Abstract
Background
Triatomines are natural vectors of Chagas disease and are mainly prevalent in the Americas. In China, previous data from decades ago showed that there were two species of triatomine bugs, Triatoma rubrofasciata and T. sinica. However, the distribution, genetic characteristics and public health implications of triatomines in China are still relatively unknown. In order to gain knowledge on the distribution, genetic characteristics and public health implications of the triatomines in Guangxi, China, an entomological-epidemiological study and genetic research was conducted.
Methods
Different methods were used to elucidate the distribution of triatomines in Guangxi including consultations with county-level Center for Disease Prevention and Control staff and village doctors, the distribution of educational material on triatomines though the internet and social media apps such as Wechat and QQ, and conducting manual inspections and light trapping to collect triatomines. The morphological characteristics of the collected triatomines were identified under light microscopy. The mitochondrial 16S rRNA, cytochrome b (cytb) genes and nuclear 28S rRNA gene were amplified, sequenced and used in phylogenetic analyses.
Results
A total of 305 triatomines were captured from 54 different sites in 13 cities in Guangxi. All collected bugs were identified as T. rubrofasciata based on morphology. Most triatomine collection sites were around or inside houses. Four triatomines bite cases were observed during the investigation indicating that triatomine bites are common, the bites can cause serious anaphylaxis and skin papules and urticaria, suggesting a systemic skin response. The 16S rRNA, 28S rRNA and cytb sequence analyses of T. rubrofasciata from Guangxi and other countries showed that T. rubrofasciata sequences from different regions exhibit a high similarity, with no geographical differences. The phylogenetic tree based on the 16S rRNA and cytb genes showed that T. rubrofasciata sequences from different regions and continents were in the same cluster, indicating no differentiation among different geographical populations.
Conclusions
Our study showed that T. rubrofasciata is widely distributed in Guangxi and that people are commonly bitten by this insect in some regions. This highlights the need to enhance surveillance for and control of T. rubrofasciata and to strengthen the monitoring of imported Trypanosoma cruzi in China. The 16S rRNA, 28S rRNA and cytb sequence analyses of T. rubrofasciata from different regions and continents suggested that T. rubrofasciata populations exhibit high similarity, and the clustering in the phylogenetic analyses indicates that T. rubrofasciata has a close ancestor originating in the Americas.
Keywords: Triatoma rubrofasciata, Guangxi, Distribution, Genetic characteristics
Background
Chagas disease, or American trypanosomiasis, is one of the 10 most seriously neglected tropical diseases [1] and currently affects 10 million people worldwide [2]. There is no vaccine or effective cure once the symptoms of the chronic disease have manifested. Chagas disease is considered limited to the Americas; however, in recent decades, it has become a global health issue [3]. Due to the growing rate of immigrants unaware of their own infection status [4], the disease can potentially spread to non-endemic countries. Moreover, Trypanosoma cruzi Chagas, 1909 (Kinetoplastida, Trypanosomatidae) can be transmitted by blood transfusion and organ transplantation. With increasing transcontinental exchanges, Chagas disease is no longer restricted to the Americas [5, 6]. Currently, it has been diagnosed in several non-endemic countries, such as New Zealand, Australia, Japan and Europe [7–9].
Chagas disease is transmitted mainly by triatomine bugs (kissing bugs) [10] belonging to 18 genera and 154 species worldwide [11–16]. Triatoma rubrofasciata (De Geer, 1773) is the only species with a worldwide distribution [17]. It is mostly found in Asia, Oceania, Africa and Central America. It is a common vector of Trypanosoma conorhini (Donovan) that infects Rattus rattus [18, 19]. T. rubrofasciata is also occasionally found naturally infected by T. cruzi [20, 21]. Additionally, T. rubrofasciata bites can cause dermatitis, anaphylactic shock, and even death [22, 23].
In China, very few records have indicated that T. rubrofasciata exists in southern China, such as in Guangxi Zhuang Autonomous Region, Guangdong and Fujian provinces [24–26]. Furthermore, the peridomestic presence of T. rubrofasciata was reported to the northeast of Hanoi, extending across the frontier well into southern China [27]. However, there have been few studies on T. rubrofasciata over the decades in China, and a very limited amount of information about its distribution has been published. Two blood-sucking triatomines, T. rubrofasciata and T. sinica Hsiao, 1965, have been reported in China over the past five decades [24]. Nine cases of T. rubrofasciata invading houses and biting people, resulting in anaphylactic shock or death, have been reported in China [23, 28, 29]. However, blood-sucking triatomines have received limited attention for decades. People are not sufficiently concerned about this insect or aware of the consequences. Several publications ranging from the characterization of the number of chromosomes [30] to the description of the genome [31] and its phylogenetic relationships [32] have contributed to the genetic, taxonomic and evolutionary knowledge of T. rubrofasciata. However, little is known about the actual distribution of this vector in China. Thus, we conducted an entomological-epidemiological study and genetic research in Guangxi, China.
Methods
Triatomines and triatomine bite investigations
Triatomine collection in the field was carried out from July 2016 to October 2018 in Guangxi, China. First, we asked the county level Center for Disease Prevention and Control staff and village doctors about the presence of T. rubrofasciata in its suspected distribution areas and showed them pictures of triatomines. The fieldwork involved inspection of old houses (houses made of clay), tree cavities, wood and rock piles, chicken coops and dog kennels. Additionally, light traps were used to collect triatomines. The light installation followed the method by Castro et al. [33] and included a mobile electric power pack and approximately a 1.8 × 2.2 m vertical aluminium frame with a white sheet. The frame was kept erect with guide ropes. On both sides of the frame, two 125 W and 250 W mercury vapor bulbs (HPL) were suspended with external ballasts. The light trap was run from sunset until midnight and the insects on the sheet were checked every hour. Geographical coordinates of the location from where the specimens were caught were recorded with a handheld GPS unit (UniStrong, Beijing, China) for each site. To obtain more information on triatomine distribution, we disseminated electronic posters and leaflets about the triatomine bugs on the internet and social media Apps such as Wechat. The electronic poster and leaflets were also distributed to the staff of the County and City Disease Prevention and Control units. People who found the bugs sent photos with locations. We identified the species of the triatomine based on the photos and went to the location to collect the specimen. In the locations where triatomines were found, we interviewed the local people about how often they get bitten by triatomines. Information from each individual who had been bitten by triatomines was collected, including personal information, symptomatic descriptions, treatment and follow-up visits.
DNA extraction, amplification and sequencing
From 13 collected triatomines, genomic DNA was extracted from two legs of each of the triatomines using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. The mitochondrial 16S rRNA and cytb gene and the nuclear ribosomal 28S rRNA gene were PCR-amplified using established primers (Table 1). PCR was conducted in a final volume of 25 μl containing 2× Taq PCR Master Mix (Takara, Dalian, China), 0.4 μM of each primer and 2 μl DNA template. The following PCR cycling conditions were employed: 95 °C for 3 min; 35 cycles of 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 1 min; and a final extension step at 72 °C for 10 min. PCR products were analysed by electrophoresis in a 1.5% agarose gel. Amplified DNA was purified from the gel using the Agarose Gel DNA Extraction Kit (Takara), and the DNA was sequenced at Sangon Biotech (Shanghai, China).
Table 1.
The primers used for 16S rRNA, 28S rRNA and cytb sequence amplification
Sequence alignment and phylogenetic analyses
The sequences were aligned by ClustalW (https://www.genome.jp/tools-bin/clustalw) and any mutations were detected. The sequences were compared with those available in the GenBank database using the Basic Local Alignment Search Tool (BLASTn http://blast.ncbi.nlm.nih.gov/Blast.cgi). The phylogenetic tree for triatomines from Guangxi, along with other homologous sequences (rubrofasciata clade) for the 16S rRNA and cytb genes were constructed using the neighbour-joining (NJ) method with 1000 bootstrap replications using MEGA7.0 [36].
Results
The distribution of triatomines in Guangxi
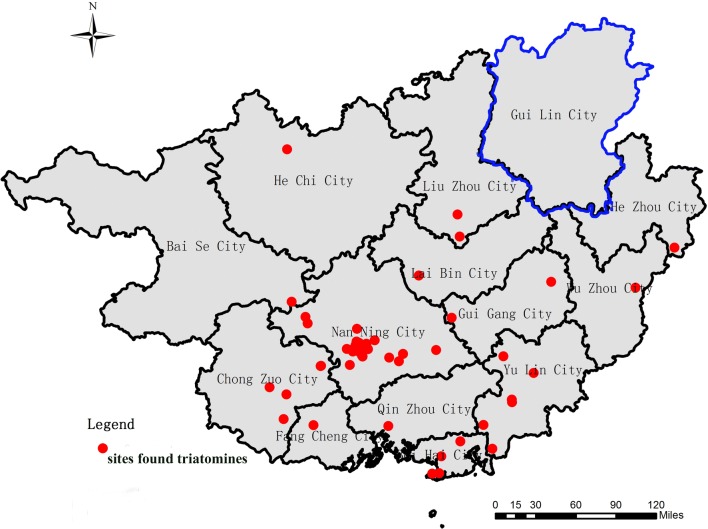
According to the field investigation results and feedback from the internet and social media apps, 54 sites across 13 different cities in Guangxi Zhuang Autonomous Region were positive for blood-sucking triatomines (Fig. 1). The most common places where triatomines were found were inside or around houses (Fig. 2a–d), such as in wood piles (Fig. 2a, b) and chicken coops (Fig. 2c), in both urban and rural locations. A total of 305 triatomines were collected, including juvenile and adult bugs (see Additional file 1: Table S1 for additional information for collected triatomines). A total of 42.62% (n = 130) of the specimens were female, 40% (n = 122) were male, 17.38% (n = 53) were nymphs, and 31.15% (n = 95) had fed on blood. The number of collected insects at a single location was very different, ranging from one to over one hundred.
Fig. 1.
The distribution of Triatoma rubrofasciata in Guangxi, China. Over the two years of study, triatomines were found in 54 cities (red dots). Except for Guilin city (highlighted with blue), 13 other cities in Guangxi Zhuang Autonomous Region were positive for T. rubrofasciata
Fig. 2.

The living habit of Triatoma rubrofasciata. a T. rubrofasciata tend to hide in woodpiles; b T. rubrofasciata found in a woodpile; c T. rubrofasciata tend to hide near chicken coops. d T. rubrofasciata found in a house when cleaning
Morphology of the triatomines
The collected juvenile and adult triatomine bugs were identified as T. rubrofasciata by morphological characteristics following Xiao et al. [24] and the website for T. rubrofasciata of the Centers for Disease Control and Prevention USA (https://www.cdc.gov/parasites/chagas/gen_info/vectors/t_rubrofasciata.html). The length of collected fourth-instar larvae was approximately 10 mm. Adult T. rubrofasciata morphology showed that the length of male bugs was 19.0–24.0 mm, and the length of females was 20–25 mm (Fig. 3a). The overall colour was dark brown to black, and its entire outer margin was delicately bordered with orange-red, with yellowish and orange-red markings on the neck, pronotum, corium and connexium. The dorsal surface was conspicuously granulose, while the head and pronotum were heavily granulose dorsally. The scutellum had the general body colour and was rugose-granulose. The posterior process was conical, subtriangular, and strongly tapered from a wide base to a point. The head was uniformly dark, with the first antennal segment distinctly projecting beyond the head (Fig. 3b).
Fig. 3.
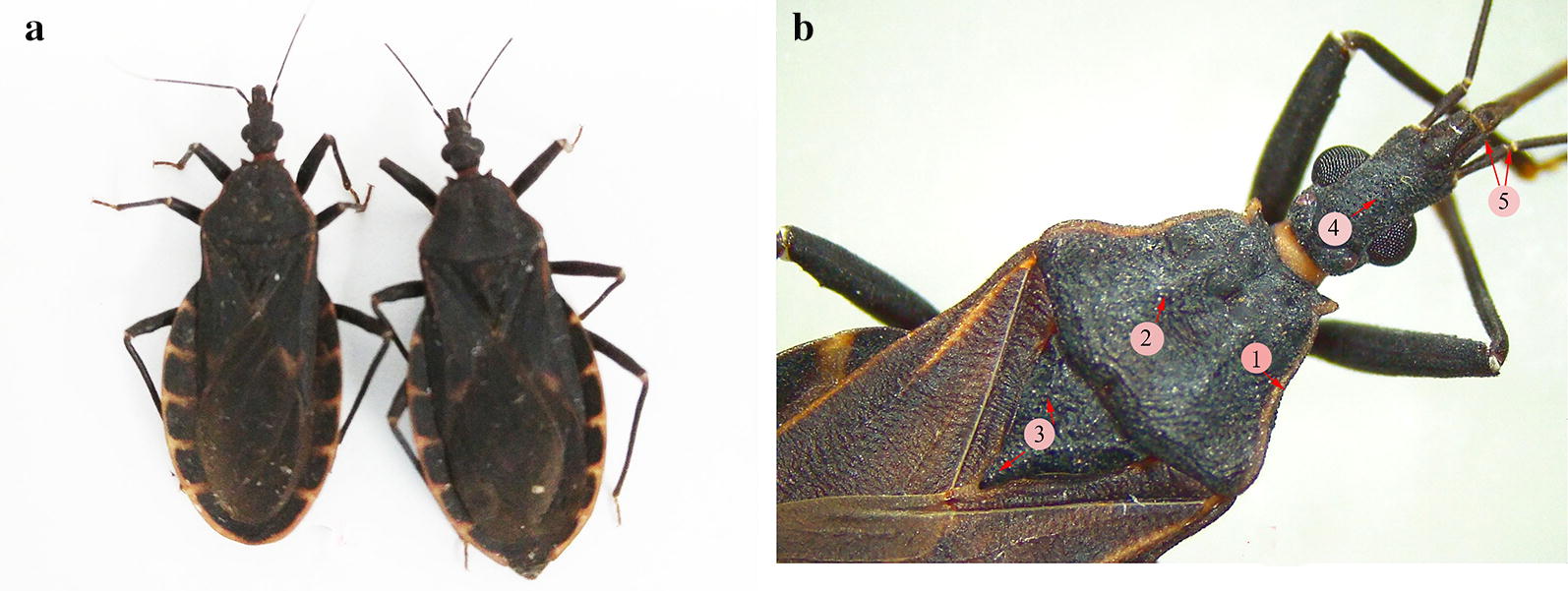
Morphology of Triatoma rubrofasciata. a An adult male (left) and female (right). b Morphological characteristics of T. rubrofasciata: 1, there is an orange-red margin along the outer edge of the abdomen as well as the side of the pronotum; 2, the pronotum is dark brown or black and conspicuously granulose; 3, the scutellum is wide at the base and tapers to the tip; 4, the head is uniformly dark and heavily granulose dorsally; 5, the 1st segment of antenna surpasses the head
People bitten by T. rubrofasciata
Our inquiry showed that over 20 people had been bitten by T. rubrofasciata in the past year, and over one hundred people said that they had experienced a bite by T. rubrofasciata. Because many of the people bitten by T. rubrofasciata did not show serious clinical signs and recovered within approximately two weeks, they did not pay attention to the bite. Four cases of T. rubrofasciata bites were observed during the investigation (Fig. 4a–d). The ages of the patients were 26, 30, 35 and 52 years-old. All of them were bitten during noon break or night sleep in their living quarters. The bite sites included the back of the hand, forearm, eyelid, shin, thigh and back. Bites can cause serious anaphylactic reactions, including skin bullae, vesicles, papules, surrounding swelling or erythema and urticaria, suggesting a systemic skin response. One patient presented generalized cutaneous symptoms, including urticaria, flushing, pruritus, and angioedema (Fig. 4a); another patient experienced a headache and muscle aches (Fig. 4c). Urticaria and other clinical signs tend to disappear within approximately two weeks. The triatomines were caught after biting and were identified as T. rubrofasciata (Fig. 4).
Fig. 4.
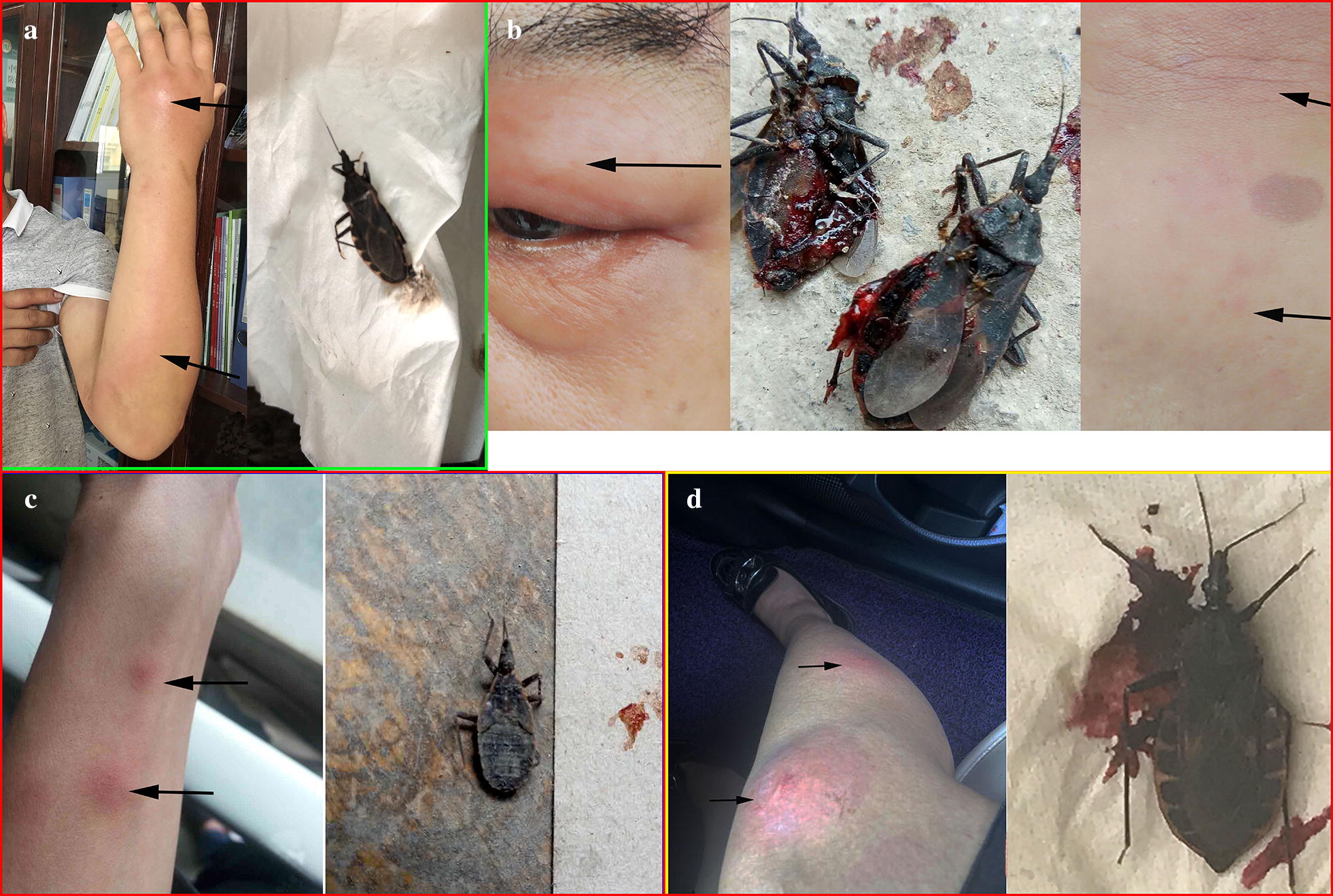
Four Triatoma rubrofasciata bite cases. a A bite by an adult T. rubrofasciata caused cutaneous symptoms including urticaria, flushing, pruritus, and angioedema. b A bite by an adult T. rubrofasciata (middle) caused palpebral oedema (left) and slight skin redness on the back (right). c A bite by a fifth-instar T. rubrofasciata (left) caused an urticarial skin reaction and slight redness on the arm (right). d A woman was bitten by an adult T. rubrofasciata on her foot, thigh and knees while sleeping
Genetic analysis and genetic relatedness
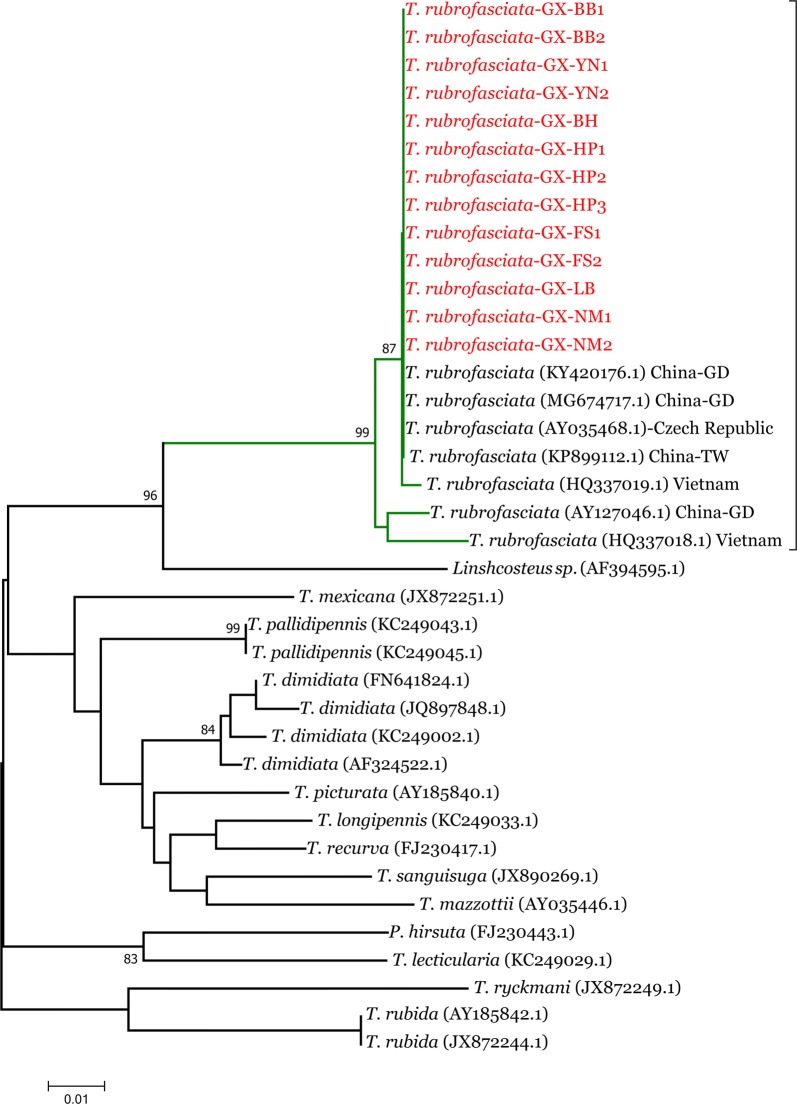
The 13 triatomines collected were from the Haicheng district (n = 1) and Hepu County (n = 3) of Beihai city; Bobai County (n = 2) of Yulin city; Yongning County (n = 2) of Nanning city; and Fusui County (n = 2), Ningming County (n = 2) and Luobai town (n = 1) of Chongzuo city. The DNA was amplified, and the mitochondrial (16S rRNA and cytb) and nuclear (28S rRNA) genes were sequenced. Multiple 499-bp fragments of the 16S rRNA gene from T. rubrofasciata were obtained, and the sequences were submitted to the GenBank database under the accession numbers MH236899.1–MH236905.1. The NCBI BLAST search indicated that the isolated triatomine bugs had over 98% identity with T. rubrofasciata from China (GenBank: MG674717.1, KY420176.1, KP899112.1 and AY127046.1), the Czech Republic (GenBank: AY035468.1) and Vietnam (GenBank: HQ337019.1 and HQ337018.1). The 16S rRNA gene sequences from 13 T. rubrofasciata from Guangxi (Infomation see Table 2) that were aligned with the sequences of T. rubrofasciata from Vietnam, the Czech Republic and other places in China showed nine polymorphic sites; seven mutations were found in a single sequence, and the other two mutated sites were detected in multiple sequences (Additional file 2: Figure S1). The phylogenetic tree based on the 16S rRNA gene showed that all T. rubrofasciata from China, Vietnam and the Czech Republic were in the same cluster (Fig. 5), with a bootstrap value of 99. The cluster could be divided into two separate branches; the first branch contained T. rubrofasciata from Guangdong Province (GenBank: AY127046.1) and Vietnam (GenBank: HQ337018.1). The other branch contained the sequences from China, Vietnam and the Czech Republic. The phylogenetic relationships of T. rubrofasciata populations were not associated with their geographical distribution. Triatoma rubrofasciata is closely related to Linshcosteus spp. (GenBank: AF394595.1) from India, the only triatomine genus exclusively from the Old World (Fig. 5).
Table 2.
Information of Triatoma rubrofasciata isolates from Guangxi used in the 16S rRNA and cytb sequence phylogenetic analyses
| Isolate | Locality | Latitude/Longitude | Date | GenBank ID |
|---|---|---|---|---|
| BB-1 | Yunlin City, Bobai County | 22°27ʹ28.95ʺN, 109°96ʹ99.93ʺE | 2017 |
MH236899.1 (16S); MH368015.1 (cytb) |
| BB-2 | Yunlin City, Bobai County | 21°74ʹ30.31ʺN, 109°75ʹ84.17ʺE | 2017 | |
| YN-1 | Nanning City, Yongning County | 22°68ʹ68.38ʺN, 108°74ʹ86.31ʺE | 2017 |
MH236900.1 (16S); MH368016.1 (cytb) |
| YN-2 | Nanning City, Yongning County | 22°68ʹ68.38ʺN, 108°.74ʹ86.31ʺE | 2017 | |
| FS-1 | Chongzuo City, Fusui County | 22°63ʹ64.84ʺN, 107°90ʹ89.09ʺE | 2017 |
MH236903.1 (16S); MH368020.1 (cytb) |
| FS-2 | Chongzuo City, Fusui County | 22°63ʹ49.75ʺN, 107°90ʹ41.06ʺE | 2017 | |
| LB | Chongzuo City, Jiangzhou District | 22°40ʹ52.00ʺN, 107°35ʹ19.25ʺE | 2017 |
MH236904.1 (16S); MH368019.1 (cytb) |
| NM-1 | Chongzuo City, Ningming County | 22°06ʹ27.24ʺN, 107°50ʹ35.50ʺE | 2017 |
MH236905.1 (16S); MH368021.1 (cytb) |
| NM-2 | Chongzuo City, Ningming County | 22°06ʹ27.24ʺN, 107°50ʹ35.50ʺE | 2017 | |
| BH | Beihai City, Haicheng District | 21°47ʹ88.52ʺN, 109°18ʹ45.86ʺE | 2017 |
MH236901.1 (16S); MH368017.1 (cytb) |
| HP-1 | Beihai City, Hepu County | 21°82ʹ13.00ʺN, 109°41ʹ40.26ʺE | 2017 |
MH236902.1 (16S); MH368018.1 (cytb) |
| HP-2 | Beihai City, Hepu County | 21°82ʹ13.00ʺN, 109°41ʹ40.26ʺE | 2017 | |
| HP-3 | Beihai City, Hepu County | 21°66ʹ09.00ʺN, 109°20ʹ725.11ʺE | 2017 |
Fig. 5.
The phylogenetic tree based on the 16S rRNA gene sequences for Triatoma rubrofasciata from Guangxi and other related species. The phylogenetic tree constructed by MEGA using the neighbour-joining (NJ) method with 1000 bootstrap replications. The sequences from this study were highlighted with red color. Abbreviations: GD, Guangdong Province; TW, Taiwan
A 666-bp fragment of the 28S rRNA gene from the collected T. rubrofasciata was amplified. The sequences were submitted to the GenBank database under the accession numbers MH356275.1-MH356281.1. The BLAST search indicated that the isolated triatomines had the greatest homology (over 99%) with T. rubrofasciata from China (GenBank: MG675575.1 and KY420177.1), Brazil (GenBank: KR632546.1), Vietnam (GenBank: KR632547.1 and KR632548.1) and the USA (GenBank: GQ853371.1). The alignment of the T. rubrofasciata 28S rDNA sequences from GenBank showed six mutations in a single sequence among the T. rubrofasciata from China, Uruguay and the USA (Additional file 2: Figure S1).
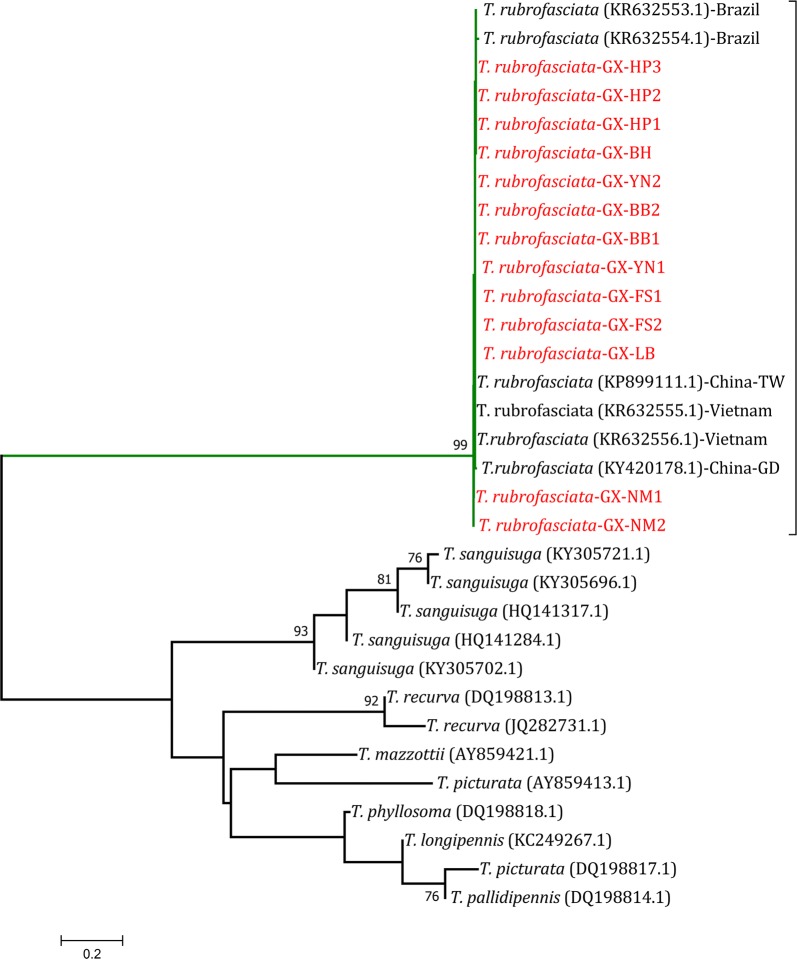
A 667-bp fragment of the cytb gene from the collected T. rubrofasciata was amplified, and the sequences were submitted to the GenBank database under the accession numbers MH368015.1-MH368021.1. The BLAST search indicated that the triatomines had 99% homology with T. rubrofasciata from China (GenBank: KP899111.1 and KY420178.1), Brazil (GenBank: KR632554.1 and KR632553.1) and Vietnam (GenBank: KR632555.1 and KR632556.1). Ten mutated sites were found after the alignment of the cytb gene of T. rubrofasciata from China, Vietnam and Brazil. Four mutations were found in a single sequence, and six other mutations were identified (Additional file 2: Figure S1). The phylogenetic tree showed that all of the T. rubrofasciata from China, Brazil and Vietnam were in the same cluster (Fig. 6).
Fig. 6.
The phylogenetic tree based on the cytb gene sequences for Triatoma rubrofasciata from Guangxi and other related species. The phylogenetic tree constructed by MEGA using the neighbour-joining (NJ) method with 1000 bootstrap replications. The sequences from this study were highlighted with red color. Abbreviations: GD, Guangdong Province; TW, Taiwan
Discussion
In China, only few studies on T. rubrofasciata have been carried out over the past fifty years [24–26], with only a limited amount of information regarding its distribution. To date, the knowledge of the distribution of triatomines in China is still scarce. In the present study, we investigated the distribution of T. rubrofasciata in Guangxi using multiple methods, including network reporting, field interviews and field trapping. We collected 305 T. rubrofasciata, including nymphs and adults, from 54 different sites across 13 cities in Guangxi. Our study showed that T. rubrofasciata, the vector of T. cruzi, exists extensively in Guangxi, South China. With increasing intercontinental activities, there is an increasing risk of imported Chagas disease. The wide distribution of T. rubrofasciata could support T. cruzi colonization, transmission and invasion in new areas.
In this study, we used various methods to survey for T. rubrofasciata, including manual inspection, light trapping, internet tools and social media apps such as Wechat and QQ. Among the three methods, light trapping was the least effective, as it did not capture any T. rubrofasciata. This could be because of the low distribution density of these kissing bugs. The internet and social media app feedback enabled us to identify most of the T. rubrofasciata distribution locations. While the use of a mobile phone is very common in Guangxi (over 90% of people are actively online) Wechat and QQ were very effective tools to communicate and receive insect information in real time; thus, Internet could be powerful tool for disease surveillance and management [37–39]. Since the triatomines had a relatively low population density and because they are active at night, manual inspections were difficult to conduct. The feedback by witnesses and reports by local people was particularly important to locate T. rubrofasciata; these reports greatly benefited the manual inspections and insect collections.
In the present study, most of the T. rubrofasciata were collected in houses or near living areas, indicating that this insect lives near human habitation and that humans are easily exposed. Our interviews also revealed that people being bitten by T. rubrofasciata is common in some regions in Guangxi. Four cases of T. rubrofasciata bites were observed during the investigation. Common clinical symptoms are skin papules and an urticaria-like systemic skin response; people will usually recover in approximately one to two weeks. Because most of the people bitten by T. rubrofasciata did not show serious clinical symptoms, people did not pay attention to this insect and its bite. However, the prevalence of T. rubrofasciata and the common biting activity of T. rubrofasciata in Guangxi Zhuang Autonomous Region could make the colonization and spread of T. cruzi possible. Moreover, triatomines, as a potential vector of other viruses, bacteria and parasites, can transmit pathogens between humans and animals [40]. Triatomines have a wide range of host blood sources. The pathogens carried by the bug can be stored and propagated in its midgut or maintained in the crop and spread to the salivary glands. Therefore, saliva and faeces are two common ways to spread pathogens to humans and animals [40]. A pathogen in the saliva can enter a hostʼs blood vessels and subcutaneous tissue through a bite. Studies have confirmed that Trypanosoma rangeli and Bartonella spp. carried by an American blood-sucking triatomine can be transmitted to humans [41–43]. The pathogen-containing faeces excreted by the triatomines can enter the human body through a skin wound or the mucosa, causing infection. Studies have shown that T. cruzi, Serratia marcescens, Mycobacterium leprae, HIV and HBV can be transmitted to humans through triatomine faeces [44–49]. In China, there have been no reports about the infectious diseases transmitted by T. rubrofasciata; future research should focus on the acquisition and transmission of pathogens by this insect.
The Triatominae is a subfamily of the Reduviidae that is mainly distributed in the Americas. The current consensus is that the Triatominae have relatively recent origins in the Americas and that the Old World species represent derivatives of an American form [33]. Triatoma rubrofasciata is the only species of the Triatominae with a worldwide distribution, and the mode of natural dispersal of T. rubrofasciata to other areas and to the Old World is unknown [34]. One hypothesis is that mice infested with T. rubrofasciata were accidentally carried to the Old World and other regions on ships, resulting in the current worldwide dispersal of T. rubrofasciata [50, 51]. The other hypothesis is that the Bering Land Bridge in the mid-Oligocene was a route for terrestrial plants and animals, as well as triatomines, that migrated between North America and Asia [51]; perhaps the land bridge provided suitable conditions to facilitate the dispersal of triatomines. In the present study, the sequence alignment of T. rubrofasciata from different regions and continents exhibited a high similarity and phylogenetic analyses showed they were in the same cluster, indicating that T. rubrofasciata has a close ancestor originating in the Americas.
Conclusions
Our study showed that T. rubrofasciata is widely distributed in Guangxi Zhuang Autonomous Region, southern China, and in some regions, people are commonly bitten by this insect. This highlights the need to enhance surveillance and control of T. rubrofasciata and strengthen the monitoring of imported T. cruzi in China. The 16S rRNA, 28S rRNA and cytb sequence analyses of T. rubrofasciata from different regions and continents suggested that T. rubrofasciata populations exhibited high similarity, and clustered in the same cluster in the phylogenetic analyses, indicating that T. rubrofasciata has a close ancestor originating in the Americas.
Supplementary information
Additional file 1: Table S1. Data for T. rubrofasciata collected in Guangxi, China.
Additional file 2: Figure S1. Alignments of 16S rRNA, 28S rRNA and cytb genes of T. rubrofasciata from Guangxi, China.
Acknowledgements
We sincerely thank the people who participated in the investigation, including Chenghui Lao from Hepu County Peopleʼs Hospital, Pei Guanghui from Qinzhou City Center for Disease Prevention and Control, Yingqiong Zhang from Chongzuo City Center for Disease Prevention and Control, Jianxun Li and Guanhuai Qiu from Ningming County Center for Disease Prevention and Control and the people who sent kissing bug information to us.
Abbreviations
- cytb
cytochrome b
- NJ
neighbour-joining
Authors’ contributions
YS and YY developed the study protocol. YS, YW, XF, JH, FO, HW, XW, GL and YY performed the field work and contributed to the data analysis. YS and YY performed the final analysis. YS wrote the first manuscript draft. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2016YFC1202000, 2016YFC1202001, 2016YFC1202004) and the Guangxi Natural Science Foundation Programme (2018GXNSFAA294092).
Availability of data and materials
The newly generated sequences were submitted to the GenBank database under the accession numbers MH236899.1–MH236905.1 (16S rRNA gene), MH356275.1-MH356281.1 (28S rRNA gene) and MH368015.1-MH368021.1 (cytb). The data from this study are available from the corresponding author upon request.
Ethics approval and consent to participate
The study was approved by the Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention Ethics Committee. The information of the bitten patients was obtained with the patient’s approval. The research aims, methods, risks and benefits of the study were explained in detail to the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yunliang Shi, Email: syunliang2008@126.com.
Yaobao Wei, Email: 805952163@qq.com.
Xiangyang Feng, Email: 13077712341@163.com.
Jianfeng Liu, Email: 89442169@qq.com.
Zhihua Jiang, Email: laojiang20@163.com.
Fangqi Ou, Email: 1204821431@qq.com.
Haiyan Wei, Email: 476489602@qq.com.
Guoli Lv, Email: 185358868@qq.com.
Xiaoling Wan, Email: Wan-xl500@163.com.
Ziyue Wang, Email: 296948339@qq.com.
Yichao Yang, Email: 531174868@qq.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-020-3903-z.
References
- 1.WHO. WHO roadmap inspires unprecedented support to defeat neglected tropical diseases. Geneva: World Health Organization; 2012. https://www.who.int/neglected_diseases/London_meeting_follow_up/en/. Accessed 3 Feb 2019.
- 2.Pereira PC, Navarro EC. Challenges and perspectives of Chagas disease: a review. J Venom Anim Toxins Incl Trop Dis. 2013;19:34. doi: 10.1186/1678-9199-19-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q, Zhou XN. Preventing the transmission of American trypanosomiasis and its spread into non-endemic countries. Infect Dis Poverty. 2015;4:60. doi: 10.1186/s40249-015-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Coura JR, Vinas PA. Chagas disease: a new worldwide challenge. Nature. 2010;465:S6–S7. doi: 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- 6.Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS One. 2011;5:1–2. doi: 10.1371/journal.pntd.0001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Jackson Y, Pinto A, Pett S. Chagas disease in Australia and New Zealand: risks and needs for public health interventions. Trop Med Int Health. 2014;19:212–218. doi: 10.1111/tmi.12235. [DOI] [PubMed] [Google Scholar]
- 9.Roure S, Valerio L, Valles X, Morales B, Garcia-Diaz MI, Pedro-Botet ML, et al. Oesophageal motility disorders in infected immigrants with Chagas disease in a non-endemic European area. United European Gastroenterol J. 2016;4:614–620. doi: 10.1177/2050640616630856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Chagas disease (American trypanosomiasis). Geneva: World Health Organisation; 2019. https://www.who.int/chagas/en. Accessed 24 May 2019.
- 11.Oliveira J, Alevi KCC. Taxonomic status of Panstrongylus herreri Wygodzinsky, 1948 and the number of Chagas disease vectors. Rev Soc Bras Med Trop. 2017;50:434–435. doi: 10.1590/0037-8682-0125-2017. [DOI] [PubMed] [Google Scholar]
- 12.Dorn PL, Justi SA, Dale C, Stevens L, Galvão C, Lima-Cordón R, et al. Description of Triatoma mopan sp. n. from a cave in Belize (Hemiptera, Reduviidae, Triatominae) ZooKeys. 2018;775:69–95. doi: 10.3897/zookeys.775.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira J, Ayala JM, Justi SA, Rosa JA, Galvão C. Description of a new species of Nesotriatoma Usinger, 1944 from Cuba and revalidation of synonymy between Nesotriatoma bruneri (Usinger, 1944) and N. flavida (Neiva, 1911) (Hemiptera, Reduviidae, Triatominae) J Vector Ecol. 2018;43:148–157. doi: 10.1111/jvec.12294. [DOI] [PubMed] [Google Scholar]
- 14.Lima-Cordón RA, Monroy MC, Stevens L, Rodas A, Rodas GA, Dorn PL, et al. Description of Triatoma huehuetenanguensis sp. n., a potential Chagas disease vector (Hemiptera, Reduviidae, Triatominae) ZooKeys. 2019;820:51–70. doi: 10.3897/zookeys.820.27258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nascimento JD, Ravazi A, Alevi KCC, Pardo-Diaz C, Salgado-Roa FC, Rosa JA, et al. Taxonomical over splitting in the Rhodnius prolixus (Insecta: Hemiptera: Reduviidae) clade: Are R. taquarussuensis (da Rosa et al. 2017) and R. neglectus (Lent 1954) the same species? PLoS One. 2019;14:e0211285. doi: 10.1371/journal.pone.0211285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poinar G. A primitive triatomine bug, Paleotriatoma metaxytaxa gen. et. sp. nov. (Hemiptera: Reduviidae: Triatominae), in mid-Cretaceous amber from northern Myanmar. Cretaceous Res. 2019;93:90–97. doi: 10.1016/j.cretres.2018.09.004. [DOI] [Google Scholar]
- 17.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull Am Mus Nat Hist. 1979;163:123–520. [Google Scholar]
- 18.Deane M. Ocorrência do Trypanosoma conorrhini em “barbeiros” e em rato na cidade de Belém, Pará, e seu cultivo em meio de NNN. Rev Serv Espec Saude Pub. 1947;1:433–448. [Google Scholar]
- 19.Dias E, Seabra C. Trypanosoma conorrhini, hemoparasito do rato transmitido pelo Triatoma rubrofasciata: presença do vector infectado na cidade do Rio de Janeiro. Mem Inst Oswaldo Cruz. 1943;39:301–329. doi: 10.1590/S0074-02761943000600006. [DOI] [Google Scholar]
- 20.Lucena D, Marques R. Subsídios para o estudo ecológico do Triatoma rubrofasciata no Brasil. An Fac Med Univ Recife. 1955;15:19–31. [Google Scholar]
- 21.Brazil RP, Da Silva AR. Triatomine vectors of Trypanosoma cruzi-like trypanosomes in urban areas of Sao Luiz, Maranhao, Brazil. Trans R Soc Trop Med Hyg. 1983;77:568. doi: 10.1016/0035-9203(83)90145-1. [DOI] [PubMed] [Google Scholar]
- 22.Anderson C, Belnap C. The kiss of death: a rare case of anaphylaxis to the bite of the “red margined kissing bug”. Hawaii J Med Public Health. 2015;74:33–35. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Peng L. Four cases of anaphylactic shocks caused by triatomine bugs. Clin Focus. 2006;21:1059–1061. [Google Scholar]
- 24.Xiao C, Ren S, Zhen L, Jing X, Zou H, Liu S. Handbook of bug identification in China (Hemiptera Heteroptera) 2. Beijing: Science Press; 1981. [Google Scholar]
- 25.Liu Q, Guo YH, Zhang Y, Zhou ZB, Zhang LL, Zhu D, et al. First records of Triatoma rubrofasciata (De Geer, 1773) (Hemiptera, Reduviidae) in Foshan, Guangdong Province, southern China. Infect Dis Poverty. 2017;6:129. doi: 10.1186/s40249-017-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YL, Huang DN, Wu WH, Yang F, Zhang XM, Wang M, et al. Identification and characterization of the causative triatomine bugs of anaphylactic shock in Zhanjiang, China. Infect Dis Poverty. 2018;7:127. doi: 10.1186/s40249-018-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dujardin JP, Lam TX, Khoa PT, Schofield CJ. The rising importance of Triatoma rubrofasciata. Memórias do Instituto Oswaldo Cruz. 2015;110:319–323. doi: 10.1590/0074-02760140446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen MX. Prevention and treatment of the dermatitis caused by Triatoma. Hainan Med. 1986;1:35. [Google Scholar]
- 29.Zhang G. Study on the medical significance of the Triatoma. Hainan Med. 1991;4:8–9. [Google Scholar]
- 30.Alevi KC, Borsatto KC, Moreira FF, Jurberg J, De Azeredo-Oliveir MT. Karyosystematic of Triatoma rubrofasciata (De Geer, 1773) (Hemiptera: Reduviidae: Triatominae) Zootaxa. 2015;3494:433–438. doi: 10.11646/zootaxa.3994.3.7. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Guo YH, Zhag Y, et al. A chromosomal-level genome assembly for the insect vector for Chagas disease, Triatoma rubrofasciata. GigaScience. 2019;8:giz89. doi: 10.1093/gigascience/giz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Justi SA, Russo CA, Mallet JR, Obara MT, Galvao C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae) Parasit Vectors. 2014;7:149. doi: 10.1186/1756-3305-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro MC, Barrett TV, Santos WS, Abad-Franch F, Rafael JA. Attraction of Chagas disease vectors (Triatominae) to artificial light sources in the canopy of primary amazon rainforest. Mem Inst Oswaldo Cruz. 2010;105:1061–1064. doi: 10.1590/S0074-02762010000800019. [DOI] [PubMed] [Google Scholar]
- 34.Diaz S, Triana-Chavez O, Gomez-Palacio A. The nuclear elongation factor-1α gene: a promising marker for phylogenetic studies of Triatominae (Hemiptera: Reduviidae) Infect Genet Evol. 2016;43:274–280. doi: 10.1016/j.meegid.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich CH, Rakitov RA, Holmes JL, Black WC. Phylogeny of the major lineages of Membracoidea (Insecta: Hemiptera: Cicadomorpha) based on 28S rDNA sequences. Mol Phylogenet Evol. 2001;18:293–305. doi: 10.1006/mpev.2000.0873. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher J, O’Donoghue J, Car J. Managing immune diseases in the smartphone era: how have apps impacted disease management and their future? Expert Rev Clin Immunol. 2015;11:431–433. doi: 10.1586/1744666X.2015.1010518. [DOI] [PubMed] [Google Scholar]
- 38.Ernsting C, Dombrowski SU, Oedekoven M, O’Sullivan JL, Kanzler M, Kuhlmey A, et al. Using smartphones and health apps to change and manage health behaviors: a population-based survey. J Med Internet Res. 2017;19:e101. doi: 10.2196/jmir.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagel J. Survey of registered dietitians’ proficiency of celiac disease and use of Twitter, Facebook, smart phone app, and internet for celiac disease management. MSc thesis. North Dakota State University, North Dakota, USA; 2014.
- 40.Vieira CB, Praca YR, Bentes K, Santiago PB, Silva SMM, Silva GDS, et al. Triatomines: trypanosomatids, bacteria, and viruses potential vectors? Front Cell Infect Microbiol. 2018;8:405. doi: 10.3389/fcimb.2018.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paim RMM, Pereira MH, Araújo RN, Gontijo NF, Guarneri AA. The interaction between Trypanosoma rangeli and the nitrophorins in the salivary glands of the triatomine Rhodnius prolixus (Hemiptera; Reduviidae) Insect Biochem Mol Biol. 2013;43:229–236. doi: 10.1016/j.ibmb.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Ocaña-Mayorga S, Aguirre-Villacis F, Pinto CM, Vallejo GA, Grijalva MJ. Prevalence, genetic characterization, and 18S small subunit ribosomal RNA diversity of Trypanosoma rangeli in triatomine and mammal hosts in endemic areas for Chagas disease in Ecuador. Vector Borne Zoonot Dis. 2015;15:732–742. doi: 10.1089/vbz.2015.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laroche M, Berenger JM, Mediannikov O, Raoult D, Parola P. Detection of a potential new Bartonella species “Candidatus Bartonella rondoniensis” in human biting kissing bugs (Reduviidae; Triatominae) PLoS Negl Trop Dis. 2017;11:e0005297. doi: 10.1371/journal.pntd.0005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azambuja P, Feder D, Garcia ES. Isolation of Serratia marcescens in the midgut of Rhodnius prolixus: impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp. Parasitol. 2004;107:89–96. doi: 10.1016/j.exppara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Ruano SM, Škochová V, Rego ROM, Schmidt JO, Roachell W, Hypša V, et al. Microbiomes of North American Triatominae: the grounds for Chagas disease epidemiology. Front Microbiol. 2018;9:1167. doi: 10.3389/fmicb.2018.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann AS, Dias FA, Ferreira JS, Fontes AN, Rosa PS, Macedo RE, et al. Experimental infection of Rhodnius prolixus (Hemiptera, Triatominae) with Mycobacterium leprae indicates potential for leprosy transmission. PLoS One. 2016;11:e0156037. doi: 10.1371/journal.pone.0156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granato HCF, de Mendonça JS, Silva Pinto PL, Neto VA, Veronesi R, Tolezano E. Papel de triatomíneos na transmissäo de infecçäo pelo vírus da hepatite tipo B, em diferentes normas clínicas da doença. Rev Hosp Clin Fac Med Sao Paulo. 1987;42:173–175. [PubMed] [Google Scholar]
- 48.Candeias JAN, Forattini OP, Vieira JG. Hepatitis B antigen (HBsAg) in wild caught Triatominae in Brazil. Rev Saúde Pública. 1976;10:267–268. doi: 10.1590/S0034-89101976000300007. [DOI] [PubMed] [Google Scholar]
- 49.Nuzzo S, Amato Neto V, Braz L, Silva M, Oliveira M, Castilho M, et al. Evaluation of presence of protein 24 from HIV in feces of Triatoma infestans fed blood from HIV positive patients. Rev Saúde Públ. 1998;32:464–466. doi: 10.1590/S0034-89101998000500009. [DOI] [PubMed] [Google Scholar]
- 50.Gorla DE, Dujardin JP, Schofield CJ. Biosystematics of Old World Triatominae. Acta Trop. 1997;63:127–140. doi: 10.1016/S0001-706X(97)87188-4. [DOI] [PubMed] [Google Scholar]
- 51.Justi SA, Cleber G, Schrago CG, et al. Geological changes of the Americas and their influence on the diversification of the Neotropical kissing bugs (Hemiptera: Reduviidae: Triatominae) PLoS Negl Trop Dis. 2016;10:e0004527. doi: 10.1371/journal.pntd.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Data for T. rubrofasciata collected in Guangxi, China.
Additional file 2: Figure S1. Alignments of 16S rRNA, 28S rRNA and cytb genes of T. rubrofasciata from Guangxi, China.
Data Availability Statement
The newly generated sequences were submitted to the GenBank database under the accession numbers MH236899.1–MH236905.1 (16S rRNA gene), MH356275.1-MH356281.1 (28S rRNA gene) and MH368015.1-MH368021.1 (cytb). The data from this study are available from the corresponding author upon request.