamma Coactivator 1-alpha (PGC-1 alpha) is a transcription factor that has a vital role in mitochondrial biogenesis and based on recent studies, decrease in PGC-1 alpha with aging may be one of the main mechanisms of NMJ instability at advanced age [33].
Muscle Ring Finger-1 (MuRF1) as the main enzyme at the protein degradation pathways may play an important role in NMJ integrity by modulation of AchR as shown in Fig. (2). A recent study implicates the significant effect of MuRF-1 in endplate membrane protein turnover during aging [34].
Fig. (2).
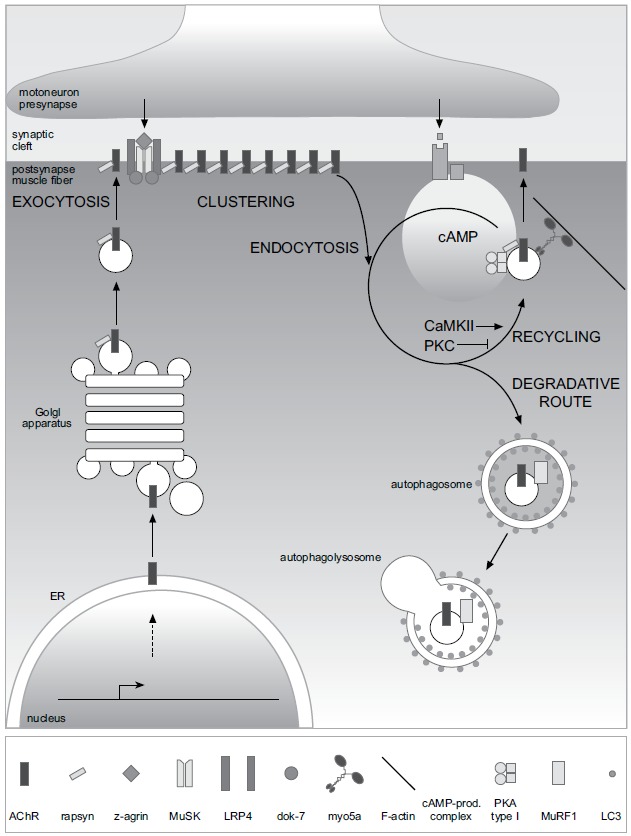
Figure from Rudolf et al. Frontiers Aging Neuroscience 2014 showing life cycle of AChR at the vertebrate NMJ.
Tyrosine kinase receptor B (TkrB) has an important role in regulation of NMJ function. A recent study on the soleus muscle of 6 to 8 months old TrkB+/- mice shows similar changes in NMJ structure compared with 24 months old wild-type mice, including expansion of post synaptic AChR area, AChR fragmentation and denervation. These results suggest an important role of reduced TkrB receptor signaling on NMJ aging [35].
Sirt1 is a Nicotinamide Adenosine Dinucleotide (NAD)-dependent deacetylase. It plays an important role in various physiological functions, including aging and neurogenesis [36]. Studies studying the role on Sirt1 have demonstrated delayed aging and extended life spans in mice with Brain-Specific Overexpression of Sirt1 (BRASTO) [37]. Similar findings were also seen in the recent study where the BRATSO mice were compared to Wild -Type (WT) control mice and showed less signs of aging when comparing NMJ components including terminal Schwann Cells (tSCs), nerve terminal, and AChRs. On the other hand, mice with Sirt1 knockdown in the dorsomedial hypothalamus of young mice showed more aged morphology of NMJ compared to controls.
10. TREATMENT
Based on the recent studies, certain types of physical exercise are effective in compensation of age-related decrease in muscle size and force. Significantly, life-long high- intensity physical exercise reduces the loss of motor unit numbers with aging. In fact, chronic exercise training has the similar effect on muscle by increasing the adaptation capacity of NMJ with aging. This remodeling of NMJ with physical training includes increase in the total length of nerve terminal branching, a greater total number of pre-synaptic Ach vesicles and a higher number of post-synaptic Ach receptors. In fact, a recent study on mice by Cheng et al. demonstrates that the morphological remodeling of NMJs with aging may be inhibited or even reversed by regular endurance type exercise [38].
The role of caloric restriction on age-related disorders has always been one of the most interesting fields for researchers. Based on one of the similar studies by Valdez et al., caloric restriction of mice from 4 to 24 months of age can lead to sparing of many NMJ in the tibialis anterior muscle with aging in comparison to control group. Also, NMJ fragmentations and denervation of post-synaptic sites were significantly reduced in caloric restricted group [39]. On the other hand, a recent study by Jang et al. concluded that dietary restriction decreases age associated atrophy by reducing oxidative stress in SOD1-/- mice and up-regulation Manganese Superoxide Dismutase (MnSOD), the main mitochondrial antioxidant enzyme [40].
Several therapeutic modalities have shown variable success in the management of NMJ aging. One such agent is Acetyl L Carnitine (ALCAR), the beneficial effects of which were reported by Clara de Angelis in the early 90s. ALCAR is known to play a vital role in the production of cellular energy via mitochondrial metabolism of fatty acids and ketone bodies. Subsequently, it creates conditions favorable for nerve metabolism by improving axonal flow with consequent restoration of nerve function. De Angelis and his team studied its morphological and physiological benefits in the soleus and extensor digitorum longus muscles of aging rats. A daily dose of 150 mg/kg/day of ALCAR for a period of 6 months physiologically restored the neuromuscular conduction velocities and morphologically increased the number of myelinated fibers with an increase in the complexity of NMJs. Additionally, it was demonstrated that treatment with ALCAR aids in preserving the biochemical properties of the neuronal membranes. Using Xenopus nerve-muscle co-cultures Wang CY et al. demonstrated that long-term application of Glial cell line-derived Neurotrophic Factor (GDNF) significantly increased the total length of neurites in the motoneurons. GDNF also caused an increase in the number and the size of synaptic vesicle clustering. Electro physiologically GDNF markedly increased the frequency of spontaneous transmission and decreased the variability of evoked transmission, suggesting an enhancement of transmitter secretion [41]. A decade ago, melatonin, the main hormone of the pineal gland, was proposed as a protective agent against macromolecular destruction associated with aging. Pedro J. Gomez-Pinilla et al. in 2006 reported the physiological benefits of melatonin in reverting age-related changes in guinea pig gallbladder neuromuscular transmission and contractility [42]. Nowadays, the role of diet and exercise is gaining significance in the management of several diseases and aging is no exception. Valdez et al. beautifully demonstrated the effect of caloric restriction and exercise in the attenuation of age-related morphological changes in mouse neuromuscular synapses [39]. Various chemical substances including resveratrol and metformin are known to produce effects similar to exercise and caloric restriction. Resveratrol has looked promising and was reported to slower down the effects of age-related changes at the NMJ in mice. Various studies in mice have also highlighted the potential role of gene therapy in delaying NMJ aging. Additionally, copper was found to prevent the synaptic defects in s- inclusion body myositis [43]. At a cellular level, free radicals like superoxides are known to be involved in the etiopathogenesis of aging. Their role in the aging of NMJ in particular, was recently demonstrated by Jang et al. thus suggesting a possibility of use of antioxidants in NMJ aging management [44].
CONCLUSION
Evaluation of NMJ dysfunction due to aging based on the observation of the pathophysiological, anatomical and biochemical alterations in human and animal studies, would certainly be the first and essential step in order to design scientific researches for the assessment of new preventive and therapeutic methods in this area. Despite the prominent progression in understanding of the NMJ morphological changes due to aging in recent years, such as remodeling of the motor unit and decrease in the number of the type 2 muscle fibers leading to excitation-contraction uncoupling, we still need more evidence to understand clearly pathophysiology behind the NMJ aging process by designing novel molecular and structural studies on both human and animals. Although current knowledge concludes that the NMJ aging process is better prevented than cured, extensive scientific researches in this area will definitely be our guide in searching for new treatments in the future.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Mishra S.K., Misra V. Muscle sarcopenia: an overview. Acta Myol. 2003;22(2):43–47. [PubMed] [Google Scholar]
- 2.Rosenberg I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997;127(5) Suppl.:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 3.Sanes J.R., Lichtman J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 4.Kim N., Stiegler A.L., Cameron T.O., Hallock P.T., Gomez A.M., Huang J.H., et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135(2):334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada K., Inoue A., Okada M., et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312(5781):1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- 6.Misgeld T., Kummer T.T., Lichtman J.W., Sanes J.R. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl. Acad. Sci. USA. 2005;102(31):11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butikofer L., Zurlinden A., Bolliger M.F., Kunz B., Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011;25(12):4378–4393. doi: 10.1096/fj.11-191262. [DOI] [PubMed] [Google Scholar]
- 8.Campanari M.L., García-Ayllón M.S., Ciura S., Sáez-Valero J., Kabashi E. Neuromuscular junction impairment in amyotrophic lateral sclerosis: Reassessing the role of acetylcholinesterase. Front. Mol. Neurosci. 2016;9:160. doi: 10.3389/fnmol.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenheimer J.L., Smith D.O. Differential changes in the end-plate architecture of functionally diverse muscles during aging. J. Neurophysiol. 1985;53(6):1567–1581. doi: 10.1152/jn.1985.53.6.1567. [DOI] [PubMed] [Google Scholar]
- 10.McMullen C.A., Andrade F.H. Functional and morphological evidence of age-related denervation in rat laryngeal muscles. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64(4):435–442. doi: 10.1093/gerona/gln074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang Y.C., Van Remmen H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011;46(2-3):193–198. doi: 10.1016/j.exger.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolf R., Khan M.M., Labeit S., Deschenes M.R. Degeneration of neuromuscular junction in age and dystrophy. Front. Aging Neurosci. 2014;6:99. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taetzsch T., Valdez G. NMJ maintenance and repair in aging. Curr Opin Physiol. 2018;4:57–64. doi: 10.1016/j.cophys.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oki S., Desaki J., Ezaki T., Matsuda Y. Aged neuromuscular junctions in the extensor digitorum longus muscle of the rat as revealed by scanning electron microscopy. J. Electron Microsc. (Tokyo) 1999;48(3):297–300. doi: 10.1093/oxfordjournals.jmicro.a023681. [DOI] [PubMed] [Google Scholar]
- 15.Beramendi A., Peron S., Casanova G., Reggiani C., Cantera R. Neuromuscular junction in abdominal muscles of Drosophila melanogaster during adulthood and aging. J. Comp. Neurol. 2007;501(4):498–508. doi: 10.1002/cne.21253. [DOI] [PubMed] [Google Scholar]
- 16.Garcia M.L., Fernandez A., Solas M.T. Mitochondria, motor neurons and aging. J. Neurol. Sci. 2013;330(1-2):18–26. doi: 10.1016/j.jns.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Fahim M.A., Holley J.A., Robbins N. Scanning and light microscopic study of age changes at a neuromuscular junction in the mouse. J. Neurocytol. 1983;12(1):13–25. doi: 10.1007/BF01148085. [DOI] [PubMed] [Google Scholar]
- 18.Arizono N., Koreto O., Iwai Y., Hidaka T., Takeoka O. Morphometric analysis of human neuromuscular junction in different ages. Acta Pathol. Jpn. 1984;34(6):1243–1249. doi: 10.1111/j.1440-1827.1984.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 19.Wokke J.H., Jennekens F.G., van den Oord C.J., Veldman H., Smit L.M., Leppink G.J. Morphological changes in the human end plate with age. J. Neurol. Sci. 1990;95(3):291–310. doi: 10.1016/0022-510x(90)90076-y. [DOI] [PubMed] [Google Scholar]
- 20.Smith D.O. Restricted diffusion of extracellular potassium at the neuromuscular junction of aged rats. Brain Res. 1982;239(2):668–673. doi: 10.1016/0006-8993(82)90548-0. [DOI] [PubMed] [Google Scholar]
- 21.Smith D.O., Rosenheimer J.L. Factors governing speed of action potential conduction and neuromuscular transmission in aged rats. Exp. Neurol. 1984;83(2):358–366. doi: 10.1016/S0014-4886(84)90104-3. [DOI] [PubMed] [Google Scholar]
- 22.Kazama H., Morimoto-Tanifuji T., Nose A. Postsynaptic activation of calcium/calmodulin-dependent protein kinase II promotes coordinated pre- and postsynaptic maturation of Drosophila neuromuscular junctions. Neuroscience. 2003;117(3):615–625. doi: 10.1016/s0306-4522(02)00923-5. [DOI] [PubMed] [Google Scholar]
- 23.Kulakowski SA, Parker SD, Personius KE. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. J Appl Physiol (1985) 2011;111(3):844-52. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- 24.Salmon A.B., Richardson A., Perez V.I. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic. Biol. Med. 2010;48(5):642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graus Y.M., Verschuuren J.J., Spaans F., Jennekens F., van Breda V.P.J., De Baets M.H. Age-related resistance to experimental autoimmune myasthenia gravis in rats. J. Immunol. 1993;150(9):4093–4103. doi: 10.1016/0896-8411(91)90111-o. [DOI] [PubMed] [Google Scholar]
- 26.Gorlewicz A., Wlodarczyk J., Wilczek E., Gawlak M., Cabaj A., Majczynski H., et al. CD44 is expressed in non-myelinating Schwann cells of the adult rat, and may play a role in neurodegeneration-induced glial plasticity at the neuromuscular junction. Neurobiol. Dis. 2009;34(2):245–258. doi: 10.1016/j.nbd.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 27.McCullough M.J., Peplinski N.G., Kinnell K.R., Spitsbergen J.M. Glial cell line-derived neurotrophic factor protein content in rat skeletal muscle is altered by increased physical activity in vivo and in vitro. Neuroscience. 2011;174:234–244. doi: 10.1016/j.neuroscience.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messi M.L., Delbono O. Target-derived trophic effect on skeletal muscle innervation in senescent mice. J. Neurosci. 2003;23(4):1351–1359. doi: 10.1523/JNEUROSCI.23-04-01351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drey M., Sieber C.C., Bauer J.M., Uter W., Dahinden P., Fariello R.G., et al. C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp. Gerontol. 2013;48(1):76–80. doi: 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Samuel M.A., Valdez G., Tapia J.C., Lichtman J.W., Sanes J.R. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PLoS One. 2012;7(10):e46663. doi: 10.1371/journal.pone.0046663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K.M., Chand K.K., Hammond L.A., Lavidis N.A., Noakes P.G. Functional decline at the aging neuromuscular junction is associated with altered laminin-alpha4 expression. Aging (Albany NY) 2017;9(3):880–899. doi: 10.18632/aging.101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatakou E., Salinas P.C. Postsynaptic assembly: A role for Wnt signaling. Dev. Neurobiol. 2014;74(8):818–827. doi: 10.1002/dneu.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gouspillou G., Picard M., Godin R., Burelle Y., Hepple R.T. Role of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha) in denervation-induced atrophy in aged muscle: Facts and hypotheses. Longev. Healthspan. 2013;2(1):13. doi: 10.1186/2046-2395-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franke B., Gasch A., Rodriguez D., Chami M., Khan M.M., Rudolf R., et al. Molecular basis for the fold organization and sarcomeric targeting of the muscle atrogin MuRF1. Open Biol. 2014;4:130172. doi: 10.1098/rsob.130172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Personius K.E., Parker S.D. TrkB expression at the neuromuscular junction is reduced during aging. Muscle Nerve. 2013;47(4):532–538. doi: 10.1002/mus.23616. [DOI] [PubMed] [Google Scholar]
- 36.Satoh A., Brace C.S., Rensing N., Cliften P., Wozniak D.F., Herzog E.D., et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder-Warwick A.K., Satoh A., Santosa K.B., Imai S.I., Jablonka-Shariff A. Hypothalamic Sirt1 protects terminal Schwann cells and neuromuscular junctions from age-related morphological changes. Aging Cell. 2018;17(4):e12776. doi: 10.1111/acel.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng A., Morsch M., Murata Y., Ghazanfari N., Reddel S.W., Phillips W.D. Sequence of age-associated changes to the mouse neuromuscular junction and the protective effects of voluntary exercise. PLoS One. 2013;8(7):e67970. doi: 10.1371/journal.pone.0067970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdez G., Tapia J.C., Kang H., Clemenson G.D., Jr, Gage F.H., Lichtman J.W., et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. USA. 2010;107(33):14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang Y.C., Liu Y., Hayworth C.R., Bhattacharya A., Lustgarten M.S., Muller F.L., et al. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell. 2012;11(5):770–782. doi: 10.1111/j.1474-9726.2012.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C.Y., Yang F., He X.P., Je H.S., Zhou J.Z., Eckermann K., et al. Regulation of neuromuscular synapse development by glial cell line-derived neurotrophic factor and neurturin. J. Biol. Chem. 2002;277(12):10614–10625. doi: 10.1074/jbc.M106116200. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Pinilla P.J., Camello-Almaraz C., Moreno R., Camello P.J., Pozo M.J. Melatonin treatment reverts age-related changes in Guinea pig gallbladder neuromuscular transmission and contractility. J. Pharmacol. Exp. Ther. 2006;319(2):847–856. doi: 10.1124/jpet.106.109256. [DOI] [PubMed] [Google Scholar]
- 43.Aldunate R., Minniti A.N., Rebolledo D., Inestrosa N.C. Synaptic defects associated with s-inclusion body myositis are prevented by copper. Biometals. 2012;25(4):815–824. doi: 10.1007/s10534-012-9553-7. [DOI] [PubMed] [Google Scholar]
- 44.Jang Y.C., Lustgarten M.S., Liu Y., Muller F.L., Bhattacharya A., Liang H., et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24(5):1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]


