Editor
Malignant hyperthermia (MH) is a life-threatening disorder triggered in susceptible individuals primarily by halogenated volatile anaesthetic agents, depolarising neuromuscular blocking agents, or both.1 A fulminant MH crisis is characterised by hyperthermia, skeletal muscle rigidity, tachycardia or arrhythmia, respiratory and metabolic acidosis.1 In most MH susceptible (MHS) patients, the disease remains subclinical until they are exposed to pharmacological triggering agents.1, 2 MH is associated with mutations in either RYR1 or CACNA1S that cause profound alterations in resting intracellular Ca2+ ([Ca2+]i) in skeletal muscle cells, even in the absence of anaesthesia.3, 4 An elevated [Ca2+]i may induce alterations in cell signalling in MHS skeletal muscles, producing other complications beyond an MH crisis.
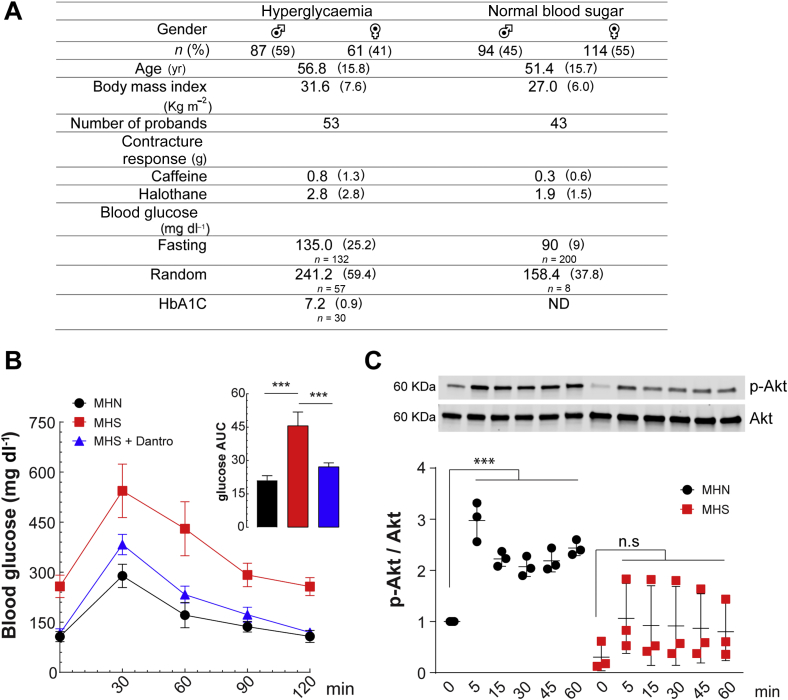
We report an unexpected increased frequency of hyperglycaemia in MHS patients (Fig. 1A). Appropriate research approvals were obtained for both humans (University of Toronto, ON, Canada) and animals (Mount Sinai Medical Center, Miami, FL, USA). We analysed fasting or random concentrations of blood glucose and glycosylated haemoglobin (HbA1c) from an MH database at the MH Investigation Unit of University of Toronto, Canada. The database includes 721 MHS patients who tested positive for MH susceptibility either with the caffeine-halothane contracture test or by carrying a diagnostic MH mutation. Blood glucose and HbA1c were available for 356 patients, and hyperglycaemia (i.e. either fasting blood glucose ≥100 mg dl−1, or random blood glucose ≥200 mg dl−1) was detected in 148 MHS patients (42%) (Fig. 1A). MHS patients with hyperglycaemia had a larger contracture response to caffeine and halothane; there was a positive correlation between fasting blood glucose values and the contracture response to both caffeine and halothane in MHS patients (Pearson's coefficient=0.17 and 0.18, respectively, both P<0.001), suggesting that the severity of [Ca2+]i alterations may correlate with alterations in glucose homeostasis.
Fig 1.
Impaired glucose homeostasis in malignant hyperthermia susceptible (MHS) individuals. (A) Retrospective study of 356 MHS patients showed increased blood glucose or hyperglycaemia in 42% of the patients. Please note the elevated values of blood glucose (fasting and random) and increased glycosylated haemoglobin (HbA1c) in patients with higher contracture response to caffeine and halothane. (B) Glucose tolerance test in MHS and MH normal (MHN) mice (n=5 per group). Mice were fasted overnight and glucose (2 g kg−1) was given by gastric gavage. Glucose was measured at the indicated times. Insert: glucose area under the curve (AUC). Dantrolene (2.0 mg kg−1 i.p.) pretreatment was given for 3 days. ***P<0.001, one-way analysis of variance (ANOVA)-Tukey. (C) Akt phosphorylation (Ser473) upon insulin (100 nM) stimulation determined by western blot in MHN and MHS myotubes (n=3 cultures for each genotype). ***P<0.001, one-way ANOVA-Dunnett compared with each control without stimulation (time point 0). Values are expressed as mean (standard deviation). Dantro, dantrolene.
To further characterise the glucose impairment observed in MHS patients, we performed glucose tolerance tests (GTTs) in an animal model of MHS (RyR1 R163C+/−)5 and MH normal (MHN) mice (WT littermates). An intragastric bolus of glucose (2 g kg−1 body weight after overnight fasting) was given by a gavage needle, and blood samples obtained from the tail vein at different time points after glucose administration. Blood glucose concentrations were measured using a glucometer (AlphaTRAK® Glucose Meter, Abbott Laboratories, Abbott Park, IL, USA).6 We observed impaired GTT in MHS mice (Fig. 1B) characterised by a higher basal value for fasting blood glucose (258 vs 107 mg dl−1, P<0.001), and increased peak amplitude (543 vs 258 mg dl−1, n=5 per group, P<0.001). Furthermore, MHS mice had slower glucose clearance than MHN mice at 60, 90, and 120 min. The area under the curve analysis demonstrated that MHS mice had blood glucose 2.2-fold higher than MHN mice (Fig. 1b, insert). Dantrolene pretreatment (2.0 mg kg−1 i.p. for 3 days) in MHS mice normalised GTT to near MHN concentrations (Fig. 1b). These results suggest that the R163C+/− RyR1 mutation, which is causative for MH, causes glucose intolerance likely because of increased resting [Ca2+], which can be reversed by dantrolene administration. To elucidate the possible mechanism causing glucose intolerance in MHS skeletal muscle, we isolated myoblasts from neonatal MHN and MHS mouse limb muscle (n=3 per genotype; euthanized sacrificed by cervical dislocation) and differentiated them into mature myotubes to study the insulin response using phosphorylation at Ser473 of Akt, a kinase that mediates insulin actions. Differentiated myotubes were exposed to insulin (100 nM) at different times and then quickly lysed on ice. Using western blot analysis, we found reduced Akt phosphorylation upon insulin stimulation in MHS myotubes compared with MHN (Fig. 1C).
We found that 42% of Canadian patients in this study diagnosed as MHS have hyperglycaemia, which was more than what would be expected in the general population. In fact, the prevalence of diabetes and prediabetes in Canada (in people ≥20 yr old for 2015) are estimated at 0.3% and 22%, respectively (www.diabetes.ca). Furthermore, we found a positive correlation between blood glucose and contracture responses to caffeine and halothane in our MHS cohort. It is well established that skeletal muscle from MHS patients or animals has a lower pharmacological threshold and an exaggerated response to submaximal concentrations of caffeine7 and halothane7 than MHN muscle, tests widely used in the clinical diagnosis of MH susceptibility in humans8 and validated experimentally in muscle from swine9 and mice.5 The molecular and cellular basis for heightened sensitivity to caffeine, halothane, and 4-chloro-m-cresol in MHS muscle appears to be related to elevated [Ca2+]i.10, 11 Glucose intolerance was also observed in MHS rodents, which was partially reversed by pretreatment with dantrolene, a specific drug to prevent and treat MH, which reduced [Ca2+]i.12 The impaired Akt phosphorylation upon insulin stimulation found in MHS mice may be one of the reasons why MHS mice have chronic hyperglycaemia.
Skeletal muscle is one of the most important tissue targets for insulin in the regulation of blood glucose, as 75–80% of the glucose removed from the blood goes into skeletal muscle and skeletal muscle insulin resistance is one of the primary characteristics of type 2 diabetes mellitus (T2D).13 MHS individuals have an increased glucose-induced insulin response,14 and in this study, we found higher blood glucose in 42% of MHS patients. Furthermore, we found that MHS skeletal muscle cells have impaired insulin cell signalling evidenced by Akt dysregulation that correlates with altered glucose tolerance in mice. The potential impact of the present findings can be quite broad considering that MH syndrome appears to be not just an operating room emergency. More studies are necessary to dissect the molecular mechanism responsible for glucose intolerance in MHS individuals and to determine whether these patients are at higher risk of developing a diabetic disequilibrium.
Declaration of interest
The authors declare that they have no conflicts of interest.
References
- 1.Rosenberg H., Pollock N., Schiemann A., Bulger T., Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015;10:93. doi: 10.1186/s13023-015-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jurkat-Rott K., Lerche H., Lehmann-Horn F. Skeletal muscle channelopathies. J Neurol. 2002;249:1493–1502. doi: 10.1007/s00415-002-0871-5. [DOI] [PubMed] [Google Scholar]
- 3.Yang T., Allen P.D., Pessah I.N., Lopez J.R. Enhanced excitation-coupled calcium entry in myotubes is associated with expression of RyR1 malignant hyperthermia mutations. J Biol Chem. 2007;282:37471–37478. doi: 10.1074/jbc.M701379200. [DOI] [PubMed] [Google Scholar]
- 4.Yang T., Esteve E., Pessah I.N., Molinski T.F., Allen P.D., Lopez J.R. Elevated resting [Ca(2+)](i) in myotubes expressing malignant hyperthermia RyR1 cDNAs is partially restored by modulation of passive calcium leak from the SR. Am J Physiol Cell Physiol. 2007;292:C1591–C1598. doi: 10.1152/ajpcell.00133.2006. [DOI] [PubMed] [Google Scholar]
- 5.Yang T., Riehl J., Esteve E. Pharmacologic and functional characterization of malignant hyperthermia in the R163C RyR1 knock-in mouse. Anesthesiology. 2006;105:1164–1175. doi: 10.1097/00000542-200612000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295:E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 7.Tong J., Oyamada H., Demaurex N., Grinstein S., McCarthy T.V., MacLennan D.H. Caffeine and halothane sensitivity of intracellular Ca2+ release is altered by 15 calcium release channel (ryanodine receptor) mutations associated with malignant hyperthermia and/or central core disease. J Biol Chem. 1997;272:26332–26339. doi: 10.1074/jbc.272.42.26332. [DOI] [PubMed] [Google Scholar]
- 8.Allen G.C., Larach M.G., Kunselman A.R. The sensitivity and specificity of the caffeine-halothane contracture test: a report from the North American malignant hyperthermia registry. The North American malignant hyperthermia registry of MHAUS. Anesthesiology. 1998;88:579–588. doi: 10.1097/00000542-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lopez J.R., Allen P.D., Alamo L., Jones D., Sreter F.A. Myoplasmic free [Ca2+] during a malignant hyperthermia episode in swine. Muscle Nerve. 1988;11:82–88. doi: 10.1002/mus.880110113. [DOI] [PubMed] [Google Scholar]
- 10.Lopez J.R., Linares N., Pessah I.N., Allen P.D. Enhanced response to caffeine and 4-chloro-m-cresol in malignant hyperthermia-susceptible muscle is related in part to chronically elevated resting [Ca2+]i. Am J Physiol Cell Physiol. 2005;288:C606–C612. doi: 10.1152/ajpcell.00297.2004. [DOI] [PubMed] [Google Scholar]
- 11.Lopez J.R., Contreras J., Linares N., Allen P.D. Hypersensitivity of malignant hyperthermia-susceptible swine skeletal muscle to caffeine is mediated by high resting myoplasmic [Ca2+] Anesthesiology. 2000;92:1799–1806. doi: 10.1097/00000542-200006000-00040. [DOI] [PubMed] [Google Scholar]
- 12.Eltit J.M., Ding X., Pessah I.N., Allen P.D., Lopez J.R. Nonspecific sarcolemmal cation channels are critical for the pathogenesis of malignant hyperthermia. FASEB J. 2013;27:991–1000. doi: 10.1096/fj.12-218354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFronzo R.A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 14.Denborough M.A., Warne G.L., Moulds R.F., Martin F.I. Insulin secretion in malignant hyperpyrexia. BMJ. 1974;3:493–495. doi: 10.1136/bmj.3.5929.493. [DOI] [PMC free article] [PubMed] [Google Scholar]