Abstract
Background
Individuals genetically susceptible to malignant hyperthermia (MH) exhibit hypermetabolic reactions when exposed to volatile anaesthetics. Mitochondrial dysfunction has previously been associated with the MH-susceptible (MHS) phenotype in animal models, but evidence of this in human MH is limited.
Methods
We used high resolution respirometry to compare oxygen consumption rates (oxygen flux) between permeabilised human MHS and MH-negative (MHN) skeletal muscle fibres with or without prior exposure to halothane. A substrate-uncoupler-inhibitor titration protocol was used to measure the following components of the electron transport chain under conditions of oxidative phosphorylation (OXPHOS) or after uncoupling the electron transport system (ETS): complex I (CI), complex II (CII), CI+CII and, as a measure of mitochondrial mass, complex IV (CIV).
Results
Baseline comparisons without halothane exposure showed significantly increased mitochondrial mass (CIV, P=0.021) but lower flux control ratios in CI+CII(OXPHOS) and CII(ETS) of MHS mitochondria compared with MHN (P=0.033 and 0.005, respectively) showing that human MHS mitochondria have a functional deficiency. Exposure to halothane triggered a hypermetabolic response in MHS mitochondria, significantly increasing mass-specific oxygen flux in CI(OXPHOS), CI+CII(OXPHOS), CI+CII(ETS), and CII(ETS) (P=0.001–0.012), while the rates in MHN samples were unaltered by halothane exposure.
Conclusions
We present evidence of mitochondrial dysfunction in human MHS skeletal muscle both at baseline and after halothane exposure.
Keywords: electron transport chain; malignant hyperthermia; mitochondria, oxidative phosphorylation; skeletal muscle; volatile anaesthetic
Editor's key points.
-
•
The effect of malignant hyperthermia susceptibility (MHS) on skeletal muscle mitochondrial function in humans is uncertain.
-
•
We compared oxygen consumption using high resolution respirometry in MHS and normal human skeletal muscle fibres.
-
•
Muscle from MHS individuals had increased mitochondrial mass but reduced oxidative capacity compared with normal individuals.
-
•
Impaired mitochondrial function in human MHS skeletal muscle may result from chronic elevation of myoplasmic Ca2+.
Malignant hyperthermia (MH) is a hypermetabolic reaction triggered by potent inhalation anaesthetics in susceptible individuals.1 Direct consequences of the hypermetabolic state are increased carbon dioxide production, increased oxygen consumption, metabolic acidosis, and hyperthermia, with reflex sympathetic stimulation.2 Ultimately, skeletal muscle adenosine triphosphate (ATP) production fails to keep up with energy requirements leading to muscle rigidity and rhabdomyolysis.2 Variants in the RYR1 gene that encodes the skeletal muscle Ca2+ release channel (ryanodine receptor, RyR1)3 or the CACNA1S gene that encodes the sarcolemmal slow voltage-gated Ca2+ channel that acts as the voltage sensor for excitation-contraction (EC) coupling (dihydropyridine receptor)4, 5 are associated with MH in 76% of UK MH families.6 Both proteins work in concert to regulate Ca2+ concentrations in skeletal muscle at rest and during EC coupling.7
Before the discovery of genetic linkage between RYR1 and MH susceptibility,8, 9 several researchers postulated an important role for mitochondria in the development of an MH reaction. Recent findings in transgenic mice with RYR1 mutation knock-in has rekindled interest in such a role for mitochondria.10, 11, 12 There is also the implication that mitochondrial dysfunction may explain why some MH-susceptible (MHS) individuals experience myopathic traits such as muscle weakness and exercise intolerance.13
Some of the earliest observations in MH mitochondria were conducted on the MH porcine model, which showed halothane-induced inhibition of complex I (CI).14, 15 Research on RYR1 mutation knock-in mouse models has found mitochondrial abnormalities with various degrees of severity depending on the RyR1 mutation. Notable observations include lower oxidative phosphorylation (OXPHOS), increased oxidative stress, and increased reactive oxygen species (ROS) production.10, 11, 12 Structural mitochondrial deformity, an indication of damage, including a loss of cristae organisation and mitochondrial swelling, has been observed in multiple reports using electron microscopy. This suggests there may be metabolic dysfunction and altered OXPHOS in muscles from both MHS mice10, 16 and humans.17, 18
In contrast to animal models, research on human MH mitochondrial function is lacking. Despite the structural abnormalities seen in MHS humans,17, 18 evidence for functional defects is conflicting. Some reports suggest normal OXPHOS and respiratory control in mitochondria of MHS humans,19 whilst others have claimed impaired OXPHOS and ATP production after bouts of training in MHS individuals.20 The discrepancies may be explained by variation in sample type, methodology, or both. We investigated whether mitochondrial dysfunction is present in ex vivo skeletal muscle from MHS humans under basal conditions and after exposure to the MH trigger agent halothane.
Methods
Patients
Patients considered for inclusion in this study were those attending for investigation of their susceptibility to MH. The reason for investigation was a clinical suspicion of increased risk either because of an adverse reaction to anaesthesia consistent with MH, or because a family member was known to be MHS. The majority of index case patients had been found not to harbour a pathogenic variant (www.emhg.org) in RYR1 and CACNA1S before attending for further investigation, which involved an open muscle biopsy and subsequent in vitro contracture testing (IVCT). Family members were either from families where no pathogenic variant had been found or who had been found not to carry a familial variant. Patients gave written informed consent to the study that was approved by Leeds (East) Research Ethics Committee (Leeds, UK, reference 10/H1306/70).
Patients were diagnosed using the IVCT as either MHS or MH negative (MHN) according to the protocol of the European MH Group.21 The laboratory classification of MHS is subdivided into MHShc (samples respond abnormally to both halothane and caffeine challenges), MHSh (samples respond abnormally to halothane but normally to caffeine challenge), or MHSc (samples respond abnormally to caffeine but normally to halothane challenge).
Muscle samples
Diagnostic muscle biopsies and IVCT were conducted according to the protocol of the European MH Group,21 and prepared by a protocol adapted from that described.22, 23 In brief, six muscle fascicles (typical dimensions 25×4×3 mm) were excised from the vastus medialis under femoral nerve block and immediately placed in oxygenated Krebs' solution (NaCl 118.1 mM, KCl 3.4 mM, MgSO4 0.8 mM, KH2PO4 1.2 mM, glucose 11.1 mM, NaHCO3 25.0 mM, CaCl2 2.5 mM, pH 7.4) at room temperature and taken to the MH laboratory. There, samples were kept at room temperature in Krebs solution, which was gassed continuously with carbogen (O2 95%, CO2 5%) until IVCT. This study required both a muscle fascicle that had not been used for the diagnostic challenge tests (baseline) and a fascicle that had been used in the static halothane test (halothane-exposed). At the end of the halothane test, the halothane-exposed and baseline samples were each placed into 1 ml of ice-cold biopsy preservation buffer, BIOPS (CaK2EGTA 2.77 mM, K2EGTA 7.23 mM, Na2ATP 5.77 mM, MgCl2·6H2O 6.56 mM, taurine 20 mM, Na2phosphocreatine 15 mM, imidazole 20 mM, dithiothreitol 0.5 mM, and MES hydrate 50 mM, pH 7.1, adjusted with KOH 5 N at 0°C). Samples were then placed in a Petri dish with ice-cold BIOPS and separated using sharp forceps to obtain small muscle fibre bundles containing ∼4–5 fibres each. These samples then underwent chemical permeabilisation in BIOPS containing saponin (50 μg ml−1) for 30 min, before being washed with 1 ml respiration medium, Mir05 (EGTA 0.5 mM, MgCl2·6H2O 3 mM, lactobionic acid 60 mM, taurine 20 mM, KH2PO4 10 mM, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid 20 mM adjusted to pH 7.1 with KOH at 37°C, D-sucrose 110 mM, and 1 g L−1 essentially fatty acid free bovine serum albumin) to remove residual saponin. The sample was then taken and blot-dried for 5 s, before being weighed and loaded into the respirometer chambers (5–10 mg in each chamber) containing Mir05 2 ml. The time between biopsy collection and assay time was ∼90–120 min.
High resolution respirometry
Oxygen consumption over time was measured using Oroboros respiratory analysers (Oroboros Instruments, Innsbruk, Austria) at 2 s intervals with polarographic oxygen sensors, and expressed as mass-specific oxygen flux (pmol s−1 mg−1). The analyser was calibrated daily in air saturated solution before experimentation. Assays were initiated by injecting oxygen into each chamber to raise the oxygen concentration to >400 nmol ml−1 before a substrate-uncoupler-inhibitor titration (SUIT) protocol. Re-oxygenation of the chambers was performed to maintain oxygen concentration at 200–500 nmol ml−1 to prevent limitation because of oxygen diffusion.23 Each assay was performed at 37°C, with chamber stirrers set at 750 rpm.
SUIT protocol
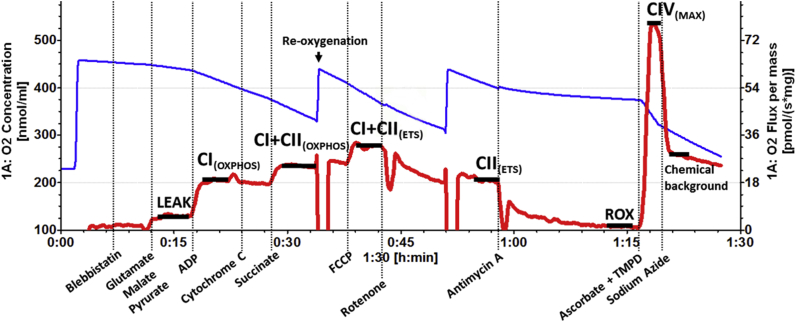
An adapted SUIT protocol was used to investigate the OXPHOS capacity of individual complexes and respiratory states22, 23 (Fig 1). The procedure was initiated with addition of blebbistatin 5 μM to prevent spontaneous contraction of muscle fibres.24 Glutamate (10 mM), malate (0.5 mM), and pyruvate (5 mM) were then added to facilitate measurements of CI respiration in the absence of adenosine diphosphate (ADP) (LEAK respiration- electron flow coupled to proton pumping to compensate for proton leaks). ADP (2.5 mM) is then added to allow measurement of maximal CI-supported OXPHOS (CI[OXPHOS]). At this point, outer mitochondrial membrane integrity was assessed by the addition of cytochrome c 10 μM. Samples with increased respiration rates of >10% after addition of cytochrome c, which indicates outer mitochondrial membrane damage, were excluded from analysis.
Fig. 1.
Example high resolution respirometry trace showing the rate of oxygen flux (red line) over time after sequential titration of substrates and inhibitors, expressed as pmol s−1 mg−1. The blue line represents oxygen concentrations within the chamber (nmol ml−1). Vertical dashed lines represent timepoints where substrates have been added. The horizontal black lines which overlay the red line plateaus indicate the position of datapoints. ADP, adenosine diphosphate; CI, complex I; CII, complex II; CIV, complex IV; ETS, electron transport system; FCCP, carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone; OXPHOS, oxidative phosphorylation; ROX, residual oxygen flux; TMPD, N,N,N′,N'-tetramethyl-p-phenylenediamine.
Once membrane integrity had been assessed, maximal activity of complex II (CII) was stimulated with addition of succinate (10 mM). Oxygen flux at this stage (CI+CII[OXPHOS]) reflects the combined activities of CI and CII, and is regarded as the maximum OXPHOS capacity in the coupled state. Next, step-wise additions of carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone (FCCP) (0.5 μM) were made until there was no further increase in oxygen flux. FCCP uncouples the electron transport system (ETS) by collapsing the proton gradient between the intermembrane space and the mitochondrial matrix providing the maximum ETS capacity (CI+CII[ETS]). After uncoupling the system, electron flow through CI was inhibited using rotenone (0.5 μM), providing maximum CII activity alone (CII[ETS]). Finally, antimycin A was introduced to inhibit complex III (CIII). CIII activity contributes to all datapoints aside from the complex IV (CIV) assay. However, its activity is not measured directly in this protocol as it obtains electrons downstream of CI and CII. Residual oxygen flux present after addition of antimycin A is a result of non-mitochondrial respiration that is subtracted from each respiratory state reading before analysis.
Complex IV assay
Measurement of mass-specific CIV oxygen flux was used as an alternative proxy marker for estimations of mitochondrial content. Ascorbate (2 mM) and N,N,N′,N'-tetramethyl-p-phenylenediamine (0.5 mM) were applied at the end of the standard SUIT protocol. Measurement of CIV flux was taken at the peak of the corresponding trace, and residual chemical background was removed from this value by applying sodium azide (10 mM).25
Data handling and analysis
Raw data in the form of oxygen flux per muscle mass (pmol s−1 mg−1) includes the confounding effects of both mitochondrial quality and quantity. Data from samples with and without halothane exposure were internally normalised to the common reference state, CI+CII(ETS) (maximum uncoupled respiration). Normalised oxygen flux per mass is presented as flux control ratios (FCR) which highlight differences in mitochondrial function, as a proportion of the reference state, which is independent of mitochondrial content.
Oxygen flux recorded from each sample trace was exported from proprietary software (DatLab5, Oroboros Instruments) into Microsoft Excel (Microsoft Inc, Redmond, Washington, USA) before analysis using IBM (Armonk, NY, USA) SPSS statistics version 21. Pairwise comparisons were performed using a combination of Wilcoxon signed-rank tests to assess the effects of halothane exposure and Mann–Whitney U-tests to assess median differences between baseline controls. Further analysis of MHS subgroups used the Kruskal–Wallis test and post hoc Dunn's procedure to identify differences between patients classified as MHN, MHSh, and MHShc. Data were summarised as boxplots using GraphPad Prism 8 (GraphPad Software Inc, San Diego, CA, USA). There are no published data on the magnitude of changes in OXPHOS variables that are associated with human pathology. Preliminary data from a transgenic mouse model of MH suggested that there was a standardised mean difference of 0.86 in baseline CI+CII(ETS) between MHS and MHN mice. Such a difference would be detected with a sample size of 12 in each group with α<0.05 and β<0.2. We sought to identify changes with a standardised difference in means of >0.75, as this is generally accepted as a relevant effect size in biological systems. On the basis that more people tested by the MH Unit prove to be MHN rather than MHS, we planned to include samples from 23 MHS and at least 35 MHN, in order to achieve α<0.05 and β<0.2. These numbers of samples enable detection of paired (with and without halothane exposure) standardised mean differences of 0.6 and 0.5 for MHS and MHN samples, respectively.
Results
Patient characteristics
A summary of patient characteristics is presented in Table 1. Eighteen of the 23 MHS group were subsequently found to carry at least one variant in the RYR1 gene and these variants are also listed in Table 1. None of the patients had clinical or histopathological features suggestive of a mitochondrial myopathy. We included 59 individuals from 52 families: the maximum number from any family was two individuals.
Table 1.
Summary of characteristics of the patients contributing samples for this study. The RYR1 variants are annotated for their likely pathogenicity using the criteria of Miller and colleaguesl6 as: *unlikely pathogenic; †potentially pathogenic; ‡likely pathogenic; and ¶pathogenic. In vitro contracture testing (IVCT) data for each MHS individual is available in Supplementary Table S1. MHN, malignant hyperthermia-negative; MHS, malignant hyperthermia-susceptible; MHSh, abnormal response in the in vitro contracture test to halothane but not caffeine; MHShc, abnormal response in the in vitro contracture test to halothane and caffeine
| MHN (n=36) | MHS (n=23) |
||||||
|---|---|---|---|---|---|---|---|
| MHSh (n=12) | MHShc (n=11) | ||||||
| Male:female | 16:20 | 5:7 | 7:4 | ||||
| Age at biopsy (yr) | (11–68) | (12–64) | (12–57) | ||||
| Individuals with at least one RYR1 variant | – | 8 | 10 | ||||
| RYR1 variants found | – | Nucleotide change | Amino acid change | Accession number | Nucleotide change | Amino acid change | Accession number |
| c.251C>T† | p.Thr84Met | rs186983396 | c.455C>A† | p.Ala152Asp | – | ||
| c.4178A>G* | p.Lys1393Arg | rs137933390 | c.1202G>A‡ | p.Arg401His | rs193922766 | ||
| c.5183C>T‡ | p.Ser1728Phe | rs193922781 | c.8729C>T† | p.Tyr2910Met | – | ||
| c.6670C>T† | p.Arg2224Cys | rs199870223 | c.10357C>T† | p.Arg3453Cys | rs1482429489 | ||
| c.6785G>A† | p.Gly2262Asp | – | c.11132C>T‡ | p.Thr3711Met | rs375915752 | ||
| c.7879G>A‡ | p.Val2627Met | – | c.11958C>G‡ | p.Asp3986Glu | rs193922842 | ||
| c.12860C>T† | p.Ala4287Val | – | c.12700G>C‡ | p.Val4234Leu | rs193922852 | ||
| c.14210G>Ac | p.Arg4737Gln | rs193922868 | c.7879G>A‡ | p.Val2627Met | – | ||
| c.4293G>A | p.Thr1431= | rs727504130 | c.1021G>A¶ | p.Gly341Arg | rs121918592 | ||
Mass-specific oxygen flux comparisons
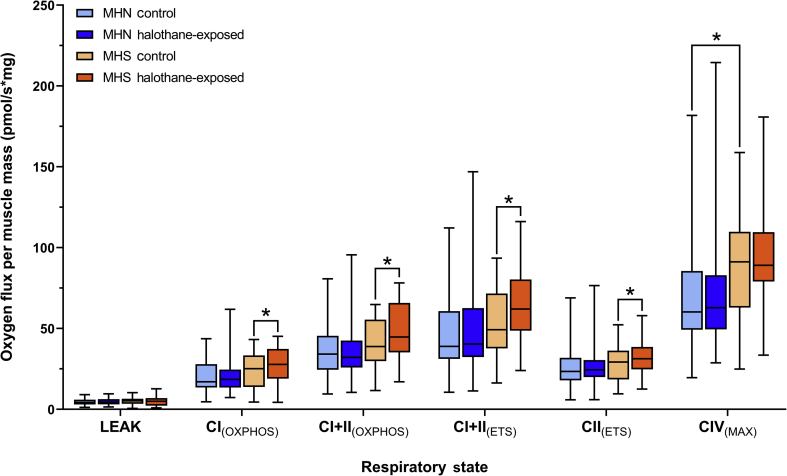
Oxygen flux per milligram of muscle was compared between MHN and MHS samples, with or without exposure to halothane (Fig 2). Comparisons of samples without halothane exposure showed no differences between the two phenotypes aside from CIV(MAX), where MHS samples had a significantly greater oxygen flux compared with MHN (P=0.021), suggesting higher mitochondrial content in MHS samples. Pairwise comparisons showed that oxygen flux was significantly elevated in MHS samples after halothane exposure for CI(OXPHOS), CI+CII(OXPHOS), CI+CII(ETS), and CII(ETS) (Table 2). The rates in MHN samples were unaltered by halothane exposure.
Fig. 2.
High resolution respirometry of permeabilised vastus medialis biopsies from MHN (n= 36) and MHS (n=23) individuals. Boxplots show median, inter-quartile range (IQR) and min-max range for each phenotype in control and halothane-exposed samples. Statistically significant pairwise comparisons (P<0.05) are labelled with an asterisk (refer to Table 2 for exact P-values). CI, complex I; CII, complex II; CIV, complex IV; ETS, electron transport system; MHN, malignant hyperthermia-negative; MHS, malignant hyperthermia-susceptible; OXPHOS, oxidative phosphorylation.
Table 2.
Summary of statistical comparisons for each data set, outlining P-values for each pairwise comparison between and within phenotype for the mass-specific oxygen flux (pmol s−1 mg−1) and flux control ratio (FCR) readings. The P-values for FCR differences between MHS subgroups are also included with post hoc statistics for each comparison. P-values reaching statistical significance are presented in bold font. ETS, electron transport system; MHN, malignant hyperthermia-negative; MHS, malignant hyperthermia-susceptible; MHSh, abnormal response in the in vitro contracture test to halothane but not caffeine; MHShc, abnormal response in the in vitro contracture test to halothane and caffeine; OXPHOS, oxidative phosphorylation
| Respiratory state | Oxygen consumption rate (pmol s−1 mg−1) |
|||
|---|---|---|---|---|
| MHN |
MHS |
MHN vs MHS |
||
| Control vs halothane-exposure (P-value) | Control comparisons (P-value) | |||
| Leak | 0.944 | 0.429 | 0.732 | |
| CI(OXPHOS) | 0.593 | 0.012 | 0.113 | |
| CI+CII(OXPHOS) | 0.789 | 0.005 | 0.071 | |
| CI+CII(ETS) | 0.157 | 0.003 | 0.050 | |
| CII(ETS) | 0.057 | 0.001 | 0.376 | |
| CIV(MAX) |
0.718 |
0.101 |
0.021 |
|
| Respiratory state | Flux control ratio (FCR) | |||
| MHN | MHS | MHN vs MHS | ||
| Control vs halothane-exposure (P-value) |
Control comparisons (P-value) |
|||
| LEAK | 0.753 | 0.362 | 0.074 | |
| CI(OXPHOS) | 0.480 | 0.007 | 0.534 | |
| CI+CII(OXPHOS) | 0.470 | 0.003 | 0.033 | |
| CI+CII(ETS) | ||||
| CII(ETS) | 0.041 | 0.001 | 0.005 | |
| CIV(MAX) |
0.307 |
0.052 |
0.913 |
|
| Respiratory state | FCR differences (halothane-exposed - control) | |||
| Kruskal-Wallis (P-value) | Post hoc (P-value) | |||
| MHN vs MHSh |
MHN vs MHShc |
MHShvs MHShc |
||
| LEAK | 0.349 | |||
| CI(OXPHOS) | 0.209 | |||
| CI+CII(OXPHOS) | 0.043 | 1.000 | 0.036 | 0.390 |
| CI+CII(ETS) | 0.086 | |||
| CII(ETS) | 0.263 | |||
| CIV(MAX) | 0.532 | |||
Flux control ratio (normalised oxygen flux)
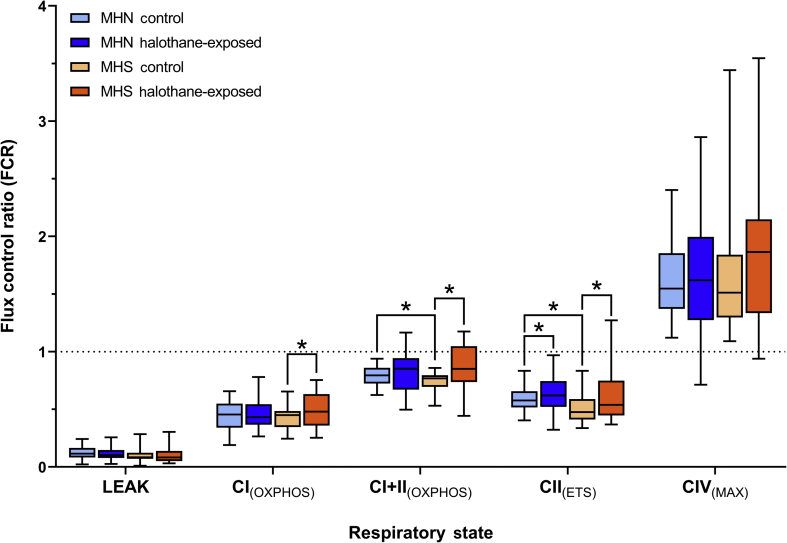
Mass-specific oxygen flux was normalised to a common reference state (control CI+CII[ETS]) to generate FCR to remove the confounding effects of mitochondrial content differences between samples (Fig 3). Comparisons of FCR at baseline revealed that CI+CII(OXPHOS) and CII(ETS) were significantly lower in MHS samples compared with MHN (P=0.033 and 0.005, respectively, Table 2), suggesting functional deficiency. Normalised CIV(MAX) showed no significant differences between MHS and MHN controls, indicating similar functional capacity of CIV. FCR comparisons between control and halothane-exposed samples support the findings seen in the mass-specific dataset, with statistically significant findings in several of the same respiratory states (Table 2). However, one exception was found in MHN CII(ETS) which increased after halothane-exposure (P=0.041).
Fig. 3.
Boxplot comparison of flux control ratios (FCR) from MHN (n=36) and MHS (n=23) control and halothane-exposed samples. FCR are generated by internally normalising oxygen flux per muscle mass to the non-halothane-exposed control CI+CII(ETS) (indicated by the horizontal dashed line). Boxplots show median, inter-quartile range (IQR) and min-max range for each phenotype in control and halothane-exposed samples. Statistically significant pairwise comparisons (P<0.05) are labelled with an asterisk (refer to Table 2 for exact P-values). CI, complex I; CII, complex II; CIV, complex IV; ETS, electron transport system; MHN, malignant hyperthermia-negative; MHS, malignant hyperthermia-susceptible; OXPHOS, oxidative phosphorylation.
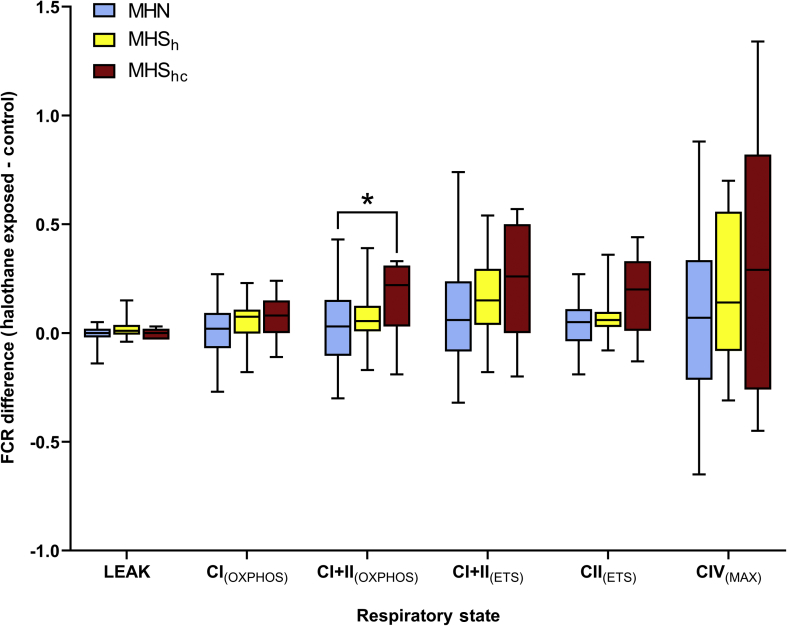
Flux control ratio responses within the subdivided MHS phenotypes
FCR were used for further analysis within the MHS phenotype, comparing the magnitude of change with and without exposure to halothane between the MHSh and MHShc phenotypes (Fig 4); there were no patients in this sample categorised as MHSc. Results from the Kruskal–Wallis test showed that CI+CII(OXPHOS) responses differed significantly between MH phenotypes after halothane exposure (Table 2). Post hoc analysis showed that these differences in response between MHN and MHS groups were largely attributable to responses of the MHShc subgroup, as there were no significant differences in response to halothane exposure between the MHN group and the MHSh subgroup.
Fig. 4.
Boxplot comparison showing the change flux control ratios (FCR) (halothane-exposed control) for each respiratory state. MHN samples (n=36) were plotted with MHS samples (n=23), further split into MHSh (n=12) and MHShc (n=11) subgroups. Boxplots show median, inter-quartile range (IQR) and min-max response range for each phenotype. Comparisons labelled with an asterisk show statistically significant post hoc pairwise comparisons (refer to Table 2 for exact P-values). CI, complex I; CII, complex II; CIV, complex IV; ETS, electron transport system; MHN, malignant hyperthermia-negative; MHS, malignant hyperthermia-susceptible; MHSh, abnormal response in the in vitro contracture test to halothane but not caffeine; MHShc, abnormal response in the in vitro contracture test to halothane and caffeine; OXPHOS, oxidative phosphorylation.
Discussion
Baseline comparisons using mass-specific flux showed higher CIV(MAX) in MHS compared with MHN muscle, which indicates a greater mass of mitochondria in MHS muscle per milligram of tissue. Alternative mitochondrial markers such as citrate synthase and mtDNA were considered for this study, but the CIV assay was chosen as it can be performed during the experiment and allows measurements on the same exact sample, avoiding issues with degradation. To normalise for differences in mitochondrial mass, we compared FCR data which showed that there were functional deficits in MHS muscle as well. The CI+CII(OXPHOS) and CII(ETS) FCR were significantly lower in MHS samples compared with MHN, which implies that there is uncoupling of mitochondria in MHS muscle in addition to CII deficiency, both of which would likely result in inefficient ATP production. The uncoupling of MHS mitochondria is perhaps a side-effect of the swelling and structural abnormalities seen in previous human studies,18 or a consequence of chronically elevated myoplasmic Ca2+concentration in MHS muscle (see below). As no significant differences in CI(OXPHOS) were found, CII deficiency seems to be the primary cause for the reduced maximum OXPHOS capacity of MHS muscle as defined by CI+CII(OXPHOS) FCR. This is of great importance, as a lower OXPHOS capacity suggests that the ETS in MHS mitochondria is less tightly coupled, and therefore less efficient at producing ATP than in MHN mitochondria. CII, also known as succinate dehydrogenase, is the only component of the ETS that is encoded entirely by nuclear genes and has roles in both OXPHOS and the Krebs cycle, indirectly affecting glycolysis.26 CII deficiency has not previously been linked to MH susceptibility and its deficiency may mean that the glycolytic function of MHS muscle is also impaired, a potential area for further research. Collectively these findings also suggest a potential compensation mechanism, in which MHS muscle upregulates mitochondrial number to counteract deficiencies in mitochondrial function, hence the similar baseline oxygen flux per unit of muscle mass.
The proposed concept of mitochondrial deficiency in MHS muscle is consistent with that seen in other studies,11, 15, 20 but some findings are conflicting. For example, Giulivi and colleagues11 found a decrease in mitochondrial content, reduced oxygen uptake using malate-glutamate and succinate, and CI, CIII, and CIV deficiency in RyR1 R163C knock-in mice when compared with wild-type. In contrast, we showed evidence of human MHS muscle having higher mitochondrial content, deficiency in succinate-facilitated oxygen flux, with activity deficits in CII only. These discrepancies may be a result of species differences, but they could also be explained by differences in methodology. Giulivi and colleagues11 used isolated mitochondria in the assessment of mitochondrial content and protein complex function, whereas we used permeabilised muscle fibres. Permeabilised muscle allows interplay between the mitochondria, sarcoplasmic reticulum, and RyR1, which we believe is crucial to accurately assess the biological effects of MH. Furthermore, the oxygen uptake assay in the mouse study was conducted at 22°C, whereas we used 37°C, as the assay temperature significantly impacts OXPHOS capacity.27
One hypothesis for the mitochondrial dysfunction in MHS muscle observed here is the result of chronic elevation in cytosolic Ca2+, as has been demonstrated in human28 and porcine29 MH muscle, and knock-in mouse models.11, 12, 30 Ca2+ increases mitochondrial activity which can stimulate higher rates of ROS production through CI, CII, and CIII of the electron transport chain.31 Increased ROS production has been observed in MH RYR1 knock-in mice11 and it is possible that this may translate into human MHS muscle. Increased ROS production, which can cause DNA damage and structural damage to organelles, could explain the mitochondrial swelling seen in previous ultrastructural studies.10, 16, 18 Ca2+ is also a positive regulator of the mitochondrial permeability transition pore (MPTP) of the inner mitochondrial membrane.32 Opening of the MPTP decreases the proton gradient across the inner mitochondrial membrane, which is associated with uncoupling of the electron transport chain from OXPHOS.33
Increased activity of several mitochondrial respiratory states is involved in the hypermetabolic response in MHS mitochondria after exposure to halothane. This could simply be a response driven by conversion of myoplasmic adenosine triphosphate (ATP) to ADP, but Díaz-Vegas and colleagues34 have recently demonstrated a more direct link between skeletal muscle mitochondrial stimulation and activation of RyR1. This may be the result of sarcoplasmic reticulum Ca2+ efflux from activated RyR1 in MHS muscle and uptake by mitochondria. Increased intramitochondrial Ca2+ stimulates dehydrogenase enzymes, which in turn increases NADH (reduced form of nicotinamide adenine dinucleotide) and ATP production.35, 36 The rhabdomyolysis and cell death that occur in a fulminant MH reaction may partly be caused by the failure of skeletal muscle mitochondria to maintain ATP production to accommodate the Ca2+-stimulated state, along with apoptotic mechanisms activated by increased intramitochondrial Ca2+. The FCR of MHN CII(ETS) also increased after exposure to halothane, suggesting a non-RyR1-mediated component of this effect of halothane. However, the clinical relevance of this phenomenon is uncertain, as it occurs in an artificially uncoupled non-physiological state.
A recent study of Canadian MHS patients found that those who responded abnormally to halothane, but not caffeine, in the caffeine-halothane contracture test, had a higher index of Ca2+-related changes in cultured skeletal muscle cells than patients who responded abnormally to halothane and caffeine.37 We compared the magnitude of FCR changes in MHSh and MHShc fibres and found a significantly greater effect of halothane exposure on (CI+CII[OXPHOS]) in MHShc muscle, which is what we expected, although it is at odds with what might have been anticipated from the work of Figueroa and colleagues.37 This potential discrepancy might be explained by the use of cultured myotubes by Figueroa and colleagues,37 whereas we studied adult muscle fibres.
Another unexpected observation was the lack of change in CI(OXPHOS) after halothane treatment. CI activity was reduced by halothane in MH pigs,11, 12 but research in other models has suggested that this effect is independent of the MH trait. Porcine heart mitochondria have shown reversible dose-dependent inhibition of CI (NADH oxidoreductase) after exposure to isoflurane, sevoflurane, or halothane; the latter was the most potent agent.38 Research using the CI mutant gas-1 Caenorhabditis elegans model has also shown evidence of decreased CI function and increased sensitivity to potent inhalation anaesthetics.39, 40 This was not observed in our MHS or MHN muscle and may be because of the inhibitory effects of halothane on CI being both acute and reversible. Such changes would not be detected using our protocol, because of the time delay between halothane exposure and measurement of mitochondrial function (∼90–120 min). In addition, previous studies with halothane treatment have used isolated mitochondria taken out of the normal cellular environment.11, 12
The time delay between halothane exposure and measurement of mitochondrial function might also have reduced the magnitude of change in other components of the electron transport chain, and could have obscured other acute and reversible changes. It is also possible that permeabilisation of the muscle fibres, which is necessary for the SUIT protocol, might have limited the responses to halothane exposure. A further potential confounding factor in our study was that the halothane-exposed samples were maintained at approximate physiological length and tension, and were electrically stimulated during the halothane contracture test, whereas the baseline samples were not. While we think that the differences observed between our halothane-exposed and baseline samples are a result of the halothane exposure rather than other interventions, we were unable to demonstrate this because of a limited supply of human muscle tissue. We plan to address this in future studies using muscle tissue from RYR1 knock-in mouse models.
In conclusion, we observed impaired mitochondrial function in permeabilised human MHS skeletal muscle which we hypothesise results from chronic elevation of cytoplasmic Ca2+ in MHS muscle that causes mitochondrial uncoupling and structural damage through increased ROS production. Exposure to halothane 2 vol% significantly increased OXPHOS and ETS capacity in MHS muscle, confirming a hypermetabolic response to halothane in MHS mitochondria with functional deficiency at baseline.
Authors' contributions
Conception and design of the study: PMH, MAS, JPB.
Conduct of experiments and data collection: LC, JPB.
Data analysis and interpretation: all authors.
Drafting of manuscript: LC, PMH.
Reviewed drafts of the manuscript and approved the final version: all authors.
Declaration of interests
PMH is an Editorial Board Member of the British Journal of Anaesthesia.
Funding
BJA/RCoA PhD studentship (MAS and PMH). National Institutes of Health (NIH) 2P01 AR-05235 (PDA, PMH).
Handling editor: H.C. Hemmings
Editorial decision: 04 February 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.02.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hopkins P.M. Malignant hyperthermia – pharmacology of triggering. Br J Anaesth. 2011;107:48–56. doi: 10.1093/bja/aer132. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins P.M. Malignant hyperthermia: advances in clinical management and diagnosis. Br J Anaesth. 2000;85:118–128. [PubMed] [Google Scholar]
- 3.Zorzato F., Fujii J., Otsu K. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]
- 4.Weiss R.G., O'Connell K.M., Flucher B.E., Allen P.D., Grabner M., Dirksen R.T. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am J Physiol Cell Physiol. 2004;287:C1094–C1102. doi: 10.1152/ajpcell.00173.2004. [DOI] [PubMed] [Google Scholar]
- 5.Eltit J.M., Bannister R.A., Moua O. Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc Natl Acad Sci USA. 2012;109:7923–7928. doi: 10.1073/pnas.1119207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller D.M., Daly C., Aboelsaod E.M. Genetic epidemiology of malignant hyperthermia in the United Kingdom. Br J Anaesth. 2018;121:944–952. doi: 10.1016/j.bja.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández-Ochoa E.O., Pratt S.J.P., Lovering R.M., Schneider M.F. Critical role of intracellular RyR1 calcium release channels in skeletal muscle function and disease. Front Physiol. 2015;6:420. doi: 10.3389/fphys.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLennan D.H., Duff C., Zorzato F. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990;343:559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy T.V., Healy J.M., Heffron J.J. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature. 1990;343:562–564. doi: 10.1038/343562a0. [DOI] [PubMed] [Google Scholar]
- 10.Durham W.J., Aracena-Parks P., Long C. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giulivi C., Ross-Inta C., Omanska-Klusek A. Basal bioenergetic abnormalities in skeletal muscle from ryanodine receptor malignant hyperthermia-susceptible R163C knock-in mice. J Biol Chem. 2011;286:99–113. doi: 10.1074/jbc.M110.153247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuen B., Boncompagni S., Feng W. Mice expressing T4826I-RYR1 are viable but exhibit sex- and genotype-dependent susceptibility to malignant hyperthermia and muscle damage. FASEB J. 2012;26:1311–1322. doi: 10.1096/fj.11-197582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riazi S., Kraeva N., Hopkins P.M. Malignant hyperthermia in the post-genomics era: new perspectives on an old concept. Anesthesiology. 2018;128:168–180. doi: 10.1097/ALN.0000000000001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britt B.A., Endrenyi L., Cadman D.L., Fan H.M., Fung H.Y. Porcine malignant hyperthermia: effects of halothane on mitochondrial respiration and calcium accumulation. Anesthesiology. 1975;42:292–300. [PubMed] [Google Scholar]
- 15.Gronert G.A., Heffron J.J. Skeletal muscle mitochondria in porcine malignant hyperthermia: respiratory activity, calcium functions, and depression by halothane. Anesth Analg. 1979;58:76–81. [PubMed] [Google Scholar]
- 16.Boncompagni S., Rossi A.E., Micaroni M. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell. 2009;20:1058–1067. doi: 10.1091/mbc.E08-07-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs H., Heffron J.J., Badenhorst M. Predictive tests for malignant hyperpyrexia. Br J Anaesth. 1975;47:1075–1080. doi: 10.1093/bja/47.10.1075. [DOI] [PubMed] [Google Scholar]
- 18.Lavorato M., Gupta P.K., Hopkins P.M., Franzini-Armstrong C. Skeletal muscle microalterations in patients carrying malignant hyperthermia-related mutations of the e-c coupling machinery. Eur J Transl Myol. 2016;26:6105. doi: 10.4081/ejtm.2016.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheah K.S., Cheah A.M., Fletcher J.E., Rosenberg H. Skeletal muscle mitochondrial respiration of malignant hyperthermia-susceptible patients. Ca2+-induced uncoupling and free fatty acids. Int J Biochem. 1989;21:913–920. doi: 10.1016/0020-711x(89)90291-7. [DOI] [PubMed] [Google Scholar]
- 20.Thompson S.J., Riazi S., Kraeva N. Skeletal muscle metabolic dysfunction in patients with malignant hyperthermia susceptibility. Anesth Analg. 2017;125:434–441. doi: 10.1213/ANE.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins P.M., Rüffert H., Snoeck M.M. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br J Anaesth. 2015;115:531–539. doi: 10.1093/bja/aev225. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsov A.V., Veksler V., Gellerich F.N., Saks V., Margreiter R., Kunz W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 23.Pesta D., Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 2012;810:25–58. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 24.Perry C.G., Kane D.A., Herbst E.A. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J Physiol. 2012;590:5475–5486. doi: 10.1113/jphysiol.2012.234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen S., Nielsen J., Hansen C.N. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezawork-Geleta A., Rohlena J., Dong L., Pacak K., Neuzil J. Mitochondrial complex II: at the crossroads. Trends Biochem Sci. 2017;42:312–325. doi: 10.1016/j.tibs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemieux H., Blier P.U., Gnaiger E. Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep. 2017;7:2840. doi: 10.1038/s41598-017-02789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López J.R., Alamo L., Caputo C., Wikinski J., Ledezma D. Intracellular ionized calcium concentration in muscles from humans with malignant hyperthermia. Muscle Nerve. 1985;8:355–358. doi: 10.1002/mus.880080502. [DOI] [PubMed] [Google Scholar]
- 29.Lopez J.R., Alamo L.A., Jones D.E. [Ca2+]i in muscles of malignant hyperthermia susceptible pigs determined in vivo with Ca2+ selective microelectrodes. Muscle Nerve. 1986;9:85–86. [PubMed] [Google Scholar]
- 30.Lopez J.R., Kaura V., Diggle C.P., Hopkins P.M., Allen P.D. Malignant hyperthermia, environmental heat stress and intracellular calcium dysregulation in a mouse model expressing the p.G2435R variant of the RYR1 gene. Br J Anaesth. 2018;121:953–961. doi: 10.1016/j.bja.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzy R.D., Sharma B., Bell E., Chandel N.S., Schumacker P.T. Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28:718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo I., Zoratti M. Mitochondrial channels: ion fluxes and more. Physiol Rev. 2014;94:519–608. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- 33.Mailloux R.J., Harper M.E. Mitochondrial proticity and ROS signaling: lessons from the uncoupling proteins. Trends Endocrinol Metab. 2012;23:451–458. doi: 10.1016/j.tem.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Díaz-Vegas A.R., Cordova A., Valladares D. Mitochondrial calcium increase induced by RyR1 and IP3R channel activation after membrane depolarization regulates skeletal muscle metabolism. Front Physiol. 2018;9:791. doi: 10.3389/fphys.2018.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormack J.G., Denton R.M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979;180:533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffiths E.J., Rutter G.A. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta. 2009;1787:1324–1333. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Figueroa L., Kraeva N., Manno C., Toro S., Ríos E., Riazi S. Abnormal calcium signalling and the caffeine–halothane contracture test. Br J Anaesth. 2019;122:32–41. doi: 10.1016/j.bja.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanley P.J., Ray J., Brandt U., Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–693. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayser E.B., Morgan P.G., Sedensky M.M. Mitochondrial complex I function affects halothane sensitivity in Caenorhabditis elegans. Anesthesiology. 2004;101:365–372. doi: 10.1097/00000542-200408000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Falk M.J., Kayser E.B., Morgan P.G., Sedensky M.M. Mitochondrial complex I function modulates volatile anesthetic sensitivity in C. elegans. Curr Biol. 2006;16:1641–1645. doi: 10.1016/j.cub.2006.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.