Abstract
Background:
Short segment myelitis (SSM, < 3 vertebral segments) is an under-recognized initial manifestation of neuromyelitis optica spectrum disorders (NMOSD). Though infrequent, failure to recognize SSM in patients with NMOSD would lead to incorrect diagnosis and treatment. Therefore, delineation of features of NMOSD-associated SSM is of paramount importance.
Objective:
Our study aimed to determine the demographic, clinical and radiological features of NMOSD-associated SSM, and compare those with NMOSD-associated longitudinally extensive transverse myelitis (LETM) and multiple sclerosis (MS)-associated SSM, respectively.
Methods:
Chinese patients presenting initially only with acute myelitis and diagnosed with NMOSD (n = 46) and MS (n = 11) were included. Clinical, serological, imaging and disability data were collected. Mann–Whitney U test or two-tailed Fisher’s exact tests were used to analyse the data.
Results:
Of the 46 enrolled NMOSD patients, 34 (74%) collectively had 38 LETM lesions, while 12 (26%) had 14 SSM lesions. When compared with LETM, NMOSD presenting with SSM were more likely to have a delayed diagnosis and a lower level of disability at nadir during the first attack. T1-weighted imaging hypointensity was more prominent in NMOSD-associated LETM lesions than NMOSD-associated SSM lesions. When compared with MS-associated SSM, NMOSD-associated SSM lesions were more likely to be centrally located, grey matter involving and transversally extensive on axial imaging and spanned no less than 2 vertebral segments on sagittal imaging.
Conclusion:
These findings suggest that SSM does not preclude the possibility of a NMOSD diagnosis. Testing for serum aquaporin-4 immunoglobulin G (AQP4-IgG) and careful study of lesions on spinal cord magnetic resonance imaging could aid in an earlier and correct diagnosis.
Keywords: aquaporin-4 immunoglobulin G, longitudinally extensive transverse myelitis, multiple sclerosis, neuromyelitis optica spectrum disorders, short segment myelitis
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory demyelinating disease affecting the central nervous system and is associated with aquaporin-4 immunoglobulin G (AQP4-IgG). Longitudinally extensive transverse myelitis (LETM), defined as myelitis with a continuous spinal cord lesion extending the length of 3 or more vertebral segments by spinal cord magnetic resonance imaging (MRI), is one of the most characteristic manifestations,1–4 and an important part of the latest diagnostic criteria for NMOSD.5 Short segment myelitis (SSM), defined as spinal cord lesions extending fewer than 3 vertebral segments, was considered non-characteristic of NMOSD and more common in multiple sclerosis (MS).6 However, though about 85% NMOSD-associated myelitis patients present with LETM,7–9 some did present with SSM.10–13 Controversy remains as to whether AQP4-IgG should be tested when SSM is the only initial presentation.
Features of NMOSD-associated SSM are less well characterized, compared with what we already know about MS-associated SSM and NMOSD-associated LETM. The under-recognition and lack of knowledge, as a result, lead to difficulty in diagnosis and delay in treatment for NMOSD patients presenting with SSM. The diagnostic process becomes more elusive when SSM is only initial manifestation of NMOSD.
Herein, our study aimed to delineate the demographic, clinical and radiographic features of SSM as the only presenting phenotype of NMOSD. We also compared NMOSD-associated SSM with NMOSD-associated LETM and MS-associated SSM, respectively, hoping to provide clues for further differential diagnosis.
Patients and methods
We retrospectively identified NMOSD and MS patients presenting with acute myelitis as the initial manifestation between January 2013 and December 2018 from the Second Affiliated Hospital School of Medicine Zhejiang University. Recruited NMOSD patients needed to meet the following criteria: (a) final diagnosis NMOSD based on the 2015 International Consensus Diagnostic Criteria and exclusion of alternative diagnoses,5 (b) acute myelitis should be the only core clinical characteristic of the first attack (without definite optic neuritis, area postrema syndrome, acute brainstem syndrome, acute diencephalic clinical syndrome or symptomatic cerebral syndrome), without a prior history of neurological symptoms or signs, (c) a spinal cord MRI performed within 30 days of myelitis onset, before high-dose steroids, (d) normal brain MRI or nonspecific lesions in the subcortical or deep white matter on the first attack, without corresponding symptoms or signs. Recruited MS patients needed to meet the following criteria: (a) final diagnosis MS based on the 2017 revision of McDonald criteria for MS,14 (b) acute myelitis should be the only clinical syndrome of the first attack, without a prior history of neurological symptoms or signs, (c) a spinal cord MRI performed within 30 days of myelitis onset, before steroids, (d) normal brain MRI or nonspecific lesions in the subcortical or deep white matter on the first attack, without corresponding symptoms or signs. This study was subject to approval by the ethics committee of the Second Affiliated Hospital School of Medicine Zhejiang University (approval number: 2019-082).
The serostatus of AQP4-IgG and myelin oligodendrocyte glycoprotein IgG (MOG-IgG) was confirmed by cell-based assay. All spinal cord MRI of the first attack was performed using a 1.5T scanner with or without contrast. Medical records were used to collect demographic, clinical (sex, age at onset and nadir disability) and laboratory parameters (white cell count, protein, and oligoclonal bands (OCBs)of cerebrospinal fluid (CSF) and autoantibodies for screening coexisting autoimmunity) at the time of the first attack. Nadir disability was measured with Expanded Disability Status Scale (EDSS) at the time of the initial myelitis episode. All the spinal cord MRIs were independently rated by 2 neurologists blinded to each other’s findings. When the nature of the lesions could not be established, a third experienced neurologist would re-evaluate and a final consensus was reached. EDSS scores were assessed independently by 2 neurologists.
Spinal cord MRI data were collected as follows: the length of each spinal cord lesion (measured as the number of vertebral segments over which each lesion extended); axial T2-weighted imaging (the axial plane with the largest lesion); gadolinium-enhanced T1-weighted spinal cord scans, if available. Lesions visually occupying half of the spinal cord area on axial T2-weighted imaging were defined as transversally extensive lesions. NMOSD patients included were divided into 2 groups based on the length of spinal cord lesion. Delay to diagnosis was defined as the NMOSD diagnosis not being made during the hospitalization for the first myelitis attack. We compared the clinical features of patients from the 2 groups and the imaging features of NMOSD-associated SSM and NMOSD-associated LETM lesions. We also compared the demographic, clinical and radiological characteristics of patients of NMOSD with SSM and MS with SSM.
Descriptive summary statistics were reported as median (range, minimum-maximum) for continuous variables and frequencies for categorical variables. Comparisons were performed using Mann–Whitney U test or two-tailed Fisher’s exact tests as appropriate using SPSS v.22.0 (IBM®).
Results
We reviewed 137 Chinese NMOSD patients with a disease onset between 2013 and 2018 in our centre. A total of 46 (33.6%) patients with acute myelitis as the only presenting feature of NMOSD met our inclusion criteria and were included in this study. Of these, 34 patients had only LETM, while 12 patients had only SSM (Figure 1). 41 patients were diagnosed as NMOSD during the hospitalization for the first myelitis attack and 5 patients had a delayed diagnosis. Serum AQP4-IgG was positive and MOG-IgG was negative for all 46 patients. 22 patients were tested for OCBs and all of them reported negative. A total of 6 out of the 46 patients had an accompanying systemic autoimmune disease, which was Sjögren’s syndrome in all 6 cases.
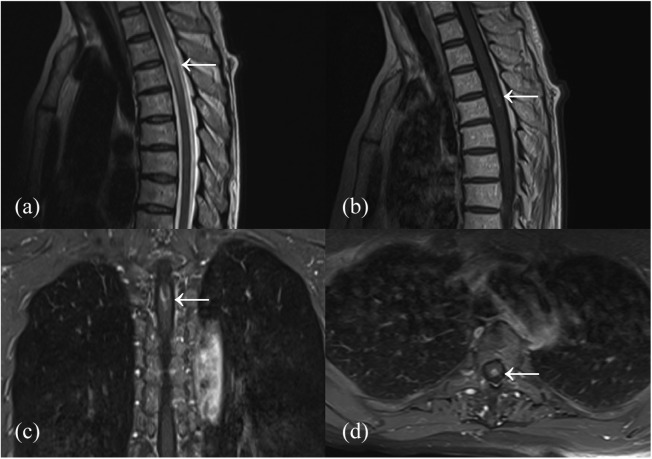
Figure 1.
A NMOSD-associated SSM lesion extending the length of 2 segments. T2-weighted imaging showing SSM lesion with swelling (a). Part of the SSM lesion showing enhancement on T1-weighted post-gadolinium images (b–d).
NMOSD, neuromyelitis optica spectrum disorder; SSM, short segment myelitis.
A total of 12 patients were included in the SSM group while 34 patients were included in the LETM group. Table 1 shows the comparison of demographic, clinical and laboratory features between NMOSD patients of SSM group and LETM group. The age of NMOSD onset, female-to-male ratio, interval from symptom onset to spinal cord MRI were similar for NMOSD patients in both groups. When compared with NMOSD-associated LETM patients (median EDSS: 3.75, range: 1–8.5), patients presenting with NMOSD-associated SSM had lower disability scores at nadir of first attack (median EDSS: 2, range: 1–7.5, p = 0.045). Furthermore, patients of SSM group were more likely to be delayed in diagnosis (4 of 12) than those in the LETM group (1 of 34) (p = 0.013). In addition, the CSF protein level in the SSM group was lower than that of the LETM group (p = 0.006). Other laboratory results were comparable between the 2 groups, including CSF white cell count and serum autoimmune antibodies.
Table 1.
Comparison of features between NMOSD patients of SSM group and LETM group.
| SSM group (n = 12) | LETM group (n = 34) | p value | |
|---|---|---|---|
| Age of onset, years, Median (range) | 58 (43–79) | 50.5 (19–81) | 0.271 |
| Female-to-male ratio | 2:1 | 7.5:1 | 0.178 |
| Time to baseline MRI, days, Median (range) | 15 (3–28) | 8 (1–28) | 0.249 |
| EDSS score at nadir of first attack, Median (range) | 2 (1–7.5) | 3.75 (1–8.5) | 0.045 |
| Delayed in diagnosis,* no. (%) | 4/12 (33%) | 1/34 (3%) | 0.013 |
| CSF white cell count (/μl), Median (range) | 5.5 (2–30) | 7 (1–160) | 0.566 |
| CSF protein (mg/dl), Median (range) | 34.8 (19.2–51.5) | 48.65 (26.3–138.3) | 0.006 |
| CSF oligoclonal bands, no. (%) | 0/7 (0%) | 0/15 (0%) | – |
| Positive serum autoimmune antibodies, no. (%) | 9/12 (75%) | 25/34 (74%) | 1.000 |
| Co-existing systemic autoimmune disease, no. (%) | 1/12 (8%) | 5/34 (15%) | 1.000 |
CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; LETM, longitudinally extensive transverse myelitis; MRI, magnetic resonance imaging; NMOSD, neuromyelitis optica spectrum disorder; SSM, short segment myelitis.
Delayed in diagnosis was defined as failure in making the NMOSD diagnosis during the hospitalization for the first myelitis attack.
A total of 38 LETM lesions (median length of 6 segments, range: 3–13) and 14 SSM lesions (median length of 2 segments, range: 1–2.5) were identified (shown in Table 2). In those with LETM lesions, 30 patients had 1 LETM lesion while 4 had 2 LETM lesions. In those with SSM lesions, 10 patients had 1 SSM lesion while 2 had 2 SSM lesions. On T1-weighted imaging (T1WI), there were more lesions with T1WI hypointensity in the LETM group than the SSM group. On sagittal imaging, the spinal cord lesions were predominantly located in cervical and thoracic cord in both groups. On axial imaging, both SSM and LETM lesions were predominantly located in the central cord area. In addition, both SSM and LETM lesions involved the central grey matter, even those not as centrally located.
Table 2.
Comparison of features between NMOSD-associated SSM lesions and NMOSD-associated LETM lesions.
| SSM lesions (n = 14) | LETM lesions (n = 38) | p value | |
|---|---|---|---|
| Median length, Median (range) | 2 (1–2.5) | 6 (3–13) | <0.001 |
| T1WI hypointensity, no. (%) | 1/14 (7%) | 18/38 (47%) | 0.009 |
| Sagittal imaging | |||
| cervical cord involved only, no. (%) | 6/14 (43%) | 11/38 (29%) | 0.506 |
| thoracic cord involved only, no. (%) | 8/14 (57%) | 16/38 (42%) | 0.366 |
| expanded over cervical and thoracic cord, no. (%) | 0/14 (0%) | 11/38 (29%) | 0.025 |
| Axial imaging* | |||
| Centrally located, no. (%) | 11/14 (79%) | 33/38 (87%) | 0.666 |
| Transversally extensive lesions, no. (%) | 12/14 (86%) | 34/38 (89%) | 0.655 |
| Grey matter involved, no. (%) | 14/14 (100%) | 38/38 (100%) | – |
| Grey matter, lateral columns and dorsal columns involved simultaneously, no. (%) | 12/14 (86%) | 33/38 (87%) | 1.000 |
| Enhancement on T1WI post-gadolinium images, no. (%) | 7/14 (50%) | 23/33 (70%) | 0.320 |
| Bright spotty lesions, no. (%) | 4/14 (29%) | 18/38 (47%) | 0.344 |
LETM, longitudinally extensive transverse myelitis; NMOSD, neuromyelitis optica spectrum disorder; SSM, short segment myelitis; T1WI, T1-weighted imaging.
If more than 1 lesion of the same patient were available, we analysed the axial plane with the largest lesion.
Gadolinium-enhanced images were available in 33 LETM lesions, of which 23 (70%) showed various degrees of enhancement (3 with ring enhancement). All the 14 SSM lesions completed gadolinium-enhanced spinal cord MRI and 7 (50%) presented as contrast-enhanced lesions (1 with ring enhancement). No significant differences were found (23/33 versus 7/14, p = 0.320).
During follow-up, five (5/12) NMOSD patients in the SSM group underwent relapses. One as LETM (Figure 2, delayed in diagnosis), another as LETM plus area postrema syndrome (delayed in diagnosis) and the remaining 3 as SSM (2 of them delayed in diagnosis).
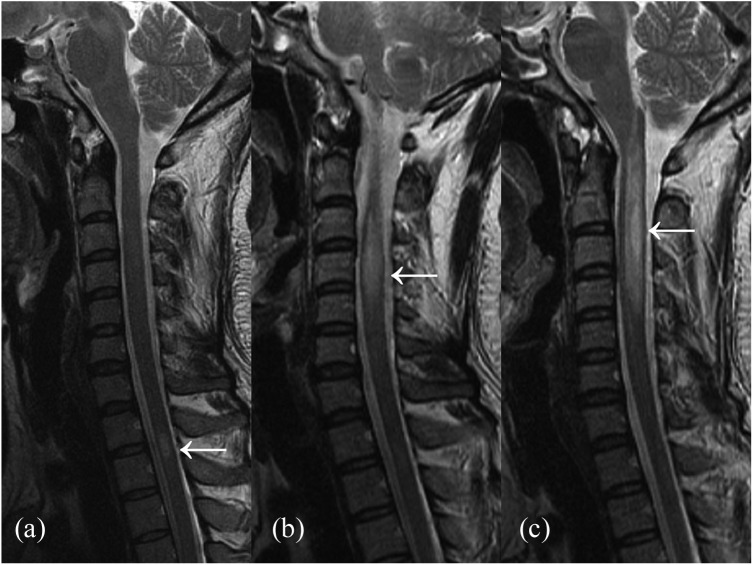
Figure 2.
The spinal cord lesions of an AQP4-IgG positive NMOSD patient’s first attack (a), second attack a year later (b) and third attack 22 months later (c) on T2-weighted imaging. The lesions of first and second attack were SSM lesions when it converted to LETM lesion when the third attack.
AQP4-IgG, aquaporin-4 immunoglobulin G; LETM, longitudinally extensive transverse myelitis; NMOSD, neuromyelitis optica spectrum disorder; SSM, short segment myelitis.
In addition, among the 82 patients diagnosed with MS according to the 2017 McDonald criteria, 11 (23%) presented with acute myelitis (all of them had SSM) during the first attack (shown in Table 3). Compared with MS patients, NMOSD-associated SSM had an older age of onset, lower incidence of positive CSF OCBs, a higher rate of both AQP4-IgG positivity and systemic autoimmune antibody positivity. Radiographically, NMOSD-associated SSM tended to have spinal cord lesions spanning no less than 2 vertebral segments on sagittal imaging and more likely to be centrally located, grey matter involving and transversally extensive on axial imaging (Figure 3).
Table 3.
Comparison of features between patients of MS-associated SSM and NMOSD-associated SSM.
| NMOSD-associated SSM (n = 12) | MS-associated SSM (n = 11) | p value | |
|---|---|---|---|
| Age of onset, years, Median (range) | 58 (43–79) | 30 (22–49) | <0.001 |
| Female-to-male ratio | 2:1 | 5:6 | 0.414 |
| Interval symptom onset to MRI, days, Median (range) | 15 (3–28) | 18 (7–30) | 0.211 |
| EDSS score at nadir of first attack, Median (range) | 2 (1–7.5) | 2 (1–4) | 0.928 |
| Serum AQP4-IgG, no. (%) | 12/12 (100%) | 0/11 (0%) | <0.001 |
| CSF white cell count (/μl), Median (range) | 5.5 (2–30) | 4 (0–8) | 0.203 |
| CSF protein (mg/dl), Median (range) | 34.8 (19.2–51.5) | 28.7 (25.5–56.2) | 0.180 |
| CSF oligoclonal bands, no. (%) | 0 /7 (0%) | 7/10 (70%) | 0.010 |
| Serological positive of autoimmune antibodies, no. (%) | 9/12 (75%) | 3/11 (27%) | 0.039 |
| Co-existing systemic autoimmune disease, no. (%) | 1/12 (8%) | 0/11 (0%) | 1.000 |
| Multifocal lesions, no. (%) | 2/12 (17%) | 5/11 (45%) | 0.193 |
| Lesions spanned ⩾2 vertebral segments,* no. (%) | 10/12 (83%) | 3/11 (27%) | 0.012 |
| T1WI hypointensity, no. (%) | 1/12 (8%) | 1/11 (9%) | 1.000 |
| Axial imaging$ | |||
| Centrally located, no. (%) | 10/12 (83%) | 3/11 (27%) | 0.012 |
| Transversally extensive lesions, no. (%) | 10/12 (83%) | 4/11 (36%) | 0.036 |
| Grey matter involved, no. (%) | 12/12 (100%) | 6/11 (55%) | 0.014 |
| Grey matter, lateral columns and dorsal columns involved simultaneously, no. (%) | 10/12 (83%) | 5/11 (45%) | 0.089 |
| Enhancement on T1WI post-gadolinium images, no. (%) | 7/12 (58%) | 5/11 (45%) | 0.684 |
| Bright spotty lesions, no. (%) | 4/12 (33%) | 0/11 (0%) | 0.093 |
AQP4-IgG, aquaporin-4 immunoglobulin G; CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; SSM, short segment myelitis; T1WI, T1-weighted imaging
If the patient had more than 1 lesion, we only analysed the image of the longest one.
If more than one axial image of the same patient was available, we analysed the axial plane with the largest lesion.
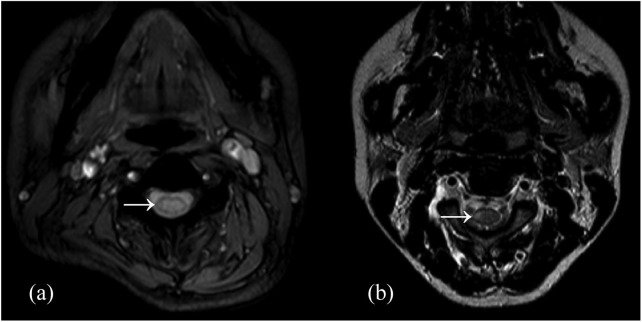
Figure 3.
The spinal cord lesions of NMOSD-associated SSM lesion (a) and MS-associated SSM lesion (b) on T2-weighted imaging. NMOSD-associated SSM lesion was more likely to be centrally located, grey matter involving and transversally extensive on axial imaging.
MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; SSM, short segment myelitis.
Discussion
Myelitis is a common initial presentation of both NMOSD and MS. In clinical practice, neurologists tend to associate LETM with NMOSD and SSM with MS.1–4 However, SSM may also be the presenting feature of NMOSD, and not so rare.10–12 In our study, among the 137 NMOSD patients, 46 presented with acute myelitis. Notably, 12 of them had SSM as the only presenting feature, accounting for 8.8% among all NMOSD patients and 26.0% among NMOSD presenting as myelitis. Previous studies reported 7.3–19.8% of NMOSD patients had SSM, either as one of the presenting feature or a symptom developed later in the disease course.10–13 When accompanied by other manifestations, a correct diagnosis of NMOSD can be facilitated by clues apart from the myelitis. However, when presented as the first and only symptom of NMOSD, SSM may very likely lead to misdiagnosis and a delay in treatment. For this reason, the present study highlights a population of NMOSD patients who presented with SSM as the initial and only manifestation of NMOSD. To our knowledge, this is the first study targeting on this population of NMOSD patients. From a demographic, clinical and radiological perspective, our study fully delineated SSM as a possible but under-recognized initial presentation of NMOSD, aiming to prompt an early diagnosis and correct treatment for this group of patients.
Acute myelitis is a frequent presentation in neurology, though the search for the underlying diagnosis has never been straightforward. For patients with LETM, it is not hard to think of NMOSD as a potential diagnosis, thus prompting further investigations of AQP4-IgG. SSM, on the other hand, often obviates our diagnosis elsewhere, most commonly MS. However, this is not always the case. SSM could also be the first and only symptom of NMOSD, posing a great diagnostic challenge in the clinical practice. In our study, 4 out of 12 patients in the SSM group were not correctly diagnosed during the first admission, much more than that in the LETM group (1/34, p = 0.013). Previous studies also found misdiagnosis and delayed treatment common in NMOSD patients presenting with SSM. In our centre, AQP4-IgG was not routinely performed in patients with SSM, and the decision was left to the discretion of the treating physician. Specifically, AQP4-IgG testing would be more likely ordered in SSM patients with a high EDSS score (⩾6) or an accompanying systemic autoimmune disease. This is based on the rationale that myelitis in NMOSD, compared with that in MS, generally have a higher level of disability and tend to have an accompanying systemic autoimmune disease.15 Notably, of the 4 NMOSD patients in the SSM group who were delayed in diagnosis, 3 of them were not tested for AQP4-IgG initially. The remaining 1 patient was AQP4-IgG-negative during the first admission and turned AQP4-IgG-positive in a relapse 3 years later. Accordingly, investigation for serum AQP4-IgG is still important for acute SSM patients even without optic neuritis, area postrema syndrome or accompanying systemic autoimmune disease.
Apart from AQP4-IgG, other clinical and paraclinical investigations may aid in a correct diagnosis for SSM patients. Clinically, patients of the SSM group had a lower degree of disability than these of the LETM group, consistent with previous studies.11 In addition, the CSF protein level was lower in the SSM group, when compared with NMOSD patients presenting as LETM. Radiologically, LETM lesions were more likely to show T1WI hypointensity than SSM lesions, possibly associated with a more severe damage to the spinal cord. The sagittal involvement, axial location and enhancement mode showed no significant difference between SSM lesions and LETM lesions in our study. Inconsistent with our study, Hu and colleagues12 found that LETM lesions were more likely to be centrally located than SSM lesions on axial image. However, their study aimed at a broader group of patients, without excluding those accompanied by other clinical characteristics apart from myelitis, which may lead to the different conclusions. One more thing to note is that SSM lesions may convert to LETM as the disease progresses, either within the first attack or in future relapses, as previous studies have suggested.12,16,17 SSM can be a transient stage in the development of LETM in NMOSD.16 By contrast, spinal cord lesions will become shorter during remission or after treatment with high-dose steroids, or in patients having an attack while receiving immunosuppressant therapy.10,18 For this reason, spinal cord MRI after high-dose steroids or other immunosuppressant therapy was excluded in our study.
Though difficult, it is important to differentiate between NMOSD from MS for patients with a demyelinating attack, given the distinct treatment for the two diseases.19–21 When SSM is the only presentation, the differentiation can be much more challenging. Our study proposed several clinical clues to distinguish NMOSD-associated SSM from MS-associated SSM. First, NMOSD-associated SSM lesions were predominantly localized to the central grey matter on cross-sectional views, which is rarely seen in MS. Second, the overall length of NMOSD-associated SSM lesions is longer than that of majority of MS-associated SSM lesions. Our study focused on the longest lesion of each patient and found whether the longest lesion spanned less than 2 segments may provide some evidence for differential diagnosis. Though both manifested as SSM, NMOSD-associated SSM lesions extended no less than 2 vertebral segments on sagittal images, while about 90% of MS spinal cord lesions spanned fewer than 2 vertebral segments.22,23 Third, CSF-specific OCBs, serum AQP4-IgG and systemic autoimmune antibodies may also help in differential diagnosis. Yonezu and colleagues24 found that bright spotty lesions can help differentiate patients with NMOSD from those with MS. In our study, bright spotty lesions seemed numerically more common in NMOSD-associated SSM than MS- associated SSM, though with no significant difference (p = 0.093). Besides MS, neurologists need to bear in mind that SSM can also occur in other conditions including vascular myelopathy, neurosarcoidosis, neoplastic or compressive etiologies.25,26 In addition, testing for MOG-IgG in SSM patients is also recommended, since previous studies have found 38.4% of SSM to be the initial presentation in MOG-IgG-associated diseases.27
Interestingly, our NMOSD cohort is a little different from the typical cohorts of former studies. The median onset age of NMOSD in our study is 51.5 (range: 19–81), which seems to be older than the usual onset age of about 40 years.28 However, a study based on Asian NMOSD patients have shown that patients with late-onset NMOSD (>50 years) had more frequent isolated spinal cord involvement at onset.29 We think our cohort, targeting patients who presented with myelitis as the initial and only manifestation of NMOSD, is prone to having an older onset age.
This study is mainly limited by its retrospective design, relatively small sample size and timing of MRI acquisition. However, the sample size is still acceptable considering the strict inclusion criteria and the low incidence of SSM in patients of NMOSD. In addition, due to the small sample size, we failed to identify SSM patients diagnosed with seronegative NMOSD or NMOSD with an unknown AQP4-IgG antibody status. In addition, MRI readers of our study were not blinded to diagnosis, which may lead to bias. Furthermore, the interval between symptom onset and the spinal cord MRI of each patient was different. We restricted the interval to 1 month to minimize this limitation and make sure there was no significant difference between the groups. What’s more, our study included only patients with NMOSD or MS, rather than all patients presenting with myelitis as the initial and only manifestation. For these limitations, further studies with a larger sample size and wider disease spectrum were needed to confirm our findings.
Conclusion
In summary, in a group of Chinese patients, our study focused on the under-recognized scenario when SSM is the only initial presentation of NMOSD. We delineated features in this group of patients and compared them with NMOSD-associated LETM and MS-associated SSM. For an early and correct diagnosis of NMOSD, we suggest SSM patients undergo spinal cord MRI and serum AQP4-IgG. Specifically, AQP4-IgG may have the highest diagnostic yield for centrally located, grey matter involving and transversally extensive lesions on axial imaging and for SSM lesions spanning no less than 2 vertebral segments on sagittal imaging.
Footnotes
Author contributions: Authors Wei Fang and Yang Zheng contributed equally to this work.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China [grant numbers 81671283 and 81701266] and the Zhejiang Provincial Natural Science Foundation of China [grant number LY17H090003].
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Mei-Ping Ding  https://orcid.org/0000-0003-3145-7778
https://orcid.org/0000-0003-3145-7778
Contributor Information
Wei Fang*, Department of Neurology, Fourth Affiliated Hospital, School of Medicine, Zhejiang University, Yiwu, China.
Yang Zheng*, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Fan Yang, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Meng-Ting Cai, Department of Neurology, Hangzhou Hospital of Traditional Chinese Medicine, Hangzhou, China.
Chun-Hong Shen, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Zhi-Rong Liu, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Yin-Xi Zhang, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, 88 Jiefang Road, Hangzhou, 310009, China.
Mei-Ping Ding, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, 88 Jiefang Road, Hangzhou, 310009, China.
References
- 1. Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–815. [DOI] [PubMed] [Google Scholar]
- 2. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012; 11: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology 2015; 84: 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinshenker BG, Wingerchuk DM, Vukusic S, et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol 2006; 59: 566–569. [DOI] [PubMed] [Google Scholar]
- 5. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim SH, Kim W, Li XF, et al. Clinical spectrum of CNS aquaporin-4 autoimmunity. Neurology 2012; 78: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 8. Hyun JW, Jeong IH, Joung A, et al. Evaluation of the 2015 diagnostic criteria for neuromyelitis optica spectrum disorder. Neurology 2016; 86: 1772–1779. [DOI] [PubMed] [Google Scholar]
- 9. Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain 2012; 135: 1834–1849. [DOI] [PubMed] [Google Scholar]
- 10. Flanagan EP, Weinshenker BG, Krecke KN, et al. Short myelitis lesions in aquaporin-4-IgG-positive neuromyelitis optica spectrum disorders. JAMA Neurol 2015; 72: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huh SY, Kim SH, Hyun JW, et al. Short segment myelitis as a first manifestation of neuromyelitis optica spectrum disorders. Mult Scler 2017; 23: 413–419. [DOI] [PubMed] [Google Scholar]
- 12. Hu H, You X, Ye J. Short transverse myelitis in Chinese patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2018; 21: 78–83. [DOI] [PubMed] [Google Scholar]
- 13. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation 2012; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 15. Ciccarelli O, Cohen JA, Reingold SC, et al. Spinal cord involvement in multiple sclerosis and neuromyelitis optica spectrum disorders. Lancet Neurol 2019; 18: 185–197. [DOI] [PubMed] [Google Scholar]
- 16. Asgari N, Skejoe HP, Lennon VA. Evolution of longitudinally extensive transverse myelitis in an aquaporin-4 IgG-positive patient. Neurology 2013; 81: 95–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asgari N, Skejoe HP, Lillevang ST, et al. Modifications of longitudinally extensive transverse myelitis and brainstem lesions in the course of neuromyelitis optica (NMO): a population-based, descriptive study. BMC Neurol 2013; 13: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krampla W, Aboul-Enein F, Jecel J, et al. Spinal cord lesions in patients with neuromyelitis optica: a retrospective long-term MRI follow-up study. Eur Radiol 2009; 19: 2535–2543. [DOI] [PubMed] [Google Scholar]
- 19. Shimizu J, Hatanaka Y, Hasegawa M, et al. IFNβ-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology 2010; 75: 1423–1427. [DOI] [PubMed] [Google Scholar]
- 20. Kim SH, Kim W, Li XF, et al. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler 2012; 18: 1480–1483. [DOI] [PubMed] [Google Scholar]
- 21. Palace J, Leite MI, Nairne A, et al. Interferon Beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol 2010; 67: 1016–1017. [DOI] [PubMed] [Google Scholar]
- 22. Tartaglino LM, Friedman DP, Flanders AE, et al. Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology 1995; 195: 725–732. [DOI] [PubMed] [Google Scholar]
- 23. Thielen KR, Miller GM. Multiple sclerosis of the spinal cord: magnetic resonance appearance. J Comput Assist Tomogr 1996; 20: 434–438. [DOI] [PubMed] [Google Scholar]
- 24. Yonezu T, Ito S, Mori M, et al. “Bright spotty lesions” on spinal magnetic resonance imaging differentiate neuromyelitis optica from multiple sclerosis. Mult Scler 2014; 20: 331–337. [DOI] [PubMed] [Google Scholar]
- 25. Zalewski NL, Krecke KN, Weinshenker BG, et al. Central canal enhancement and the trident sign in spinal cord sarcoidosis. Neurology 2016; 87: 743–744. [DOI] [PubMed] [Google Scholar]
- 26. Zalewski NL, Flanagan EP, Keegan BM. Evaluation of idiopathic transverse myelitis revealing specific myelopathy diagnoses. Neurology 2018; 90: e96–e102. [DOI] [PubMed] [Google Scholar]
- 27. Ciron J, Cobo-Calvo A, Audoin B, et al. Frequency and characteristics of short versus longitudinally extensive myelitis in adults with MOG antibodies: a retrospective multicentric study. Mult Scler. Epub ahead of print 31 May 2019. DOI: 10.1177/1352458519849511. [DOI] [PubMed] [Google Scholar]
- 28. Bruscolini A, Sacchetti M, La Cava M, et al. Diagnosis and management of neuromyelitis optica spectrum disorders - An update. Autoimmun Rev 2018; 17: 195–200. [DOI] [PubMed] [Google Scholar]
- 29. Seok JM, Cho HJ, Ahn SW, et al. Clinical characteristics of late-onset neuromyelitis optica spectrum disorder: a multicenter retrospective study in Korea. Mult Scler 2017; 23: 1748–1756. [DOI] [PubMed] [Google Scholar]