Abstract
Despite their wide use in academia as metal-carbene precursors, diazo compounds are often avoided in industry owing to concerns over their instability, exothermic decomposition, and potential explosive behavior. The stability of sulfonyl azides and other diazo transfer reagents is relatively well understood, but there is little reliable data available for diazo compounds. This work first collates available sensitivity and thermal analysis data for diazo transfer reagents and diazo compounds to act as an accessible reference resource. Thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and accelerating rate calorimetry (ARC) data for the model donor/acceptor diazo compound ethyl (phenyl)diazoacetate are presented. We also present a rigorous DSC dataset with 43 other diazo compounds, enabling direct comparison to other energetic materials to provide a clear reference work to the academic and industrial chemistry communities. Interestingly, there is a wide range of onset temperatures (Tonset) for this series of compounds, which varied between 75 and 160 °C. The thermal stability variation depends on the electronic effect of substituents and the amount of charge delocalization. A statistical model is demonstrated to predict the thermal stability of differently substituted phenyl diazoacetates. A maximum recommended process temperature (TD24) to avoid decomposition is estimated for selected diazo compounds. The average enthalpy of decomposition (ΔHD) for diazo compounds without other energetic functional groups is −102 kJ mol–1. Several diazo transfer reagents are analyzed using the same DSC protocol and found to have higher thermal stability, which is in general agreement with the reported values. For sulfonyl azide reagents, an average ΔHD of −201 kJ mol–1 is observed. High-quality thermal data from ARC experiments shows the initiation of decomposition for ethyl (phenyl)diazoacetate to be 60 °C, compared to that of 100 °C for the common diazo transfer reagent p-acetamidobenzenesulfonyl azide (p-ABSA). The Yoshida correlation is applied to DSC data for each diazo compound to provide an indication of both their impact sensitivity (IS) and explosivity. As a neat substance, none of the diazo compounds tested are predicted to be explosive, but many (particularly donor/acceptor diazo compounds) are predicted to be impact-sensitive. It is therefore recommended that manipulation, agitation, and other processing of neat diazo compounds are conducted with due care to avoid impacts, particularly in large quantities. The full dataset is presented to inform chemists of the nature and magnitude of hazards when using diazo compounds and diazo transfer reagents. Given the demonstrated potential for rapid heat generation and gas evolution, adequate temperature control and cautious addition of reagents that begin a reaction are strongly recommended when conducting reactions with diazo compounds.
Keywords: differential scanning calorimetry, diazo compound, diazo transfer, sulfonyl azide, process safety
Introduction
The choice of synthetic route in process development is multifactorial and often governed by reagent costs, product yield and purity, environmental impact, robustness, and, most importantly, safety.1,2 Chemical transformations from commercial reagents to the desired product are evaluated holistically with these principles in mind, and a route is devised using available synthetic methods. Certain classes of reagents are essentially excluded from a process chemist’s toolkit due to safety concerns. To determine whether reagents are acceptable to use in a scaled process requires the practical assessment of specific hazardous properties.3
Energetic materials pose specific explosive hazards, resulting from the high quantities of heat and gas that can be released upon rapid decomposition. There are several metrics commonly used to assess a reagent.4 These can be grouped into risk assessment categories determining the likelihood or severity of an unwanted decomposition. The likelihood will be proportional to the activation energy term of an Arrhenius equation for the decomposition reaction. Thermal stability is a measure of the temperature at which a compound is observed to pyrolyze. Sensitivity to several stimuli can be exhibited by energetic materials, defined by specialist tests with results quoted in comparison to well-known sensitive materials. The cause of sensitive behavior is a complex phenomenon, and there is currently no theoretical way to predict sensitivity.5 Impact (or shock) sensitivity (IS)6 can be the most relevant for a chemical process, but friction, electrostatic discharge (ESD), or other sensitivities such as to light may also be relevant.7 On the other hand, metrics related to severity will be primarily concerned with the magnitude of the heat produced in an exothermic process (ΔHR). Other important considerations include whether a material can sustain a detonation8 and the amount of pressure generated by evolved gases.
These concepts are defined from an experimental basis, with reference to well-studied materials; so, there is no inherent property to determine. 1,3,5-Trinitro-1,3,5-triazinane (RDX) is often used as a reference compound by explosive material scientists;9 for organic or process chemists, 1,3-dinitrobenzene (m-DNB) is a more appropriate, conservative reference.10 These tests are notorious for reproducibility as a large number of factors can alter the result: from test methodology to the presence of air bubbles in liquid samples,10 or the crystal structure5b or particle morphology of a solid sample.4 The measurement obtained will therefore depend upon the idiosyncrasies of the specific method used, and as such, full and accurate reporting of all experimental aspects is vital. Ideally, measurements should be conducted on the same setup and performed at the same time. Such tests require specialist test equipment not commonly available outside energetic material labs and consume decagram quantities of material.
Differential scanning calorimetry (DSC) is a valuable screening tool in process safety, providing information on both likelihood and severity characteristics related to a reaction or compound. The energy change of a sample crucible is monitored vs an inert reference crucible as the temperature is controlled, usually following a steady temperature ramp. Thermal stability and exotherm magnitude can be easily and rapidly obtained using this technique from small quantities of a sample (∼mg). However, DSC lacks the high sensitivity of other techniques and does not provide valuable pressure data, such as with accelerating rate calorimetry (ARC). Energetic compounds, which evolve gas upon decomposition, must be analyzed in a sealed DSC crucible capable of withstanding the generated pressure to avoid misleading results.11
DSC results generally include the enthalpy of decomposition (ΔHD), which indicates the energetic yield of the decomposition, as well as initiation (Tinit) and/or extrapolated onset temperatures (Tonset), which represent thermal stability. Tinit is defined by the temperature at which the heat flow is measured to be >0.01 W g–1 from the baseline. Tonset is defined by the intersection of the extrapolated maximum peak slope and the baseline, often determined by DSC software. Typically, for process safety purposes, only Tinit is used, and to complicate matters, it is often named Tonset. It is important to note that unlike melting points, decomposition reactions have a kinetic factor and decomposition will occur at lower temperatures than Tinit, albeit at an exponentially slower rate.
Diazo compounds are energetic materials that find extensive application in chemical synthesis as versatile synthetic intermediates, but almost exclusively in academic labs.12 Such compounds present powerful bond-forming potential via the (catalytic) generation of metal carbenes and offer important synthetic disconnections, relevant to the preparation of pharmaceutically relevant motifs. The most valuable transformation is arguably the reaction with alkenes to form cyclopropanes.13,14 In a rare large-scale example, a styryl-diazoester was employed by Bristol-Myers Squibb in an enantioselective cyclopropanation in a multikilogram synthesis of Beclabuvir,15 using the Rh2(S-DOSP)4 catalyst reported by Davies.16 Carbene C–H insertion processes are becoming increasingly controlled, with potential to streamline synthetic routes.17 Similarly, heteroatom X–H insertion reactions (X = O, N, S, and P)18−20 and cycloaddition reactions21 can install important pharmaceutically relevant functionality and heterocycles. Despite these available transformations, the diazo functional group is essentially avoided for reactions at process scale, due to the potential explosion hazard.22
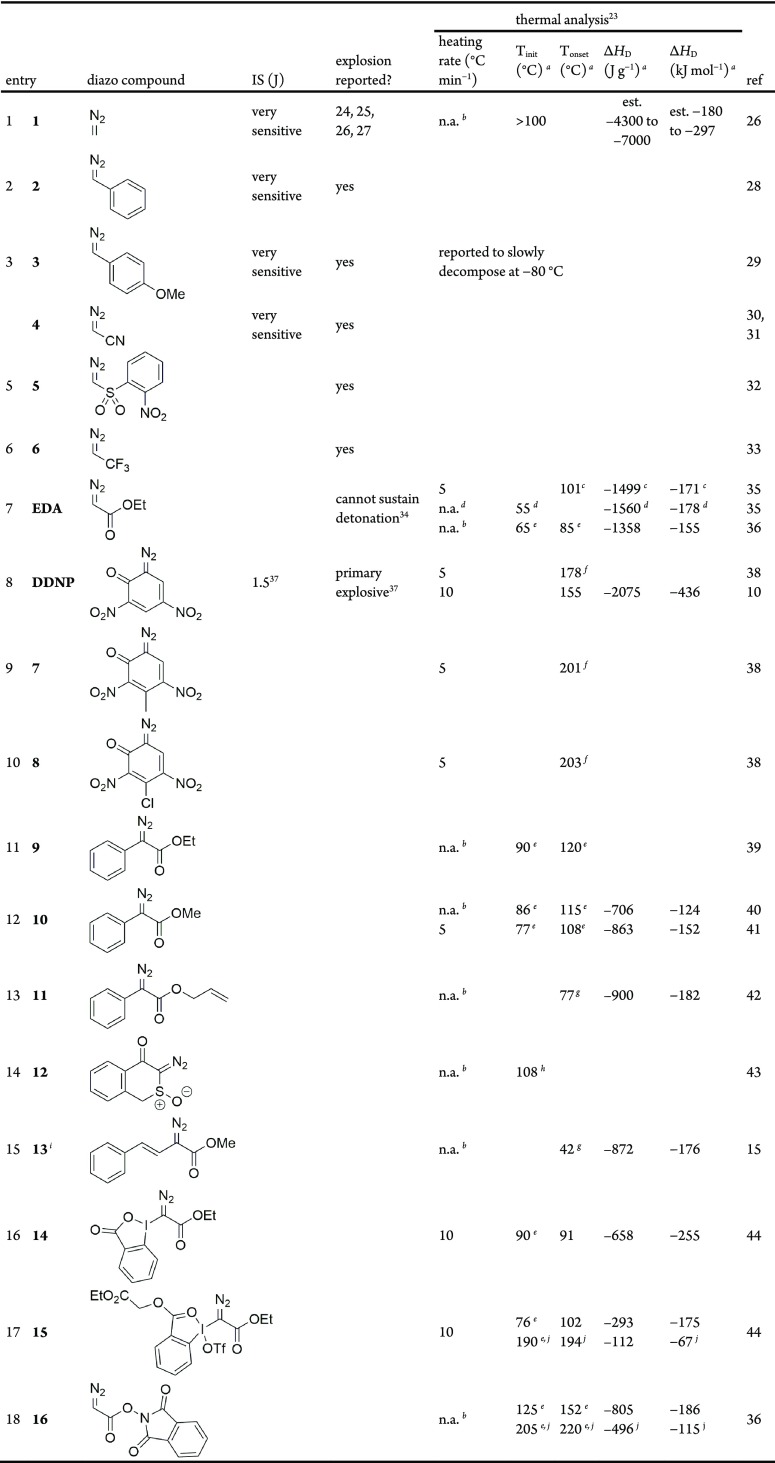
The available data on the thermal hazards of diazo compounds is limited and disparate. Rarely is thermal hazard data presented, and it is common in the academic literature to make general comments as to the potential stability hazards, along the lines, of “Caution: while we never experienced any problems, diazo compounds are presumed to be potentially explosive”. Here, we have collated all available data on diazo compounds from the literature (Table 1). This data is largely from DSC experiments, though other results are quoted where this is not available. We have included ΔHD in J g–1 and in kJ mol–1. For process safety, the ΔH value is often reported in J g–1, as this can be divided by the specific heat capacity (cp in J g–1 K–1) to gauge the adiabatic temperature rise (ΔTad) of a defined mixture to give an idea of the severity of a runaway exotherm.2 Impact sensitivity (IS) is given where it is available. If an explosion has been reported, it is noted though the root cause is often unclear and an absence of reports should not be interpreted as an absence of explosive behavior. The data presented in Table 1 should be treated with caution as exact details of experimental methods are often not reported and there are inconsistencies in the use of terminology within the reported literature.
Table 1. Impact Sensitivity (IS), Thermal Stability, and Exotherm Data Previously Reported for Diazo Compounds10,15,25−43.
Measured by DSC unless otherwise noted.
Heating rate not given.
DSC of 40% weight ethyl diazoacetate (EDA) in toluene. ΔHD reported was −602 J g–1, which was extrapolated to 100% weight EDA assuming a linear relationship.
Measured by ARC on neat EDA, a much more sensitive instrument and slower heating rate and so Tonset is lower and ΔHD is higher than a typical DSC experiment.
Estimated from DSC plot given in Supporting Information.
DSC peak maxima temperatures.
Distinction between Tinit and Tonset was unclear; so, we have assumed Tinit as defined in this work.
Measured by thermogravimetric analysis (TGA).
Interestingly, this compound is reported to cyclize into a pyrazole rather than evolving N2 gas.
Distinct second decomposition is observed.
The extremely unstable and explosive nature of alkyl diazo compounds has been evident since their discovery in the late 1890s,23 and they are rarely isolated as a result. Diazomethane 1 is the simplest diazo compound and is a highly sensitive, explosive gas.24 Widely known to explode at the slightest provocation, including exposure to direct sunlight, chipped glassware, or ground-glass joints,25 its extreme sensitivity and gaseous nature are a barrier to precisely investigating the initiation of the decomposition using standard techniques. While generally agreed to decompose above 100 °C,26 the difficulty in ensuring the reaction is triggered thermally makes the accurate determination of the thermal stability impractical.27 Given the well-known hazards, the specter of diazomethane is perhaps one reason that diazo compounds are often avoided in the pharmaceutical industry. There are a few examples of industrial interest in diazomethane, leveraging continuous flow processes to generate and consume the reagent in situ with increased safety.28
Pure phenyl diazomethane 2,29 the 4-methoxyphenyl-analogue 3,30 and diazoacetonitrile 4(31,32) are reported to explode spontaneously, although this list is not exhaustive. When required, these monosubstituted, low-molecular-weight diazo compounds are generally prepared in situ for immediate use in one-pot protocols or continuous flow processes.33 Due to their violently short-lived nature, the isolation of quantities sufficient for stability or sensitivity testing is impractical. The so-called “semistabilized” diazo compounds with a conjugated aryl or alkenyl π-system are similarly handled in solution for brief periods of time.34
Ethyl diazoacetate (EDA) is the most commonly used diazo compound, and DSC data is available on a dilute solution35 and neat sample.36 Diazodinitrophenol (DDNP) is a well-studied primary explosive,37 which, along with its derivatives (7 and 8), is less appropriate for fine chemical synthesis.50 Disubstituted donor/acceptor diazo compounds are often isolated, depending on the structure, but there remains very little data on their stability in the literature. Data were reported for aryl diazoacetates 9–11, and α-diazo-β-keto sulfoxide 12; however, in each case, full experimental details of the thermal analysis were not reported.38−42 Recently, hypervalent iodine diazoacetates 14 and 15 for photoredox chemistry and redox-active N-hydroxyphthalimidoyl diazoacetate (NHPI-DA) 16 were reported with accompanying DSC data.36,43
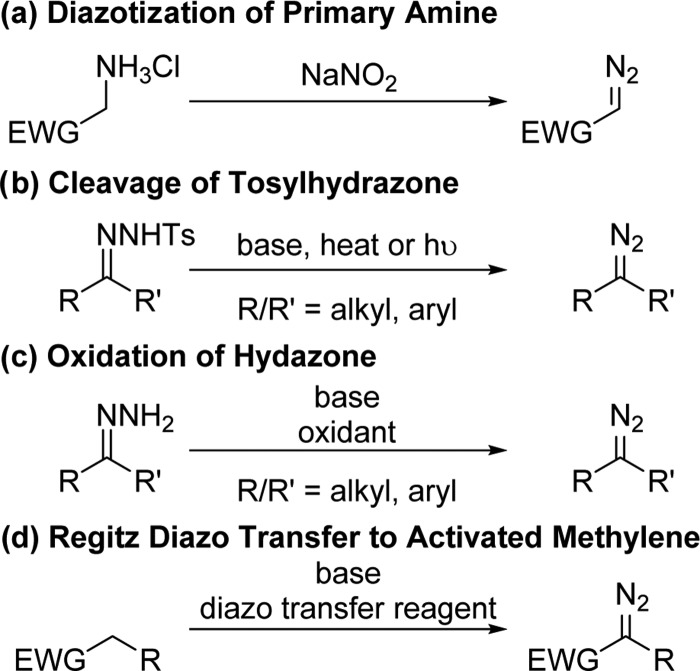
There are several possible routes for the preparation of diazo compounds, which themselves involve energetic reagents (Figure 1). Diazotization is limited to simple diazo compounds.51 Aryl or alkyl diazo compounds can be prepared from N-sulfonylhydrazones, which are cleaved with base,52 thermolytically or photolytically53 though forcing conditions may decompose the diazo product. Unsubstituted hydrazones can be dehydrogenated using oxidizers.54 Regitz diazo transfer to an activated methylene compound is the most widely used method to access complex diazo compounds with at least one electron-withdrawing group. The N2 transfer is typically effected by sulfonyl azide reagents, though recently, other azides have been developed that effect the same reaction.55,56
Figure 1.
General synthetic methods to prepare diazo compounds.
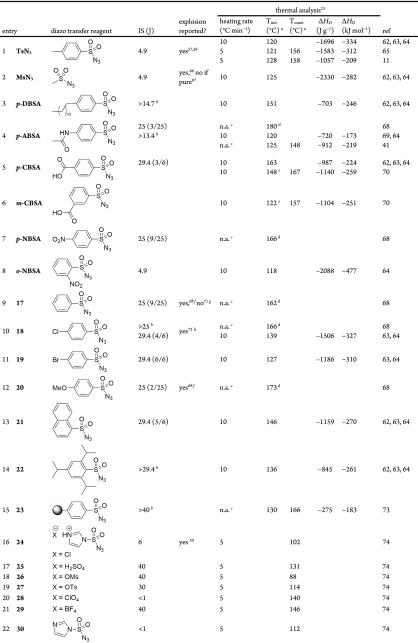
Sulfonyl azides are commonly used diazo transfer reagents that have known hazardous properties.57 Several explosions have been reported,58−62 and so, these compounds have been relatively well studied. Available literature thermal analysis and sensitivity data for sulfonyl azide and other diazo transfer reagents are collated in Table 2. Though generally more thermally stable than diazo compounds, the sulfonyl azide functional group confers a significantly higher energetic yield. Sulfonyl azides have been reported to be impact-sensitive;63−65 however, another report claimed that impurities were the cause, i.e., pure MsN3 was not impact-sensitive but a slightly impure sample was highly sensitive.66 Pure TsN3 has been reported to explode,58,59 but the root cause of these incidents was not conclusively demonstrated to be the azide, and the only report of explosive power comes from an unpublished private correspondence.63 It has been noted that preparations of sulfonyl azide using an alcohol as solvent lead to impurities, which may be the cause of the observed impact sensitivity, and so, the preparation of sulfonyl azides in acetone/water is recommended to avoid this possibility.67
Table 2. Impact Sensitivity, Thermal Stability, and Exotherm Data Previously Reported for Sulfonyl Azide and Other Diazo Transfer Reagents11,44,39,55,56,58−82.
Measured by DSC unless otherwise noted.
Sample reached the upper test limit with no reaction.
Heating rate not given.
Method not clear from the report.
Estimated from the DSC plot given in the report.
Explosion judged by the report70 of audible sound effect upon thermal decomposition, indicative of a supersonic detonation.
Pure 17 reported to decompose “quietly” at approx 105 °C but crude product exploded.
Explosion occurred on the addition of a spiro azepanoindole species to neat azide.
The root cause of this explosion is unclear.
Reported from TGA as DSC was not run to decomposition.
No data has been published, but the pure form is reported to spontaneously explode and so testing would be difficult.
Initially reported as +30 kJ mol–1 endothermic decomposition due to DSC measurement in open crucibles.
p-Acetamidobenzenesulfonyl azide (p-ABSA) is considered to be a “safer” diazo transfer reagent and generally preferred over mesyl (MsN3) or tosyl azide (TsN3) for batch diazo preparation. The key reasons for this can be seen in Table 2; while the thermal stability (Tinit and Tonset) is similar, it is significantly less sensitive to impact and has an apparently lower ΔHD. It has not been demonstrated that p-ABSA can sustain a detonation, as the only result is unclear.70p-Dodecylbenzenesulfonyl azide (p-DBSA) and 4,6-dimethoxy-1,3,5-triazine (ADT) have been demonstrated to be nonimpact-sensitive alternative diazo transfer reagents.56,63−65 Polystyrene-supported benzenesulfonyl azide 23 has been suggested as another safer, if costly, option.75 Both TsN3 and MsN3 have been prepared and used in flow,83 where the sulfonyl azide is maintained in a solution throughout the protocol and thus the explosive properties of the neat material are avoided.84 Among the more reactive diazo transfer reagents, NfN3,81 2-azido-1,3-dimethylimidazolinium hexafluorophosphate (ADMP),55 and 25(76) have been demonstrated to be insensitive alternatives to the explosive TfN3. Friction and ESD sensitivity data are available for the imidazole-1-sulfonyl azide salts 24–2976 and NfN3.81
Diazo compounds and diazo transfer reagents are valuable synthetic tools, but the question of explosive potential discourages their use. Table 2 compiles a large amount of thermal and explosive hazard data on diazo transfer reagents available in the literature. It is apparent that similar information is scarce for diazo compounds, which are generally regarded to pose similar hazards in the chemical laboratory. Here, we present a rigorous DSC dataset of diazo compounds and offer a direct comparison to diazo transfer reagents and other energetic materials to provide a clear reference work to the academic and industrial chemistry communities. Thermal stability data on 44 diazo compounds using DSC is reported, and the effects of various substituents on these properties are explored. Onset temperatures were found to be 75–160 °C, with electron-rich substituents generally giving lower thermal stability. Energetic yields were around 102 kJ mol–1 unless other energetic functional groups were included. Furthermore, the Yoshida correlation is applied to the DSC data to predict the impact sensitivity and explosivity of the tested diazo compounds. None are predicted to be explosive, but many are predicted to exhibit impact sensitivity with exothermic decomposition. The implications of these results are that the scale-up of reactions using sulfonyl azides or diazo compounds should be undertaken with caution, ensuring that adequate thermal control and overpressure relief mechanisms are in place and that the initiating reagent is added at an appropriate rate.
Results and Discussion
DSC Analysis of Diazo Compounds
In this study, the thermal stability and energetic yield of 44 diazo compounds were measured using DSC. In a typical measurement, 5 mg of the diazo compound was weighed into a high-pressure crucible, sealed, and equilibrated at 25 °C. The sample was then heated at 5 °C min–1 while the heat flow was monitored vs an empty reference crucible. The use of high-pressure crucibles ensured containment of all gaseous decomposition products, which otherwise led to erroneous endothermic peaks.11,85 The results for Tinit, Tonset, and ΔHD were measured for two samples and averaged (Table 3). For selected results, this analysis was repeated on different DSC equipment at GlaxoSmithKline (GSK) using a slightly different method with a 2 °C min–1 temperature ramp, which resulted in lower Tinit and Tonset due to the higher resolution. Repeatability criteria were 2% for Tinit and Tonset and 10% for ΔHD,3a though the majority of results were well within this range; any results falling outside this range were repeated.
Table 3. Averaged DSC Results for Diazo Compounds.
Results using DSC method A and average results, where n = 2 unless otherwise noted.
n = 6.
Using DSC method B and n = 1.
Tinit given as the start of the endothermic melting point, which overlapped the exothermic decomposition. This makes calorimetric data less reliable as more than one transition is being measured.
Data reported was obtained by excluding endothermic melting to more accurately determine calorimetric data, which shifts Tonset slightly. See Experimental Section for more details.
Result altered by an overlapping transition.
EDA in 13 wt % CH2Cl2.
ΔHD divided by 0.87 to exclude the unreactive mass of CH2Cl2.
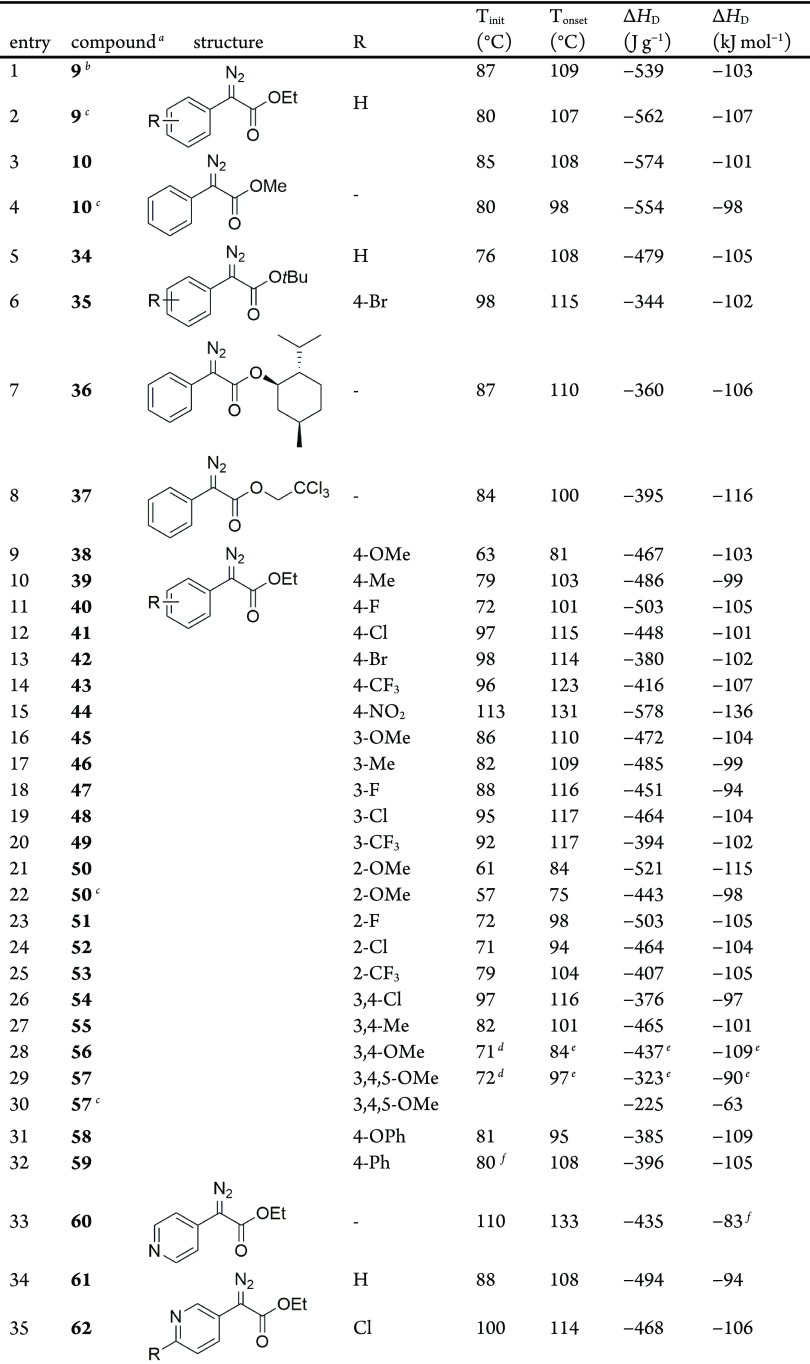
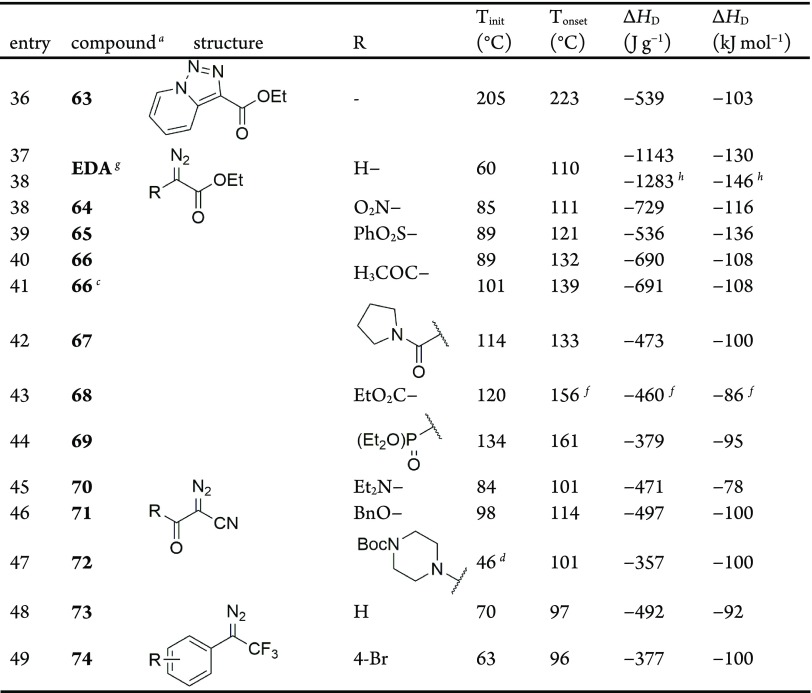
The DSC plots for the donor/acceptor diazo compound ethyl (phenyl)diazoacetate 9 and analogous ethyl (phenyl)acetate 33 are shown in Figure 2.86 Both compounds are liquid at 25 °C and thus do not exhibit melting points, typically observed as a sharp endothermic peak for solids. A clear exothermic decomposition is observed in the DSC plot of 9, which corresponds to the dissociation of the diazo to form N2 gas and a highly reactive carbene. This decomposition is also evident in the thermogravimetric analysis (TGA) plot of 9 (Figure 3), which clearly shows a sharp mass loss initiating around 85 °C (99.6% of the starting mass) and corresponding to a 14.8% mass loss by 126 °C. The molecular mass of N2 (28.01 g mol–1) is exactly 14.73% of the molecular mass of 9 (190.2 g mol–1). The average DSC for 9 (Table 3, entry 1) shows an agreement with both our result (Table 3, entry 2) and existing literature report (Table 1, entry 10; heating rate and ΔHD were not reported).
Figure 2.
Comparison of the DSC plot for ethyl (phenyl)acetate 33 and ethyl (phenyl)diazoacetate 9.
Figure 3.
TGA plot for ethyl (phenyl)diazoacetate 9.
Diazo compound 9 was then compared to a variety of other aryl diazoacetates to investigate the effect of substituents on thermal stability. Tonset was used for this comparison, as it was found to give more consistent results. Changing the ester alkyl group did not have a significant effect on Tonset, which represents thermal stability. tert-Butyl (phenyl)diazoacetate 34 and tert-butyl (4-bromophenyl)diazoacetate 35 have almost identical DSC results to their ethyl ester analogues 9 and ethyl (4-bromophenyl)diazoacetate 42. A bulkier alkyl ester such as menthol derivative 36 did not significantly impact thermal stability. A small reduction in thermal stability was observed for trichloroethyl ester 37.
The effect of different aryl substituents was explored with ethyl (phenyl) diazoacetates 38–59 (Table 3, entries 9–35). It was apparent that the more electron-donating substituents led to lower Tinit and Tonset. Indeed, there was a correlation of thermal stability with the Hammett substitution constants (Figure 4): σm is used for meta substituents and σp+ for para substituents.87 Chlorine at the 4-position 41 resulted in almost identical Tonset to 4-bromo 42; these substituents have very similar σp+ constants (4-Cl = 0.11, 4-Br = 0.15). For chloro-substituted ethyl (phenyl)diazoacetates, Tonset is similar for 4-Cl 42 and 3-Cl 48. Fluorine has a greater difference between σm and σp+ (0.41 vs 0.26) as it is significantly more electronegative, and so for fluoro-substituted ethyl (phenyl)diazoacetates, we observed a more exaggerated difference between the electron-poor 3-F 47 and 4-F 40, which is destabilized by π-donation into the conjugated aryl-diazo system. The methoxy group is a strong π-donor, and PMP 38 gave the lowest Tonset. 3-OMe 45 had lower Tonset than 3-Cl or 3-F, indicating that the weaker σ-withdrawing effect confers a smaller benefit to the diazo compound thermal stability. Both strong σ-acceptor (such as CF343, 49) and π-acceptor substituents such as NO2 (44) serve to stabilize the diazo compound leading to Tonset of approx 120 and 130 °C, respectively.
Figure 4.
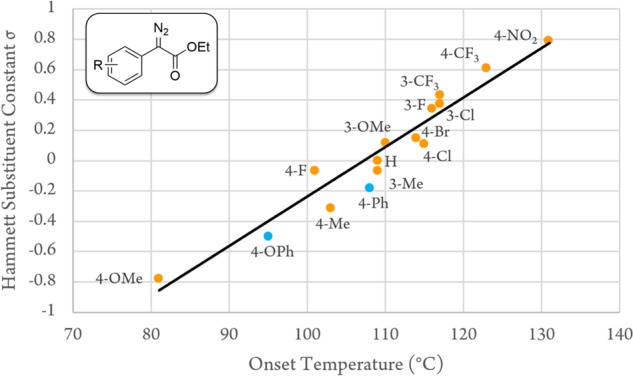
Plot of σm/σp+ against the onset temperature for compounds 38–49 (from Table 3), detailing correlation. Compounds 58 and 59 included in blue are described later.
Using the data from Table 3, we developed a statistical model to predict the thermal stability of novel diazo compounds of the substructure shown in Figure 5, using only the Hammett parameter of substituent R1 or R2 (eq 1). We propose that this model could be used as a prediction of stability (as Tonset with a 5 °C min–1 ramp) on novel diazo compounds of this type, to provide an estimate of the thermal stability before synthesis. The model was tested by the synthesis of previously unknown phenoxyaryl-substituted diazo compound 58, which was predicted by eq 1 to have a Tonset of 93 °C (σp+ = −0.50, experimental result 95 °C; Table 1, entry 31) and biphenyl diazoacetate 59, with a predicted Tonset of 102 °C (σp+ = −0.18, experimental result 108 °C; Table 1, entry 32). While phenoxy 58 had excellent predictive accuracy, biphenyl 59 was slightly more stable than expected. This data is also plotted in Figure 4 for comparison. It is possible that this difference is due to the resonance stabilization of the diazo moiety, as the enhanced delocalization of charge is not captured in the Hammett parameter
| 1 |
Ortho-substituted examples were found to be less thermally stable than meta or para analogues, depending on the steric bulk of the substituent. Chlorophenyl diazo 52 and trifluoromethylphenyl diazo 53 gave a significant reduction in Tinit and Tonset, with onset temperatures approximately 20 °C lower, whereas less bulky substituents such as methoxyphenyl diazo 50 and fluorophenyl diazo 51 had comparable thermal stability to para-substituted 38 and 40, respectively. This suggests a steric clash with the larger functional groups, reducing the conjugation of the aryl ring with the diazoester due to the out-of-plane twisting of the aromatic ring despite the proximity of electron-withdrawing aryl substituents.
Figure 5.
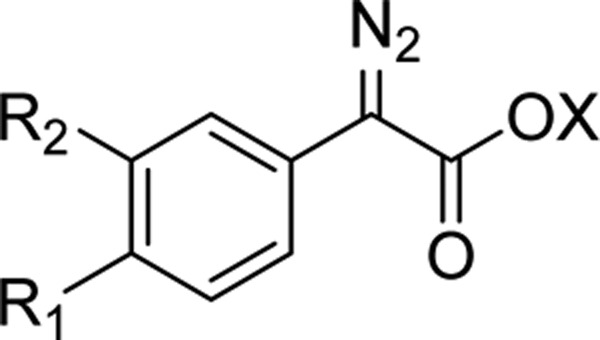
Model diazo compound.
In multisubstituted systems, similar trends in thermal stability are observed. Ethyl (3,4-dichlorophenyl)diazoacetate 54 and ethyl (3,4-dimethylphenyl)diazoacetate 55 have a similar Tonset to their 3- and 4-substituted analogues. Ethyl (3,4-dimethoxyphenyl) diazoacetate 56 is significantly stabilized compared to 4-OMe 38, due to meta-methoxy being σ-electron-withdrawing. Indeed, the presence of another methoxy group in 3,4,5-trimethoxyphenyl example 57 conferred another improvement to thermal stability.88
Ethyl (pyridin-4-yl)diazoacetate 60 was more thermally stable due to the electron-poor 4-pyridyl substituent. On the other hand, ethyl (pyridin-3-yl)diazoacetate 61 and 6-chloropyridin-3-yl 62, which cannot delocalize a negative charge of a potential carbene onto the nitrogen atom, displayed no significant difference vs benzo-analogues 9 and 41. Interestingly, the pyridin-2-yl diazo compound 63 adopts the triazolo tautomer and has a thermal stability far surpassing the diazo compounds in this study.
Acceptor/acceptor-type diazo compounds can be contrasted to the donor/acceptor compounds. Ethyl diazoacetate (EDA) has comparable thermal stability to ethyl (phenyl)diazoacetate 9, though the initiation is observed at a lower temperature. While another electron-withdrawing substituent clearly increases the thermal stability of the diazo compound for diazoesters (64–69), cyanodiazo compounds (70–72) have comparable or lower onset temperatures even with an α-carbonyl. The onset temperature for benzyl ester 71 is comparable to that for ethyl esters with electron-poor aromatics such as 62. Donor/acceptor aryl trifluoromethyldiazo compounds 73 and 74 also have lower onset temperatures than their aryl ester analogues 9 and 42.
The mean ΔHD for diazo compounds 9, 10, and 34–74 (excluding EDA, 44, 64, and 65) is −102 kJ mol–1, which confirms that diazo compound energetic yield is significantly lower than sulfonyl azides. Sulfone 65 and nitro-containing 44 and 64 are exceptions, where adding additional energetic functional groups clearly increases the enthalpy of decomposition of the single main transition, indicating that the other energetic functional groups concurrently or sequentially pyrolyze during the diazo decomposition, increasing the measured enthalpy. This average value is on the order of 50 kJ mol–1 lower than previous reports of similar diazo compounds 9 and 10, which could be attributed to differences in equipment and methodology, particularly the heating rate and how the exothermic peak is integrated, and in the case of α-vinyl diazo compound 13, a different decomposition pathway. EDA has a significantly higher ΔHD than the average diazo compound, as was also previously reported, indicating that the decomposition involves both the loss of N2 and additional processes.
DSC Analysis of Sulfonyl Azides and Other Diazo Transfer Reagents
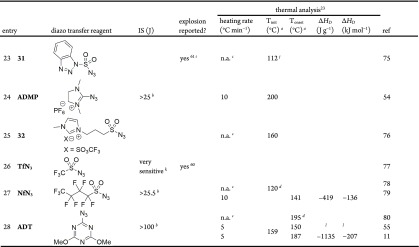
DSC data for thermal stability and energetics of decomposition were also collected for several common diazo transfer reagents (Table 4). They are generally more thermally stable than diazo compounds, but the decomposition has a larger energetic yield. The data were broadly similar to previous reports (cf. Table 2) although both TsN3 and ortho-nitrobenzenesulfonyl azide (o-NBSA) were found to be almost 30% less energetic. For the same decomposition of an energetic functional group, the ΔHD per mole might be expected to be quite similar, which is not seen in the broad range of sulfonyl azide results in the literature (Table 2, −334 to −136 kJ mol–1). With DSC data, it is important to make comparisons between compounds using the same technique, ideally on the same machine. The sulfonyl azide data in Table 4 has an average of −201 kJ mol–1 (excluding o-NBSA, ADMP, and ADT), similar to all of the literature sulfonyl azide DSC data (average −226 kJ mol–1); however, the literature ΔHD data has a much broader range and higher sample standard deviation (139.6 vs 10.7 for the data in Table 4), which is likely to be a reflection of the different methods and equipment used. This dataset therefore provides a more standardized set of measurements for comparison of the thermal stability of these reagents.
Table 4. DSC Analysis of Selected Diazo Transfer Reagents.
| entry | compounda | Tinit (°C) | Tonset (°C) | ΔHD (J g–1) | ΔHD (kJ mol–1) |
|---|---|---|---|---|---|
| 1 | TsN3 | 128 | 158 | –1057 | –208 |
| 2 | p-ABSA | 130 | 156 | –790 | –190 |
| 3 | p-ABSAb | 120 | 138 | –928 | –223 |
| 4 | p-CBSA | 148 | 167 | –923 | –210 |
| 5 | m-CBSA | 93c | 155 | –920 | –209 |
| 6 | o-NBSA | 123 | 151 | –1541 | –352 |
| 7 | ADMP | 167 | 202 | –793 | –226 |
| 8 | NfN3 | 102 | 136 | –582 | –189 |
| 9 | ADT | 159 | 186 | –1135 | –207 |
Results using DSC method A and average results, where n = 2 unless otherwise noted.
Using DSC method B and n = 1.
Reported as the initiation of melting point, which obscures exotherm initiation.
Prediction of Sensitivity and Explosion Hazards: Yoshida Correlation
In a seminal work, Yoshida conducted impact sensitivity and explosive propagation (EP) experiments on a number of energetic materials and developed a correlation to predict these properties from DSC results.10,89 The Yoshida correlations (eqs 2 and 3) use Tonset (in °C) and ΔHD (as Q, an inverse value in cal g–1 representing energy output) and give a dimensionless number. A value >0 predicts that a material will exhibit impact sensitivity (IS) and/or explosive propagation (EP), while a value <0 predicts it will not. Additionally, even a sample that gives a null prediction could still result in a safety incident if the exothermic decomposition is not respected
| 2 |
| 3 |
Process safety scientists at Pfizer recently published an assessment of peptide coupling reagents, where the authors used a more conservative version of this correlation (eqs 4 and 5), where the Q term was reduced by 25% and Tinit was used instead of Tonset(3a)
| 4 |
| 5 |
We applied both correlations to the literature DSC data for diazo transfer reagents from Table 2 to predict impact sensitivity and explosive properties, which will give an indication of sensitivity and explosivity hazards for compounds for which DSC is reported, but the specialist tests have not been undertaken. For those with specialist data reported, the IS and EP predictions were compared with those reports (Table S2).
The Yoshida correlation correctly flags all diazo transfer reagents, which are reported to be impact-sensitive with the exception of naphthalene-2-sulfonyl azide 21. The more conservative Pfizer-modified correlation on the other hand flags NfN3 and ADT, both of which have been experimentally demonstrated to be insensitive to impact.56,81para-Carboxybenzenesulfonyl azide (p-CBSA) and p-ABSA are predicted to be insensitive to impact; however, there are reports of some impact sensitivity and they are categorized as “less sensitive” and infrequently react to impact loadings of almost 30 J.65,70
Both correlations correctly predict explosivity for reagents that are known to detonate; the Pfizer-modified correlation may be overly conservative as it flags several commonly used compounds that do not appear to be explosive, such as p-ABSA. Any reagent flagged by the less conservative Yoshida EP correlation, which from the literature data is only p-bromobenzenesulfonyl azide 19, should be regarded as explosive unless further testing is undertaken. p-DBSA, m-CBSA, naphthalene-2-sulfonyl azide 21, triisopropylbenzenesulfonyl azide 22, polystyrene-supported benzenesulfonyl azide 23, and ADT are predicted to be nonexplosive.90
Application of the correlations to literature DSC data for diazo compounds highlighted that both allyl (phenyl)diazoacetate 11 and vinyl diazoester 13 are predicted to be impact-sensitive and explosive by the Yoshida correlation, primarily due to the reportedly low onset temperatures. Hypervalent iodine diazoacetates 14 and 15 and NHPI-DA 16 are not predicted to be impact-sensitive or explosive.
Prediction of the Sensitivity and Explosion Hazards of Diazo Compounds
Both correlations were applied to all diazo compound DSC results from Table 3 to predict impact sensitivity or explosive properties (see Table S3 for data). Figure 6 plots the DSC results and compares the Yoshida correlation and the more conservative Pfizer-modified prediction. While the Yoshida correlation predicts that none of the tested compounds are impact-sensitive, the Pfizer-modified correlation predicts many of the diazo compounds to be impact-sensitive. Only 35, 36, 42, 54, triazolo 63, and 69 are predicted to be insensitive using the Pfizer-modified IS correlation. Interestingly, the compounds flagged as insensitive contain more “molecular ballast”, i.e., compounds with bromo-, dichloro-, phosphonyl-, or menthyl-functionality, with a lower proportion of the overall molecular weight being due to the energetic functional group. This reduces the energetic yield per unit mass, while the energetic yield per unit mole remains the same. Triazolo 63 is significantly stabilized in the dominant aromatic tautomer. None of the peptide-coupling reagents assessed by Sperry et al. were flagged by the Yoshida IS correlation, while six were flagged by the Pfizer-modified IS correlation.3a Of these, four compounds exhibited IS. Based on this, we suggest that the Yoshida correlation may not flag some impact-sensitive materials and the Pfizer-modified prediction more accurately predicts impact sensitivity for diazo compounds.
Figure 6.
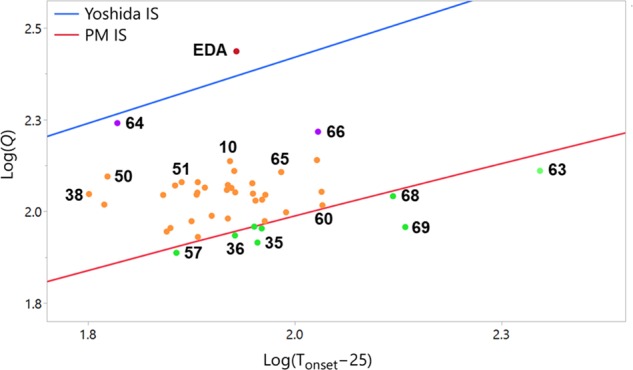
Plot of log(Q) vs log(Tonset – 25) for diazo compounds 9, 10, and 34–74 compared to the IS correlation limits, with selected compounds highlighted. See SI for further details.
Figure 7 plots the same DSC results with both the Yoshida and Pfizer-modified EP predictions (see SI) and highlights that only ketodiazoacetate 66 is predicted to be capable of detonation by only the more conservative PM correlation. The Pfizer study determined that all compounds flagged by the modified EP correlation but not the Yoshida correlation were not considered explosive. We therefore do not predict explosivity for any of the tested diazo compounds.
Figure 7.
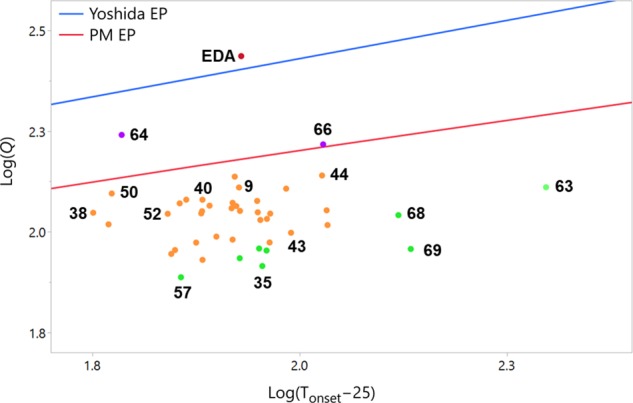
Plot of log(Q) vs log(Tonset – 25) for diazo compounds 9, 10, and 34–74 compared to the EP correlation limits, with selected compounds highlighted. See SI for further details.
Estimation of Maximum Recommended Process Temperature To Avoid Hazardous Thermal Decomposition
Exotherms of the magnitude typically observed for diazo compounds and sulfonyl azides are a cause for concern. To initially determine safe process operating conditions at GSK, the formula given in eq 6 is used,2 with Tinit (in °C) from a DSC experiment collected at a 2 °C min–1 heating rate. This allows for a conservative first approximation of safe operating temperature with a reasonable margin of safety, from a simple experiment using readily available DSC equipment.
| 6 |
TD24 is the temperature at which the time to maximum rate under adiabatic conditions (TMRad) becomes >24 h. This equation relies on the assumptions that at the detection limit of an exotherm in the DSC experiment (Tinit), the conversion is close to zero and thus the heat release rate is equal to the detection limit, as well as the conservative assumptions of a relatively low activation energy (50 kJ mol–1) and zero-order kinetics for the decomposition. Because of the limited sensitivity of the DSC test, values are given to the nearest 5 °C.
TD24 was determined for selected diazo compounds using DSC data generated by method B (Table 5). For the donor/acceptor diazo compounds 9, 10, 50, and 57, the TD24 was found to be below 20 °C, which means that these compounds should not be processed above ambient temperature as some amount of decomposition may occur. If temperature control is not applied, it could lead to a thermal runaway and explosion, depending on the quantity of the diazo compound. Acceptor/acceptor diazo compound 66 (Table 5, entry 5) had a TD24 of 25 °C, indicative of its higher thermal stability.
Table 5. Estimated Maximum Recommended Process Temperatures for Selected Diazo Compounds.
| entry | compound | TD24 (°C) |
|---|---|---|
| 1 | 9 | 10 |
| 2 | 10 | 10 |
| 3 | 50 | –5 |
| 4 | 57 | 0 |
| 5 | 66 | 25 |
Though the formula is not directly applicable to DSC data using a 5 °C min–1 heating rate, which gives higher Tinit than the 2 °C min, it can be used to give an indication of a safe operating window. We found that despite the higher TD24 using this data, most diazo compounds in Table 3 had a value below 25 °C; so, it is not recommended to prepare or use these compounds with heating and that additional cooling measures should be considered. Only triazolo 63, ethyl (4-nitrophenyl)diazoacetate 44, acceptor/acceptor amide/ester 67, malonate 68, and phosphonyl/ester 69 were found to have a TD24 above ambient temperature using the 5 °C min–1 data.
It is important to note that for a process scale reaction, more complex and reliable thermal safety assessments may be conducted. For example, using ARC or reaction calorimeters on a case-by-case basis, which would seek to understand other process and reaction parameters and interactions. This enables a suitable basis of safety to be defined for the process to be scaled up safely.
Accelerating Rate Calorimetry (ARC)
DSC is used as an initial screening test by process safety at GSK when considering a reaction that is intended to be undertaken on a laboratory scale. Any sample exhibiting an exotherm above 300 J g–1 may be classed as self-reactive as defined by the United Nations Globally Harmonized System of Classification and Labelling of Chemicals and would therefore be submitted for further testing. ARC gives an approximately 10-fold more sensitive determination of Tinit by operating in a heat-wait-search mode: a sample is heated in predefined temperature steps (typically 5 °C), stabilized at each temperature where the ARC searches for an exotherm at a predefined sensitivity. When the sample begins exothermic decomposition, the ARC heating matches the self-heating rate of the sample to simulate adiabatic conditions. The rate of pressure and temperature rise is measured. Compared to DSC, the technique is more sensitive but requires significantly more time and sample (typically between 3 and 7 g).
To provide highly accurate thermal stability data, ethyl (phenyl)diazoacetate 9 (3.355 g) was tested in an ARC using the heat-wait-search mode, and the temperature and pressure plots were recorded (Figure 8). The decomposition initiated at 60 °C, which was followed by a long incubation time where the temperature rise rate remained below 0.1 °C min–1; the TMRad was calculated to be 183 min. The maximum rate of temperature rise was 4110 °C min–1, and the maximum rate of pressure rise was 3094 bar min–1.91 The maximum temperature was 340 °C, and the maximum pressure was 202 bar. Sperry suggests that materials exhibiting a rate of pressure rise of >17 000 bar min–1 should be considered for further explosivity testing, albeit using the faster modified “ScanARC” method.3a,8a This adds further evidence that diazo compound 9 is not predicted to be explosive, but the significant rate of heat and pressure generation must be respected.
Figure 8.
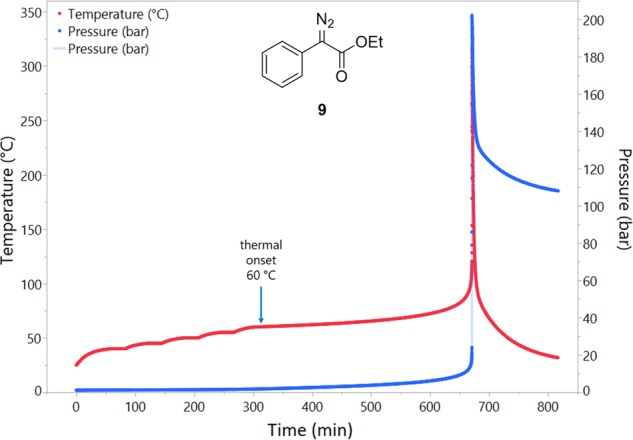
Plot of temperature and pressure during the ARC experiment with 9.
Diazo transfer reagent p-ABSA (3.699 g) was analyzed using the same ARC protocol (Figure 9). The decomposition initiated at 100 °C, and the initial decomposition was much less smooth than the diazo compound, exhibiting small jumps in temperature due to the initially very slow rate. The experiment had to be stopped as the decomposition developed to protect the ARC equipment and only the initial stages were measured. Using the same ARC protocol, another diazo transfer reagent ADT (2.107 g) was also analyzed (Figure S104); the thermal onset was found to be 125 °C, and the TMRad was calculated to be 123 min; the maximum temperature was 350 °C, with a maximum rate of temperature rise of 2314 °C min–1, and the maximum pressure was 284 bar, with a maximum rate of pressure rise of 13,461 bar min–1.91
Figure 9.
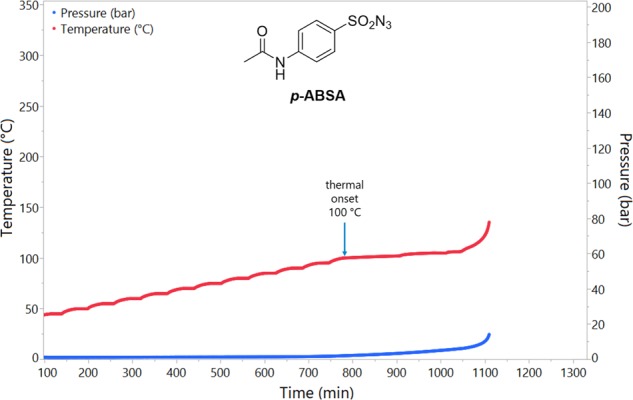
Plot of temperature and pressure during the ARC experiment with p-ABSA.
Contextualizing the Hazards of Diazo Compounds
The energy release from an uncontrolled decomposition of sulfonyl azides or diazo compounds is typically rapid and results in thermal runaway under adiabatic conditions. Sulfonyl azides are clearly more energetic than diazo compounds, which have a similar ΔHD to the decomposition of hydrogen peroxide (see Figure 10 for comparisons).3a,10,92−94 In the context of a reaction, any process that intends to decompose these compounds to effect the desired chemistry would be best conducted with adequate cooling and slow addition of initiator to manage exotherm development.
Figure 10.
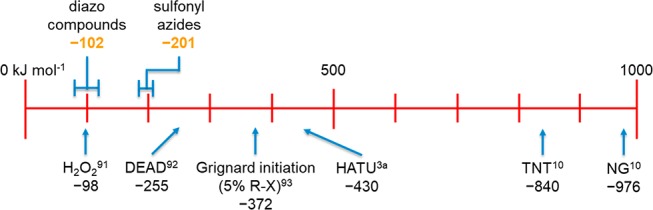
Indicative scale of ΔHD for known exothermic reagents.
From the literature diazo transfer reagent IS data in Table 2, it can be seen that some reagents are classed as sensitive and should be handled with extreme care when neat (TsN3, MsN3, o-NBSA, TfN3) if at all; most other reagents fall into the less sensitive category (including p-ABSA) or are insensitive to impact (ADMP, NfN3, ADT). In the lab, impacts to neat reagent are most likely to be from manipulation during reaction preparation: handling with spatula, agitation with a stirrer or dropping a container. From our Yoshida correlation IS predictions, we recommend that neat diazo compounds are handled with similar care as impact sensitivity is a strong possibility for all but the most stable examples. Some impact sensitivities of well-defined energetic materials are given in Table S4 for comparison.
The principal hazard of using diazo compounds (ignoring toxicity), sulfonyl azides, and indeed many other exothermic reactions involving potentially unstable reagents is the risk of thermal runaway and pressure generation. Thermal runaway occurs because as the temperature of a reaction mixture increases, the rate of reaction (and thus heat output for exothermic processes) increases exponentially, whereas the rate of cooling remains linear. For this reason, scale-up should always be approached with appropriate caution. Metal-carbene chemistry relies upon the decomposition of the diazo compound, which is similarly exothermic to thermally induced decomposition and will evolve the same amount of N2 gas. For exotherms of the magnitude observed, on small scales (for example <1 mmol), the likelihood of thermal runaway is low due to effective cooling. Furthermore, the heat and pressure generated from a runaway decomposition are unlikely to present a major hazard due to the small reaction mass. Additionally, at dilute concentrations (for example, <0.3 M), the solvent mass acts as a heatsink for the energy generated. At larger scales, although there may be sufficient heatsink to prevent a hazard, there may be potential for serious consequences. More concentrated reaction mixtures present the possibility for the solvent mass to be heated rapidly and vaporized. Thus, any significant scale-up would require its own detailed thermal hazard assessment to enable safe reaction design.
Any chemist conducting this type of reaction should pay careful attention to the scale, concentration, temperature control of reaction vessel, and rate of addition when initiating the reaction and to err on the side of caution if in doubt. The addition of the initiating reagent should be slow to allow the effective management of the developing exotherm by the temperature control unit. Reaction vessel choice should seek to maximize surface-area-to-volume ratio and have an appropriate overpressure relief mechanism to manage the evolved N2.
Conclusions
Diazo compounds offer powerful synthetic routes to complex molecules but are often avoided due to justifiable concerns about the potential hazards. Though the perils of diazomethane are well known, there is little data available to describe the hazards of more complex diazo compounds despite their frequent appearance in the literature; when results are presented, vital experimental detail is often absent. We have presented consistent DSC data for 44 donor/acceptor and acceptor/acceptor diazo compounds. Onset temperatures were found to be in the range of 75–160 °C, and the thermal stability was significantly affected by substituents. Generally, electron-withdrawing groups were found to improve thermal stability and increase Tinit and Tonset, whereas electron-donating groups have the opposite effect. More complex resonance effects were also noted, as steric clashes resulted in out-of-plane twisting and therefore reduced the conjugation of the diazo functional group with the aryl ring, leading to a destabilizing effect. A statistical model was demonstrated to reasonably predict Tonset for hypothetical alkyl (phenyl)diazoacetates based on the Hammett parameter σm or σp+ of the aryl substituent.
A formula was used to estimate the maximum recommended process temperature to avoid unwanted decomposition; for most of the tested compounds, to avoid decomposition, it is not recommended to use above 25 °C or store above 0 °C, excepting particularly stabilized acceptor/acceptor diazo compounds. The diazo compounds were found to have a mean ΔHD of −102 kJ mol–1, and it is expected that a similar ΔHD would be observed for similar compounds where the diazo functional group is the only source of exothermic decomposition. Several diazo transfer reagents were also tested using the same DSC method and found to have generally higher thermal stability in agreement with the reported data and, for sulfonyl azides, a mean ΔHD of −201 kJ mol–1. Considerable variation in Tonset and ΔHD results was found in the literature, likely due to different (and unreported) heating rates, highlighting the importance of exhaustive experimental detail in reporting DSC results. High-quality thermal hazard data from the ARC is presented on ethyl (phenyl)diazoacetate 9, which was found to initiate decomposition at 60 °C. The diazo transfer reagent p-ABSA was found to initiate decomposition at 100 °C. Significant pressure development was measured in addition to the exotherm and constitutes a considerable explosion hazard if the compounds are heated without an overpressure relief.
Yoshida’s predictive correlation and a more conservative modified form from Pfizer were applied to the DSC data to screen for impact sensitivity and explosive propagation. Many of the diazo compounds tested are predicted to be impact-sensitive as a neat substance, though all are predicted to be less sensitive than the borderline compound m-DNB. It is therefore recommended to treat diazo compounds with particular caution when neat, particularly to avoid impacts or physical processing at scale, especially for lower-molecular-weight examples. None of the diazo compounds tested are predicted to be explosive. All of the compounds still demonstrate a significant exothermic decomposition, which must be respected, particularly when utilizing the reaction of diazo compounds with a metal catalyst for metal-carbene chemistry. As such, the addition of the “initiator” in the preparation and use of diazo compounds should always be conducted slowly and with adequate temperature control. While some of these observations may be in line with the perceived knowledge, we believe that this study will provide a valuable reference and standard set of experimental conditions for those working with these reagents. While on a small scale, diazo compounds will continue to be widely used, there are clear challenges to adapting these reagents to process scales. Part of the evaluation of the industrial viability and safety of any diazo transfer reagent or diazo compound should include DSC (and follow-up ARC) to determine safe operating conditions.
Experimental Section
DSC Experimental Terminology
Tinit is defined by the temperature at which the heat flow is measured to be >0.01 W g–1 from the baseline. Tonset is defined by the intersection of the extrapolated maximum peak slope and the baseline. ΔHD is obtained by integration of the peak. Averaged DSC results reported where individual results have <2% difference from the average for Tinit and Tonset and <10% difference from the average for ΔHD. Where noted, if a melting point overlaps with the decomposition, the melt endotherm was removed and blended with adjacent data to allow Tonset and ΔHD to be more accurately determined. In these cases, if Tinit cannot be determined due to the melt endotherm, the temperature where the melt commences is used.
DSC Method A
DSC analysis was performed using a TA Instruments Q2000 DSC with a liquid nitrogen cooling system (LNCS). Approx 5 mg was accurately weighed to 1 d.p. into a reusable PerkinElmer B0182901 stainless steel high-pressure crucible with a disposable gold-plated copper seal rated to 150 bar and sealed under air with the appropriate B0182864 sealing tool. After equilibrating at 25 °C, samples were heated at 5 °C min–1 to a maximum temperature of 250 °C for diazo compounds and 300 °C for other compounds. Data analysis was conducted using TA Instruments Universal Analysis 2000 software version 4.5.0.5.
DSC Method B
DSC analysis was performed using a Mettler Toledo DSC 823e. Approx 5 mg was accurately weighed to 1 d.p. into a Mettler Toledo ME-26731 gold-plated 40 μL high-pressure crucible with seal insert and lid rated to 150 bar and sealed under air with the appropriate sealing press. After equilibrating at 25 °C, samples were heated at 2 °C min–1 to a maximum temperature of 350 °C. Data analysis was conducted using a Mettler Toledo STARe software version 12.00 build 5342.
TGA Method
TGA analysis was performed using a Mettler Toledo TGA/DSC 1LF/UMX. A tare weight was obtained for a Mettler Toledo 70 μL platinum crucible (open, medium, product no. 51119654) in the TGA before approx 30 mg was weighed into the crucible and reweighed in the TGA. A purge gas flow rate of 50 mL min–1 N2 was used. After equilibrating at 25 °C, the sample was heated at 5 °C min–1 to a maximum temperature of 300 °C. The data was visualized with JMP Pro version 14.3.0.
ARC Method
Approx 3.5 g of sample was accurately weighed into a Hastelloy ARC test cell (or “bomb”). The ARC test cell was placed in a Thermal Hazard Technology ES ARC and thermocouple attached by an equatorial clip. The experiment was performed using a heat-wait-search method starting at 40 °C, and the test cell was heated in 5 °C increments to a maximum temperature of 280 °C, a pressure of 35 bar, or user intervention. At the end of the experiment, the ARC cools the test cell with compressed air. Data analysis was performed using Thermal Hazard Technology’s ARCCal+ software version 1.6.2, which applies the Φ factor to correct the ARC thermal onset temperature and adiabatic temperature rise (ATR) results for the thermal inertia of the test cell using eq 7
| 7 |
where M is the mass (g), C is the specific heat capacity (J kg–1 K–1), b is the bomb (test cell), cl is the clip attached to the bomb, and s is the sample. The time to maximum rate under adiabatic conditions (TMRad) was calculated from the ARC data at the exotherm onset using eq 8
| 8 |
where tmax is the time at the maximum self-heat rate, and t0 is the time at the start of the exotherm.
The data was visualized with JMP Pro version 14.3.0.
Acknowledgments
For financial support, the authors gratefully acknowledge GlaxoSmithKline, the Pharmacat Consortium, and the EPSRC for iCASE studentship funding, and The Royal Society [University Research Fellowship (UF140161, to JAB) and Research Grants (RG150444 and RGF\EA\180031)]. The authors thank Alexander J. Boddy for preparing many of the compounds assessed, as well as Dr Owen A. Davis, Dr Dominic P. Affron, Anna Dobre, and Roland Li.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.oprd.9b00422.
DSC plots for all compounds, synthetic details, full table of Hammett σ parameters correlated to DSC results, abnormalities observed in DSC plots, full table of Yoshida correlation output for all compounds, and 1H and 13C NMR spectra for 49 and 58 (PDF)
Full table of Yoshida and Pfizer-modified correlation output (XLSX)
The authors declare no competing financial interest.
Notes
All characterization data for synthesized compounds, raw DSC data for all compounds, raw TGA data for 9 and raw ARC data for 9, p-ABSA, and ADT can be found at https://doi.org/10.14469/hpc/6274.
Supplementary Material
References
- a Zhang T. Y. Process Chemistry: The Science, Business, Logic, and Logistics. Chem. Rev. 2006, 106, 2583–2595. 10.1021/cr040677v. [DOI] [PubMed] [Google Scholar]; b Butters M.; Catterick D.; Craig A.; Curzons A.; Dale D.; Gillmore A.; Green S. P.; Marziano I.; Sherlock J.; White W. Critical Assessment of the Pharmaceutical Processes – A Rationale for Changing the Synthetic Route. Chem. Rev. 2006, 106, 3002–3027. 10.1021/cr050982w. [DOI] [PubMed] [Google Scholar]
- Stoessel F.Thermal Safety of Chemical Processes; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008. [Google Scholar]
- For recent examples of studying hazardous properties of reagents or reactions, see:; a Sperry J. B.; Minteer C. J.; Tao J.; Johnson R.; Duzguner R.; Hawksworth M.; Oke S.; Richardson P. F.; Barnhart R. W.; Bill D. R.; Giusto R. A.; Weaver J. D. Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used In Pharmaceutical Manufacturing. Org. Process Res. Dev. 2018, 22, 1262–1275. 10.1021/acs.oprd.8b00193. [DOI] [Google Scholar]; b Richardson M. B.; Brown D. B.; Vasquez C. A.; Ziller J. W.; Johnston K. M.; Weiss G. A. Synthesis and Explosion Hazards of 4-Azido-l-phenylalanine. J. Org. Chem. 2018, 83, 4525–4536. 10.1021/acs.joc.8b00270. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Alazet S.; Preindl J.; Simonet-Davin R.; Nicolai S.; Nanchen A.; Meyer T.; Waser J. Cyclic Hypervalent Iodine Reagents for Azidation: Safer Reagents and Photoredox-Catalyzed Ring Expansion. J. Org. Chem. 2018, 83, 12334–12356. 10.1021/acs.joc.8b02068. [DOI] [PubMed] [Google Scholar]; d Dallaston M. A.; Bettencourt C. J.; Chow S.; Gebhardt J.; Spangler J.; Johnston M. R.; Wall C.; Brusnahan J. S.; Williams C. M. Ranking Oxidant Sensitiveness: A Guide for Synthetic Utility. Chem. – Eur. J. 2019, 25, 9614–9618. 10.1002/chem.201902036. [DOI] [PubMed] [Google Scholar]
- For comprehensive works on energetic materials, terminology and their characterization see; a Klapötke T. M.Chemistry of High-Energy Materials, 3rd ed.; Walter de Gruyter GmbH: Berlin, 2015. [Google Scholar]; b Matyáš R.; Pachman J.. Primary Explosives, 1st ed.; Springer-Verlag: Berlin, Heidelberg, Germany, 2012. [Google Scholar]
- a Nefati H.; Diawara B.; Legendre J. J. Predicting the Impact Sensitivity of Explosive Molecules Using Neuromimetic Networks. SAR QSAR Environ. Res. 1993, 1, 131–136. 10.1080/10629369308028824. [DOI] [PubMed] [Google Scholar]; b Tian B.; Xiong Y.; Chen L.; Zhang C. Relationship between the Crystal Packing and Impact Sensitivity of Energetic Materials. CrystEngComm 2018, 20, 837–848. 10.1039/C7CE01914A. [DOI] [Google Scholar]
- Sensitivity to energy imparted by an impact is generally considered to be dependent on electron-deficient regions occurring over covalent bonds.; a Rice B. M.; Hare J. J. A Quantum Mechanical Investigation of the Relation between Impact Sensitivity and the Charge Distribution in Energetic Molecules. J. Phys. Chem. A 2002, 106, 1770–1783. 10.1021/jp012602q. [DOI] [Google Scholar]; There are a number of tests available to measure this property, from the rudimentary ‘hammer-and-anvil test’; b Frurip D. J.; Gorman D. G.; Klosin J.; Buske G. R. Evaluating Hazards of Reactive Chemicals, Especially Azides. Chem. Eng. News 2012, 12, 1287–1292. [Google Scholar]; c Weaver M.; Blair L. H.; Flood N.; Stennett C.. A Review of the Mallet Impact Test for Small Scale Explosive Formulations. In Proceedings of the 19th Seminar on New Trends in Research of Energetic Materials, Part 2; Pachmáň J.; Šelešovský J., Eds.; NTREM, University of Pardubice: Czech Republic, April 20−22, 2016; pp 1037–1043; [Google Scholar]; to more rigorous tests used for determination of transportation classes. See ref (9). The drop (or fall) hammer tests involve a weight dropped onto a sample imparting a known amount of kinetic energy. If a reaction is observed (judged by a flash, sound or discoloration) the weight is decreased until a minimum energy can be determined.
- Though thermal stability or impact sensitivity are most commonly used to define an unstable subtance, there are a number of other sensitivities, which can be considered. Friction sensitivity is measured by placing the material between two plates and applying a defined load in a back-and-forth motion. Electrostatic discharge (ESD) sensitivity is measured with an applied electrical flow. Both tests are less common than impact sensitivity and suffer from more reproducibility issues. See ref (4). Some energetic compounds can also exhibit instability to light. Experimental values will be reported in the SI where they exist but due to the lack of available data and variability concerns will not be commented on further.
- If a combustion reaction is autocatalytic (or self-sustaining) a decomposition front is observed, where the energy released from exothermic decomposition of a molecule triggers decomposition of adjacent molecules, propagating through the material. Both TNT and wood can be considered materials with high energy content but differ in the velocity of a decomposition front. The decomposition is defined as deflagration (burning) if the front is subsonic, or detonation if the front moves faster than the speed of sound, creating a shockwave. This is measured by a variety of specialist tests, such as the Mk. III Ballistic Mortar; a sample of primary explosive such as PETN is placed in a shell containing the test material and detonated. If the shockwave can initiate detonation of the test material, it can be considered an explosive. As most chemists will not have access to this testing, Bodman and Chervin developed an alternative screening test using a modified ARC with ultra-fast pressure sensing in the sample capsule.; a Bodman G. T.; Chervin S. Use of ARC in Screening for Explosive Properties. J. Hazard. Mater. 2004, 115, 101–105. 10.1016/j.jhazmat.2004.06.022. [DOI] [PubMed] [Google Scholar]; Upon decomposition, the maximum rate of pressure rise can be measured and considered versus known explosive samples: >20 000 bar/min would be considered worthy of further testing, specified in the UN Treaty on the Transport of Dangerous Goods. See refs (3a) and (9).
- UN. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria (ST/SG/AC.10/11.Rev.6), 2015. ISBN 978-92-1-139155-8, link: www.unece.org/trans/areas-of-work/dangerous-goods/legal-instruments-and-recommendations/un-manual-of-tests-and-criteria/rev6-files.html.
- Yoshida T.; Yoshizawa F.; Itoh M.; Matsunaga T.; Watanabe M. Prediction of Fire and Explosion Hazard for Reactive Chemicals (I): Estimation of Explosive Properties of Self-Reactive Chemicals from SC-DSC Data. Kogyo Kayaku 1987, 48, 311–316. [Google Scholar]
- Green S. P.; Payne A. D.; Wheelhouse K. M.; Hallett J. P.; Miller P. W.; Bull J. A. Diazo Transfer Reagent 2-Azido-4,6-Dimethoxy-1,3,5-Triazine (ADT) Displays Highly Exothermic Decomposition Comparable to Tosyl Azide. J. Org. Chem. 2019, 84, 5893–5898. 10.1021/acs.joc.9b00269. [DOI] [PubMed] [Google Scholar]
- Selected reviews:; a Ye T.; McKervey M. A. Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 1994, 94, 1091–1160. 10.1021/cr00028a010. [DOI] [PubMed] [Google Scholar]; b Ford A.; Miel H.; Ring A.; Slattery C. N.; Maguire A. R.; McKervey M. A. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. 10.1021/acs.chemrev.5b00121. [DOI] [PubMed] [Google Scholar]; c Fructos M. R.; Díaz-Requejo M. M.; Pérez P. J. Gold and Diazo Reagents: A Fruitful Tool for Developing Molecular Complexity. Chem. Commun. 2016, 52, 7326–7335. 10.1039/C6CC01958G. [DOI] [PubMed] [Google Scholar]; d Mix K. A.; Aronoff M. R.; Raines R. T. Diazo Compounds: Versatile Tools for Chemical Biology. ACS Chem. Biol. 2016, 11, 3233–3244. 10.1021/acschembio.6b00810. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ciszewski Ł. W.; Rybicka-Jasińska K.; Gryko D. Recent Developments in Photochemical Reactions of Diazo Compounds. Org. Biomol. Chem. 2019, 17, 432–448. 10.1039/C8OB02703J. [DOI] [PubMed] [Google Scholar]
- a Lebel H.; Marcoux J. F.; Molinaro C.; Charette A. B. Stereoselective Cyclopropanation Reactions. Chem. Rev. 2003, 103, 977–1050. 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]; b Maas G. Ruthenium-Catalysed Carbenoid Cyclopropanation Reactions with Diazo Compounds. Chem. Soc. Rev. 2004, 33, 183–190. 10.1039/b309046a. [DOI] [PubMed] [Google Scholar]; c Davies H. M. L.; Antoulinakis E. G.. Intermolecular Metal-Catalyzed Carbenoid Cyclopropanations. Organic Reactions; John Wiley & Sons, Ltd.: Hoboken, NJ, 2001; Vol. 57, pp 1–326. [Google Scholar]
- For selected recent developments, including formation of pharmaceutically relevant motifs, see:; a Morandi B.; Carreira E. M. Iron-Catalyzed Cyclopropanation with Trifluoroethylamine Hydrochloride and Olefins in Aqueous Media: in situ Generation of Trifluoromethyl Diazomethane. Angew. Chem., Int. Ed. 2010, 49, 938–941. 10.1002/anie.200905573. [DOI] [PubMed] [Google Scholar]; b Lindsay V. N. G.; Nicolas C.; Charette A. B. Asymmetric Rh(II)-Catalyzed Cyclopropanation of Alkenes with Diacceptor Diazo Compounds: p-Methoxyphenyl Ketone as a General Stereoselectivity Controlling Group. J. Am. Chem. Soc. 2011, 133, 8972–8981. 10.1021/ja201237j. [DOI] [PubMed] [Google Scholar]; c Xu X.; Zhu S.; Cui X.; Wojtas L.; Zhang X. P. Cobalt(II)-Catalyzed Asymmetric Olefin Cyclopropanation with α-Ketodiazoacetates. Angew. Chem., Int. Ed. 2013, 52, 11857–11861. 10.1002/anie.201305883. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chawner S. J.; Cases-Thomas M. J.; Bull J. A. Divergent Synthesis of Cyclopropane-Containing Lead-Like Compounds, Fragments and Building Blocks through a Cobalt Catalyzed Cyclopropanation of Phenyl Vinyl Sulfide. Eur. J. Org. Chem. 2017, 2017, 5015–5024. 10.1002/ejoc.201701030. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Wei Y.; Tinoco A.; Steck V.; Fasan R.; Zhang Y. Cyclopropanations via Heme Carbenes: Basic Mechanism and Effects of Carbene Substituent, Protein Axial Ligand, and Porphyrin Substitution. J. Am. Chem. Soc. 2018, 140, 1649–1662. 10.1021/jacs.7b09171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien J.; Davulcu A. H.; DelMonte A. J.; Fraunhoffer K. J.; Gao Z.; Hang C.; Hsiao Y.; Hu W.; Katipally K.; Littke A.; Pedro A.; Qiu Y.; Sandoval M.; Schild R.; Soltani M.; Tedesco A.; Vanyo D.; Vemishetti P.; Waltermire R. E. The First Kilogram Synthesis of Beclabuvir, an HCV NS5B Polymerase Inhibitor. Org. Process Res. Dev. 2018, 22, 1393–1408. 10.1021/acs.oprd.8b00214. [DOI] [Google Scholar]; To our knowledge, this is the only recently reported process scale application of a diazo compound as a metal-carbene precursor and required controlling diazo to <10 wt % in heptane.
- a Davies H. M. L.; Huby N. J. S.; Cantrell W. R.; Olive J. L. α-Hydroxy Esters as Chiral Auxiliaries in Asymmetric Cyclopropanations by Rhodium(II)-Stabilized Vinylcarbenoids. J. Am. Chem. Soc. 1993, 115, 9468–9479. 10.1021/ja00074a012. [DOI] [Google Scholar]; b Davies H. M. L.; Bruzinski P. R.; Lake D. H.; Kong N.; Fall M. J. Asymmetric Cyclopropanations by Rhodium(II) N-(Arylsulfonyl)Prolinate Catalyzed Decomposition of Vinyldiazomethanes in the Presence of Alkenes. Practical Enantioselective Synthesis of the Four Stereoisomers of 2-Phenylcyclopropan-1-Amino Acid. J. Am. Chem. Soc. 1996, 118, 6897–6907. 10.1021/ja9604931. [DOI] [Google Scholar]
- a Demonceau A.; Noels A. F.; Hubert A. J.; Teyssié P. Transition-Metal-Catalysed Reactions of Diazoesters. Insertion into C–H Bonds of Paraffins by Carbenoids. J. Chem. Soc., Chem. Commun. 1981, 14, 688–689. Selected reviews: 10.1039/C39810000688. [DOI] [Google Scholar]; First described:; b Davies H. M. L.; Beckwith R. E. J. Catalytic Enantioselective C–H Activation by Means of Metal-Carbenoid-Induced C–H Insertion. Chem. Rev. 2003, 103, 2861–2903. 10.1021/cr0200217. [DOI] [PubMed] [Google Scholar]; c Davies H. M. L.; Manning J. R. Catalytic C–H Functionalization by Metal Carbenoid and Nitrenoid Insertion. Nature 2008, 451, 417–424. 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Davies H. M. L.; Morton D. Guiding Principles for Site Selective and Stereoselective Intermolecular C–H Functionalization by Donor/Acceptor Rhodium Carbenes. Chem. Soc. Rev. 2011, 40, 1857. 10.1039/c0cs00217h. [DOI] [PubMed] [Google Scholar]; e Ye F.; Qu S.; Zhou L.; Peng C.; Wang C.; Cheng J.; Hossain M. L.; Liu Y.; Zhang Y.; Wang Z. X.; Wang J. Palladium-Catalyzed C–H Functionalization of Acyldiazomethane and Tandem Cross-Coupling Reactions. J. Am. Chem. Soc. 2015, 137, 4435–4444. 10.1021/ja513275c. [DOI] [PubMed] [Google Scholar]; f Xia Y.; Qiu D.; Wang J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. 10.1021/acs.chemrev.7b00382. [DOI] [PubMed] [Google Scholar]; Other selected asymmetric examples:; g Davies H. M. L.; Hansen T.; Churchill M. R. Catalytic Asymmetric C–H Activation of Alkanes and Tetrahydrofuran. J. Am. Chem. Soc. 2000, 122, 3063–3070. 10.1021/ja994136c. [DOI] [Google Scholar]; h Davies H. M. L.; Grazini M. V. A.; Aouad E. Asymmetric Intramolecular C–H Insertions of Aryldiazoacetates. Org. Lett. 2001, 3, 1475–1477. 10.1021/ol0157858. [DOI] [PubMed] [Google Scholar]; i Fu J.; Ren Z.; Bacsa J.; Musaev D. G.; Davies H. M. L. Desymmetrization of Cyclohexanes by Site- and Stereoselective C–H Functionalization. Nature 2018, 564, 395–399. 10.1038/s41586-018-0799-2. [DOI] [PubMed] [Google Scholar]
- a Gillingham D.; Fei N. Catalytic X–H Insertion Reactions Based on Carbenoids. Chem. Soc. Rev. 2013, 42, 4918–4931. 10.1039/c3cs35496b. [DOI] [PubMed] [Google Scholar]; For selected recent examples, see:; b Xu B.; Zhu S. F.; Xie X. L.; Shen J. J.; Zhou Q. L. Asymmetric N–H Insertion Reaction Cooperatively Catalyzed by Rhodium and Chiral Spiro Phosphoric Acids. Angew. Chem., Int. Ed. 2011, 50, 11483–11486. 10.1002/anie.201105485. [DOI] [PubMed] [Google Scholar]; c Keipour H.; Jalba A.; Delage-Laurin L.; Ollevier T. Copper-Catalyzed Carbenoid Insertion Reactions of α-Diazoesters and α-Diazoketones into Si–H and S–H Bonds. J. Org. Chem. 2017, 82, 3000–3010. 10.1021/acs.joc.6b02998. [DOI] [PubMed] [Google Scholar]; d Bartrum H. E.; Blakemore D. C.; Moody C. J.; Hayes C. J. Continuous-Flow Generation of Diazoesters and Their Direct Use in S–H and P–H Insertion Reactions: Synthesis of α-Sulfanyl, α-Sulfonyl, and α-Phosphono Carboxylates. Tetrahedron 2013, 69, 2276–2282. 10.1016/j.tet.2013.01.020. [DOI] [Google Scholar]
- For process scale intramolecular N–H insertions, see:; a Ratcliffe R. W.; Salzmann T. N.; Christensen B. G. A Novel Synthesis of the Carbapen-2-em Ring System. Tetrahedron Lett. 1980, 21, 31–34. 10.1016/S0040-4039(00)93616-5. [DOI] [Google Scholar]; b Karady S.; Amato J. S.; Reamer R. A.; Weinstock L. M. Stereospecific Conversion of Penicillin to Thienamycin. J. Am. Chem. Soc. 1981, 103, 6765–6767. 10.1021/ja00412a048. [DOI] [Google Scholar]
- For X–H insertion and cyclization, see for example:; a Davis O. A.; Bull J. A. Synthesis of Di-, Tri-, and Tetrasubstituted Oxetanes by Rhodium-Catalyzed O–H Insertion and C–C Bond-Forming Cyclization. Angew. Chem., Int. Ed. 2014, 53, 14230–14234. 10.1002/anie.201408928. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Davis O. A.; Croft R. A.; Bull J. A. Synthesis of Diversely Functionalised 2,2-Disubstituted Oxetanes: Fragment Motifs in New Chemical Space. Chem. Commun. 2015, 51, 15446–15449. 10.1039/C5CC05740J. [DOI] [PubMed] [Google Scholar]; c Davis O. A.; Croft R. A.; Bull J. A. Synthesis of Substituted 1,4-Dioxenes through O–H Insertion and Cyclization Using Keto-Diazo Compounds. J. Org. Chem. 2016, 81, 11477–11488. 10.1021/acs.joc.6b02134. [DOI] [PubMed] [Google Scholar]; d Nicolle S. M.; Lewis W.; Hayes C. J.; Moody C. J. Stereoselective Synthesis of Highly Substituted Tetrahydrofurans through Diverted Carbene O–H Insertion Reaction. Angew. Chem., Int. Ed. 2015, 54, 8485–8489. 10.1002/anie.201502484. [DOI] [PubMed] [Google Scholar]; e Nicolle S. M.; Lewis W.; Hayes C. J.; Moody C. J. Stereoselective Synthesis of Functionalized Pyrrolidines by the Diverted N–H Insertion Reaction of Metallocarbenes with β-Aminoketone Derivatives. Angew. Chem., Int. Ed. 2016, 55, 3749–3753. 10.1002/anie.201511433. [DOI] [PubMed] [Google Scholar]; f Boddy A. J.; Affron D. P.; Cordier C. J.; Rivers E. L.; Spivey A. C.; Bull J. A. Rapid Assembly of Saturated Nitrogen Heterocycles in One-Pot: Diazo-Heterocycle “Stitching” by N–H Insertion and Cyclization. Angew. Chem., Int. Ed. 2019, 58, 1458–1462. 10.1002/anie.201812925. [DOI] [PubMed] [Google Scholar]; g Choi S.; Ha S.; Park C.-M. α-Diazo Oxime Ethers for N-Heterocycle Synthesis. Chem. Commun. 2017, 53, 6054–6064. 10.1039/C7CC02650A. [DOI] [PubMed] [Google Scholar]
- a Aggarwal V. K.; De Vicente J.; Bonnert R. V. A Novel One-Pot Method for the Preparation of Pyrazoles by 1,3-Dipolar Cycloadditions of Diazo Compounds Generated in situ. J. Org. Chem. 2003, 68, 5381–5383. 10.1021/jo0268409. [DOI] [PubMed] [Google Scholar]; b Babinski D. J.; Aguilar H. R.; Still R.; Frantz D. E. Synthesis of Substituted Pyrazoles via Tandem Cross-Coupling/Electrocyclization of Enol Triflates and Diazoacetates. J. Org. Chem. 2011, 76, 5915–5923. 10.1021/jo201042c. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wu L. L.; Ge Y. C.; He T.; Zhang L.; Fu X. L.; Fu H. Y.; Chen H.; Li R. X. An Efficient One-Pot Synthesis of 3,5-Disubstituted 1H-Pyrazoles. Synthesis 2012, 44, 1577–1583. 10.1055/s-0031-1290772. [DOI] [Google Scholar]; d Mykhailiuk P. K.; Ishchenko A. Y.; Stepanenko V.; Cossy J. Synthesis of Fluoroalkyl Pyrazoles from In-Situ-Generated C2F5CHN2 and Electron-Deficient Alkenes. Eur. J. Org. Chem. 2016, 2016, 5485–5493. 10.1002/ejoc.201600947. [DOI] [Google Scholar]; e Mykhailiuk P. K. New Life for Diazoacetonitrile (N2CHCN): in situ Generation and Practical Synthesis of CN-Pyrazoles. Eur. J. Org. Chem. 2015, 2015, 7235–7239. 10.1002/ejoc.201501027. [DOI] [Google Scholar]
- Simpson J. H.; Kotnis A. S.; Deshpande R. P.; Kacsur D. J.; Hamm J.; Kodersha G.; Merkl W.; Domina D.; Wang S. Y.. Development of a Multi-Kilogram Procedure To Prepare, Use, and Quench Ethyl Diazoacetate. In Managing Hazardous Reactions and Compounds in Process Chemistry; Pesti J. A.; Abdel-Magid A. F., Eds.; American Chemical Society: Washington DC, 2014; Chapter 9, pp 235–244. [Google Scholar]
- For the definitions of Tinit and Tonset used here and how they are obtained, see Experimental Section: DSC Experimental Terminology. Note that Tinit is sometimes referred to as Tonset, and if no definition is given, it is safer to assume Tonset is Tinit as defined here.
- de Boer T. J.; Backer H. J. Diazomethane. Org. Synth. 1956, 36, 16 10.15227/orgsyn.036.0016. [DOI] [Google Scholar]
- Bretherick L.Bretherick’s Handbook of Reactive Chemical Hazards, 4th ed.; Butterworth-Heinemann, 1990; p 693, entry 2533. [Google Scholar]
- Steacie E. W. R. The Thermal Decomposition of Diazomethane. J. Phys. Chem. A 1931, 35, 1493–1495. 10.1021/j150323a030. [DOI] [Google Scholar]
- Paulett G. S.; Ettinger R. Mass Spectra and Appearance Potentials of Diazirine and Diazomethane. J. Chem. Phys. 1963, 39, 825–827. 10.1063/1.1734330. [DOI] [Google Scholar]
- Aggarwal V. K.; Alonso E.; Hynd G.; Lydon K. M.; Palmer M. J.; Porcelloni M.; Studley J. R. Catalytic Asymmetric Synthesis of Epoxides from Aldehydes Using Sulfur Ylides with in situ Generation of Diazocompounds. Angew. Chem., Int. Ed. 2001, 40, 1430–1433. . [DOI] [PubMed] [Google Scholar]
- Closs G. L.; Moss R. A. Carbenoid Formation of Arylcyclopropanes from Olefins, Benzal Bromides, and Organolithium Compounds and from Photolysis of Aryldiazomethanes. J. Am. Chem. Soc. 1964, 86, 4042–4053. 10.1021/ja01073a029. [DOI] [Google Scholar]
- Phillips D.; Champion W. C. An Explosion during the Preparation of Diazoacetonitrile. J. Am. Chem. Soc. 1956, 78, 5452. 10.1021/ja01601a085. [DOI] [Google Scholar]
- Mykhailiuk P. K. New Life for Diazoacetonitrile (N2CHCN): in situ Generation and Practical Synthesis of CN-Pyrazoles. Eur. J. Org. Chem. 2015, 2015, 7235–7239. 10.1002/ejoc.201501027. [DOI] [Google Scholar]
- Wagenaar A.; Kransen G.; Engberts J. B. F. N. Synthesis and Acid-Catalyzed Decomposition of o-Nitrophenylsulfonyldiazomethane. J. Org. Chem. 1974, 39, 411–414. 10.1021/jo00917a031. [DOI] [Google Scholar]
- Fields R.; Tomlinson J. P. Preparation of Trifluoromethyl-Pyrazoles and -Pyrazolines by the Reaction of 2,2,2-Trifluorodiazoethane with Carbon-Carbon Multiple Bonds. J. Fluorine Chem. 1979, 13, 147–158. 10.1016/S0022-1139(00)81083-0. [DOI] [Google Scholar]
- Clark J. D.; Shah A. S.; Peterson J. C.; Patelis L.; Kersten R. J. A.; Heemskerk A. H. Detonation Properties of Ethyl Diazoacetate. Thermochim. Acta 2002, 386, 73–79. 10.1016/S0040-6031(01)00761-4. [DOI] [Google Scholar]
- Clark J. D.; Shah A. S.; Peterson J. C.; Patelis L.; Kersten R. J. A.; Heemskerk A. H.; Grogan M.; Camden S. The Thermal Stability of Ethyl Diazoacetate. Thermochim. Acta 2002, 386, 65–72. 10.1016/S0040-6031(01)00760-2. [DOI] [Google Scholar]
- Montesinos-Magraner M.; Costantini M.; Ramírez-Contreras R.; Muratore M. E.; Johansson M. J.; Mendoza A. General Cyclopropane Assembly by Enantioselective Transfer of a Redox-Active Carbene to Aliphatic Olefins. Angew. Chem., Int. Ed. 2019, 58, 5930–5935. 10.1002/anie.201814123. [DOI] [PubMed] [Google Scholar]
- Meyer R.; Köhler J.; Homburg A.. Explosives, 6th ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007. [Google Scholar]
- Spear R. J.; Maksacheff M. The Relationship between Ignition Temperature and Thermal Stability for Selected Primary Explosives. Thermochim. Acta 1986, 105, 287–293. 10.1016/0040-6031(86)85245-5. [DOI] [Google Scholar]
- Nicolle S. M.; Hayes C. J.; Moody C. J. Alkyl Halide-Free Heteroatom Alkylation and Epoxidation Facilitated by a Recyclable Polymer-Supported Oxidant for the in-Flow Preparation of Diazo Compounds. Chem. – Eur. J. 2015, 21, 4576–4579. 10.1002/chem.201500118. [DOI] [PubMed] [Google Scholar]
- Bartrum H. E.; Blakemore D. C.; Moody C. J.; Hayes C. J. Rapid Access to α-Alkoxy and α-Amino Acid Derivatives through Safe Continuous-Flow Generation of Diazoesters. Chem. – Eur. J. 2011, 17, 9586–9589. 10.1002/chem.201101590. [DOI] [PubMed] [Google Scholar]
- Müller S. T. R.; Murat A.; Maillos D.; Lesimple P.; Hellier P.; Wirth T. Rapid Generation and Safe Use of Carbenes Enabled by a Novel Flow Protocol with In-Line IR Spectroscopy. Chem. – Eur. J. 2015, 21, 7016–7020. 10.1002/chem.201500416. [DOI] [PubMed] [Google Scholar]
- Müller S. T. R.; Murat A.; Hellier P.; Wirth T. Toward a Large-Scale Approach to Milnacipran Analogues Using Diazo Compounds in Flow Chemistry. Org. Process Res. Dev. 2016, 20, 495–502. 10.1021/acs.oprd.5b00308. [DOI] [Google Scholar]
- McCaw P. G.; Buckley N. M.; Eccles K. S.; Lawrence S. E.; Maguire A. R.; Collins S. G. Synthesis of Cyclic α-Diazo-β-Keto Sulfoxides in Batch and Continuous Flow. J. Org. Chem. 2017, 82, 3666–3679. 10.1021/acs.joc.7b00172. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Herraiz A. G.; Del Hoyo A. M.; Suero M. G. Generating Carbyne Equivalents with Photoredox Catalysis. Nature 2018, 554, 86–91. 10.1038/nature25185. [DOI] [PubMed] [Google Scholar]
- von Pechmann H. Ueber Diazomethan. Ber. Dtsch. Chem. Ges. 1894, 27, 1888–1891. 10.1002/cber.189402702141. [DOI] [Google Scholar]
- Gutsche C. D. The Reaction of Diazomethane and Its Derivatives with Aldehydes and Ketones. Org. React. 1954, 8, 364–429. 10.1002/0471264180.or008.08. [DOI] [Google Scholar]
- For a review:; a Yang H.; Martin B.; Schenkel B. On-Demand Generation and Consumption of Diazomethane in Multistep Continuous Flow Systems. Org. Process Res. Dev. 2018, 22, 446–456. For selected examples, see: 10.1021/acs.oprd.7b00302. [DOI] [Google Scholar]; b Proctor L. D.; Warr A. J. Development of a Continuous Process for the Industrial Generation of Diazomethane. Org. Process Res. Dev. 2002, 6, 884–892. 10.1021/op020049k. [DOI] [Google Scholar]; c Rossi E.; Woehl P.; Maggini M. Scalable in situ Diazomethane Generation in Continuous-Flow Reactors. Org. Process Res. Dev. 2012, 16, 1146–1149. 10.1021/op200110a. [DOI] [Google Scholar]; d Koolman H. F.; Kantor S.; Bogdan A. R.; Wang Y.; Pan J. Y.; Djuric S. W. Automated Library Synthesis of Cyclopropyl Boronic Esters Employing Diazomethane in a Tube-in-Tube Flow Reactor. Org. Biomol. Chem. 2016, 14, 6591–6595. 10.1039/C6OB00715E. [DOI] [PubMed] [Google Scholar]; e Hock K. J.; Koenigs R. M. The Generation of Diazo Compounds in Continuous-Flow. Chem. – Eur. J. 2018, 10571–10583. 10.1002/chem.201800136. [DOI] [PubMed] [Google Scholar]
- For a minireview:; a Deadman B. J.; Collins S. G.; Maguire A. R. Taming Hazardous Chemistry in Flow: The Continuous Processing of Diazo and Diazonium Compounds. Chem. – Eur. J. 2015, 21, 2298–2308. 10.1002/chem.201404348. [DOI] [PubMed] [Google Scholar]; b Sarabia-García F.; Jorge López-Herrera F.; Pino González M. S. Unstabilized Diazo Derivatives from Carbohydrates. Application to the Synthesis of 2-Deamino-Tunicamine and Products Related to C-Disaccharides. Tetrahedron 1995, 51, 5491–5500. 10.1016/0040-4020(95)00210-Y. [DOI] [Google Scholar]; c McGuiness M.; Shechter H. Conversions of Hydrazones to Diazo Compounds by N-Butyllithium and Azidotris(Diethylamino)Phosphonium Bromide. Tetrahedron Lett. 2002, 43, 8425–8427. 10.1016/S0040-4039(02)01443-0. [DOI] [Google Scholar]; d Furrow M. E.; Myers A. G. A General Procedure for the Esterification of Carboxylic Acids with Diazoalkanes Generated in situ by the Oxidation of N-Tert- Butyldimethylsilylhydrazones with (Difluoroiodo)Benzene. J. Am. Chem. Soc. 2004, 126, 12222–12223. 10.1021/ja0459779. [DOI] [PubMed] [Google Scholar]; e Morandi B.; Carreira E. M. Rhodium-Catalyzed Cyclopropenation of Alkynes: Synthesis of Trifluoromethyl-Substituted Cyclopropenes. Angew. Chem., Int. Ed. 2010, 49, 4294–4296. 10.1002/anie.201000787. [DOI] [PubMed] [Google Scholar]; f Morandi B.; Carreira E. M. Iron-Catalyzed Cyclopropanation in 6 M KOH with in situ Generation of Diazomethane. Science 2012, 335, 1471–1474. 10.1126/science.1218781. [DOI] [PubMed] [Google Scholar]; g Rendina V. L.; Kingsbury J. S. Titration of Nonstabilized Diazoalkane Solutions by Fluorine NMR. J. Org. Chem. 2012, 77, 1181–1185. 10.1021/jo202214e. [DOI] [PubMed] [Google Scholar]; h Greb A.; Poh J.-S.; Greed S.; Battilocchio C.; Pasau P.; Blakemore D. C.; Ley S. V. A Versatile Route to Unstable Diazo Compounds via Oxadiazolines and Their Use in Aryl-Alkyl Cross-Coupling Reactions. Angew. Chem., Int. Ed. 2017, 56, 16602–16605. 10.1002/anie.201710445. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Dingwall P.; Greb A.; Crespin L. N. S.; Labes R.; Musio B.; Poh J.-S.; Pasau P.; Blakemore D. C.; Ley S. V. C–H Functionalisation of Aldehydes Using Light Generated, Non-Stabilised Diazo Compounds in Flow. Chem. Commun. 2018, 54, 11685–11688. 10.1039/C8CC06202A. [DOI] [PubMed] [Google Scholar]; See also references (21c), (40−42), (34h), (53), and (83).
- a Aggarwal V. K.; De Vicente J.; Pelotier B.; Holmes I. P.; Bonnert R. V. A Simple, User-Friendly Process for the Homologation of Aldehydes Using Tosylhydrazone Salts. Tetrahedron Lett. 2000, 41, 10327–10331. 10.1016/S0040-4039(00)01856-6. [DOI] [Google Scholar]; b Fulton J. R.; Aggarwal V. K.; De Vicente J. The Use of Tosylhydrazone Salts as a Safe Alternative for Handling Diazo Compounds and Their Applications in Organic Synthesis. Eur. J. Org. Chem. 2005, 2005, 1479–1492. 10.1002/ejoc.200400700. [DOI] [Google Scholar]; c Javed M. I.; Brewer M. Diazo Preparation via Dehydrogenation of Hydrazones with “Activated” DMSO. Org. Lett. 2007, 9, 1789–1792. 10.1021/ol070515w. [DOI] [PubMed] [Google Scholar]; d Lévesque É.; Goudreau S. R.; Charette A. B. Improved Zinc-Catalyzed Simmons-Smith Reaction: Access to Various 1,2,3-Trisubstituted Cyclopropanes. Org. Lett. 2014, 16, 1490–1493. 10.1021/ol500267w. [DOI] [PubMed] [Google Scholar]; e Chu W. D.; Zhang L.; Zhang Z.; Zhou Q.; Mo F.; Zhang Y.; Wang J. Enantioselective Synthesis of Trisubstituted Allenes via Cu(I)-Catalyzed Coupling of Diazoalkanes with Terminal Alkynes. J. Am. Chem. Soc. 2016, 138, 14558–14561. 10.1021/jacs.6b09674. [DOI] [PubMed] [Google Scholar]; f Liu Z.; Li Q.; Liao P.; Bi X. Silver-Catalyzed [2+1] Cyclopropenation of Alkynes with Unstable Diazoalkanes: N-Nosylhydrazones as Room-Temperature Decomposable Diazo Surrogates. Chem. – Eur. J. 2017, 23, 4756–4760. 10.1002/chem.201605335. [DOI] [PubMed] [Google Scholar]; g Allouche E. M. D.; Al-Saleh A.; Charette A. B. Iron-Catalyzed Synthesis of Cyclopropanes by in situ Generation and Decomposition of Electronically Diversified Diazo Compounds. Chem. Commun. 2018, 54, 13256–13259. 10.1039/C8CC07060A. [DOI] [PubMed] [Google Scholar]; h Pang Y.; He Q.; Li Z. Q.; Yang J. M.; Yu J. H.; Zhu S. F.; Zhou Q. L. Rhodium-Catalyzed B–H Bond Insertion Reactions of Unstabilized Diazo Compounds Generated in situ from Tosylhydrazones. J. Am. Chem. Soc. 2018, 140, 10663–10668. 10.1021/jacs.8b05946. [DOI] [PubMed] [Google Scholar]
- Regitz M.; Maas G.. Diazo Compounds—Properties and Synthesis; Academic Press, Inc.: London, 1986. [Google Scholar]
- Bamford W. R.; Stevens T. S. The Decomposition of Toluene-p-Sulphonylhydrazones by Alkali. J. Chem. Soc. 1952, 4735. 10.1039/jr9520004735. [DOI] [Google Scholar]
- a Lévesque É.; Laporte S. T.; Charette A. B. Continuous Flow Synthesis and Purification of Aryldiazomethanes through Hydrazone Fragmentation. Angew. Chem., Int. Ed. 2017, 56, 837–841. 10.1002/anie.201608444. [DOI] [PubMed] [Google Scholar]; b Gérardy R.; Winter M.; Vizza A.; Monbaliu J.-C. M. Assessing Inter- and Intramolecular Continuous-Flow Strategies towards Methylphenidate (Ritalin) Hydrochloride. React. Chem. Eng. 2017, 2, 149–158. 10.1039/C6RE00184J. [DOI] [Google Scholar]
- Nicolle S. M.; Moody C. J. Potassium N-Iodo p-Toluenesulfonamide (TsNIK, Iodamine-T): A New Reagent for the Oxidation of Hydrazones to Diazo Compounds. Chem. – Eur. J. 2014, 20, 4420–4425. 10.1002/chem.201304656. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work has a comprehensive introduction to previous methods of oxidizing hydrazones to generate diazo compounds.
- a Kitamura M.; Kato S.; Yano M.; Tashiro N.; Shiratake Y.; Sando M.; Okauchi T. A Reagent for Safe and Efficient Diazo-Transfer to Primary Amines: 2-Azido-1,3-Dimethylimidazolinium Hexafluorophosphate. Org. Biomol. Chem. 2014, 12, 4397. 10.1039/c4ob00515e. [DOI] [PubMed] [Google Scholar]; b Kitamura M. Azidoimidazolinium Salts: Safe and Efficient Diazo-Transfer Reagents and Unique Azido-Donors. Chem. Rec. 2017, 17, 653–666. 10.1002/tcr.201600118. [DOI] [PubMed] [Google Scholar]
- Xie S.; Yan Z.; Li Y.; Song Q.; Ma M. Intrinsically Safe and Shelf-Stable Diazo-Transfer Reagent for Fast Synthesis of Diazo Compounds. J. Org. Chem. 2018, 83, 10916–10921. 10.1021/acs.joc.8b01587. [DOI] [PubMed] [Google Scholar]
- Bräse S.; Gil C.; Knepper K.; Zimmermann V. Organic Azides: An Exploding Diversity of a Unique Class of Compounds. Angew. Chem., Int. Ed. 2005, 44, 5188–5240. 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- Hazen G. G.; Bollinger F. W.; Roberts F. E.; Russ W. K.; Seman J. J.; Staskiewicz S. 4-Dodecylbenzenesulfonyl Azides. Org. Synth. 1996, 73, 144 10.15227/orgsyn.073.0144. [DOI] [Google Scholar]
- Rewicki D.; Tuchscherer C. 1-Diazoindene and Spiro[Indene-1,7′-Norcaradiene]. Angew. Chem., Int. Ed. 1972, 11, 44–45. 10.1002/anie.197200441. [DOI] [Google Scholar]
- Goddard-Borger E. D.; Stick R. V. Correction: An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-Sulfonyl Azide Hydrochloride. Org. Lett. 2011, 13, 2514. 10.1021/ol2007555. [DOI] [PubMed] [Google Scholar]
- Cavender C. J.; Shiner V. J. Trifluoromethanesulfonyl Azide. Its Reaction with Alkyl Amines to Form Alkyl Azides. J. Org. Chem. 1972, 37, 3567–3569. 10.1021/jo00795a052. [DOI] [Google Scholar]
- Katritzky A. R. Chemical Safety: Benzotriazole-1-Sulfonyl Azide. Chem. Eng. News 2012, 90, 4. [Google Scholar]
- Hazen G. G.; Weinstock L. M.; Connell R.; Bollinger F. W. A Safer Diazotransfer Reagent. Synth. Commun. 1981, 11, 947–956. 10.1080/00397918108065754. [DOI] [Google Scholar]
- Tuma L. D. Identification of a Safe Diazotransfer Reagent. Thermochim. Acta 1994, 243, 161–167. 10.1016/0040-6031(94)85051-8. [DOI] [Google Scholar]
- Bollinger F. W.; Tuma L. D. Diazotransfer Reagents. Synlett 1996, 1996, 407–413. 10.1055/s-1996-5440. [DOI] [Google Scholar]
- Cardillo P.; Gigante L.; Lunghi A.; Fraleoni-Morgera A.; Zanirato P. Hazardous N-Containing System: Thermochemical and Computational Evaluation of the Intrinsic Molecular Reactivity of Some Aryl Azides and Diazides. New J. Chem. 2008, 32, 47–53. 10.1039/B707931C. [DOI] [Google Scholar]
- Nikolaev V. A.; Chiba J.; Tomohiro T.; Hatanaka Y.. Methanesulfonyl Azide. EEROS Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Chichester, U.K., 2015; Vol. 2, pp 1–4. [Google Scholar]
- Breslow D. S.; Sloan M. F.; Newburg N. R.; Renfrow W. B. Thermal Reactions of Sulfonyl Azides. J. Am. Chem. Soc. 1969, 91, 2273–2279. 10.1021/ja01037a016. [DOI] [Google Scholar]
- Balabanov G. P.; Dergunov Y. I. Comparative Evaluation of the Sensitivity of Arenesulfonic Azides. J. Appl. Chem. USSR (Zh. Prikl. Khim.) 1968, 41, 2578–2580. [Google Scholar]
- Baum J. S.; Shook D. A.; Davies H. M. L.; Smith H. D. Diazotransfer Reactions with p-Acetamidobenzenesulfonyl Azide (p-ABSA). Synth. Commun. 1987, 17, 1709–1716. 10.1080/00397918708063988. [DOI] [Google Scholar]
- O’Mahony R. M.; Broderick C. M.; Lynch D.; Collins S. G.; Maguire A. R. Synthesis and Use of a Cost-Effective, Aqueous Soluble Diazo Transfer Reagent – m-Carboxybenzenesulfonyl Azide. Tetrahedron Lett. 2019, 60, 35–39. 10.1016/j.tetlet.2018.11.051. [DOI] [Google Scholar]
- Dermer O. C.; Edmison M. T. Orientation in Aromatic Substitution by the Benzenesulfonimido Radical. J. Am. Chem. Soc. 1955, 77, 70–73. 10.1021/ja01606a021. [DOI] [Google Scholar]
- Bailey A. S.; Vandrevala M. H. The Reactions of Some Tetrahydro-β-Carbolines, of Hexahydroazepino[3,4-b]Indoles, and of Tetrahydrocarbazolones with Arenesulphonyl Azides. J. Chem. Soc., Perkin Trans. 1 1980, 1512–1515. 10.1039/P19800001512. [DOI] [Google Scholar]
- Green G. M.; Peet N. P.; Metz W. A. Polystyrene-Supported Benzenesulfonyl Azide: A Diazo Transfer Reagent That Is Both Efficient and Safe. J. Org. Chem. 2001, 66, 2509–2511. 10.1021/jo005738d. [DOI] [PubMed] [Google Scholar]
- Fischer N.; Goddard-Borger E. D.; Greiner R.; Klapötke T. M.; Skelton B. W.; Stierstorfer J. Sensitivities of Some Imidazole-1-Sulfonyl Azide Salts. J. Org. Chem. 2012, 77, 1760–1764. 10.1021/jo202264r. [DOI] [PubMed] [Google Scholar]
- Katritzky A. R.; El Khatib M.; Bolshakov O.; Khelashvili L.; Steel P. J. Benzotriazol-1-Yl-Sulfonyl Azide for Diazotransfer and Preparation of Azidoacylbenzotriazoles. J. Org. Chem. 2010, 75, 6532–6539. 10.1021/jo101296s. [DOI] [PubMed] [Google Scholar]
- Muthyala M. K.; Choudhary S.; Kumar A. Synthesis of Ionic Liquid-Supported Sulfonyl Azide and Its Application in Diazotransfer Reaction. J. Org. Chem. 2012, 77, 8787–8791. 10.1021/jo301529b. [DOI] [PubMed] [Google Scholar]
- Bernet B.; Vasella A.; Liu Q.; Tor Y. Trifluoromethanesulfonyl Azide. Encycl. Reagents Org. Synth. 2009, 1–6. 10.1002/047084289X.rn00114.pub2. [DOI] [Google Scholar]
- Zhu S.-Z. Synthesis and Reactions of Fluoroalkanesulfonyl Azides and N,N-Dichlorofluoroalkanesulfonamides. J. Chem. Soc., Perkin Trans. 1 1994, 113, 2077–2081. 10.1039/p19940002077. [DOI] [Google Scholar]
- Suárez J. R.; Collado-Sanz D.; Cárdenas D. J.; Chiara J. L. Nonafluorobutanesulfonyl Azide as a Shelf-Stable Highly Reactive Oxidant for the Copper-Catalyzed Synthesis of 1,3-Diynes from Terminal Alkynes. J. Org. Chem. 2015, 80, 1098–1106. 10.1021/jo5025909. [DOI] [PubMed] [Google Scholar]
- Kayama R.; Hasunuma S.; Sekiguchi S.; Matsui K. The Thermal Reactions of Azido-1,3,5-Triazines. Bull. Chem. Soc. Jpn. 1974, 47, 2825–2829. 10.1246/bcsj.47.2825. [DOI] [Google Scholar]
- Curphey T. J. Preparation of p-Toluenesulfonyl Azide. A Cautionary Note. Org. Prep. Proced. Int. 1981, 13, 112–115. 10.1080/00304948109356105. [DOI] [Google Scholar]
- a Deadman B. J.; O’Mahony R. M.; Lynch D.; Crowley D. C.; Collins S. G.; Maguire A. R. Taming Tosyl Azide: The Development of a Scalable Continuous Diazo Transfer Process. Org. Biomol. Chem. 2016, 14, 3423–3431. 10.1039/C6OB00246C. [DOI] [PubMed] [Google Scholar]; b O’Mahony R. M.; Lynch D.; Hayes H. L. D. D.; Ní Thuama E.; Donnellan P.; Jones R. C.; Glennon B.; Collins S. G.; Maguire A. R. Exploiting the Continuous in situ Generation of Mesyl Azide for Use in a Telescoped Process. Eur. J. Org. Chem. 2017, 2017, 6533–6539. 10.1002/ejoc.201700871. [DOI] [Google Scholar]
- Phlegmatization (or desensitization) is when a sensitive material is diluted in solvent or plasticizer to reduce the sensitivity. In this case, the sulfonyl azide is never handled out of solution and thus at far lesser risk of detonation.
- Distinct endotherms were observed from gas loss when the DSC experiment was conducted in an unsealed crucible.
- The initial drop in heat flow is an artefact from using the heavier high-pressure crucibles, which are required to avoid energy loss when gas is evolved. For more details, see: Thomas L. C.Interpreting Unexpected Events and Transitions in DSC Results; TA039, 2018; pp 1–5. Retrieved from: http://www.tainstruments.com/pdf/literature/TA039.pdf (August 2018).
- Hansch C.; Leo A.; Taft R. W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. 10.1021/cr00002a004. [DOI] [Google Scholar]
- Both di- and trimethoxy compounds 56 and 57 have melting points, which overlap the decomposition. As a result, Tinit is reported as the start of the melt, Tonset must be extrapolated over the endothermic melt to be comparable and ΔHD is unreliable as it will incorporate the ΔHfus.
- The threshold was defined based on borderline sensitive m-DNB with a degree of conservatism. This plot was repeated for substances known to propagate an explosion (detonate) and those that cannot, defined by borderline explosives ammonium nitrate and 90% benzoyl peroxide in water.
- It is important to note this prediction only relates to the neat substance and does not preclude these compounds exploding if decomposition is trigged in a sealed vessel or reaction mixture, due to the significant amounts of heat and gas evolved.
- The rates of temperature and pressure rise are exponentially linked to sample size, so the rate data is only comparable to other ARC tests with the same sample size. For rates above 1000 °C min–1, there is a large margin of error due to the limits of detection speed. The same is true for large pressure rise rates.
- Tatsuoka T.; Koga N. Energy Diagram for the Catalytic Decomposition of Hydrogen Peroxide. J. Chem. Educ. 2013, 90, 633–636. 10.1021/ed400002t. [DOI] [Google Scholar]
- Berger A.; Wehrstedt K. D. In Azodicarboxylates: Explosive Properties and Thermal Hazards, Mary Kay O’Connor Process Safety Center 12th Annual Symposium “Beyond Regulatory Compliance: Making Safety Second Nature”; Texas A&M, 2009; pp 192–202. [Google Scholar]
- am Ende D. J.; Clifford P. J.; DeAntonis D. M.; SantaMaria C.; Brenek S. J. Preparation of Grignard Reagents: FTIR and Calorimetric Investigation for Safe Scale-Up. Org. Process Res. Dev. 1999, 3, 319–329. 10.1021/op9901801. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.