Abstract
Airway wall thickening and mucus plugging are important characteristics of cystic fibrosis (CF) lung disease in the first 5 years of life.The aim of this study is to investigate the association of lung disease in preschool children (age, 2‐6) with bronchiectasis and other clinical outcome measures in the school age (age >7). Deidentified computed tomography‐scans were annotated using Perth‐Rotterdam annotated grid morphometric analysis for CF. Preschool %disease (a composite score of %airway wall thickening, %mucus plugging, and %bronchiectasis) and %MUPAT (a composite score of %airway wall thickening and %mucus plugging) were used as predictors for %bronchiectasis and several other school‐age clinical outcomes. For statistical analysis, we used regression analysis, linear mixed‐effects models and two‐way mixed models. Sixty‐one patients were included. %Disease increased significantly with age (P < .01). Preschool %disease and %MUPAT were significantly associated with school‐age %bronchiectasis (P < .01 and P < .01, respectively). No significant association was found between preschool %disease and %MUPAT and school‐age forced expiratory volume 1 (FEV1%) predicted and quality of life (P > .05). Cross‐sectional, %disease in school‐age was associated with a low FEV1% predicted and low quality of life (P = .01 and P = .007, respectively). %Disease can be considered an early marker of diffuse airways disease and is a risk factor for school‐age bronchiectasis.
Keywords: bronchiectasis, computed tomography, cystic fibrosis, imaging, preschool children
1. INTRODUCTION
Cystic fibrosis (CF) lung disease is characterized by chronic airway inflammation and infection, leading to early structural changes of the airways, such as airway wall thickening and mucus plugging.1, 2, 3 These changes are thought to lead to the development of bronchiectasis, which is defined as an irreversible widening of the airway.2
Chest computed tomography (CT) is currently the most sensitive method to detect structural changes of the airways in children.1, 4 Bronchiectasis on CT in children is diagnosed when the outer diameter of the airway is larger than the adjacent artery and/or when a lack of tapering can be observed.5, 6, 7 Multiple image analysis systems have been developed to quantify structural lung changes in CT. Using these systems, bronchiectasis was validated as a clinically relevant CT related outcome measures in many studies.8, 9, 10, 11, 12 In addition, bronchiectasis in school‐age children is considered a clinically important outcome measure in CF lung disease.2, 9, 13 It was shown that the extent of bronchiectasis is associated with more pulmonary exacerbations and lower quality of life in school‐aged children.8, 9, 14, 15, 16, 17 Furthermore, it has been well established that spirometry parameters have poor sensitivity to detect and monitor bronchiectasis.18, 19 In today's CF populations that have well‐preserved lung function, bronchiectasis still increases over time in school‐age children as well as in adults, which can occur even when forced expiratory volume 1 (FEV1) does not show significant changes.9, 19, 20 However, to date in preschool children, the usefulness of a bronchiectasis score as an outcome measure for CF lung disease is less clear. Even though bronchiectasis is present in 60% to 80% of the children with CF in the school‐age,21 the extent and severity of bronchiectasis in preschool children are generally lower (29.3%‐61.5%) and therefore not considered a sensitive outcome measure for clinical studies and patient care in this age group.4, 14, 22 However, diffuse airway abnormalities such as airway wall thickening, and mucus plugging are observed in many preschool children.23 It is hypothesized that these preschool airway changes reflect diffuse airway disease that eventually will result in bronchiectasis in the school age.
To quantify early structural lung changes, the Perth‐Rotterdam annotated grid morphometric analysis for CF (PRAGMA‐CF) was developed for the sensitive assessment of structural airway abnormalities in CF children below the age of 6 years.4 An important PRAGMA‐CF outcome measure is the composite score %disease which includes airway wall thickening, mucus plugging, and bronchiectasis all three reflecting airway disease, but which are often difficult to differentiate in the preschool age. It was shown that %disease is a reproducible and precise way of assessing airway disease.4 To validate %disease it was compared in school‐age children to two other established image analysis methods, namely the CF‐CT method and the airway‐artery (AA) ratio method.13 %Disease correlated well to both CF‐CT outcomes and AA‐outcomes, reflecting airway disease.13
To further validate %disease as a clinically relevant outcome measure for preschool CF lung disease it is important to investigate whether it is associated with school‐age bronchiectasis.
The overall aim of this study was to investigate the association of %disease in the preschool age with the development of bronchiectasis and other clinical outcome measures at school age. We hypothesized that %disease assessed by PRAGMA‐CF in the preschool age is a risk factor for the development of later bronchiectasis, for a lower FEV1% predicted, for a lower quality of life and for the development of exacerbations. To do so we studied the association of %disease on routinely acquired chest CTs in preschool children with the prevalence of bronchiectasis on routine CTs of these children in school age. In addition, we studied the association value of %disease with pulmonary exacerbations, FEV1% predicted, and health‐related quality of life. Finally, we investigated the cross‐sectional association between %disease in school‐age and FEV1 and quality of life.
2. METHODS
2.1. Study population
For this longitudinal and retrospective study, we used clinical data acquired at the routine annual evaluations of patients with CF who were followed at the Erasmus Medical Center (MC)—Sophia Children's Hospital (Rotterdam, The Netherlands) which is a tertiary care university hospital. The CF center takes care on average of 150 children with CF up to the age of 19 years. Newborn screening was introduced in May 2011. In annual benchmarking studies as part of the Dutch and European CF registry, our center ranks in the upper quartile for outcome measures such as FEV1 and body mass index.
The following inclusion criteria were applied: (a) a confirmed CF diagnosis by sweat test and genetic testing, (b) informed consent is given by the patient and/or the parent or legal guardian for using clinical data (protocol MEC2013‐338), (c) availability of at least 2 volumetric biennials CT scans acquired at the annual examination: a preschool CT scan made between the age of 2 and 6 years and the most recent spirometer controlled or technician guided school‐age CT scan made at the age of 7 years or older. Patients that had a respiratory exacerbation at the time of CT scan were excluded. The data used for this study was collected retrospectively, from the routinely acquired data of the Sophia Children's cohort. This study was approved by the ethical review board of the Erasmus MC—Sophia Children's Hospital (MEC2013‐338).
2.2. Chest CT protocol and evaluation
From 1999 up to 2004 a biennial sequential CT protocol was used for monitoring the patients with CF. This protocol was replaced by a volumetric CT protocol in 2004. CTs were performed on different CT scanners using low‐dose protocols. From 2007 and onwards, a new CT‐scanner became available, that was fast enough to obtain near motion free volumetric CT scans in nearly all free‐breathing preschool children. From 2007 and onwards most of the school‐aged volumetric CT scans were spirometer controlled. This protocol was introduced in our hospital to optimize and standardize volume during acquisition.24 In short, all school‐age patients are trained to undertake the CT breath‐hold maneuvers before the CT scan and the instructions during the scan are given by the same lung function technician who provided the training. Spirometer controlled CT scans are composed of a volumetric inspiratory scan and a volumetric expiratory scan. Inspiratory scans are performed during a breath‐hold at maximum inspiration. Expiratory scans are performed during a breath‐hold at maximum expiration. When due to logistic reasons CT could not be spirometer controlled, breath‐hold maneuvers were trained and guided by the lung function technician during the CT, but without the spirometer. For those children in the preschool age not able to do a breath‐hold maneuver, a CT scan was obtained while the child was free breathing. CT scanning was done in a supine position, with arms positioned over the head. CT scans in preschool children were free‐breathing in 57 out of the 61 eligible children, spirometer controlled in 1 and technician guided in 3 children. CT scans in school‐age children were technician guided in 4 children and spirometer controlled in 57 children. The first biennial CT scans were executed at the age of 2 years until 2007 after which we changed to execute the first scan at the age of 1 year based on the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST‐CF) findings. Biennial scanning is continued until children reach adulthood and are transferred to the CF team for adults in the Erasmus MC or elsewhere.
2.3. Image analysis
Before annotating the CT scans, all scans were deidentified and randomized. All CT scans were annotated by one certified observer (NB) with more than 2 years of scoring experience. To assess intraobserver agreement, a subset of 20 randomly selected CT scans was rescored by the observer 1 month after scoring the complete CT dataset. To assess interobserver agreement, a subset of 20 randomly selected CT scans were scored by a different observer (CM).
The PRAGMA‐CF scoring system has been extensively described elsewhere.4 In short, a grid is overlaid on 10 equally spaced axial slices of a volumetric chest CT.4 The size of the grid cells overlaying the CT scan is adjusted for the patient's size by measuring the horizontal distance of the lung parenchyma on the axial slice closest to the carina. This distance is divided by 20 to set the grid size. All grid cells on each slice are annotated using a hierarchical system in the following order: (a) healthy, (b) bronchiectasis, (c) mucus plugging, (d) abnormal, (e) atelectasis, (f) trapped air. Abnormal representing airway wall thickening. annotating of each grid cell is only allowed when the grid cell contains at least 50% lung parenchyma.4 For each of the scored items, the volume fraction of the scored component is expressed as a percentage of total lung volume, %disease is computed as the sum of %bronchiectasis, %mucus plugging, and %airway wall thickening, reflecting all components related to airways disease. For this study, we also computed an additional PRAGMA‐CF composite score: %MUPAT. This score consists of the sum of %mucus plugging, and %airway wall thickening, which was used to study the association with school‐age bronchiectasis of airway disease components, without the confounding contribution of %bronchiectasis. Important to note is that as %bronchiectasis, %mucus plugging, %airway wall thickening, and %disease represent % of total lung volume they are small but they can represent a large number of affected airways.
2.4. Clinical outcome measures
For all patients the following clinical data were collected from the electronic patient records.
2.4.1. Spirometry
Spirometry was executed using the ERS‐ATS guidelines. FEV1 was expressed as %predicted using the global lung function 2012 equations.8, 25, 26 The spirometry data that was closest to the last CT scan (maximum 2 months apart) was used for further analysis.
2.4.2. Exacerbations
Because no universally accepted definition of a pulmonary exacerbation is available,27 we selected the same conservative approach as used in previous similar studies, defining an exacerbation as the number of episodes of treatment with IV antibiotics for increased pulmonary symptoms. Using this definition, a positive correlation was observed in several studies between bronchiectasis as scored using the CF‐CT scoring method and the number of exacerbations in patients with CF.8, 15, 28 For our cohort the number of exacerbations was counted from the date of the preschool CT up to the date of the school‐age CT scan. For our statistical analysis, we also divided patients into two groups, one group that had one or more exacerbations and the other group that had no exacerbations between CT scans.
2.4.3. Quality of life
To measure the quality of life at the time of the school‐age CT we used the CF quality of life revised (CFQ‐R) tool29: the child version for children aged 6 to 13; the teen/adult version for patients above 14 years old.27, 30 For our analysis we evaluated only the respiratory domain of the CFQ‐R, as it is most directly related to the severity of lung disease.16, 28 The CFQ‐R scores can vary between 0 and 100, with a higher score reflecting a better health‐related quality of life.
2.5. Statistical analysis
The descriptive statistics were done using SPSS/PC Statistics 21.0 (SPSS Inc Chicago, IL), and the statistical software package R (free download from http://www.rproject.org) version 3.3.1. Continuous variables are presented as mean and categorical variables as percentages.
Regression analysis was performed to investigate the association between the covariates and the outcomes of interest. In particular, we investigated
The association between %disease at preschool age, the CT‐scanner used at preschool age, the age at preschool, with the outcomes: FEV1% predicted, quality of life, and bronchiectasis (linear regression).
The association between %MUPAT, the CT‐scanner used at preschool age, the age at preschool, with the outcomes: FEV1% predicted, quality of life, and bronchiectasis (linear regression).
The cross‐sectional association between %disease at school age, with the outcomes: FEV1% predicted, quality of life, and bronchiectasis (linear regression).
The dataset consists of some outliers, therefore sensitivity analysis was performed to investigate whether the outliers will influence the results. We, furthermore, investigated some transformations of the outcomes, in the example the Wilcoxon rank‐sum test to check sensitivity to the assumptions of the other models. From the analysis with and without the outliers and the different transformations of the outcomes, we obtained the same conclusions.
The relationship between %disease at preschool age and whether a patient experienced no or at least one exacerbation episode from preschool until school age was assessed using a logistic regression model. Linear mixed‐effects models were used for the %disease outcome to assess changes between preschool and one school‐age measurement. The advantage of these models is that they can work with unbalanced data collection times (as in our case datasets with varying times between repeated measurements of each subject).31 Furthermore, they take into account that measurements from the same patient may be more correlated than measurements from different patients. Two‐way mixed‐effects models were used to determine the intraclass correlation, a score above 0.70 was considered to be a good agreement.31 Correction for multiple testing was not performed. Two‐sided P < .05 were considered to be statistically significant.
3. RESULTS
3.1. Study population
The Sophia Children's cohort includes follow‐up data of 187 patients available at the time of data collection. Sixty‐one patients were included in our current study having met all inclusion criteria (Figure 1). Eighty‐two patients were excluded, because they did not have a volumetric CT scan available in the preschool age. Furthermore, 36 patients were excluded, because they were still too young (<7 years old) at the time of data collection and therefore did not have a school‐age volumetric CT scan available. Eight patients were excluded, because they had less than one CT scan available at the time of inclusion (for various reasons, such as changing hospitals). Patient characteristics are shown in Table 1.
Figure 1.
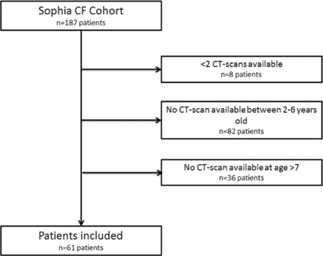
Study flowchart. CF, cystic fibrosis; CT, computed tomography
Table 1.
Patient characteristics
| Characteristics | Preschool | School age |
|---|---|---|
| Number of patients | 61 | |
| Sex, male (%) | 32 (52.4) | |
| Age, y | 4.07 (3.25, 4.67) | 10.65 (8.75, 11.83) |
| Time between scans, y | 6.58 (5.08, 8.08) | |
| Respiratory subscores | ||
| %Disease | 0.7 (0, 0.98) | 1.73 (0.28, 2.86) |
| %MUPAT | 0.46 (0, 0.51) | 0.58 (0, 0.82) |
| %Bronchiectasis | 0.23 (0, 0.29) | 1.15 (0, 1.43) |
| FEV1 (%) predicted | NA | 89.93 (76.5, 97.3) |
| Quality of life (CFQ‐R score) | NA | 76.26 (67, 92) |
| Number of exacerbations | – | 0.72 (0, 1) |
| Genotype | ||
| F508 del/F508 del (%) | 61 | |
| F508 del/other (%) | 38 | |
| Other/other (%) | 2 | |
| Pancreatic insufficiency (%) | 92 | |
| Pseudomonas infection (%) ever | 28 | 42 |
| Mean courses of IV antibiotics (range) | ||
| Birth to preschool CT | 0.44 (0, 5) | 0.75 (0, 9) |
| Preschool CT to school‐age CT | ||
Note: Patient characteristics at the time of the preschool and last school‐age CT scan. Data are presented as no. (%) or mean (interquartile range).
Abbreviations: CF, cystic fibrosis; CT, computed tomography; CFQ‐R, CF quality of life revised; FEV1, forced expiratory volume 1; NA, not available.
In total 122 CT scans were annotated using the PRAGMA‐CF method. CTs were made on six different CT scanners (Table S2a+b online supplement [OLS]). CFQ‐R data and spirometry at the last school‐age CT were available for all 61 patients. The intraclass correlation coefficients (ICC) for intraobserver and interobserver agreement for the PRAGMA‐CF score for %disease and %bronchiectasis were, respectively, 0.91 and 0.70, and 0.93 and 0.83.
3.2. PRAGMA‐CF scores and clinical outcomes
Between preschool and school‐age, %disease increased significantly. In addition, positive associations between preschool CT scores %disease and %MUPAT, and school‐age %bronchiectasis score were observed (Table 2). Also, linear mixed models showed a significant increase (P < .01) in %disease over time (Figure 2). Preschool %disease and %MUPAT were not significantly associated with school‐age FEV1% predicted and CFQ‐R.
Table 2.
Multivariable linear regression analysis
| %Bronchiectasis school age | FEV1% predicted school age | CFQ‐R school age | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | |
| %Disease preschool | 1.18 | 0.82 to 1.54 | <.001 | −2.12 | −7.35 to 3.10 | .42 | −1.57 | −6.19 to 3.05 | .50 |
| %MUPAT preschool | 1.58 | 1.12 to 2.03 | <.001 | −3.45 | −10.20 to 3.30 | .31 | −2.09 | −8.03 to 3.86 | .49 |
| Cross‐sectional analysis | |||||||||
| %Disease school age | NA | NA | NA | −2.79 | −4.98 to −0.60 | .01 | −2.81 | −4.82 to −0.80 | .007 |
Note: A multivariable linear regression was used, where we corrected for the CT‐scanner used at preschool age and for the age at school age.
Abbreviations: CF, cystic fibrosis; CI, confidence interval; CT, computed tomography; CFQ‐R, CF quality of life revised; FEV1, forced expiratory volume 1; NA, not available.
Figure 2.
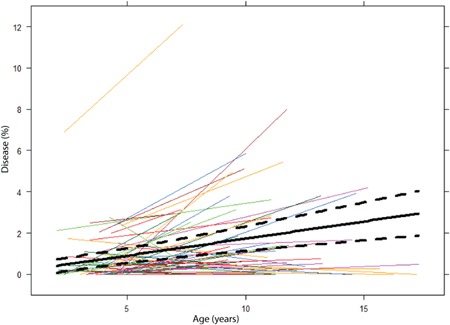
%Disease over time. This figure shows the progression of %disease of individual patients from their preschool CT up to the moment of their last school‐age CT. The black solid line represents the average evolution over time obtained by the linear regression model, while the dashed lines represent the confidence intervals. CT, computed tomography [Color figure can be viewed at wileyonlinelibrary.com]
Multivariable linear regression models showed a significant association after correcting for age at school age and type of CT‐scanner used at preschool between %disease at preschool and %bronchiectasis at school age (Table 2). An increase of 1% in %disease at the preschool age, resulted in an increase of 1.18% (P < .001) in %bronchiectasis at school age. The effects of age at preschool age and the CT‐scanner used at preschool had no significant effect on the outcomes.
When the bronchiectasis component was excluded from %disease the association between %MUPAT and %bronchiectasis at school age remained significant (P < .001; Table 2). Cross‐sectional analysis at school age showed significant associations between %disease and FEV1%predicted (P = .01) and CFQ‐R (P = .007; Table 2).
Of the 61 patients, 19 patients had one or more pulmonary exacerbations that required a course of IV antibiotics in the period between preschool and school age. No significant association between %disease at preschool and exacerbations could be found (P = .19; Table S4 of the OLS).
4. DISCUSSION
Our key finding is that %disease in the preschool age established by PRAGMA‐CF is associated with bronchiectasis in school age. Importantly, the association was still significant for %MUPAT excluding preschool %bronchiectasis. These findings are in line with previous studies in school‐aged children that showed, that mucus plugging is associated with inflammation and airway wall thickening and that these are thought to be risk factors for later bronchiectasis.2
Our findings are also in line with previous findings using a sensitive image analysis method for the objective measurement of AA dimensions (AA‐method) of all visible AA pairs on a CT scan.6, 13 In preschool children the AA‐method showed diffuse widening and progressive thickening of the airways in patients with CF in the Australian AREST‐CF cohort. On the basis of these studies, it was suggested that bronchiectasis represents the tip of the iceberg of underlying diffuse airway disease that starts early in life. In school‐age children, it was shown that bronchiectasis, as detected by the AA‐method, correlated well to bronchiectasis detected by the PRAGMA‐CF scoring method.32 %Disease is a composite score that includes all key morphological changes visible on CT related to airway disease. Thickening of the airway wall is visible on chest CT may be the result of inflammation, and/or mucous attached to the airway wall and both features are captured in %MUPAT. On the basis of our findings, we suggest that %disease and %MUPAT could be used as a clinically relevant outcome measure in clinical studies in preschool patients with CF, as these measures predict later bronchiectasis. %Disease may be preferred as it captures all the principal features of CF airways disease including bronchiectasis. %Bronchiectasis by itself, however, is a less suitable outcome measure in clinical studies in preschool children, as the extent of bronchiectasis in these children is low. In addition, in early CF lung disease in preschool children, it can be difficult to distinguish by eyeballing between a widened and thickened bronchiectatic airway and an airway showing only airway wall thickening.
As supported by our data, %disease captures all the relevant morphological changes of airways in one composite score.
It is important to notice that the %bronchiectasis component of %disease increased over time. This increase in absolute number might seem to be small but that is related to the fact that %bronchiectasis represents the absolute volume fraction of the lung occupied by bronchiectatic airways (Figure S3, OLS).
Similarly, %disease represents the volume fraction of the lung occupied by diseased airways. For example for a patient with a low %disease score of 0.2% at the preschool age, 121 grid cells contained airways, only 3 out of these 121 airways (=2.5%) contained abnormal airways. While at follow‐up, %disease was 0.6% and 230 grid cells contained airways, 11 out of these 230 airways (=4.7%) were considered to be abnormal. For a patient with a higher %disease score of 3.4% at the preschool age, 146 grid cells contained airways, 50 out of these 146 airways (=30%) were considered to be abnormal. At the school‐age %disease increased to 5.8% and 186 grid cells contained airways, 100 out of these 186 airways (=53%) were considered to be abnormal. Hence, %disease captures well the extend of airways disease and tracks progression over time, making it an important outcome measure for randomized controlled trials investigating the effectiveness of drugs that aim to halt progression or reverse CF lung disease. However, %disease as a number might not be right away meaningful for the clinician. Unfortunately, currently, the PRAGMA‐CF software does not automatically generate the absolute number of cells containing normal airways or airways showing bronchiectasis, airway wall thickening, or mucus plugging. For future development of the software, it would be meaningful for clinicians to generate in addition to %disease also the total number of airways counted and the percentage of abnormal airways. For an example of the progression of structural lung disease over time see Figure S4 of the OLS.
We did not observe an association between preschool %disease and later FEV1. This is in line with previous studies in older children where over 2 year time intervals it was shown that chest CT scores showed the significant progression of structural abnormalities, while spirometry outcomes such as FEV1, did not show significant changes.9, 18
For clinical studies in early or milder CF disease, it will, therefore, be important to include a direct measurement of lung structure such as chest CT. Whether progression of structural lung abnormalities from the preschool age into school age can be tracked more sensitively using lung clearance index relative to spirometry outcomes needs to be further investigated in longitudinal studies that include both multiple breath washout and routine chest CT.
No association between preschool %disease and the occurrence of pulmonary exacerbations was found. In previous studies including a larger number of patients, we have observed a correlation between bronchiectasis as scored by CF‐CT and exacerbations defined as courses of IV antibiotics.33 Due to the inclusion criteria, our current cohort is smaller and of a more recent period in time. These patients, therefore, have less advanced disease relative to previous cohorts and require less courses of IV antibiotics. Hence, it is no surprise that we did not observe a correlation between %disease and courses of IV antibiotics.
The regression analysis showed that %disease in the preschool age was not significantly associated with school‐age CFQ‐R respiratory domain scores. This contrasts with earlier longitudinal studies in school‐aged children, where it was shown that bronchiectasis scores were a predictor of later lower quality of life as measured by CFQ‐R. This may be related to the fact that our cohort was younger and had less severe airway disease compared with older and less recent cohorts from previous studies.28, 34
However, in the cross‐sectional analysis at the time of school‐age CT, we did observe a negative association between %disease and %predicted FEV1, and the CFQ‐R respiratory domain scores. This was to be expected as children with more advanced structural damage on CT are more likely to have a lower lung function and quality of life.
We acknowledge some limitations to our study. First, this study was executed in a single‐center, which could mean that the population we studied is not representative for the general CF population. In the European Cystic Fibrosis Society Patient Registry, our center has clinical outcome measures, in the upper range (thus healthier) and structural abnormalities are therefore likely to be less severe compared with other centers. Second, the analysis includes 61 subjects out of the full cohort of 179 subjects. These were all available consecutive subjects that met all inclusion criteria. Most patients were excluded because of them not having a volumetric CT scan at the right age available (Figure 1). Also, there were differences in the CT protocol and CT scanners used for the preschool and school‐age CT scans. At preschool CT scans, most CT scans were acquired supervised by a lung function technician, but without the use of a spirometer. Most school‐age CTs were technician and spirometer controlled, thus optimizing inspiratory lung volume. Therefore, the scans taken at the school‐age were likely to be more sensitive for the detection of bronchiectasis, compared with preschool CT scans. These differences in technique might have influenced the observed progression of %disease. On the one hand, the ability to pick up bronchiectasis in children between 1 and 5 years at functional residual capacity (FRC) has been shown to be reduced relative to total lung capacity (TLC) CTs.7 On the other hand, in preschool and in school‐age children the severity of bronchiectasis on expiratory scans in school‐age children correlates well to the severity of bronchiectasis on scans taken at full inspiration.32, 35 More importantly, it has been shown in preschool children that the sensitivity to pick up airway wall thickening is better on FRC scans relative to TLC scans.6 Even though differentiation between its components is proven difficult, %disease is a composite score that includes both airway wall thickening and bronchiectasis, so differentiation between its components seems to be less relevant to capture relevant airway disease leading to later bronchiectasis. It is likely that we underestimate the true association between preschool %disease and school‐age %bronchiectasis in our real‐life study due to differences in CT scanners and imaging and reconstruction protocols over the 13 years observational period. However, visual scoring methods such as PRAGMA‐CF are considered less affected by such technical influences. In addition, the lower level of lung volume standardization for the preschool CT might have reduced our ability to identify bronchiectasis diseased airways at low lung volume levels. However, the sensitivity to detect airway wall thickening at lower lung volumes is better relative to high lung volumes.6 %Disease has been identified as a reproducible and sensitive PRAGMA‐CF outcome measure for assessing early CF lung disease as it sums up the three most important components of early airways disease being: %airway wall thickening, %mucus plugging, and %bronchiectasis making it less dependent on variation in lung volume level.4
In this study we decided not to include trapped air as an outcome measure as the primary aim of in this cohort was to investigate the predictive value of early visible airway changes (%disease) for later bronchiectasis. However, for future studies, it may be worth investigating if this could also be a sensitive marker for the future development of bronchiectasis.
The use of routine biennial chest CT outcomes for monitoring CF lung disease in young children includes a small risk related to the repeated exposure to ionizing radiation.36 As CF is a genetic disease with high morbidity and mortality, the use of CT imaging in patients with CF is thought to have a favorable risk‐benefit ratio. The findings of this study add further evidence in support of this view.
In conclusion, in preschool children, %disease measured by PRAGMA‐CF on chest CT allows quantification of early clinically relevant morphological features of CF airway disease and it is associated with later school‐age bronchiectasis. In addition, the progression of %disease was observed in this cohort. These findings support the use of %disease as a clinical relevant outcome measure in early CF lung disease.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors like to thank Prof Claire Wainwright (Queensland Children's Medical Research Institute, University of Queensland, Brisbane, Australia) for her critical reading of the manuscript and for her useful comments. We also thank the Netherlands CF Society (NCFS) for their unrestricted financial support as part of the HIT‐CF program that allows to run the AREST‐CF protocol in preschool children in the Erasmus Medical Center‐Sophia. We would also like to thank Merlijn Bonte, Mariette Kemner, Badies Manai, and Els van der Wiel of our research group for all their help with collecting the data for this article.
Bouma NR, Janssens HM, Andrinopoulou E‐R, Tiddens HAWM. Airway disease on chest computed tomography of preschool children with cystic fibrosis is associated with school‐age bronchiectasis. Pediatric Pulmonology. 2020;55:141–148. 10.1002/ppul.24498
References
REFERENCES
- 1. Mott LS, Park J, Murray CP, et al. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67(6):509‐516. [DOI] [PubMed] [Google Scholar]
- 2. Tepper LA, Caudri D, Rovira AP, Tiddens HA, de Bruijne M. The development of bronchiectasis on chest computed tomography in children with cystic fibrosis: can pre‐stages be identified? Eur Radiol. 2016;26(12):4563‐4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flume PA. Pulmonary complications of cystic fibrosis. Respir Care. 2009;54(5):618‐627. [DOI] [PubMed] [Google Scholar]
- 4. Rosenow T, Oudraad MC, Murray CP, et al. PRAGMA‐CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med. 2015;191(10):1158‐1165. [DOI] [PubMed] [Google Scholar]
- 5. de Jong PA, Nakano Y, Hop WC, et al. Changes in airway dimensions on computed tomography scans of children with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(2):218‐224. [DOI] [PubMed] [Google Scholar]
- 6. Kuo W, de Bruijne M, Petersen J, et al. Diagnosis of bronchiectasis and airway wall thickening in children with cystic fibrosis: objective airway‐artery quantification. Eur Radiol. 2017;27:4680‐4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mott LS, Graniel KG, Park J, et al. Assessment of early bronchiectasis in young children with cystic fibrosis is dependent on lung volume. Chest. 2013;144(4):1193‐1198. [DOI] [PubMed] [Google Scholar]
- 8. Loeve M, Gerbrands K, Hop WC, Rosenfeld M, Hartmann IC, Tiddens HA. Bronchiectasis and pulmonary exacerbations in children and young adults with cystic fibrosis. Chest. 2011;140(1):178‐185. [DOI] [PubMed] [Google Scholar]
- 9. Tepper LA, Caudri D, Utens EM, van der Wiel EC, Quittner AL, Tiddens HA. Tracking CF disease progression with CT and respiratory symptoms in a cohort of children aged 6‐19 years. Pediatr Pulmonol. 2014;49(12):1182‐1189. [DOI] [PubMed] [Google Scholar]
- 10. Brody AS, Kosorok MR, Li Z, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thor Imaging. 2006;21(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 11. Davis SD, Fordham LA, Brody AS, et al. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175(9):943‐950. [DOI] [PubMed] [Google Scholar]
- 12. Brody AS, Molina PL, Klein JS, Rothman BS, Ramagopal M, Swartz DR. High‐resolution computed tomography of the chest in children with cystic fibrosis: support for use as an outcome surrogate. Pediatr Radiol. 1999;29(10):731‐735. [DOI] [PubMed] [Google Scholar]
- 13. Kuo W, Andrinopoulou ER, Perez‐Rovira A, Ozturk H, de Bruijne M, Tiddens HA. Objective airway artery dimensions compared to CT scoring methods assessing structural cystic fibrosis lung disease. J Cyst Fibros. 2017;16(1):116‐123. [DOI] [PubMed] [Google Scholar]
- 14. Stick SM, Brennan S, Murray C, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155(5):623‐628. [DOI] [PubMed] [Google Scholar]
- 15. Brody AS, Sucharew H, Campbell JD, et al. Computed tomography correlates with pulmonary exacerbations in children with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(9):1128‐1132. [DOI] [PubMed] [Google Scholar]
- 16. Sawicki GS, Rasouliyan L, McMullen AH, et al. Longitudinal assessment of health‐related quality of life in an observational cohort of patients with cystic fibrosis. Pediatr Pulmonol. 2011;46(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 17. Bortoluzzi CF, Volpi S, D'Orazio C, et al. Bronchiectases at early chest computed tomography in children with cystic fibrosis are associated with increased risk of subsequent pulmonary exacerbations and chronic pseudomonas infection. J Cyst Fibros. 2014;13(5):564‐571. [DOI] [PubMed] [Google Scholar]
- 18. de Jong PA, Nakano Y, Lequin MH, et al. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J. 2004;23(1):93‐97. [DOI] [PubMed] [Google Scholar]
- 19. de Jong PA, Lindblad A, Rubin L, et al. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax. 2005;61(1):80‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loeve M, Krestin GP, Rosenfeld M, de Bruijne M, Stick SM, Tiddens HA. Chest computed tomography: a validated surrogate endpoint of cystic fibrosis lung disease? Eur Respir J. 2013;42(3):844‐857. [DOI] [PubMed] [Google Scholar]
- 21. Sly PD, Wainwright CE. Diagnosis and early life risk factors for bronchiectasis in cystic fibrosis: a review. Expert Rev Respir Med. 2016;10(9):1003‐1010. [DOI] [PubMed] [Google Scholar]
- 22. Sly PD, Gangell CL, Chen L, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963‐1970. [DOI] [PubMed] [Google Scholar]
- 23. Mott LS, Park J, Gangell CL, et al. Distribution of early structural lung changes due to cystic fibrosis detected with chest computed tomography. J Pediatr. 2013;163(1):243‐248. [DOI] [PubMed] [Google Scholar]
- 24. Salamon E, Lever S, Kuo W, Ciet P, Tiddens HA. Spirometer guided chest imaging in children: it is worth the effort!: spirometer guided chest imaging. Pediatr Pulmonol. 2017;52(1):48‐56. [DOI] [PubMed] [Google Scholar]
- 25. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3‐95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zacharasiewicz A, Renner S, Haderer F, et al. Early detection of lung function decrements in children and adolescents with cystic fibrosis using new reference values. Wien Klin Wochenschr. 2017;129:533‐539. [DOI] [PubMed] [Google Scholar]
- 27. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research , U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research , U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health . Guidance for industry: patient‐reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tepper LA, Utens EM, Caudri D, et al. Impact of bronchiectasis and trapped air on quality of life and exacerbations in cystic fibrosis. Eur Respir J. 2013;42(2):371‐379. [DOI] [PubMed] [Google Scholar]
- 29. Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of the cystic fibrosis questionnaire in the United States. Chest. 2005;128(4):2347‐2354. [DOI] [PubMed] [Google Scholar]
- 30. Rosenfeld M. An overview of endpoints for cystic fibrosis clinical trials: one size does not fit all. Proc Am Thorac Soc. 2007;4(4):299‐301. [DOI] [PubMed] [Google Scholar]
- 31. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. NY: Springer; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuo W, Soffers T, Andrinopoulou ER, et al. Quantitative assessment of airway dimensions in young children with cystic fibrosis lung disease using chest computed tomography. Pediatr Pulmonol. 2017;52(11):1414‐1423. [DOI] [PubMed] [Google Scholar]
- 33. Sanders DB, Li Z, Brody AS. Chest computed tomography predicts the frequency of pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc. 2015;12(1):64‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanders DB, Li Z, Brody AS, Farrell PM. Chest computed tomography scores of severity are associated with future lung disease progression in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;184(7):816‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loeve M, Lequin MH, de Bruijne M, et al. Cystic fibrosis: are volumetric ultra‐low‐dose expiratory CT scans sufficient for monitoring related lung disease? Radiology. 2009;253(1):223‐229. [DOI] [PubMed] [Google Scholar]
- 36. Kuo W, Ciet P, Tiddens HAWM, Zhang W, Guillerman RP, van Straten M. Monitoring cystic fibrosis lung disease by computed tomography. Radiation risk in perspective. Am J Respir Crit Care Med. 2014;189(11):1328‐1336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information


