Abstract
This article provides an overview of the current knowledge on medical complications, health characteristics, and psychosocial issues in adults with achondroplasia. We have used a scoping review methodology particularly recommended for mapping and summarizing existing research evidence, and to identify knowledge gaps. The review process was conducted in accordance with the PRISMA‐ScR guidelines (Preferred Reporting Items for Systematic reviews and Meta‐Analyses Extension for Scoping Reviews). The selection of studies was based on criteria predefined in a review protocol. Twenty‐nine publications were included; 2 reviews, and 27 primary studies. Key information such as reference details, study characteristics, topics of interest, main findings and the study author's conclusion are presented in text and tables. Over the past decades, there has only been a slight increase in publications on adults with achondroplasia. The reported morbidity rates and prevalence of medical complications are often based on a few studies where the methodology and representativeness can be questioned. Studies on sleep‐related disorders and pregnancy‐related complications were lacking. Multicenter natural history studies have recently been initiated. Future studies should report in accordance to methodological reference standards, to strengthen the reliability and generalizability of the findings, and to increase the relevance for implementing in clinical practice.
Keywords: achondroplasia, adults, health‐related quality of life, health status, medical complications, review
1. INTRODUCTION
Achondroplasia is the most common skeletal dysplasia resulting in disproportional short stature, and is in about 99% of the cases caused by a mutation (Gly380Arg) in the gene coding for the fibroblast growth factor receptor 3 (FGFR3).1 A high prevalence of medical complications has been reported in children with achondroplasia, including increased mortality, foramen magnum stenosis, hydrocephalus, sleep apnea, recurrent ear infections, impaired hearing, and leg and spine deformities.2, 3, 4 Several authors have reviewed the existing literature regarding children (0‐16 years) with achondroplasia, and have proposed recommendations for follow‐up and surveillance.2, 3, 4, 5, 6 This includes a comprehensive review recently (2019) published by Pauli.7 We have not identified previous systematic reviews specifically focusing on medical complications, health characteristics, and psychosocial issues in adults with achondroplasia.
Systematic reviews are the reference standard for synthesizing evidence in health care, and can also be used to support the development of clinical practice guidelines and decision‐making.8 A scoping review is one type of systematic literature review based on the framework proposed by Arksey and O'Malley, and further refined by the Joanna Briggs Institute.8, 9 The scoping review methodology is particularly recommended for mapping existing literature, including different study designs, to summarize existing research evidence, clarify key concepts, and identify knowledge gaps.8, 10 The PRISMA‐ScR guidelines (Preferred Reporting Items for Systematic reviews and Meta‐Analyses extension for Scoping Reviews) have recently been published with the aim to improve the quality of scoping reviews.11
The objective of the present study was to systematically review the present research knowledge on medical complications, health characteristics, and psychosocial issues in adults with achondroplasia using a scoping review methodology.
2. METHODS
2.1. Study design
The study was conducted in accordance with the PRISMA‐ScR guidelines and the methods outlined by the Joanna Briggs Institute Methods Manual for scoping reviews, and includes the following stages: (a) identifying the research questions, (b) identifying relevant studies, (c) study selection, (d) extracting and charting the data (e) collating, summarizing and reporting the results in relation to the research questions(s), and (f) (optional) consultations with experts in the field.8, 10, 11 A study protocol was developed (Appendix S1), in accordance with the PRISMA‐ScR guidelines.8, 11
2.2. Search strategy
A systematic literature search was conducted on the 16th February 2017 in MEDLINE, PubMed, Embase, Cinahl, Psychinfo, SweMed+, and the Cochrane Library. We used the following MeSH words and Boolean operators: achondroplasia mp. tw. OR dwarf* mp. tw. OR dwarfism*.mp. tw. AND adult*.mp. OR adult/ OR young adult/ OR adult care.mp. OR adult*.tw.; LIMIT to (humans). We applied no time limit. Two experienced librarians were consulted and approved the search strategy. References in included papers and relevant excluded papers were examined for additional studies, and a non‐systematic search in Google Scholar was conducted as well. We also consulted leading experts in the field of skeletal dysplasia for additional literature. Supplementary literature searches for recently published papers were conducted up to 10th January 2019.
2.3. Inclusion and exclusion criteria
The population of interest was adults, aged 16 years or older, with achondroplasia. In relevant studies of mixed populations, we accepted results on a population of at least 80% of adults with achondroplasia for inclusion, or results presented separately on adults with achondroplasia. Contents of interests were medical complications and health characteristics, including psychosocial issues. Topics of interest were: mortality, neurologic symptoms and spinal stenosis, orthopedic complications, respiratory disorders, sleep apnea, body composition, hearing and vision, obstetric and gynecologic issues, pain, physical functioning, and psychosocial issues. We excluded studies regarding diagnostics, genetics, anatomy, pathophysiology and treatment. Both qualitative and quantitative studies were included, and reviews using a systematic literature search strategy. We excluded case‐studies and/or studies with less than six participants, guidelines, book chapters, letters to the editor and conference abstracts. We restricted the inclusion to publications in English and the Scandinavian languages.
2.4. Study selection, data extraction and synthesizing
Two reviewers (S.O.F. and I.B.L.) independently screened the identified titles and abstracts for potentially eligible articles. Full‐text articles were selected and assessed for eligibility by two independent reviewers (S.O.F. and H.J./H.S./G.M.). We applied an inclusion‐form (available as Supporting Information), and the judgments were compared to determine the final study selection. A third reviewer (IBL) verified the inclusion or exclusion. One reviewer (SOF) conducted the data extraction and another reviewer (IBL) verified the results to ensure comprehensiveness and accuracy of the synthesis. We used an a priori data extraction form to collect information (available as Supporting Information). Any disagreements in the process of study selection and data extraction were resolved by discussion with the other reviewers.
According to the scoping review methodology, an assessment of the quality (risk of bias) of the included studies was not performed.8 We did not exclude any studies because of the methodological quality.
The included articles are presented in text and tables. We organized our review results in the following way: first, included reviews are presented. Next, primary studies are presented with results according to the following headings: mortality, neurology and spinal stenosis, orthopedics and bone density, obesity and body composition, respiratory disorders and sleep apnea, obstetric and gynecologic issues, hearing, voice and vision, pain, physical functioning and health‐related quality of life (HRQOL), and education and work participation. Because some of the primary studies have broad aims and cover several issues, they are reported within more headings.
3. RESULTS
3.1. Search results, inclusion and exclusion
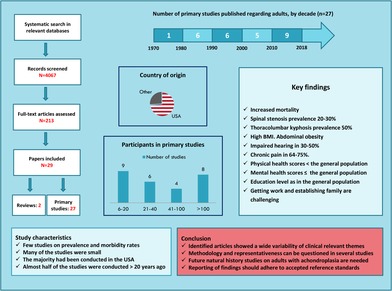
Figure 1 presents a flow chart of the search results and selection of eligible publications. The literature search generated a total of 4067 records after duplicates had been removed, including an additional 28 articles identified from reference lists of reviews and included studies, and from a non‐systematically Google Scholar search. We assessed 213 full‐text articles of which 29 met the eligibility criteria; 2 reviews and 27 primary studies.
Figure 1.
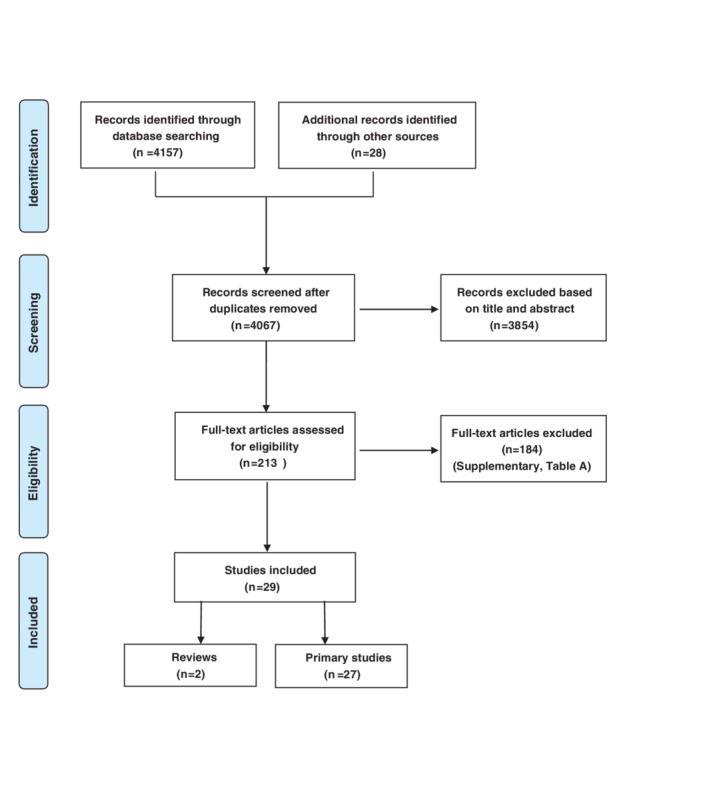
PRISMA Flow‐diagram: Search and selection process [Colour figure can be viewed at http://wileyonlinelibrary.com]
Key information and findings of the reviews, including the study author's conclusions, are presented in Table 1. Characteristics of the primary studies are presented in Table 2, including the year of publication, first author, topics, study design, whether standardized instruments were used, the reporting of inclusion and exclusion criteria and limitations, adult study population, and country of origin.
Table 1.
Main findings of included reviews
| Reference details, title | Design and methods | Materials | Main results and primary author's conclusion |
|---|---|---|---|
| Engberts et al12 | |||
| The prevalence of thoracolumbar kyphosis in achondroplasia: a systematic review | A systematic literature review in PubMed, Embase and Thompson Reuters Web of Knowledge. Selection and quality assessment of included studies by the Newcastle‐Ottawa Quality Assessment Scale for cohort studies | Seven primary studies were included |
The thoracolumbar kyphosis prevalence rate could not be assessed because of differences in definition of thoracolumbar kyphosis and population Author's conclusion: There was very little information available on the prevalence of thoracolumbar kyphosis in achondroplasia. The quality of existing studies was low, and sample sizes were small |
| Thompson et al13 | |||
| Medical and social aspects of the life course for adults with a skeletal dysplasia: a review of current knowledge | Literature search in relevant databases on medical, psychological and social issues up to August 2004, supplied by recent material the following 18 months (≈ February 2006) | Twenty‐two primary studies were included |
Reported on the following issues: Adolescence and transition to adulthood, stature, employment, independence, partnership and marriage, identity and the “disability label,” quality of life, medical and health aspects, living with the attitude of others, and older life. An appendix summarized the main findings Author's conclusion: There were serious gaps in the available literature, and research evidence was sparse and often based on biased samples and limited numbers. There is a clear need for future research in the area with a more stringent methodological approach |
Table 2.
| Year | Reference details | Topics | Study design | Standardized instruments | Inclusion(I) or exclusion (E) criteria provided |
Study limitations discussed |
Adult study population (n) | Country of origin | |
|---|---|---|---|---|---|---|---|---|---|
| I | E | ||||||||
| 2017 | Brooks et al14 | Orthopedics: knee ligament injuries | Retrospective | X | X | 430 | USA | ||
| 2017 | Dhiman et al15 | Pain and HRQOL | Cross‐sectional | SF‐12 | X | X | 106 | USA | |
| 2016 | Khan et al16 | Orthopedics and spine | Retrospective | X | 39 | USA | |||
| 2015 | Matsushita et al17 | Bone density | Cross‐sectional | X | 10 | Japan | |||
| 2013 | Alade et al18 | Pain and mobility | Cross‐sectional | BPI, Bleck Scale | X | X | X | 159 | USA |
| 2012 | Arita et al19 | Bone density | Case series | 11 | Brazil | ||||
| 2012 | Tunkel et al20 | Hearing | Cross‐sectional | X | 29 | USA | |||
| 2011 | Cortinovis et al21 | Psychosocial health | Mixed method |
Flow Questionnaire, The Life Theme Questionnaire |
X | 18 | Italy | ||
| 2010 | Ain et al22 | Spinal stenosis and pain | Cross‐sectional | Symptomatic lumbar spinal stenosis assessment, SCL90R, BDI, STAI | X | X | 181 | USA | |
| 2008 | Modi et al23 | Spinal stenosis, spinal canal morphology | Cross‐sectional | X | X | X | 17 | South‐Korea | |
| 2007 | Johansen et al24, 25, a | Physical functioning and health status | Cross‐sectional | SF‐36 | X | X | X | 19 | Norway |
| 2007 | Wynn et al26 | Mortality | Retrospective | 307 | USA | ||||
| 2006 | Jeong et al27 | Spinal stenosis, spinal canal morphology | Cross‐sectional | X | X | X | 15 | South‐Korea | |
| 2003 | Gollust et al28 | Physical functioning and QOL | Cross‐sectional | X | X | 189 | USA | ||
| 1998 | Hunter et al29 | Medical complications | Retrospective | X | X | 43 | USA/Canada/UK | ||
| 1998 | Mahomed et al30 | Medical complications | Cross‐sectional | SF‐36 | X | X | 437 | USA | |
| 1995 | Heuer et al31 | Voice abnormalities | Cross‐sectional | 6 | USA | ||||
| 1990 | Owen et al32 | Body composition and metabolism | Cross‐sectional | 27 | USA/Canada | ||||
| 1990 | Roizen et al33 | Education and work | Cross‐sectional | 20 | USA | ||||
| 1990 | Stokes et al34 | Respiration and lung function | Cross‐sectional | 11 | USA | ||||
| 1988 | Stokes et al35 | Respiration and lung function | Cross‐sectional | X | 66 | USA | |||
| 1987 | Hecht et al36 | Mortality | Retrospective | X | 287 | USA | |||
| 1986 | Allanson and Hall37 | Obstetric and gynecologic issues | Cross‐sectional | 87 | USA/Canada | ||||
| 1982 | Kahanovitz et al38 | Orthopedics and spine | Retrospective | X | 47 | USA | |||
| 1981 | Stace and Danks39 | Education and work | Mixed method | 25 | Australia | ||||
| 1980 | Griffin et al40 | Vision | Cross‐sectional | 27 | USA | ||||
| 1970 | Bailey41 | Orthopedics: spine and arthritis | Cross‐sectional | 39 | USA | ||||
Johansen et al: one study, two papers.
Abbreviations: BDI, Beck depression inventory; BenDebba, instrument for evaluating lumbar spine disorders, validated by BenDebba et al; BPI, brief pain inventory; HRQOL, health‐related quality of life; LPA, Little People of America; QOL, quality of life; SCL90R, symptom check list; SF‐12 or SF‐36, Medical Outcomes Study, short form 12 or 36; STAI, state–trait anxiety inventory; UK, United Kingdom; US, The United States of America.
Eight studies reported on more than 100 participants, while nine small studies included between 6 and 20 persons. The distribution of year of publication was: one in the 70s, six in each of the decades 80s and 90s, five during the period from 2000 to 2009, and nine studies from 2010 to 2018. Reference details, methods and materials, main findings, and the primary study author's conclusion are summarized and presented for each study in Table 3.
Table 3.
Main findings of primary studies
| Reference details, title | Design and methods |
Materials N: Number NR: Data not reported ACH: Achondroplasia |
Main results and primary authors’ conclusion |
|---|---|---|---|
|
Brooks et al14 Low prevalence of anterior and posterior cruciate ligament injuries in patients with achondroplasia |
Cross‐sectional chart review (2002‐2014) of medical records (n = 430) and telephone interview (n = 148) with ACH patients recruited from a hospital register. History of ACL and PCL injuries and level of physical activity |
N: 430 (148 interviewed) Age: 35 ± 18 y Females (n): 212 (49%) Response rate, interview: 148/430 |
No ACL or PCL injuries were found on chart review. One patient reported ACL injury on telephone interview. Self‐reported level of physical activity: Low: 29%, moderate: 51%, high:17%
Author's conclusion: ACL and PCL injuries seem to be rare in patients with ACH, and cannot be completely ascribed to a low level of physical activity. Anatomical differences (increased anterior tibial slope) may be a possible explanation that protect the ACL |
|
Dhiman et al15 Factors associated with health‐related quality of life (HRQOL) in adults with short stature skeletal dysplasias |
Cross‐sectional online survey. Patients recruited from LPA. Questionnaires for physical and mental health (SF‐12), demographic data, pain, surgery, health insurance and social support |
N (total): 189 Age (all): >18 y Females (N, all): 114 (60%) N (ACH): 106 Response rate: NR |
SF‐12 Physical Component Summary: 41 had lower scores than median, 65 had higher SF‐12 Mental Component Summary: 51 had lower scores than median, 55 had higher Prevalence of pain was high in ACH (74.5%) compared with the US average (19%). Results on education, employment, pain location and surgery were not reported separately on ACH.
Author's conclusion: Prevalence of pain was high in ACH and increased with age. Physical and mental mean scores were lower than the national average |
|
Khan et al16 Prevalence of scoliosis and thoracolumbar kyphosis in patients with achondroplasia |
Retrospective chart review (1999‐2013) regarding Cobbs angel measured on lateral and posterio‐anterior radiographs in patients recruited from a hospital register |
N (all ages): 326 N adults (≥20 y): 98 Age (mean all): 18 y Males (n): 176 Females (n): 150 Response rate: Not relevant |
Prevalence of scoliosis in adults, defined as any curvature >10°: 20‐40 y: mild (>10°‐25°): 21/43; moderate to severe: (>25°): 4/43 > 40 y: mild (>10°‐25°): 34/55; moderate to severe: (>25°): 8/55 Thoracolumbar kyphosis in adults, defined as any curvature >10° with apex between T11‐L2: 20‐40 y: mild (>10°‐25°): 13/43; moderate to severe: (>25°): 18/43 > 40 y: mild (>10°‐25°): 13/55; moderate to severe: (>25°): 27/55
Author's conclusion: Patients with ACH have a high prevalence of scoliosis and thoracolumbar kyphosis, and much higher than reported in the literature for the general population. |
|
Matsushita et al17 Low bone mineral density in achondroplasia and hypochondroplasia |
Cross‐sectional study. BMD was measured by DXA at level L1‐L4 in ACH patients and compared with HCH |
N (all): 22 N (adults ACH): 10 Age (mean): 24.8 ya Males (n): 4, Females (n): 6 Response rate: NR |
BMI (mean adults): 26.5a, BMD (mean): 0.86a, z‐score: −1,1a
Author's conclusion: Based on overall results (both ACH/HCH and all age‐groups) the average z‐scores was −1.1 in ACH and HCH, indicating osteopenia |
|
Alade et al18 Cross‐sectional assessment of pain and physical function in skeletal dysplasia patients |
Cross‐sectional online survey. Patients recruited from LPA. The participants answered questionnaires, The Brief Pain Inventory and the Bleck scale, regarding pain intensity, pain interference with daily function, physical function and quality of life |
N (all): 361 Age (all): mean 35.7 y (±16.7) N (adult ACH): 159 Males (n): 60 Females (n): 99 Response rate (all): 361/3000 |
Chronic pain prevalence in adults with ACH: 153 (64%) vs 25%‐35% in the US population. Pain intensity (0‐10): mild (0‐3): 61 (68.5%), moderate (4‐6): 26 (29.2%), and severe (7‐10): 2 (2.3%). Females reported more pain than males. Ambulation: poor walking: 20 (13%), good walking: 133 (87%). ADL: can bath/dress self: 142 (89.3%), can toilet independently: 141 (88.7%), can cook/do housework: 134 (84.3%), can grocery shop: 133 (83.6%). BMI (mean) for all ACH adults: 34.4.
Author's conclusion: Chronic pain is prevalent in short stature patients, and is higher than in general US adult populations. Pain prevalence increased with age, reaching a plateau in the 50s, and markedly impaired independent ambulation and daily function |
|
Arita et al19 Assessment of osteoporotic alterations in achondroplastic patients: a case series |
Case‐series. Patients recruited from hospital registers. Spinal BMD measured by DXA at the lumbar region (L1‐L4) and dental panoramic radiographs |
N: 11 Age, range: 25‐53 y Males (n): 6 Females (n): 5 Response rate: NR |
BMD: 5/11 had low bone density (ostepenia). Panoramic radiographs: 8/11 had cortical erosions
Author's conclusion: The diagnosis of osteoporosis may have a special clinical relevance in cases of bone tissue disorders, such as ACH |
|
Tunkel et al20 Hearing loss in skeletal dysplasia patients |
Cross‐sectional study. Patients recruited from LPA in 2010. Measurements: Audiometry and otoacustic emissions (in 2 adults). Screening threshold 35 dB. Tympanometry and otoscopy |
N (all ACH): 73 A (all ACH): 20,5 y ± 18,3 N (ACH adults): 29 Males/females: NR Response rate: NR |
Audiometry: 16 (55%) failed hearing screening in one or both ears, 9 (31%) in one ear, and 7 (24%) in both ears. 3% (of all) used hearing aids. Tympanometry: Results for ACH are not reported separately
Author's conclusion: Hearing loss is common in skeletal dysplasia, and the prevalence increases with age |
|
Cortinovis et al21 The daily experience of people with achondroplasia |
Mixed method. Patients recruited from the AISAC. Applied the Experience Sampling Method and two questionnaires: Flow Questionnaire and The Life Theme Questionnaire |
N: 18 Age: 23‐48 y (mean 35) Males (n): 8 Females (n): 10 Response rate: NR |
Most participants were unmarried. In particular men spent a large percentage of their time alone. Work was a key resource to achieve well‐being for both men and women, but also a major challenge and future goal. Building one's own family was a major future goal.
Author's conclusion: Challenging and qualified work opportunities are crucial in promoting the personal growth and social integration. Promoting socialization and removing social and communication barriers should be major issues for policy makers, health professionals, and associations |
|
Ain et al22 Progression of low back pain and lower extremity pain in a cohort of patients with achondroplasia |
Cross‐sectional cohort study with 1‐year follow‐up. Patients recruited from LPA. Questionnaires sent by mail, collected by telephone or in person. Several psychological distress instruments and instrument for pain assessment |
N: 181 Age: mean 42.9 years (range 18‐77) Males (n): 86 Females (n): 95 Response rate: 181/480 |
Pain: baseline vs >1 year follow up: Back pain only: 26 (14%) vs 14 (8%), back pain and proximal leg pain: 68 (38%) vs 55 (30%), back, proximal and distal leg pain: 51 (28%) vs 62 (34%), leg only: 36 (20%) vs 50 (28%). BMI (mean): 35.3. Work participation (n = 45): 24.9% had stopped working or changed their type of work within 1 year of follow‐up. Back pain severity, functional disability, psychological distress and presence of other physical symptoms had not changed significantly
Author's conclusion: Individuals with ACH and symptomatic spinal stenosis often experience back pain, which may progress to lower extremity pain and debilitating consequences |
|
Modi et al23 Lumbar nerve root occupancy in the foramen in achondroplasia: a morphometric analysis |
Prospective cross‐sectional study. MRI‐scans of the lumbar spine. Patients were divided into three groups: Symptomatic ACH, non‐symptomatic ACH and control group (non‐ACH with backache) |
N: ACH: 17 Age (ACH): Gr 1:35.6 y, Gr 2:24.2 y, Gr 3:35.9 y N (controls): 20 Males ACH (n): 7 Females ACH (n): 10 Response rate: NR |
The foramen area and root area were reduced in all levels from L1‐L5 in ACH compared with non‐ACH. Nerve root occupancy in patients with ACH was similar or lower than in patients without ACH
Author's conclusion: Symptomatic lumbar stenosis in ACH is primarily a central stenosis rather than a foraminal stenosis, and may be arising from degenerative disc disease |
|
(one study, two papers) Health status of adults with short stature: a comparison with the normal population and one well‐known chronic disease (rheumatoid arthritis) |
Cross‐sectional, postal survey sent to patients registered in the database of the Norwegian Resource Centre for Rare Disorders in 2004. Instruments: SF‐36 and demographic data. Results compared with the general Norwegian population and rheumatoid arthritis (RA) |
N (all): 44 N (ACH): 19 Age: (median): 38 y Females (n): 12 Response rate (all): 44/72 |
Married or being cohabitant: 8 (42%), had own children: 5 (26%), had higher education (>12 y): 7 (37%), currently working full time: 6 (32%) and currently worked part time: 3 (16%). Bodily pain most commonly reported: back pain: 18 (95%), neck pain: 12 (63%), shoulder: 12 (63%), hips: 9 (47%), knees: 9 (47%) and ankles: 9 (47%). Physical health was impaired in all SF‐36 subscales, most in physical functioning, and equal score with RA. Mental health and social functioning were reduced in the short‐stature group, included ACH, and was lower than in RA. BMI (median): 33
Author's conclusion: People with short stature (including ACH) reported impaired health status in all SF‐36 subscales, indicating that they had health problems that influenced their daily living. Health status seemed to decline with increasing age, and earlier than in the general population. Education level in ACH was comparable with the general population |
|
Wynn et al26 Mortality in achondroplasia study: a 42‐year follow‐up |
Retrospective cohort study. Three databases and LPA deceased members registry from the period 1960 to 2003 were used to assess the vital status of ACH individuals and causes and age of death |
N (all): 793 N (adults > 15 y): 307 Males (n): 126 Females (n): 181 Response rate: Not relevant |
Total number of adult deaths: 133. Causes of deaths (adults): heart disease: 50, neurological disease: 6, malignancy: 15, accidents: 12, other: 40, unknown: 10. Number of cardiovascular deaths in the age‐group 25‐35 y: 4, age‐group 35‐45 y: 8, age‐group 45‐55 y: 12
Author's conclusion: Higher rates of mortality and heart‐disease‐related deaths were reported in ACH. Overall survival and average life expectancy were decreased by 10 years in ACH. Accidental, neurological and heart‐disease‐related deaths were increased in adults. Heart‐disease‐related mortality was 10 times higher (32% of all deaths) in ages between 25‐35 years compared with the US general population |
|
Jeong et al27 MRI study of the lumbar spine in achondroplasia. A morphometric analysis for the evaluation of stenosis of the canal |
Cross‐sectional study. 15 patients with ACH were divided into two groups based on having lumbar spine symptoms or not |
N:15 Age (mean): Symptom group: 32 y Asymptomatic group: 26 y Males (n): 5 Females (n): 10 Response rate: NR |
Symptomatic (n = 8), asymptomatic (n = 7). Most common level affected: L1‐L2 and L3‐L4. Cross‐section area was significantly different between symptomatic and asymptomatic patients. The degree of constriction of the spinal canal needed to produce symptoms was unclear. All symptomatic patients had stenosis at the level of the intervertebral disc, suggesting that the stenosis was degenerative
Author's conclusion: A developmental narrow canal plus early or accelerated degenerative changes are important factors for developing spinal stenosis. There is no agreement on exact clinical or radiological definitions of lumbar spinal stenosis. Both clinical and radiological assessment are necessary to establish the diagnosis of spinal stenosis |
|
Gollust et al28 Living with achondroplasia in an average‐sized world: an assessment of quality of life |
Cross‐sectional survey. 750 questionnaires were mailed to individuals with ACH, recruited from LPA, and 750 questionnaires mailed to unaffected parents and/or siblings (FDR) of affected individuals. A qualitative part asking for seriousness and advantages/ disadvantages of ACH was included |
N: 189 + 136 unaffected relatives (FDR) Age: ACH: 40.5 y (range 19‐89) FDR: 43.5 y (range 20‐84) Females (n): ACH: 127, FDR: 103 Response rate: ACH: 25%, FDR: 18% |
Married: ACH 91 (49%), FDR: 121 (89%). Completed college or graduate school: ACH: 86 (46%), FDR: 80 (59%). Employed full time: ACH: 100 (53%), FDR: 65 (48%). Income > $50.000: ACH: 55 (31%), FDR: 98 (73%). Religious attendance: ACH: 89 (47%), FDR: 86 (63%). QOL was lower for ACH in all domains investigated
Author's conclusion: ACH had lower income, less education and were less likely to be married than their unaffected relatives. Disadvantages related to social barriers were as likely to be cited as barriers related to health and functioning |
|
Hunter et al29 Medical complications of achondroplasia: A multicentre patient review |
Retrospective cross‐sectional data, multicenter study. Data were abstracted from hospital records at 5 departments of genetics in Canada, US, UK and Australia. About 40% of cases were supplemented by direct interview |
N (total): 193 N (ACH adults): 43 Age (range, all): 1 y ‐ late 50s Response rate: NR |
Medical complications were reported in children and adults >20 y (n = 43) and presented as cumulative percentage of all ages. There were too few reports (n < 6) on adults on tonsillectomy, speech delay, shunts, apnea, osteotomy, cervicomedullar decompression and cervical neurological signs. Cumulative rates of hearing loss in adults were 38.3%, orthodontic problems 53.8%, and tibial bowing 41.6%. Of 43 adults followed in the age group 20‐30 y, 19.8% reported back pain, increasing to 69.9% at the age ≥ 50 y (n = 5 patients followed). Leg neurologic signs were reported in 40.9% at age 20‐30 y (n = 43), increasing to 77.9% at the age ≥ 50 y (n = 5 patients followed).
Author's conclusion: A significant number of patients have neurological complaints by their teens, and that becomes the majority in adulthood |
|
Mahomed et al30 Functional health status of adults with achondroplasia |
Cross‐sectional study. A mailed questionnaire, including SF‐36, demographic data, general‐ and disease‐specific comorbidities, was sent to ACH members of LPA |
N (all): 816 N (adults): 437 Age: mean 38 y (range 18‐90) Females (%): 59.3 Response rate (all): 816/4000 |
Most common health complaints (n = 437): Chronic back problems 178 (41%), allergies or sinus problems: 167 (38%), arthritis: 146 (33%), hearing impairment: 143 (33%), deformity of spine: 132 (30%), sleeping difficulty: 125 (29%), neck problems: 89 (20%), paralysis or weakness of arm/leg: 86 (20%), chronic ear infection: 73 (17%). Surgery: 2/3 had undergone surgery, most common: tonsillectomy 203 (47%), laminectomy lumbar spine: 101 (23%), osteotomy 84 (19%) SF‐36: Physical Component Summary (PCS): Significantly lower in the fourth decade (from 30 y) SF‐36: Mental Component Summary (MCS): No difference from the general population
Author's conclusion: Functional health status of adults with ACH, measured by the SF‐36, is not drastically reduced in comparison with that of the US population. Physical health declines from 30 years of age and appears to plateau from 50 years of age. Problems related to spinal deformity, pain and neurological manifestations are the most significant determinants of overall physical function |
|
Heuer et al31 Voice abnormalities in short stature syndromes |
Cross‐sectional study. Otolaryngologic and audiologic assessment in patients with short stature recruited from a hospital clinic. |
N (all): 16 N (ACH): 6 Age, ACH mean: 34 y (19–54) ACH males/females: NR Response rate: NR |
5/6 patients with ACH had voice abnormalities: laryngeal abnormalities, hoarse or breathy voice, low pitch
Author's conclusion: Voice and laryngeal abnormality are common in people with short stature. |
|
Owen et al32 Resting metabolic rate and body composition of achondroplastic dwarfs |
Cross‐sectional study. Anthropometric measures (height, weight, skinfold thickness, body circumference and abdominal‐hip ratio), densitometry, indirect calorimetry and fasting blood samples were performed in 27 adults with ACH and compared with 103 lean and obese adults of average height |
N (all): 32 Age, range (all): 18‐54 y N (ACH): 27 Males (n): 16 Females (n): 11 Response rate: NR |
About half had android (abdominal) obesity: abdominal‐hip‐ratio was >1.0 for 5 of 16 males and > 0.8 for 7 of 11 females. Skinfold thickness: the spread of measured densitometric values and predicted skinfold thickness values were wide. Measured RMR: 0.67‐1.27 kcal/min or 962‐1823 kcal/day
Author's conclusion: The study indicates that ACH has greater resting caloric requirements per unit body weight than average statured individuals. BMI were worthless and skinfold thickness and other anthropometric measurements were of very limited value in predicting body fat of dwarfs. Increased abdominal‐hip‐ratios were prevalent in dwarfs, but these ratios do not reflect body fat. None of the ACH individuals had elevated triglycerides or diabetes mellitus |
|
Roizen et al33 Comparison of education and occupation of adults with achondroplasia with same‐sex sibs |
Cross‐sectional study. Patients recruited from LPA. 10 participants were face‐to‐face interviewed, 10 were mailed the same standardized questionnaire regarding education and occupation. Factors related to employment in ACH were compared to their unaffected sibs |
N: 20 Age (mean): Males: 43 y (±14) Females: 33.9 y (±9.3) Males (n): 8 Females (n): 12 Response rate: 20/89 |
Formal education: Male ACH: 14.9 y (±2), sibs: 14.4 (±3). Female ACH: 14.7 y (±2.6), sibs: 14.6 y (±2.1), no significant difference. Mean occupation score: Male ACH: 5.0 (±1.7), not significantly different from their unaffected brothers. Female ACH: 5.3 (±2.1), significantly lower than their unaffected sisters
Author's conclusion: Education appears to be the most important variable determining occupational level for men and women with ACH, but alone cannot explain the differences between occupational attainment of affected men and women. |
|
Stokes et al34 The lungs and airways in achondroplasia. Do little people have little lungs? |
Cross‐sectional study. Patients recruited from an LPA meeting. Measurements: anthropometrics (height, sitting‐height and weight), chest diameter, spirometry and plethysmography |
N (all): 12 N (adults): 11 Age,range: 16‐53 y (median 29) Males (n): 7 Females (n): 4 Response rate: NR |
Chest dimensions: Males: 91% of predicted. Females: 96% of predicted. Vital capacity (FVC) was reduced
Author's conclusion: Lung function was normal: lung volume was reduced, but also oxygen demands and therefore not physiologically relevant. Muscle strength was normal. AP‐diameter was slightly reduced in men |
|
Stokes et al35 Spirometry and chest wall dimensions in achondroplasia |
Cross‐sectional study. Participants recruited from LPA meetings and Johns Hopkins Hospital. Measurements: Anthropometrics (height, sitting‐height, weight), chest diameter and spirometry |
N: 66 A (mean): 28 y Males (n): 26 Females (n): 40 Response rate: NR |
Chest dimensions: only AP‐diameter of males was significantly reduced Spirometry: analysis based on sitting height: FVC: significantly reduced (about 25%‐30%) for both males and females. FEV1/FVC% was normal
Author's conclusion: ACH appears to result in a relative reduction in vital capacity, possibly reflecting effects of the skeletal dysplasia on the chest wall or lung growth |
|
Hecht et al36 Mortality in achondroplasia |
Retrospective historical cohort study. Medical record review on vital status of ACH patients registered at two medical genetic clinics in the US. Causes of death reported in death certificates were compared with the US GP in specific age‐groups. Standardized mortality ratios (SMRs) were calculated |
N (all ages): 701 N (adults ≥ 15 y): 287 Age: all Males/females: NR Response rate: Not relevant |
733 patients detected, 287 adults were included. Number of adult deaths: 36. Main causes of death (adults): cardiovascular: 19, cancer: 3, accidents 3. Number of cardiovascular deaths in the age‐group 25‐34 y: 2, age‐group 35‐44 y: 2, age‐group 45‐54 y: 6
Author's conclusion: Mortality was increased at all ages in ACH and mean life expectancy was about 10 years less in ACH compared with the general US population. Cardiovascular death was increased in the 25‐54‐year‐old age group, accounting for 10 of 17 deaths. |
|
Allanson and Hall37 Obstetric and gynecologic problems in women with chondrodystrophies |
Cross‐sectional study. Questionnaires distributed to women at two patient organizations' meetings in the US and Canada and through their local chapters |
N (total): 150 N (ACH): 87 Age: NR Males/females: NR Response rate: NR |
ACH menarche: 13.3 y (US mean: 12.8 y), menstrual cycle length: 30.2 days (US mean: 28.4), menopause (n = 3): 47.3 y (US mean: 51.4). 26 ACH women had 47 pregnancies, mean age at conception was 26.7 y (US mean: 25.7 y). Complications of pregnancy: 4/26 had symptoms of nerve root compression (lower limbs), 4/26 had respiratory difficulties during pregnancy.
Author's conclusion: Menstrual cycle, menarche and menopause in ACH women did not differ much from US mean. There was a high degree of deliveries by Cesaeran section in pregnancies of short stature women (all). Prevalence of spontaneous abortion was not increased |
|
Kahanovitz et al.38 The clinical spectrum of lumbar spine disease in achondroplasia |
Retrospective review of medical records of patients 15 years or older with ACH having had an assessment of lumbar spine disease |
N = 47 Age (mean): 27,6 y Males (n): 21 Females (n): 26 Response rate: NR |
1. No symptoms: 13 (28%), mean age: 23.5 y, 2. Lumbar pain: 13 (28%), mean age: 24 y 3. Clinical symptoms of disc herniation: 3 (6%), mean age 42 y, 4. Spinal claudication, no neurologic findings: 10 (21%), mean age 32 y. 5. Spinal claudication and objective neurologic findings: 8 (17%), mean age 32 y. TLK was present at the thoracolumbar junction in 50% of all the patients
Author's conclusion: 91% of symptomatic patients had symptom onset ≤30 years of age. The presence of TLK correlates with the severity of symptoms. Higher risk patients should be followed more closely and treated aggressively when disability of neurologic symptoms arise |
|
Stace and Danks39 A social study of dwarfing conditions III. The social and emotional experiences of adults with bone dysplasias |
Mixed method. Only the cross‐sectional part met the inclusion criteria. Patients recruited from different hospital registers, state institutions, the patient association LPAA, and by press and television publicity. Methods not described |
N (total): 57 Age (all): ≥19 y ACH: 25 Males (n): 11 Females (n): 14 Response rate: NR |
Occupation ACH: Employed: 12/25 (48%), general Australian population (GP): 61%, unemployed: 2/25 (8%), GP 1%, invalid/age pension: 10/25 (40%), GP: 9%. The study also reports on obtaining and keeping jobs, job satisfaction, insurance and economy, marital status, children/offspring, social activities, contact with other dwarfed people, membership in LPAA, friendships, use of community facilities and use of specialist services and transport, but the data are not reported separately on ACH
Author's conclusion: No definite conclusion provided |
|
Griffin et al40 Optometric screening in achondroplasia, diastrophic dysplasia, and spondylo‐epiphyseal dysplasia congenital |
Cross‐sectional study. Visual screening (visual acuity, determination of refractive errors, opthalmoscopy, cover test and tonometry) performed on 27 adults with ACH |
N (all): 61 N (adult ACH): 27 Age: ≥ 21 y (mean 38) Males/females: NR Response rate: NR |
Mean spherical refractive error: o.d: + 0.37 (−4.00 − +2.75) o.s: +0.36 (−4.00 − +3.37). 19 of 27 had astigmatism. 6 individuals had strabismus.
Author's conclusion: Refractive error distribution was approximately the same in adults with ACH compared with the general population. The frequency of strabismus was higher than expected in ACH |
|
Bailey 41 Orthopedic aspects of achondroplasia |
Cross‐sectional study. Patients recruited from LPA and hospital clinics. Clinical examination of 63 patients of all ages (3 days ‐ 72 years), radiological findings of 87 patients of all ages, and review of medical charts |
Clinical material: N (>15 y): 39 Radiological material: N (>15 y): 41 Males (all) >15 y (n): 34 Females (all) > 15 y (n): 29 Response rate: NR |
The clinical study: Spinal stenosis/neurological signs: 5/39, orthopedic problems: lateral tibial bowing, hip flexion contracture. The radiological study: 25 had mild scoliosis <20° and 7 had moderate scoliosis (20°‐25°) mainly in the T‐L‐region. Anterior wedging was observed mainly in T12 and L1. Arthritis in the hips (n = 19), knees (n = 14) and ankles (n = 9) was not observed in any of the adult participants
Author's conclusion: Kyphosis and scoliosis are usually mild. Degenerative arthritis in major weight‐bearing joints does not appear to be a problem in ACH adults |
Results are calculated for adults (≥16 y) based on the reported measures in Table 1 in the original paper.
Abbreviations: ACH, Achondroplasia; ACL, Anterior crucial ligament; ADL, Activities of daily living; AISAC, The Italian Association for the Knowledge and Study of Achondroplasia; BMD, Bone mineral density; DTD, Diastrophic dysplasia; DMC, Dyggve‐Melchior‐Clausen dwarfism; DXA, Dual X‐ray absorptiometry; FDR, First degree relatives; FEV1, Forced expiratory volume in 1 second; FVC, Forced vital capacity; GP, General population/average‐statured population; HRQOL, health‐related quality of life; HCH, hypochondroplasia, L, lumbar; LPA; Little People of America; LPAA, Little People's Association of Australasia; MRI, magnetic resonance Imaging; PCL, posterior crucial ligament; PSACH, pseudoachondroplasia; RA, rheumatoid arthritis; RMR, resting metabolic rate; SF‐12/SF‐36, medical outcomes score, Short Form 12 or 36; SD, skeletal dysplasia; T, thoracal; TLK, thoracolumbar kyphosis; UK, United Kingdom, US, The Unites States of America.
Of the publications read in full‐text, 184 articles did not meet the eligibility criteria for inclusion in this scoping review. Excluded articles and reasons for exclusion are presented as Table S1. The most frequent reasons for exclusion were: (a) the study design was not a primary study or a systematic review, (b) the study's principle aim was not within the scope of the present review, (c) the study population was predominantly children, not adults with achondroplasia, (d) the study population was mixed, and data were not reported separately on adults with achondroplasia, (e) case reports with less than six participants, and (f) the paper did not provide sufficient data relevant for the aim of our scoping review.
Some of the excluded papers provide valuable information on adults with achondroplasia and important aspects of living with a skeletal dysplasia, but did not meet the inclusion criteria for the present scoping review. The most relevant papers2, 3, 4, 7, 42, 43 are presented in Table 4.
Table 4.
Selected papers not meeting the inclusion criteria, but providing information on adults with achondroplasia
| Reference details | Title | Study design |
|---|---|---|
| Pauli7 | Achondroplasia: a comprehensive clinical review | Review |
| Unger et al3 | Current care and investigational therapies in achondroplasia | Review |
| Doherty et al42 | Neurological symptoms, evaluation and treatment in Danish patients with achondroplasia and hypochondroplasia | Primary study |
| Ireland et al2 | Optimal management of complications associated with achondroplasia | Review |
| Wright and Irving4 | Clinical management of achondroplasia | Review |
| Shakespeare et al43 | No laughing matter: medical and social experiences of restricted growth | Primary study |
3.2. Reviews
Engberts et al was the only review paper applying a systematic review approach.12 The authors reviewed the literature on thoracolumbar kyphosis in achondroplasia published in the period from 1975 to July, 2010. They identified seven studies, of which one study38 met the inclusion criteria for our scoping review. The other six studies dealt with children or surgical treatment, and were therefore not within the purpose of our scope. The authors found little information available on the prevalence of thoracolumbar kyphosis, that the results were based on small sample sizes and the quality of existing studies was low12; Table 1.
Thompson et al reported on a systematic literature search up to February 2006 on medical, health and social aspects of life in adults with skeletal dysplasia conditions13; Table 1. The authors did not specifically focus on adults with achondroplasia. They identified 22 studies, of which seven have also been identified and included in our scoping review.28, 29, 30, 33, 36, 37, 39 The other 15 studies did not meet the eligibility criteria for our review. Thompson et al reported substantial gaps in knowledge regarding adults, and that the research evidence was based on biased samples and limited numbers. They found little information on social aspects of living with a skeletal dysplasia, on general rates of morbidity, rates of surgical and orthopedic treatment and obesity.13
3.3. Primary studies
Table 2 shows the characteristics of the 27 primary studies included. The studies were published in the period from 1970 to 2017, and included 2657 adult individuals with achondroplasia. The majority were observational studies; six with a retrospective design, 18 were cross‐sectional, and one study was a case series. Two studies applied a mixed method approach, including a qualitative part,21, 39 but only the qualitative results from Cortinovis et al21 were reported separately on adults with achondroplasia.
The diagnosis of achondroplasia was in most studies based on clinical and/or radiological findings, and some studies relied on self‐reports. None of the included studies required a genetically verified diagnosis of achondroplasia. Five studies had clearly defined inclusion and exclusion criteria, all published in 2006 or later (Table 2). Nine studies provided either inclusion or exclusion criteria, but not both. The other 13 primary studies did not report on inclusion or exclusion criteria. Twelve of the 27 primary studies discussed study limitations.
Regarding country of origin, 20 of the 27 original studies mainly recruited from US populations, in particular, the patient association Little People of America (LPA) and Johns Hopkins Hospital. The studies conducted outside the United States were all rather small, comprising between 10 and 33 participants (Table 2). All studies with more than 100 participants were based on US populations. However, their response rates were low; between 20% and 30%. Half of the included primary studies were conducted more than 20 years ago. Six papers reported on the use of standardized instruments regarding HRQOL (Table 2).
3.4. Medical complications, health characteristics and psychosocial issues
Table 5 provides a summary of the key findings based on the included papers in this scoping review.
Table 5.
Summary of key findings based on included studies of medical complications, health characteristics and psychosocial issues in adults with achondroplasia
| Medical complications and health characteristics |
Mortality26, 36
|
| Neurological symptoms and spinal stenosis |
| Orthopedic complications and bone density |
| Obesity and body composition |
| Respiratory disorders and sleep apnea |
| Hearing, voice and vision |
| Obstetric and gynecologic issues |
| Psychosocial issues |
| Pain, physical functioning and HRQOL |
| Education and work participation |
Paper not included in the scoping review.
Small study sample.
3.4.1. Mortality
Two large US studies found that heart disease‐related deaths were high in the age groups 25 to 35 years and 25 to 54 years in achondroplasia.26, 36 Wynn et al reported that heart disease‐related mortality was more than 10 times increased in the ages between 25 and 35 years compared with the general US population, and overall survival and average life expectancy in achondroplasia were decreased by 10 years.26 Of 50 adult deaths, four deceased of heart disease in the age‐group 25 to 35 years, and another 20 in the age group 35 to 55 years. Hecht et al reported on 36 adult achondroplasia deaths, including 10 deaths of cardiovascular disease in the age group 25 to 54 years.36
3.4.2. Neurological symptoms and spinal stenosis
Six studies investigated spinal stenosis and back pain in adults with achondroplasia.22, 23, 27, 29, 30, 38 Hunter et al found that 70% reported back pain at the age of 50 years or older.29 The cumulative prevalence of spinal stenosis increased with age, but these observations were based on a small number of adult participants (n = 11) over 40 years of age.29 Mahomed et al found that 178 (41%) of 437 adults with achondroplasia reported chronic back problems, 101 (23%) had undergone lumbar spine laminectomy, and 32 (7%) cervical spine laminectomy.30 Ain et al found a marked progression of symptoms of spinal stenosis within 1 year of follow‐up of 181 adults with achondroplasia having back pain or lower extremity pain.22 Because of the rapid progression of symptoms, the authors recommended early intervention and close follow‐up after onset of spinal stenotic symptoms. Kahanovitz et al found that in 47 individuals with lumbar spine disease, the majority reported symptom onset before 30 years of age.38
Two studies investigated nerve root occupancy and morphometric analysis of the lumbar spine by using magnetic resonance imaging.23, 27 Both concluded that a developmental narrow canal plus early or accelerated degenerative changes are important factors for developing spinal stenosis. Modi et al suggested that symptomatic spinal stenosis in achondroplasia primarily is a central stenosis rather than a foraminal stenosis.23 Jeong et al found no agreement in the literature on exact clinical or radiological definitions of lumbar spinal stenosis in achondroplasia, and recommended that both clinical and radiological assessment should be required for establishing the diagnosis of spinal stenosis.27
3.4.3. Orthopedic complications and bone density
Six studies reported on orthopedic complications in adults.14, 16, 29, 30, 38, 41 Three of them concerned thoracolumbar kyphosis and scoliosis16, 38 and spine deformity,30 of which one38 was described in the systematic review by Engberts et al.12 Two studies reported on leg deformities and osteotomy,29, 30 another two on prevalence of arthritis,30, 41 and one study reported on ligament injuries in the knee.14
Kahanovitz et al found that kyphosis at the thoracolumbar junction was present in half of the 47 included patients.38 The thoracolumbar kyphosis correlated with the severity of neurologic symptoms, but not all patients with a kyphosis became symptomatic.38 Khan et al showed a much higher prevalence of scoliosis and thoracolumbar kyphosis in 98 adults with achondroplasia than reported in the general US population.16 Mild scoliosis, defined as Cobbs angel >10°, was found in 55 of 98 adults, and moderate to severe scoliosis (Cobbs angel >25°) was found in 12/98 adults. Mild thoracolumbar kyphosis, defined as the curvature >10° to 25° at the thoracolumbar junction (T11‐L2), was seen in 26/98 adults, while severe thoracolumbar kyphosis (curvature >25°) was seen in 45/98 adult individuals.16
Bailey also found high prevalence of scoliosis and thoracolumbar kyphosis, but arthritis in hips, knees and ankles was not observed.41 Conversely, in Mahomed et al (1998) 146 of 437 included adults reported arthritis, and 132 of 437 reported deformity of the spine.30
Brooks et al demonstrated a very low prevalence of anterior and posterior cruciate ligament injuries in 430 adults, indicating that anatomical differences may protect from this kind of injuries in achondroplasia.14
Two small studies, of 10 and 11 adults, respectively, found reduced bone density measured by dual X‐ray absorptiometry.17, 19
3.4.4. Obesity and body composition
Several of the included studies reported on high body mass index (BMI) and obesity in adult achondroplasia populations.18, 22, 24, 32 Owen et al found that half of the participants had increased abdominal‐hip ratio, indicating abdominal obesity. BMI, skinfold thickness and other anthropometric measurements were found to be of very limited value in predicting body fat of dwarfs.32 None of the 27 adults in the study sample had elevated triglycerides or diabetes mellitus. Resting metabolic rates were increased per unit body weight compared to average statured individuals.32
3.4.5. Respiratory disorders and sleep apnea
In two studies, Stokes et al reported on chest diameter, pulmonary function and reference values for spirometry in adults with achondroplasia.34, 35 The lung volume and vital capacity (FVC) were reduced. As the oxygen demands were also reduced, the authors concluded that the reduction in FVC was not physiologically relevant.34 The authors recommended applying sitting height in achondroplasia when comparing spirometry results with average‐statured individuals.35
We did not identify any papers investigating sleep apnea in adults with achondroplasia.
3.4.6. Hearing, voice and vision
In the survey by Mahomed et al 146 of 437 respondents (33%) reported impaired hearing.30 A clinical study by Tunkel et al found that 16 of 29 participants (55%) failed hearing screening in one or both ears, but few reported the use of hearing aids.20 Heuer et al reported voice abnormalities in 5 of 6 adults with achondroplasia.31
Regarding vision, only one study was identified.40 The findings by Griffin et al indicated that strabismus could be more prevalent in achondroplasia (6 of 27 adults) than in the general US population, but the study sample was small.40
3.4.7. Obstetric and gynecologic issues
One study investigated obstetric and gynecologic issues in women with chondrodystrophies, including 87 participants with achondroplasia.37 Menstrual cycle, menarche and menopause in achondroplasia women did not differ much from the US mean. The authors reported a high degree of deliveries by Cesarean section in pregnancies of all short stature women, but results were not reported explicitly on achondroplasia.37
3.4.8. Pain, physical functioning and HRQOL
Dhiman et al found that 79 of 106 (75%) adults with achondroplasia reported pain, compared with 19% in the average‐statured US population.15 Alade et al found that 64% of the 153 respondents reported pain vs 25% to 35% in the general US population.18 Pain prevalence increased with age, and reached a plateau in the 50s, and markedly impaired independent ambulation and daily functioning. However, more than 80% reported to be independent in activities of daily living.18 Dhiman et al found that mean physical component scores, measured by SF‐12, were lower than the US mean in 41 individuals (39%), and decreased with the age of 40 years and older.15 Mahomed et al and Johansen et al reported similar findings.24, 25, 30 Johansen et al found impaired health status in adults with achondroplasia (n = 19), most reduced on physical health subscales, and SF‐36 scores equivalent to individuals with rheumatoid arthritis.24, 25 Physical health scores declined with increasing age, and earlier than in the general average‐statured Norwegian population.24, 25
Three studies found lower mean mental component scores in adults with achondroplasia compared with the general populations.15, 24, 25, 28 These findings were in contrast to Mahomed et al who found lower physical component scores, but no difference in mean mental component scores.30
3.4.9. Education and work participation
Five studies reported on education, work participation, family establishment and social activities.21, 24, 25, 28, 33, 39 Gollust et al found that individuals with achondroplasia had lower income, less education and were less probably to be married than their unaffected relatives.28 Despite 189 respondents, the response rate was low. Cortinovis et al conducted a mixed method study on 18 Italian adults with achondroplasia.21 They found work participation to be crucial in promoting the personal growth and social integration, but also a major challenge and a future goal for many of the respondents. Building one's own family was another reported major future goal. In particular, men (n = 8) spent a large percentage of their time alone.21 Johansen et al found that the education level was comparable to the general Norwegian population, but fewer were married and had children.24, 25
4. DISCUSSION
In this first scoping review on adults with achondroplasia, we document only a slight increase in publications on medical complications, health characteristics, and psychosocial issues over the past decades. The identified articles showed a wide variability of themes relevant for clinical practice, and also to be verified in future studies.
4.1. Characteristics of included papers and the selection process
We identified two reviews and 27 primary studies reporting on a broad range of medical topics and health issues in adults with achondroplasia. Fifteen of the 27 studies had less than 40 participants and 13 had been conducted more than 20 years ago. The majority was conducted on populations recruited from the US patient association LPA. We did not find any population‐based studies. The representativeness and generalizability of some of the included primary studies might therefore be questioned, and the primary author's conclusions, as presented in Table 3, must be interpreted with this in mind.
A number of review papers were identified,2, 3, 4, 7, 46, 47, 48, 49, 50, 51 but were excluded as they were mainly concerning children or because of their non‐systematic methodology.11 However, we have examined their reference lists for potential eligible original papers. Not surprisingly, our literature review did not identify any randomized controlled studies or clinical trials as we did not include treatment as eligibility criteria.
The diagnosis of achondroplasia has until recently been based on characteristic phenotypical and radiological findings.1, 46, 48 Recent studies have reported that the overlap between achondroplasia and the phenotypically milder hypochondroplasia might be more prevalent than previously expected, implicating the need of molecular testing if clinical uncertainty.1, 52 None of the included studies required genetically verified diagnosis of achondroplasia for their inclusion of participants. Some patients with other skeletal disorders, especially hypochondroplasia, might therefore have been included in the populations studied.
4.2. Medical complications, health characteristics and psychosocial issues
Our systematic literature search identified a large body of literature regarding achondroplasia (Figure 1). The majority concerned genetics, diagnostics, pathophysiology, children and treatment, including different surgical procedures, which were not within the aim of the present scoping review. The literature regarding natural history, medical complications and morbidity rates in adults remains rather limited, as also reported by Thompson et al in 2008.13 However, some larger studies have more recently been conducted on pain, HRQOL, orthopedics and spine, as shown in Table 2.14, 15, 16, 18, 22 In the following, we will discuss some of the key findings of the identified studies, as also presented in Tables 3 and 5, including study limitations and identified knowledge gaps.
The mortality rate in achondroplasia has in two large studies been shown to be significantly increased at all ages, both in males and females.26, 36 Heart disease, neurological complications and accidents were the leading causes of death in adults.26 The heart disease‐related mortality was reported to be particularly high in the age group 25 to 35 years, and more than 10 times higher than in the average‐statured population.26 However, the actual recorded number of cardiovascular deaths in this age group was low, the causes were unknown, and only some cases were confirmed by autopsy.26, 36 Coronary heart disease is a common cause of death in the general population as well.53 Further research is therefore needed before we can draw definite conclusions on increased risk of cardiovascular death in adults with achondroplasia.
Spinal stenosis and back pain are frequently reported in adults with achondroplasia.22, 29, 30, 44, 47 Characteristic symptoms are low back pain or lower extremity pain induced by prolonged walking or standing.22, 38, 54 Symptoms are typically relieved by rest or lumbar flexion. In the average‐statured population, symptoms rarely start before 60 years of age, and the progression is slow.54, 55 In achondroplasia, symptoms can start already in late teenage years, increases with age, and the progression seems to be much faster than in average‐statured individuals.22, 38, 56 However, the exact prevalence of spinal stenosis in adults with achondroplasia is uncertain, and based on a relative small number of adult patients or a low response‐rate, giving risk of selection bias.29, 30, 38 Thoracolumbar kyphosis and mild scoliosis were found in more than half of the studied adults with achondroplasia,16 and the presence of thoracolumbar kyphosis was correlated with the severity of symptomatic spinal stenosis.38
Leg deformities, in particular, tibia bowing, are common in children with achondroplasia.29, 57 A high prevalence of arthritis in the hips and knees in adults could therefore be expected.58 However, there are few studies reporting on arthritis in achondroplasia, and the results are somewhat contradictory. While one paper41 did not find an increased prevalence of arthritis in major weight‐bearing joints, a third of the respondents in another study30 reported arthritis. We did not find other studies specifically investigating arthritis in adults. Interestingly, recent studies on mice have indicated that the FGFR3‐mutation might protect from the development of arthritis in achondroplasia.59 Two studies reported on osteopenia in achondroplasia,17, 19 but the studies are small and the findings need to be confirmed in future studies.
Obesity and high BMI are frequently reported in achondroplasia.18, 22, 32, 60, 61, 62, 63 However, BMI and other anthropometric measurements are poor predictors of body fat and fat distribution,64 and even more in individuals of disproportionate short stature, such as achondroplasia.32, 60, 65 In a recently published paper, the authors found an atypical obesity with preferential abdominal obesity in achondroplasia children.66 The obesity was not associated with classical complications, such as diabetes or hypercholesterolemia, suggesting an uncommon energy metabolism in achondroplasia.66 This corresponds with previous findings by Owen et al, who found normal triglycerides and no incidents of diabetes in a cohort of adults with achondroplasia with abdominal obesity.32 More research is needed to explore body composition and fat distribution in adults with achondroplasia, including clinical and metabolic implications.
Respiratory and pulmonary disorders may arise in achondroplasia in early infancy,67, 68 but the literature does not reflect pulmonary disorders as a common problem in adults.35
On the contrary, obstructive sleep apnea (OSA) is reported to occur in more than half of the children.69, 70, 71 We found no studies explicitly investigating OSA in adults. Obesity is associated with increased risk of OSA in the general population,72 indicating that adults with achondroplasia might be at a particular risk of OSA.
Recurrent otitis and impaired hearing are other well‐documented complications in children with achondroplasia.29, 73, 74, 75 However, there are few studies reporting on hearing in adults,20, 30 and we only identified one clinical study.20 The authors found that about half of the study population had impaired hearing, but few used hearing aids.20 The study had some methodological weaknesses, as also pointed out by the primary study authors, being conducted under non‐standardized conditions and with risk of selection bias.
Abnormal pelvic anatomy and spine deformities might predispose for complications in pregnancy of females with achondroplasia.45, 76, 77 Nevertheless, there are surprisingly few studies investigating management of pregnancy in achondroplasia. We found one study reporting on obstetric and gynecologic issues in women with chondrodystrophies, but the study was not explicitly focusing on achondroplasia.37 Consensus‐based best practice guidelines for prenatal evaluation and delivery of patients with skeletal dysplasia have recently been published.45 As a consequence of the abnormal pelvic anatomy, the guidelines recommend Cesarean delivery for most skeletal dysplasia disorders, including achondroplasia.45
A high prevalence of chronic pain (64%‐75%) in adults with achondroplasia was reported in two relatively large studies,15, 18 corresponding with other publications included in this review.24, 25, 30 Physical health declined much earlier than in the average‐statured population, often starting in the fourth decade.15, 24, 25, 30 Regarding mental health, the findings were more diverging. While three studies found lower mental health scores in adults with achondroplasia,15, 24, 25, 28 another study did not find any difference compared with the general population.30
Several studies found that education and work participation were crucial for social integration and personal development in achondroplasia,21, 28, 33, 39 but gaining and keeping work was challenging, including finding a partner and establishing family.21, 28, 39 Gollust et al concluded that individuals with achondroplasia experience social challenges living in an average‐sized world.28 Social barriers were reported to be as challenging as barriers related to health and functioning, as also pointed out in a systematic review on quality of life in rare genetic conditions.78
4.3. Strengths and limitations of this scoping review
The present scoping review article provides an overview of the current knowledge of medical complications, health characteristics and psychosocial issues in adults with achondroplasia. The main strength of this review is the methodological approach. We used a systematic framework as recommended in the PRISMA checklist for scoping reviews (PRISMA‐ScR)8, 11 to investigate a broad research question. We regard this methodology as expedient to research fields with small and relatively few studies, and as a possible precursor to systematic reviews and to identify knowledge gaps.
The development of a predefined scoping review protocol is also a strength. The preparation of such protocols promotes consistency and transparency through the review process, and aid prevention of selection bias.8, 79 Moreover, reviews will become dated and need to be revised. A protocol will simplify subsequent revisions and could also be a template for a future systematic review.
Although we conducted a thorough search for literature in many databases and hand‐searches, and also consulted leading experts in the field, there is a possibility that we have failed to identify all studies relevant for our scope. We did not contact the authors of the primary studies to clarify questions we may have had in the review process. In some circumstances, additional information from authors could have enlightened the extracted data. We decided to limit our inclusion to studies with at least six participants. This might be a limitation to the results. However, this decision was made to ensure that our review was not a review of case studies, which is a separate methodology.80
4.4. Implications and future directions
Several identified studies were excluded as they have been conducted on mixed populations of skeletal dysplasia, or on populations of mixed age‐groups, without reporting separately on the results regarding adults with achondroplasia. When not able to identify explicitly the findings related to achondroplasia or adults, it is difficult to draw general conclusions on the population of interest.
The health services provided should be based on recommendations from research evidence.8 Despite achondroplasia being one of the best described skeletal dysplasias, known for centuries,7 the research field needs to be brought forward in a way that makes topics, methodology and findings stronger and relevant for implementing in clinical practice. Several large multicenter natural history studies on adults with achondroplasia have recently been initiated (https://clinicaltrials.gov), including a natural history registry.81 We will encourage future studies to follow reporting guidelines, as proposed by the EQUATOR network, to enhance the quality and consistency of published papers.82 New treatment options will affect the natural history of achondroplasia in children and adults.3, 83 Good clinical descriptions of natural history are necessary for monitoring and evaluating long term outcomes in medical trials and for decision‐making regarding implementation of new treatment options.
5. CONCLUSION
This scoping review showed a modest increase in peer‐reviewed studies regarding medical complications, health characteristics and psychosocial issues in adults with achondroplasia over the past decades. A broad variety of study topics were identified, many with relevance for clinical practice, but most documentation needs to be elucidated in future research. Studies on sleep‐related disorders in adults and pregnancy‐related complications were lacking. A number of studies on mixed populations were excluded as the results were not presented separately on adults with achondroplasia. Methodological issues and representativeness can also be questioned in several of the included studies, and some of them are also dated. Multicenter natural history studies have recently been initiated. Future studies should report in accordance to methodological reference standards, to strengthen the reliability and generalizability of the findings, and to increase the relevance for implementing in clinical practice.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
Data are available on request.
Supporting information
APPENDIX S1 Current knowledge of medical complications in adults with achondroplasia: protocol for a scoping review
Table S1 Excluded papers (n = 184) read in full‐text, and reason for exclusion
ACKNOWLEDGEMENTS
TRS National Resource Centre for Rare Disorders for facilitating the present work with the scoping review. (SOF) has received funding from the Norwegian ExtraFoundation for Health and Rehabilitation to complete the work with this study.
Fredwall SO, Maanum G, Johansen H, Snekkevik H, Savarirayan R, Lidal IB. Current knowledge of medical complications in adults with achondroplasia: A scoping review. Clin Genet. 2020;97:179–197. 10.1111/cge.13542
Funding information Norwegian ExtraFoundation for Health and Rehabilitation
Peer ReviewThe peer review history for this article is available at https://publons.com/publon/10.1111/cge.13542/
REFERENCES
- 1. Pauli RM, Legare JM. Achondroplasia. Seattle: University of Washington; 1998. https://www.ncbi.nlm.nih.gov/books/NBK1152/. Accessed 15th August 2018. [Google Scholar]
- 2. Ireland PJ, Pacey V, Zankl A, Edwards P, Johnston LM, Savarirayan R. Optimal management of complications associated with achondroplasia. Appl Clinical Genet. 2014;7:117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unger S, Bonafe L, Gouze E. Current care and investigational therapies in Achondroplasia. Curr Osteoporos Rep. 2017;15(2):53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wright MJ, Irving MD. Clinical management of achondroplasia. Arch Dis Child. 2012;97(2):129‐134. [DOI] [PubMed] [Google Scholar]
- 5. White KK, Bompadre V, Goldberg MJ, et al. Best practices in the evaluation and treatment of foramen magnum stenosis in achondroplasia during infancy. Am J Med Genet A. 2016;170(1):42‐51. [DOI] [PubMed] [Google Scholar]
- 6. Trotter TL, Hall JG. American Academy of Pediatrics Committee on G. Health supervision for children with achondroplasia. Pediatrics. 2005;116(3):771‐783. [DOI] [PubMed] [Google Scholar]
- 7. Pauli RM. Achondroplasia: a comprehensive clinical review. Orphanet J Rare Dis. 2019;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141‐146. [DOI] [PubMed] [Google Scholar]
- 9. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19‐32. [Google Scholar]
- 10. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. [DOI] [PubMed] [Google Scholar]
- 12. Engberts AC, Jacobs WC, Castelijns SJ, Castelein RM, Vleggeert‐Lankamp CL. The prevalence of thoracolumbar kyphosis in achondroplasia: a systematic review. J Child Orthop. 2012;6(1):69‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson S, Shakespeare T, Wright MJ. Medical and social aspects of the life course for adults with a skeletal dysplasia: a review of current knowledge. Disabil Rehabil. 2008;30(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 14. Brooks JT, Ramji AF, Lyapustina TA, Yost MT, Ain MC. Low prevalence of anterior and posterior cruciate ligament injuries in patients with Achondroplasia. J Pediatr Orthop. 2017;37(1):e43‐e47. [DOI] [PubMed] [Google Scholar]
- 15. Dhiman N, Albaghdadi A, Zogg CK, et al. Factors associated with health‐related quality of life (HRQOL) in adults with short stature skeletal dysplasias. Qual Life Res. 2017;26(5):1337‐1348. [DOI] [PubMed] [Google Scholar]
- 16. Khan BI, Yost MT, Badkoobehi H, Ain MC. Prevalence of scoliosis and thoracolumbar kyphosis in patients with Achondroplasia. Spine Deformity. 2016;4(2):145‐148. [DOI] [PubMed] [Google Scholar]
- 17. Matsushita M, Kitoh H, Mishima K, et al. Low bone mineral density in achondroplasia and hypochondroplasia. Pediatr Int. 2015; 58(8): 705‐708. [DOI] [PubMed] [Google Scholar]
- 18. Alade Y, Tunkel D, Schulze K, et al. Cross‐sectional assessment of pain and physical function in skeletal dysplasia patients. Clin Genet. 2013;84(3):237‐243. [DOI] [PubMed] [Google Scholar]
- 19. Arita ES, Pippa MG, Marcucci M, et al. Assessment of osteoporotic alterations in achondroplastic patients: a case series. Clin Rheumatol. 2013;32(3):399‐402. [DOI] [PubMed] [Google Scholar]
- 20. Tunkel D, Alade Y, Kerbavaz R, Smith B, Rose‐Hardison D, Hoover‐Fong J. Hearing loss in skeletal dysplasia patients. Am J Med Genet A. 2012;158a(7):1551‐1555. [DOI] [PubMed] [Google Scholar]
- 21. Cortinovis I, Luraschi E, Intini S, Sessa M, Fave AD. The daily experience of people with achondroplasia. Appl Psychol Health Well Being. 2011;3(2):207‐227. [Google Scholar]
- 22. Ain MC, Abdullah MA, Ting BL, et al. Progression of low back and lower extremity pain in a cohort of patients with achondroplasia. J Neurosurg Spine. 2010;13(3):335‐340. [DOI] [PubMed] [Google Scholar]
- 23. Modi HN, Suh SW, Song HR, Yang JH. Lumbar nerve root occupancy in the foramen in achondroplasia: a morphometric analysis. Clin Orthop Relat Res. 2008;466(4):907‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johansen H, Andresen IL, Naess EE, Hagen KB. Health status of adults with short stature: a comparison with the normal population and one well‐known chronic disease (rheumatoid arthritis). Orphanet J Rare Dis. 2007;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johansen H. Challenges of Being Short Statured. A Cross‐sectional Study of Adults Regarding Participation in Paid Employment, Physical Complaints and the use of Health Service and the Welfare System. [master thesis]. Oslo, Norway: 2007. [Norwegian]. Available from: https://www.duo.uio.no/handle/10852/28524
- 26. Wynn J, King TM, Gambello MJ, Waller DK, Hecht JT. Mortality in achondroplasia study: a 42‐year follow‐up. Am J Med Genet A. 2007;143A(21):2502‐2511. [DOI] [PubMed] [Google Scholar]
- 27. Jeong ST, Song HR, Keny SM, Telang SS, Suh SW, Hong SJ. MRI study of the lumbar spine in achondroplasia. A morphometric analysis for the evaluation of stenosis of the canal. J Bone Joint Surg Br. 2006;88(9):1192‐1196. [DOI] [PubMed] [Google Scholar]
- 28. Gollust SE, Thompson RE, Gooding HC, Biesecker BB. Living with achondroplasia in an average‐sized world: an assessment of quality of life. Am J Med Genet A. 2003;120A(4):447‐458. [DOI] [PubMed] [Google Scholar]
- 29. Hunter AG, Bankier A, Rogers JG, Sillence D, Scott CI. Medical complications of achondroplasia: a multicentre patient review. J Med Genet. 1998;35(9):705‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahomed NN, Spellmann M, Goldberg MJ. Functional health status of adults with achondroplasia. Am J Med Genet. 1998;78(1):30‐35. [DOI] [PubMed] [Google Scholar]
- 31. Heuer RJ, Sataloff RT, Spiegel JR, Jackson LG. Voice abnormalities in short stature syndromes. Ear, Nose and Throat J. 1995;74(9):622‐628. [PubMed] [Google Scholar]
- 32. Owen OE, Smalley KJ, D'Alessio DA, et al. Resting metabolic rate and body composition of achondroplastic dwarfs. Medicine (Baltimore). 1990;69(1):56‐67. [DOI] [PubMed] [Google Scholar]
- 33. Roizen N, Ekwo E, Gosselink C. Comparison of education and occupation of adults with achondroplasia with same‐sex sibs. Am J Med Genet. 1990;35(2):257‐260. [DOI] [PubMed] [Google Scholar]
- 34. Stokes DC, Wohl ME, Wise RA, Pyeritz RE, Fairclough DL. The lungs and airways in achondroplasia. Do little people have little lungs? Chest. 1990;98(1):145‐152. [DOI] [PubMed] [Google Scholar]
- 35. Stokes DC, Pyeritz RE, Wise RA, Fairclough D, Murphy EA. Spirometry and chest wall dimensions in achondroplasia. Chest. 1988;93(2):364‐369. [DOI] [PubMed] [Google Scholar]
- 36. Hecht JT, Francomano CA, Horton WA, Annegers JF. Mortality in achondroplasia. Am J Hum Genet. 1987;41(3):454‐464. [PMC free article] [PubMed] [Google Scholar]
- 37. Allanson JE, Hall JG. Obstetric and gynecologic problems in women with chondrodystrophies. Obstet Gynecol. 1986;67(1):74‐78. [PubMed] [Google Scholar]
- 38. Kahanovitz N, Rimoin DL, Sillence DO. The clinical spectrum of lumbar spine disease in achondroplasia. Spine (Phila pa 1976). 1982;7(2):137‐140. [DOI] [PubMed] [Google Scholar]
- 39. Stace L, Danks DM. A social study of dwarfing conditions. III. The social and emotional experiences of adults with bone dysplasias. Aust Paediatr J. 1981;17(3):177‐182. [DOI] [PubMed] [Google Scholar]
- 40. Griffin JR, Ault JE, Sillence DO, Rimoin DL. Optometric screening in achondroplasia, diastrophic dysplasia, and spondyloepiphyseal dysplasia congenita. Am J Optom Physiol Opt. 1980;57(2):118‐123. [DOI] [PubMed] [Google Scholar]
- 41. Bailey JA 2nd. Orthopaedic aspects of achondroplasia. J Bone Joint Surg am. 1970;52(7):1285‐1301. [PubMed] [Google Scholar]
- 42. Doherty MA, Hertel NT, Hove HB, Haagerup A. Neurological symptoms, evaluation and treatment in Danish patients with achondroplasia and hypochondroplasia. J Rare Dis Res Treat. 2017;2(4):25‐32. [Google Scholar]
- 43. Shakespeare T, Thompson S, Wright M. No laughing matter: medical and social experiences of restricted growth. Scand J Disabil Res. 2010;12(1):19‐31. [Google Scholar]
- 44. Hall JG. The natural history of achondroplasia. Basic Life Sci. 1988;48:3‐9. [DOI] [PubMed] [Google Scholar]
- 45. Savarirayan R, Rossiter JP, Hoover‐Fong JE, et al. Best practice guidelines regarding prenatal evaluation and delivery of patients with skeletal dysplasia. Am J Obstet Gynecol. 2018;219(6):545‐562. [DOI] [PubMed] [Google Scholar]
- 46. Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370(9582):162‐172. [DOI] [PubMed] [Google Scholar]
- 47. Hecht JT, Bodensteiner JB, Butler IJ. Neurologic manifestations of achondroplasia. Handb Clin Neurol. 2014;119:551‐563. [DOI] [PubMed] [Google Scholar]
- 48. Baujat G, Legeai‐Mallet L, Finidori G, Cormier‐Daire V, Le Merrer M. Achondroplasia. Best Pract Res Clin Rheumatol. 2008;22(1):3‐18. [DOI] [PubMed] [Google Scholar]
- 49. Shirley ED, Ain MC. Achondroplasia: manifestations and treatment. J am Acad Orthop Surg. 2009;17(4):231‐241. [DOI] [PubMed] [Google Scholar]
- 50. Richette P, Bardin T, Stheneur C. Achondroplasia: from genotype to phenotype. Joint Bone Spine. 2008;75(2):125‐130. [DOI] [PubMed] [Google Scholar]
- 51. Carter EM, Davis JG, Raggio CL. Advances in understanding etiology of achondroplasia and review of management. Curr Opin Pediatr. 2007;19(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 52. Xue Y, Sun A, Mekikian PB, et al. FGFR3 mutation frequency in 324 cases from the international skeletal dysplasia registry. Mol Genet Genomic Med. 2014;2(6):497‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Joseph P, Leong D, McKee M, et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res. 2017;121(6):677‐694. [DOI] [PubMed] [Google Scholar]
- 54. Ammendolia C, Stuber K, de Bruin LK, et al. Nonoperative treatment of lumbar spinal stenosis with neurogenic claudication: a systematic review. Spine. 2012;37(10):E609‐E616. [DOI] [PubMed] [Google Scholar]
- 55. Nerland US, Jakola AS, Solheim O, et al. Minimally invasive decompression versus open laminectomy for central stenosis of the lumbar spine: pragmatic comparative effectiveness study. BMJ. 2015;350:h1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carlisle ES, Ting BL, Abdullah MA, et al. Laminectomy in patients with achondroplasia: the impact of time to surgery on long‐term function. Spine. 2011;36(11):886‐892. [DOI] [PubMed] [Google Scholar]
- 57. Ain MC, Shirley ED, Pirouzmanesh A, Skolasky RL, Leet AI. Genu varum in achondroplasia. J Pediatr Orthop. 2006;26(3):375‐379. [DOI] [PubMed] [Google Scholar]
- 58. Chapple CM, Nicholson H, Baxter GD, Abbott JH. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res. 2011;63(8):1115‐1125. [DOI] [PubMed] [Google Scholar]
- 59. Tang J, Su N, Zhou S, et al. Fibroblast growth factor receptor 3 inhibits osteoarthritis progression in the knee joints of adult mice. Arthritis Rheumatol. 2016;68(10):2432‐2443. [DOI] [PubMed] [Google Scholar]
- 60. Merker A, Neumeyer L, Hertel NT, et al. Growth in achondroplasia: development of height, weight, head circumference, and body mass index in a European cohort. Am J Med Genet A. 2018;176(8):1723‐1734. [DOI] [PubMed] [Google Scholar]
- 61. Hoover‐Fong J, McGready J, Schulze K, Alade AY, Scott CI. A height‐for‐age growth reference for children with achondroplasia: expanded applications and comparison with original reference data. Am J Med Genet A. 2017;173(5):1226‐1230. [DOI] [PubMed] [Google Scholar]
- 62. Hoover‐Fong JE, Schulze KJ, McGready J, Barnes H, Scott CI. Age‐appropriate body mass index in children with achondroplasia: interpretation in relation to indexes of height. Am J Clin Nutr. 2008;88(2):364‐371. [DOI] [PubMed] [Google Scholar]
- 63. Hecht JT, Hood OJ, Schwartz RJ, Hennessey JC, Bernhardt BA, Horton WA. Obesity in achondroplasia. Am J Med Genet. 1988;31(3):597‐602. [DOI] [PubMed] [Google Scholar]
- 64. Thomas EL, Fitzpatrick JA, Malik SJ, Taylor‐Robinson SD, Bell JD. Whole Body Fat: Content and Distribution Progress in nuclear magnetic resonance spectroscopy. 2013; 73:56‐80. [DOI] [PubMed] [Google Scholar]
- 65. Schulze KJ, Alade YA, McGready J, Hoover‐Fong JE. Body mass index (BMI): the case for condition‐specific cut‐offs for overweight and obesity in skeletal dysplasias. Am J Med Genet A. 2013;161a(8):2110‐2112. [DOI] [PubMed] [Google Scholar]
- 66. Saint‐Laurent C, Garcia S, Sarrazy V, et al. Early postnatal soluble FGFR3 therapy prevents the atypical development of obesity in achondroplasia. PLoS One. 2018;13(4):e0195876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hunter AGW, Reid CS, Pauli RM, Scott CI. Standard curves of chest circumference in achondroplasia and the relationship of chest circumference to respiratory problems. Am J Med Genet. 1996;62(1):91‐97. [DOI] [PubMed] [Google Scholar]
- 68. Tasker RC, Dundas I, Laverty A, Fletcher M, Lane R, Stocks J. Distinct patterns of respiratory difficulty in young children with achondroplasia: a clinical, sleep, and lung function study. Arch Dis Child. 1998;79(2):99‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tenconi R, Khirani S, Amaddeo A, et al. Sleep‐disordered breathing and its management in children with achondroplasia. Am J Med Genet A. 2017;173(4):868‐878. [DOI] [PubMed] [Google Scholar]
- 70. Afsharpaiman S, Sillence DO, Sheikhvatan M, Ault JE, Waters K. Respiratory events and obstructive sleep apnea in children with achondroplasia: investigation and treatment outcomes. Sleep & Breathing = Schlaf & Atmung. 2011;15(4):755‐761. [DOI] [PubMed] [Google Scholar]
- 71. Mogayzel PJ Jr, Carroll JL, Loughlin GM, Hurko O, Francomano CA, Marcus CL. Sleep‐disordered breathing in children with achondroplasia. J Pediatr. 1998;132(4):667‐671. [DOI] [PubMed] [Google Scholar]
- 72. Kohler M. Risk factors and treatment for obstructive sleep apnea amongst obese children and adults. Curr Opin Allergy Clin Immunol. 2009;9(1):4‐9. [DOI] [PubMed] [Google Scholar]
- 73. Collins WO, Choi SS. Otolaryngologic manifestations of achondroplasia. Arch Otolaryngol Head Neck Surg. 2007;133(3):237‐244. [DOI] [PubMed] [Google Scholar]
- 74. Chen G, Fu S, Dong J, Zhang L. Otologic and audiologic characteristics of children with skeletal dysplasia in Central China. Acta Otolaryngol. 2013;133(7):728‐732. [DOI] [PubMed] [Google Scholar]
- 75. Glass L, Shapiro I, Hodge SE, Bergstrom L, Rimoin DL. Audiological findings of patients with achondroplasia. Int J Pediatr Otorhinolaryngol. 1981;3(2):129‐135. [DOI] [PubMed] [Google Scholar]
- 76. Dubiel L, Scott GA, Agaram R, McGrady E, Duncan A, Litchfield KN. Achondroplasia: anaesthetic challenges for caesarean section. Int J Obstet Anesth. 2014;23(3):274‐278. [DOI] [PubMed] [Google Scholar]
- 77. Vivanti AJ, Cordier AG, Baujat G, Benachi A. Abnormal pelvic morphology and high cervical length are responsible for high‐risk pregnancies in women displaying achondroplasia. Orphanet J Rare Dis. 2016;11(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cohen JS, Biesecker BB. Quality of life in rare genetic conditions: a systematic review of the literature. Am J Med Genet A. 2010;152a(5):1136‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus‐based clinical case reporting guideline development. BMJ Case Rep. 2013;2(5): 38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hoover‐Fong JE, Bober M, Hashmi S, Hecht J, Legare J, Little M, Pauli R, Serna E, Smid C, Alade AY. Achondroplasia Natural History: the Power of a Multi‐Center Clinical Study. Bruges: International Skeletal Dysplasia Society; 2017. [Google Scholar]
- 82. Moher D. Reporting guidelines: doing better for readers. BMC Med. 2018;16(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yap P, Savarirayan R. Emerging targeted drug therapies in skeletal dysplasias. Am J Med Genet A. 2016;170(10):2596‐2604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Current knowledge of medical complications in adults with achondroplasia: protocol for a scoping review
Table S1 Excluded papers (n = 184) read in full‐text, and reason for exclusion
Data Availability Statement
Data are available on request.


