Abstract
Background
There is substantial variation in how menopausal vasomotor symptoms are reported and measured among intervention studies. This has prevented meaningful comparisons between treatments and limited data synthesis.
Objectives
To review systematically the outcome reporting and measures used to assess menopausal vasomotor symptoms from randomised controlled trials of treatments.
Search strategy
We searched MEDLINE, Embase, and Cochrane Central Register of Controlled Trials from inception to May 2018.
Selection criteria
Randomised controlled trials with a primary outcome of menopausal vasomotor symptoms in women and a sample size of at least 20 women per study arm.
Data collection and analysis
Data about study characteristics, primary vasomotor‐related outcomes and methods of measuring them.
Main results
The search identified 5591 studies, 214 of which were included. Forty‐nine different primary reported outcomes were identified for vasomotor symptoms and 16 different tools had been used to measure these outcomes. The most commonly reported outcomes were frequency (97/214), severity (116/214), and intensity (28/114) of vasomotor symptoms or a composite of these outcomes (68/214). There was little consistency in how the frequency and severity/intensity of vasomotor symptoms were defined.
Conclusions
There is substantial variation in how menopausal vasomotor symptoms have been reported and measured in treatment trials. Future studies should include standardised outcome measures which reflect the priorities of patients, clinicians, and researchers. This is most effectively achieved through the development of a Core Outcome Set. This systematic review is the first step towards development of a Core Outcome Set for menopausal vasomotor symptoms.
Tweetable summary
Menopausal hot flushes and night sweats have been reported in 49 different ways in clinical research. A core outcome set is urgently required.
Keywords: Core outcomes, menopause, randomised clinical trials, vasomotor symptoms
Tweetable summary
Menopausal hot flushes and night sweats have been reported in 49 different ways in clinical research. A core outcome set is urgently required.
Introduction
There is general agreement that vasomotor symptoms (hot flushes and night sweats) are the most common and problematic menopausal symptom.1, 2 Vasomotor symptoms are also the leading patient priority for treatment.3 Oestrogen‐containing menopausal hormone therapy (MHT) is an effective treatment for menopausal vasomotor symptoms; however, use of MHT has fallen substantially following concerns about safety.4 There is a growing focus on the development and evaluation of nonpharmacological and nonhormonal treatments for vasomotor symptoms.5 In addition, MHT is contraindicated in women with a personal history of breast cancer who may report more severe vasomotor symptoms than women experiencing natural menopause.6 Enhanced understanding of the central mechanisms regulating vasomotor symptoms is driving the development and evaluation of novel targeted therapies,7 but the interpretation and implementation of these studies is hampered by lack of consensus about how vasomotor symptoms should be reported and measured. This limits the potential to compare treatments and to synthesise the evidence, which in turn compromises decision‐making by clinicians and patients.
Current National Institute for Health and Care Excellence (NICE) guidelines on the management of menopause8 highlight the need for greater standardisation of outcome reporting and measures for treatment trials in menopause, and the consequent difficulty in evidence synthesis. There is an urgent need to determine what outcomes are most important to patients, clinicians, and researchers in order to increase the relevance of future intervention studies and facilitate comparisons between treatments.9, 10 The Core Outcomes in Effectiveness (COMET) initiative is leading protocols for the development of Core Outcome Sets. These are well defined, condition‐specific, and feasible outcomes which should be included as a minimum set of outcomes in intervention studies.11 To advance the development of Core Outcome sets in women’s health,12 80 editors of women’s health journals have formed a consortium to support the development, dissemination, and implementation of core outcome sets within the reproductive field (Core Outcomes in Women's and Newborn Health‐CROWN, http://www.crown-initiative.org).13
The Core Outcomes in Menopause (COMMA) initiative is an international consortium of clinicians, researchers, and consumers developing a Core Outcome Set for menopausal symptoms. Following a standardised process, we have first systematically reviewed all randomised controlled trials (RCTs) of interventions for menopausal vasomotor symptoms to determine what outcomes have been reported and how they have been measured. We will then repeat this process for vaginal symptoms at menopause. This information will then be used to inform a Delphi survey by clinicians, researchers, and patients to identify priorities for inclusion in the final Core Outcome Set.12
Methods
Study eligibility
We included all RCTs with a primary outcome of female menopausal vasomotor symptoms and a sample size of at least 20 women per study arm to minimise the likelihood of including feasibility or pilot studies14. We excluded studies that assessed menopausal vasomotor symptoms as a secondary outcome, quasi‐randomised studies, secondary analyses of previously published RCTs, conference abstracts of RCTs, observational, analytical, or diagnostic studies and feasibility/pilot studies. We also excluded studies primarily aiming to assess pharmacokinetics, the mechanism of drug action, or tolerability and intervention studies with no explicit sample size calculations.
Search strategy
We searched MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) until May 2018. We hand‐searched the reference lists of the included trials or other keynote publications. Search terms included menopause, menopausal, menopausal symptoms, climacteric, hot flush or flash, night sweat, vasomotor, and a search filter for RCTs (Appendix S1). There was no language restriction.
Data extraction
Two reviewers (W.W.L. and S.I.) independently assessed the studies using the predefined criteria described above. Disagreement was resolved by discussion with the steering committee. Full articles were obtained and data were extracted using a prespecified extraction sheet.
Quality assessment
Jadad scoring was used for assessing the methodological quality of the included trials.15 The 5‐point validated scoring system assesses the following: whether the trial (1) was described as randomised (1 point), (2) used an appropriate method of randomisation (1 point), (3) was blinded (1 point), (4) used an appropriate method of blinding (1 point), and (5) accounted for all patients randomised (1 point); ≤2 points was considered low quality and ≥3 was considered medium to high quality.
The quality of describing and reporting the outcome was assessed using the 6‐point Management of Otitis Media with Effusion in Cleft Palate (MOMENT) scoring criteria,16 which has been used previously in the context of quality assessment of studies for the development of a core outcome set and a cut‐off of ≥4 to indicate a high‐quality trial. The following points were considered: whether the primary outcome was (1) clearly stated (1 point), (2) clearly defined (1 point); whether the secondary outcomes were (3) clearly stated (1 point), (4) clearly defined (1 point); (5) whether the authors explained the use of the outcomes they selected (1 point); (6) whether specific methods were used to enhance the quality of outcome measurement (1 point).
Patient involvement
Patients were not involved in the stage of conducting the systematic review but they will be involved in the subsequent steps (Delphi and consensus meeting) of developing the core outcome set.
Core outcomes
Core outcomes do not exist in this research field and were therefore not used in the systematic search. Our aim is to develop and disseminate core outcomes of menopausal symptoms and this systematic review is the first step of the process.
Funding
This study was funded by an MRC postdoctoral fellowship (S.I., MR/N015177/1), NHMRC Practitioner Fellowship (MH, APP 1058935) and an NHMRC Principal Research Fellowship (G.D.M., APP1121844). The funders were not involved in any stage of conducting this systematic review.
Results
The search identified 5591 studies, of which 2711 duplicates were removed. We screened 2880 titles and abstracts and excluded 2372 records which did not meet the inclusion criteria. In all, 544 studies were read in full. Of these, 330 were excluded; 59 included fewer than 20 women per study arm and 54 were not an RCT; 126 did not clearly state a sample size calculation; 52 did not measure vasomotor symptoms as the primary outcome; 39 were secondary analysis. Following these exclusions, 214 RCT were included17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230 (Figure 1). Table S1 describes the 214 studies with a total of 22 682 participants. The studies were published between 1994 and 2018. More than one‐third of the trials (77/214, 36%) were conducted in USA. The follow‐up period ranged from 4 to 52 weeks, with half the included studies following up participants for 12 weeks (108/214, 50%).
Figure 1.
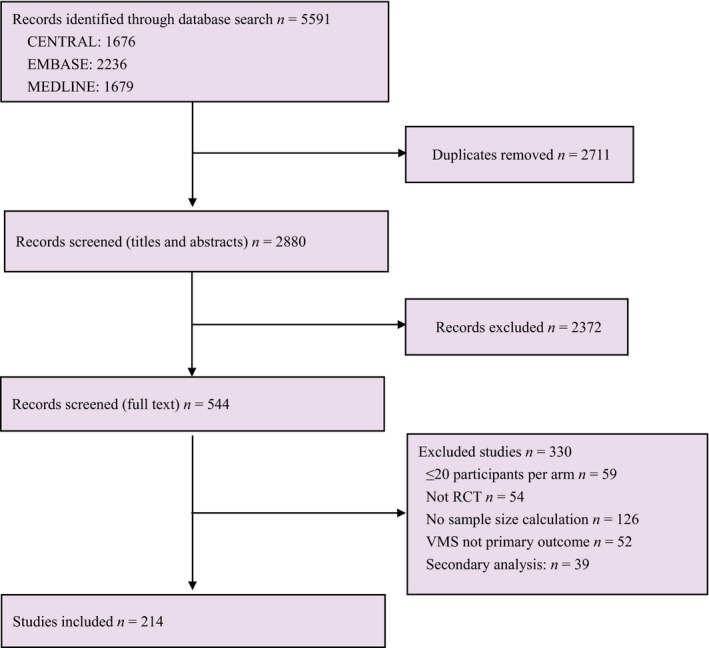
Flowchart of search strategy.
Reported outcomes
The included trials reported 31 different interventions for menopausal vasomotor symptoms: 68 (32%) included hormone therapies and the remaining 146 nonhormonal therapies; 46 were prescription therapies; and 100 were nonprescription therapies. Among these interventions, estrogen and/or progestogen therapies (61/214, 29%) and over‐the‐counter dietary or herbal supplements (68/214, 32%) were the most common interventions (Table 1).
Table 1.
Interventions for menopausal vasomotor symptoms
| Interventions | Trials, n (%) |
|---|---|
| Hormone therapy | 68 (32) |
| Estrogen alone | 45 |
| Estrogen + progestogen | 12 |
| Estrogen + bazedoxifene | 1 |
| Progestogen alone | 4 |
| Tibolone | 6 |
| Nonhormonal therapy | |
| Prescription therapies | 46 (21) |
| SSRIs/SNRIs | 26 |
| Antiepileptic | 10 |
| ERr 731 | 2 |
| BRN‐01 | 1 |
| Cinnarizin | 1 |
| Gamolenic acid | 1 |
| L‐isoleucine | 1 |
| MF 101 | 1 |
| Neurokinin 3 | 1 |
| Oxybutynin | 1 |
| RAD 1901 | 1 |
| Nonprescription therapies | 100 (47) |
| Over‐the‐counter dietary and/or herbal products | 68 |
| Acupuncture | 8 |
| Exercise | 5 |
| Lifestyle education | 4 |
| Relaxation | 3 |
| Paced respiration | 3 |
| Cognitive therapy/cognitive behaviour therapy | 4 |
| Mindfulness training | 2 |
| Guasha | 1 |
| Local thermal therapy | 1 |
| Clinical hypnosis | 1 |
| Total RCTs | 214 |
MF 101, Menopausal Formula 101; RCTs, randomised controlled trials; SSRIs, selective serotonin reuptake inhibitors; SNRIs, serotonin and norepinephrine reuptake inhibitors.
ERr 731: A special extract from the roots of Rheum rhaponticum, referred to as ERr 731 (tradename Phytoestrol N)
BRN‐01: A homeopathic medicine registered in France for menopausal hot flushes, combining the five homeopathic medications: Actaea racemosa (4 centesimal dilutions [4CH]), Arnica montana (4CH), Glonoinum (4CH), Lachesis mutus (5CH), and Sanguinaria canadensis (4CH).
RAD1901: An orally available, selective estrogen receptor degrader (SERD) and selective estrogen receptor modulator (SERM).
Forty‐nine primary outcomes were identified from 214 RCTs including 22 682 women. Almost half of the RCTs (94/214, 44%) only included postmenopausal women, 12% (26/214) only included women with a history of breast cancer, and 5% (12/214) included peri‐ and postmenopausal women. Around a quarter of the RCTs (56/214, 26%) included both surgical and naturally peri‐ and postmenopausal women. We categorised the primary outcomes into four domains: (1) purely vasomotor‐related outcomes (183/214, 86%); (2) quality‐of‐life‐related outcomes (9/214, 4%); (3) composite outcomes (17/214, 8%); and (4) functional impact, specifically how bothersome, interfering and problematic vasomotor symptoms are; for this review we will refer to the latter category as 'interference' outcomes (5/214, 2.3%). The largest group was purely vasomotor‐related outcomes, comprising 33 individual outcomes. The second largest group was composite outcomes, all of which included vasomotor symptoms as one of the parameters. Nine trials assessed quality of life28, 77, 98, 122, 135, 163, 181, 197, 228 and five trials assessed interference33, 60, 66, 92, 138 as primary outcomes (Table 2).
Table 2.
Vasomotor‐related outcome categories
| Outcome categories | The number of ways the outcomes is expressed | Trials, n (%) |
|---|---|---|
|
Purely vasomotor symptoms Frequency of HF Frequency of HF/NS Frequency of moderate to severe HF Frequency of moderate to severe HF/NS Number of HF Number of HF/NS Number of moderate to severe HF Number of severe HF/NS Severity of HF Severity of HF/NS Severity of moderate to severe HF Severity of moderate to severe HF/NS Intensity of HF Intensity of HF/NS Incidence of HF HF (composite/severity) score 41% reduction in HF 44% reduction in HF 50% reduction in HF 75% reduction in HF Frequency of awakenings resulting from nocturnal vasomotor symptoms More than 50% patients halved the distress of HF/NS Moderate to severe rate of HF Percentage of HF reported Proportion of patients responding about vasomotor symptoms Vasomotor complaints Percentage change in HF score Vasomotor symptoms (assessed with the Blatt‐Kupperman Index) HF (assessed with the Greene climacteric) Sweating at night assessed with the Greene climacteric) Vasomotor symptoms per day (HF and NS, assessed with the Wiklund scale) Vasomotor symptom intensity (assessed with the Wiklund scale) Simplified Menopausal Index score |
33 | 177 (83) |
| Quality of life (QOL) | 4 | 9 (4) |
| Interference | 5 | 11 (5) |
|
The extent HF/NS regarded as problem during last week How distressed one feels about HF during last week How much HF interfered with daily routine over the last week Bothersomeness of HF/NS Perceived perimenopausal disturbances scale score |
||
| Composite | 7 | 17 (8) |
| VMS + QOL | 5 | |
| VMS + urogenital symptoms | 4 | |
| VMS + sleep quality | 2 | |
| VMS + side‐effect | 3 | |
| VMS + endocrine symptoms | 1 | |
| VMS + pharmacodynamic markers | 1 | |
| VMS + QOL + satisfaction | 1 | |
| Total RCTs | 214 |
HF, Hot flushes; NS, night sweats; QoL, quality of life; VMS, vasomotor symptoms.
Measurement tools
Seven different measurement tool categories were used to measure purely vasomotor‐related outcomes. Most (158/214, 74%,) included trials used diary records of vasomotor symptoms and 24.7% (53/214) used menopause‐specific subscales. Of these subscales, the Kupperman Menopausal Index (25/57, 44%), Greene Climacteric Scale (15/57, 26%), and Menopause Rating Scale (MRS) (10/57, 18%) were the three most frequently used measurement tools. The Hot Flush Rating Scale (HFRS)33, 60, 66, 92, 138 measures how vasomotor symptoms interfere with daily routine and activities231, 232. The Hot Flush Related Daily Interference scales (HFRDIS) and the shortened Hot Flush Interference (HFI) scale233, 234 have been used to measure interference due to vasomotor symptoms but were not the primary outcome of eligible RCTs, and so were not included in the systematic review. However, they will still be used for the subsequent Delphi process. Other subjective vasomotor symptoms measurement tools included a 20‐item structured symptom checklist; two of the items asking about the presence of hot flushes and cold/night sweats,128 a 5‐point (from none to very severe) scoring system about the severity of hot flushes and night sweats,103 Interactive Voice Response System to record the number and severity of the hot flushes,157 and self‐reported surveys.156 Objective measures of vasomotor symptoms such as skin conductance were used in five trials in addition to subjective measures33, 68, 118, 138, 167 (Table 3).
Table 3.
Tools for measuring vasomotor‐related outcomes
| Tools | n |
|---|---|
| Purely vasomotor symptoms | |
| Hot flushes diary/electronic diary | 158 |
| Menopausal‐specific scale | 53 |
| Kupperman Menopausal Index (KMI) | 25 |
| Greene Climacteric Scale (GCS) | 15 |
| Menopause Rating Scale (MRS) | 10 |
| Wiklund Vasomotor Symptom Subscale score | 1 |
| Perceived Perimenopausal Disturbances Scale | 1 |
| Simplified Menopausal Index (SMI) | 1 |
| Structured menopausal‐specific checklist | 1 |
| Skin conductance monitor system | 5 |
| Interactive voice system | 1 |
| Symptoms scoring system | 1 |
| Self‐reported validated survey instruments | 1 |
| Quality of life | 15 |
| MENQOL | 12 |
| WHQ | 2 |
| EORTC QLQ‐C30 | 1 |
| Interference | |
| The Hot Flush Rating Scale (HFRS) | 5 |
Diaries were used to record 25 different types of menopausal vasomotor‐related outcomes and accounted for 57% (25/44) of all primary outcomes. The number, frequency, severity, and intensity of menopausal vasomotor symptoms were the most commonly reported vasomotor‐related outcomes assessed by diaries. However, there was substantial variation in the definitions of each outcome. For example, for frequency, the majority of included studies (72/214) reported the ‘number of vasomotor symptoms per 24 h’ (8 retrospectively and 64 prospectively), and 39/214 measured the ‘number of vasomotor symptoms per week’. Table S2 shows the complete list of vasomotor‐related outcomes recorded by diaries and how often they have been reported in RCTs of intervention studies for vasomotor symptoms.
Quality of life outcomes was measured by three scales: Menopausal‐Specific Quality of Life (MENQOL),28, 77, 90, 98, 109, 122, 130, 141, 181, 197, 198, 228 Women’s Health Questionnaire (WHQ),135, 163 and the European Organisation for Research and Treatment of Cancer Quality of Life‐Care30 (EORTC QLQ‐C30).136 Of these, 14 of 15 trials chose menopausal‐specific scales (MENQOL, WHQ). Only one trial including women with vasomotor symptoms after breast cancer used a general QOL scale (EORTC QLQ‐C30)136 (Table 3).
Variation in the definitions of vasomotor‐related outcomes
There was substantial heterogeneity in the definition of vasomotor‐related outcomes. Three different definitions were used to measure the frequency of vasomotor symptoms. Most studies (79/97, 81%) defined frequency as the number of hot flushes or night sweats, whereas 18 studies did not define how frequency was measured. The severity of vasomotor symptoms was defined in nine different ways and the intensity in seven different ways (Table S3). The 68 studies reporting composite outcomes for vasomotor symptoms utilised 11 different ways of defining the composite score. The most commonly used approach (27/68, 40%) measured the number of hot flushes and night sweats, and calculated a composite score weighted by severity rating. There was considerable overlap between composite score definitions.
Quality assessment of trials
Regarding methodological quality, 34% (73/214) of included RCTs scored 5 out of 5 points on the Jadad scale. More than half of the trials (118/214, 55%) scored 6 out of 6 points on the MOMENT scale (Table S1).
Discussion
Main findings
This is the first systematic review of outcomes used to measure vasomotor symptoms in randomised controlled trials of interventions. Our findings demonstrate major inconsistencies in how treatments for the same symptoms have been evaluated. For example, the severity of hot flushes and night sweats had nine different definitions. Overall, the most commonly used outcomes for vasomotor symptoms (based on 214 RCTs including 22 682 women) were the frequency and intensity of vasomotor symptoms or a compound measure of these (n = 59). A smaller number of studies (n = 5) took a different approach and measured the interference due to vasomotor symptoms. It remains unclear which measures of vasomotor symptoms best reflect the priorities of patients, clinicians, and researchers, and this will be addressed by the development of a Core Outcome Set. Inclusion of the Core Outcome Set in future intervention studies for vasomotor symptoms will enhance the quality and relevance of trials and facilitate decision‐making by clinicians and patients.
Strengths and limitations
We conducted a comprehensive search strategy with a robust methodological design to include all large (>20 participants per arm) RCTs of interventions for vasomotor symptoms. Two researchers independently evaluated the available evidence to minimise overlooking relevant evidence. To our knowledge, this is the first time that reported vasomotor‐related outcomes have been synthesised, a necessary step to inform the Delphi process for key stakeholders to rate the components of the core outcome set.12 Although most included trials were of medium or high methodological standard, the diverse nature of the outcome measures used diminishes the value of these trials to inform patient choices and clinical decision‐making.
This study focused on menopausal vasomotor symptoms in the first instance. We recognise that personal, ethnic, cultural, and geographical factors influence the nature and experience of menopause, and that not all women experience vasomotor symptoms at menopause.235 We comprehensively searched three major databases and it is unlikely we have missed an RCT published elsewhere. We acknowledge we did not search CINAHL, but we doubt that additional RCTs could only be identified in that database. We included randomised trials with vasomotor‐related symptoms as a primary outcome with over 20 participants in each arm, excluding observational studies and pilot studies. However, given the large number of included studies, we do not anticipate that we have missed outcomes not captured in larger trials. We recognise that this systematic review was limited to trials where vasomotor symptoms were the primary outcome. However, given the large number of studies included, we do not considered that we missed important outcomes. The expert panel and Delphi process will highlight any additional important outcomes that may have been overlooked because they were not included in treatment studies or were reported as secondary outcomes. We also appreciate that our findings may be skewed towards FDA‐driven outcomes, as many studies were conducted in USA. We have only listed the range of outcome measures used and have not applied any qualitative assessment of the value or importance of these measures for women or clinicians. Only a few relatively recent RCTs have measured the impact of interventions on the interference caused in women by vasomotor symptoms and it is uncertain whether the frequency/severity or interference of symptoms best reflects women’s treatment priorities. These issues will be addressed by the Delphi survey and the subsequent consensus meeting. Most published RCTs of interventions for vasomotor symptoms focused on caucasian women who may experience menopause differently from other ethnicities.236 The COMMA consortium includes representation from a wide range of geographical areas and ethnic groups to ensure that the Core Outcome Set reflects variations in stakeholder priorities.
Interpretation
Inconsistency in measures used for the evaluation of treatments for vasomotor symptoms limits comparisons between treatments and the interpretation of findings for clinical practice.237 Understanding the efficacy of new treatments and how they compare with existing approaches requires the use of standardised outcome measures that are meaningful to patients, and feasible for clinicians and researchers.238
A Core Outcome Set does not preclude the inclusion of additional outcome measures, but sets a minimum standard of outcomes that should be reported in all interventional trials. This systematic review is the first step towards the development of meaningful consensus by identifying how vasomotor symptoms have been measured to inform consensus through the Delphi process.239
Conclusion
Most intervention studies for vasomotor symptoms have measured frequency or severity of symptoms, or a combination of both. Some have measured the interference caused in daily life due to symptoms. There is a need for consensus around the optimum outcomes and how these should be measured to facilitate comparisons between interventions and ensure patient‐centred clinical practice.
Disclosure of interests
No conflict of interest to disclose. Completed disclosure of interest forms are available to view online as supporting information.
Contribution of authorship
MH and SI conceived the idea and set the protocol. GM, RN, and MAL refined the protocol. SI and WW conducted the systematic search, and SI wrote the first draft of the paper with contribution from WW in writing up the methods. MSH provided useful insight regarding the bothersome aspect of menopausal symptoms. All authors edited and accepted the manuscript prior to submission.
Details of ethics approval
No ethics approval was required as we have summarised already published data.
Funding
This study was funded by an MRC postdoctoral fellowship (S.I., MR/N015177/1), NHMRC Practitioner Fellowship (M.H., APP 1058935), and an NHMRC Principal Research Fellowship (G.D.M., APP1121844). The funders were not involved in any stage of this systematic review.
Supporting information
Table S1. Study characteristics and quality assessment scoring of the included studies.
Table S2. Vasomotor‐related outcomes measured by diary.
Table S3. Different definitions for frequency, severity, intensity, and vasomotor scores of vasomotor‐related outcomes.
Appendix S1. Search strategy.
Iliodromiti S, Wang W, Lumsden MA, Hunter MS, Bell R, Mishra G, Hickey M, the International COMMA (Core Outcomes in Menopause) Consortium . Variation in menopausal vasomotor symptoms outcomes in clinical trials: a systematic review. BJOG 2020;127:320–333.
Linked article This article is commented on by S Logan, p. 334 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.16027.
References
- 1. Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health 2006;96:1226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Institutes of Health National Institutes of Health State‐of‐the‐Science Conference statement: management of menopause‐related symptoms. Ann Intern Med 2005;142:1003–13. [PubMed] [Google Scholar]
- 3. Carpenter JS, Woods NF, Otte JL, Guthrie KA, Hohensee C, Newton KM, et al. MsFLASH participants' priorities for alleviating menopausal symptoms. Climacteric 2015;18:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrett‐Connor E, Grady D, Stefanick ML. The rise and fall of menopausal hormone therapy. Annu Rev Public Health 2005;26:115–40. [DOI] [PubMed] [Google Scholar]
- 5. Velentzis LS, Banks E, Sitas F, Salagame U, Tan EH, Canfell K. Use of menopausal hormone therapy and bioidentical hormone therapy in Australian women 50 to 69 years of age: results from a national cross‐sectional study. PLoS One 2016;11:e0146494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marino JL, Saunders CM, Emery LI, Green H, Doherty DA, Hickey M. How does adjuvant chemotherapy affect menopausal symptoms, sexual function, and quality of life after breast cancer? Menopause 2016;23:1000–8. [DOI] [PubMed] [Google Scholar]
- 7. Rance NE, Dacks PA, Mittelman‐Smith MA, Romanovsky AA, Krajewski‐Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol 2013;34:211–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence . Menopause: diagnosis and management NICE guidelines [NG23] 2015. https://www.nice.org.uk/Guidance/NG23 [PubMed]
- 9. Newton KM, Carpenter JS, Guthrie KA, Anderson GL, Caan B, Cohen LS, et al. Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause 2014;21:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) . Guidance for Industry: Estrogen and Estrogen/Progestin Drug Products To Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms—Recommendations for Clinical Evaluation. 2003. https://www.fda.gov/downloads/Drugs/DrugSafety/informationbyDrugClass/UCM135338.pdf
- 11. Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khan K. The CROWN Initiative: journal editors invite researchers to develop core outcomes in women's health. BJOG 2014;121:1181–2. [DOI] [PubMed] [Google Scholar]
- 14. Hirsch M, Duffy JMN, Kusznir JO, Davis CJ, Plana MN, Khan KS, et al. Variation in outcome reporting in endometriosis trials: a systematic review. Am J Obstet Gynecol 2016;214:452–64. [DOI] [PubMed] [Google Scholar]
- 15. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 16. Harman NL, Bruce IA, Callery P, Tierney S, Sharif MO, O'Brien K, et al. MOMENT—Management of Otitis Media with Effusion in Cleft Palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials 2013;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aghamiri V, Mirghafourvand M, Mohammad‐Alizadeh‐Charandabi S, Nazemiyeh H. The effect of Hop (Humulus lupulus L.) on early menopausal symptoms and hot flashes: A randomized placebo‐controlled trial. Complement Ther Clin Practice 2016;23:130–5. [DOI] [PubMed] [Google Scholar]
- 18. Aguirre W, Chedraui P, Mendoza J, Ruilova I. Gabapentin vs. low‐dose transdermal estradiol for treating post‐menopausal women with moderate to very severe hot flushes. Gynecol Endocrinol 2010;26:333–7. [DOI] [PubMed] [Google Scholar]
- 19. Al‐Akoum M, Maunsell E, Verreault R, Provencher L, Otis H, Dodin S. Effects of Hypericum perforatum (St. John's wort) on hot flashes and quality of life in perimenopausal women: a randomized pilot trial. Menopause 2009;16:307–14. [DOI] [PubMed] [Google Scholar]
- 20. Al‐Azzawi F, Buckler H. Comparison of a novel vaginal ring delivering estradiol acetate versus oral estradiol for relief of vasomotor menopausal symptoms. Climacteric 2003;6:118–27. [PubMed] [Google Scholar]
- 21. Allameh Z, Rouholamin S, Valaie S. Comparison of gabapentin with estrogen for treatment of hot flashes in post‐menopausal women. J Res Pharm Pract 2013;2:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson DJ, Seib C, McCarthy AL, Yates P, Porter‐Steele J, McGuire A, et al. Facilitating lifestyle changes to manage menopausal symptoms in women with breast cancer: a randomized controlled pilot trial of The Pink Women's Wellness Program. Menopause 2015;22:937–45. [DOI] [PubMed] [Google Scholar]
- 23. Andrikoula M, Baker D, Nesic J, Liao L, Duka T, Prelevic G. The effects of micronutrient supplementation on vasomotor symptoms in postmenopausal women. Climacteric 2011;14:544–50. [DOI] [PubMed] [Google Scholar]
- 24. Archer D, Schmelter T, Schaefers M, Gerlinger C, Gude K. A randomized, double‐blind, placebo‐controlled study of the lowest effective dose of drospirenone with 17β‐estradiol for moderate to severe vasomotor symptoms in postmenopausal women. Menopause 2014;21:227–35. [DOI] [PubMed] [Google Scholar]
- 25. Archer D, Seidman L, Constantine G, Pickar J, Olivier S. A double‐blind, randomly assigned, placebo‐controlled study of desvenlafaxine efficacy and safety for the treatment of vasomotor symptoms associated with menopause. Am J Obstet Gynecol 2009;200:e1–10. [DOI] [PubMed] [Google Scholar]
- 26. Archer DF, DeAbate CA, Einhaus KB, Heuer MA, Kirkegarrd LW, Miller JD, et al. Percutaneous 17beta‐estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause 2003;10:516–21. [DOI] [PubMed] [Google Scholar]
- 27. Archer DF, Dupont CM, Constantine GD, Pickar JH, Olivier S. Desvenlafaxine for the treatment of vasomotor symptoms associated with menopause: a double‐blind, randomized, placebo‐controlled trial of efficacy and safety. Am J Obstet Gynecol 2009;200:238.e1‐.e10. [DOI] [PubMed] [Google Scholar]
- 28. Asghari M, Mirghafourvand M, Mohammad‐Alizadeh‐Charandabi S, Malakouti J, Nedjat S. Effect of aerobic exercise and nutrition education on quality of life and early menopause symptoms: A randomized controlled trial. Women Health 2017;57:173–88. [DOI] [PubMed] [Google Scholar]
- 29. Aslan E, Bagis T, Kilicdag E, Tarim E, Erkanli S, Kuscu E. How best is to discontinue postmenopausal hormone therapy: immediate or tapered? Maturitas 2007;56:78–83. [DOI] [PubMed] [Google Scholar]
- 30. Aso T, Uchiyama S, Matsumura Y, Taguchi M, Nozaki M, Takamatsu K, et al. A natural S‐equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women. J Women's Health 2012;21:92–100. [DOI] [PubMed] [Google Scholar]
- 31. Auerbach L, Rakus J, Bauer C, Gerner C, Ullmann R, Wimmer H, et al. Pomegranate seed oil in women with menopausal symptoms: a prospective randomized, placebo‐controlled, double‐blinded trial. Menopause 2012;19:426–32. [DOI] [PubMed] [Google Scholar]
- 32. Avis N, Coeytaux R, Isom S, Prevette K, Morgan T. Acupuncture in Menopause (AIM) study: a pragmatic, randomized controlled trial. Menopause 2016;23:626–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ayers B, Smith M, Hellier J, Mann E, Hunter MS. Effectiveness of group and self‐help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): A randomized controlled trial. Menopause 2012;19:749–59. [DOI] [PubMed] [Google Scholar]
- 34. Bacchi‐Modena A, Bolis P, Campagnoli C, De C, Meschia M, Pansini F, et al. Efficacy and tolerability of (R)Estraderm MX, a new estradiol matrix patch. Maturitas 1997;27:285–92. [DOI] [PubMed] [Google Scholar]
- 35. Bachmann G, Crosby U, Feldman R, Ronkin S, Constantine G. Effects of bazedoxifene in nonflushing postmenopausal women: a randomized phase 2 trial. Menopause 2011;18:508–14. [DOI] [PubMed] [Google Scholar]
- 36. Bachmann G, Schaefers M, Uddin A, Utian W. Lowest effective transdermal 17beta‐estradiol dose for relief of hot flushes in postmenopausal women: a randomized controlled trial. Bstet Gynecol 2007;110:771–9. [DOI] [PubMed] [Google Scholar]
- 37. Bai W, Henneicke‐von Zepelin HH, Wang S, Zheng S, Liu J, Zhang Z, et al. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: a randomized, double blind, parallel‐controlled study versus tibolone. Maturitas 2007;58:31–41. [DOI] [PubMed] [Google Scholar]
- 38. Barton DL, LaVasseur BI, Sloan JA, Stawis AN, Flynn KA, Dyar M, et al. Phase III, placebo‐controlled trial of three doses of citalopram for the treatment of hot flashes: NCCTG trial N05C9. J Clin Oncol 2010;28:3278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benster B, Carey A, Wadsworth F, Vashisht A, Domoney C, Studd J. A double‐blind placebo‐controlled study to evaluate the effect of progestelle progesterone cream on postmenopausal women. Menopause Int 2009;15:63–9. [DOI] [PubMed] [Google Scholar]
- 40. Biglia N, Sgandurra P, Peano E, Marenco D, Moggio G, Bounous V, et al. Non‐hormonal treatment of hot flushes in breast cancer survivors: gabapentin vs. vitamin E. Climacteric 2009;12:310–8. [DOI] [PubMed] [Google Scholar]
- 41. Bokmand S, Flyger H. Acupuncture relieves menopausal discomfort in breast cancer patients: a prospective, double blinded, randomized study. Breast 2013;22:320–3. [DOI] [PubMed] [Google Scholar]
- 42. Borud E, Alraek T, White A, Fonnebo V, Eggen A, Hammar M, et al. The acupuncture on hot flushes among menopausal women (ACUFLASH) study, a randomized controlled trial. Menopause 2009;16:484–93. [DOI] [PubMed] [Google Scholar]
- 43. Bouchard P, Panay N, de Villiers TJ, Vincendon P, Bao W, Cheng RJ, et al. Randomized placebo‐ and active‐controlled study of desvenlafaxine for menopausal vasomotor symptoms. Climacteric 2012;15:12–20. [DOI] [PubMed] [Google Scholar]
- 44. Burke G, Legault C, Anthony M, Bland D, Morgan T, Naughton M, et al. Soy protein and isoflavone effects on vasomotor symptoms in peri‐ and postmenopausal women: the Soy Estrogen Alternative Study. Menopause 2003;10:147–53. [DOI] [PubMed] [Google Scholar]
- 45. Buster J, Koltun W, Pascual M, Day W, Peterson C. Low‐dose estradiol spray to treat vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008;111:1343–51. [DOI] [PubMed] [Google Scholar]
- 46. Butt D, Lock M, Lewis J, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized controlled trial. Menopause 2008;15:310–8. [DOI] [PubMed] [Google Scholar]
- 47. Carmignani LO, Pedro AO, Costa‐Paiva LH, Pinto‐Neto AM. The effect of dietary soy supplementation compared to estrogen and placebo on menopausal symptoms: a randomized controlled trial. Maturitas 2010;67:262–9. [DOI] [PubMed] [Google Scholar]
- 48. Carmody JF, Crawford S, Salmoirago‐Blotcher E, Leung K, Churchill L, Olendzki N. Mindfulness training for coping with hot flashes: results of a randomized trial. Menopause 2011;18:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carpenter J, Burns D, Wu J, Otte J, Schneider B, Ryker K, et al. Paced respiration for vasomotor and other menopausal symptoms: a randomized, controlled trial. J Gen Intern Med 2013;28:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carpenter JS, Guthrie KA, Larson JC, Freeman EW, Joffe H, Reed SD, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril 2012;97:1399–404.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cezarino PY, Bagnoli VR, Fonseca AM, Soares JM, Baracat EC. The effects of cinnarizine on menopausal symptoms in women. Climacteric. 2011;14:492–6. [DOI] [PubMed] [Google Scholar]
- 52. Chandeying V, Sangthawan M. Efficacy comparison of Pueraria mirifica (PM) against conjugated equine estrogen (CEE) with/without medroxyprogesterone acetate (MPA) in the treatment of climacteric symptoms in perimenopausal women: phase III study. J Med Assoc Thai 2007;90:1720–6. [PubMed] [Google Scholar]
- 53. Chang A, Kwak B, Yi K, Kim J. The effect of herbal extract (EstroG‐100) on pre‐, peri‐ and post‐menopausal women: a randomized double‐blind, placebo‐controlled study. Phytother Res 2012;26:510–6. [DOI] [PubMed] [Google Scholar]
- 54. Chenoy R, Hussain S, Tayob Y, O'Brien PM, Moss MY, Morse PF. Effect of oral gamolenic acid from evening primrose oil on menopausal flushing. BMJ 1994;308:501–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chien L, Liu S, Chang Y, Liu C. Local thermal therapy effects on menopausal symptoms and bone mineral density. J Altern Complement Med 2011;17:1133–40. [DOI] [PubMed] [Google Scholar]
- 56. Chung DJ, Kim HY, Park KH, Jeong KA, Lee SK, Lee YI, et al. Black cohosh and St. John's wort (GYNO‐Plus) for climacteric symptoms. Yonsei Med J 2007;48:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cohen L, Joffe H, Guthrie K, Ensrud K, Freeman M, Carpenter J, et al. Efficacy of omega‐3 for vasomotor symptoms treatment: a randomized controlled trial. Menopause 2014;21:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Colau JC, Vincent S, Marijnen P, Allaert FA. Efficacy of a non‐hormonal treatment, BRN‐01, on menopausal hot flashes: a multicenter, randomized, double‐blind, placebo‐controlled trial. Drugs R D 2012;12:107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cortés‐Bonilla M, Bernardo‐Escudero R, Alonso‐Campero R, Francisco‐Doce M, Hernández‐Valencia M, Celis‐González C, et al. Treatment of menopausal symptoms with three low‐dose continuous sequential 17b‐estradiol/progesterone parenteral monthly formulations using novel non‐polymeric microsphere technology. Gynecol Endocrinol 2015;31:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Daley A, Thomas A, Roalfe A, Stokes‐Lampard H, Coleman S, Rees M, et al. The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomised controlled trial. BJOG 2015;122:565–75. [DOI] [PubMed] [Google Scholar]
- 61. Davinelli S, Scapagnini G, Marzatico F, Nobile V, Ferrara N, Corbi G. Influence of equol and resveratrol supplementation on health‐related quality of life in menopausal women: A randomized, placebo‐controlled study. Maturitas 2017;96:77–83. [DOI] [PubMed] [Google Scholar]
- 62. Davis SR, Briganti EM, Chen RQ, Dalais FS, Bailey M, Burger HG. The effects of Chinese medicinal herbs on postmenopausal vasomotor symptoms of Australian women. A randomised controlled trial. Med J Aust 2001;174:68–71. [DOI] [PubMed] [Google Scholar]
- 63. de Luca AC, da Fonseca AM, Lopes CM, Bagnoli VR, Soares JM, Baracat EC. Acupuncture‐ameliorated menopausal symptoms: single‐blind, placebo‐controlled, randomized trial. Climacteric 2011;14:140–5. [DOI] [PubMed] [Google Scholar]
- 64. de Vrijer B, Snijders MP, Troostwijk AL, The S, Iding RJ, Friese S, et al. Efficacy and tolerability of a new estradiol delivering matrix patch (Estraderm MX) in postmenopausal women. Maturitas 2000;34:47–55. [DOI] [PubMed] [Google Scholar]
- 65. del Giorno C, Fonseca AM, Bagnoli VR, Assis JS, Soares JM Jr, Baracat EC. Effects of Trifolium pratense on the climacteric and sexual symptoms in postmenopause women. Rev Assoc Med Bras 2010;56:558–62. [DOI] [PubMed] [Google Scholar]
- 66. Duijts SF, van Beurden M, Oldenburg HS, Hunter MS, Kieffer JM, Stuiver MM, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment‐induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol 2012;30:4124–33. [DOI] [PubMed] [Google Scholar]
- 67. Ee C, Xue C, Chondros P, Myers SP, French SD, Teede H, et al. Acupuncture for menopausal hot flashes: a randomized trial. Ann Intern Med 2016;164:146–54. [DOI] [PubMed] [Google Scholar]
- 68. Elkins GR, Fisher WI, Johnson AK, Carpenter JS, Keith TZ. Clinical hypnosis in the treatment of postmenopausal hot flashes: A randomized controlled trial. Menopause 2013;20:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Endrikat J, Graeser T, Mellinger U, Ertan K, Holz C. A multicenter, prospective, randomized, double‐blind, placebo‐controlled study to investigate the efficacy of a continuous‐combined hormone therapy preparation containing 1mg estradiol valerate/2mg dienogest on hot flushes in postmenopausal women. Maturitas 2007;58:201–7. [DOI] [PubMed] [Google Scholar]
- 70. Evans M, Elliott JG, Sharma P, Berman R, Guthrie N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: a multi‐center, randomized, placebo‐controlled study. Maturitas 2011;68:189–96. [DOI] [PubMed] [Google Scholar]
- 71. Evans ML, Pritts E, Vittinghoff E, McClish K, Morgan KS, Jaffe RB. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol 2005;105:161–6. [DOI] [PubMed] [Google Scholar]
- 72. Faure ED, Chantre P, Mares P. Effects of a standardized soy extract on hot flushes: a multicenter, double‐blind, randomized, placebo‐controlled study. Menopause 2002;9:329–34. [DOI] [PubMed] [Google Scholar]
- 73. Fenlon DR, Corner JL, Haviland JS. A randomized controlled trial of relaxation training to reduce hot flashes in women with primary breast cancer. J Pain Symptom Manage 2008;35:397–405. [DOI] [PubMed] [Google Scholar]
- 74. Ferrari A. Soy extract phytoestrogens with high dose of isoflavones for menopausal symptoms. J Obstet Gynaecol Res 2009;35:1083–90. [DOI] [PubMed] [Google Scholar]
- 75. Freeman EW, Guthrie KA, Caan B, Sternfeld B, Cohen LS, Joffe H, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Frei‐Kleiner S, Schaffner W, Rahlfs VW, Bodmer C, Birkhauser M. Cimicifuga racemosa dried ethanolic extract in menopausal disorders: a double‐blind placebo‐controlled clinical trial. Maturitas 2005;51:397–404. [DOI] [PubMed] [Google Scholar]
- 77. Fu S, Zhao Y, Ren M, Zhang J, Wang Y, Han L, et al. A randomized, double‐blind, placebo‐controlled trial of Chinese herbal medicine granules for the treatment of menopausal symptoms by stages. Menopause 2016;23:311–23. [DOI] [PubMed] [Google Scholar]
- 78. Garcia J, Gonzaga F, Tan D, Ng T, Oei P, Chan C. Use of a multibotanical (Nutrafem) for the relief of menopausal vasomotor symptoms: a double‐blind, placebo‐controlled study. Menopause 2010;17:303–8. [DOI] [PubMed] [Google Scholar]
- 79. Gelfand MM, Moreau M, Ayotte NJ, Hilditch JR, Wong BA, Lau CY. Clinical assessment and quality of life of postmenopausal women treated with a new intermittent progestogen combination hormone replacement therapy: a placebo‐controlled study. Menopause 2003;10:29–36. [DOI] [PubMed] [Google Scholar]
- 80. Geller S, Shulman L, Breemen R, Banuvar S, Zhou Y, Epstein G, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause 2009;16:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Good WR, John VA, Ramirez M, Higgins JE. Double‐masked, multicenter study of an estradiol matrix transdermal delivery system (Alora) versus placebo in postmenopausal women experiencing menopausal symptoms. Alora Study Group. Clin Ther 1996;18:1093–105. [DOI] [PubMed] [Google Scholar]
- 82. Good WR, John VA, Ramirez M, Higgins JE. Comparison of Alora estradiol matrix transdermal delivery system with oral conjugated equine estrogen therapy in relieving menopausal symptoms. Alora Study Group. Climacteric 1999;2:29–36. [DOI] [PubMed] [Google Scholar]
- 83. Goodwin J, Green S, Moinpour C, Bearden J, Giguere J, Jiang C, et al. Phase III randomized placebo‐controlled trial of two doses of megestrol acetate as treatment for menopausal symptoms in women with breast cancer: southwest Oncology Group Study 9626. J Clin Oncol 2008;26:1650–6. [DOI] [PubMed] [Google Scholar]
- 84. Gordon PR, Kerwin JP, Boesen KG, Senf J. Sertraline to treat hot flashes: a randomized controlled, double‐blind, crossover trial in a general population. Menopause 2006;13:568–75. [DOI] [PubMed] [Google Scholar]
- 85. Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol 2007;109:823–30. [DOI] [PubMed] [Google Scholar]
- 86. Grady D, Sawaya GF, Johnson KC, Koltun W, Hess R, Vittinghoff E, et al. MF101, a selective estrogen receptor beta modulator for the treatment of menopausal hot flushes: a phase II clinical trial. Menopause 2009;16:458–65. [DOI] [PubMed] [Google Scholar]
- 87. Guttuso T Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin's effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45. [DOI] [PubMed] [Google Scholar]
- 88. Guttuso T, McDermott MP, Su H, Kieburtz K. Effects of L‐isoleucine and L‐valine on hot flushes and serum homocysteine: a randomized controlled trial. Obstet Gynecol 2008;112:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Haimov‐Kochman R, Barak‐Glantz E, Arbel R, Leefsma M, Brzezinski A, Milwidsky A, et al. Gradual discontinuation of hormone therapy does not prevent the reappearance of climacteric symptoms: a randomized prospective study. Menopause 2006;13:370–6. [DOI] [PubMed] [Google Scholar]
- 90. Haines C, Lam P, Chung T, Cheng K, Leung P. A randomized, double‐blind, placebo‐controlled study of the effect of a Chinese herbal medicine preparation (Dang Gui Buxue Tang) on menopausal symptoms in Hong Kong Chinese women. Climacteric 2008;11:244–51. [DOI] [PubMed] [Google Scholar]
- 91. Haines C, Yu S, Hiemeyer F, Schaefers M. Micro‐dose transdermal estradiol for relief of hot flushes in postmenopausal Asian women: a randomized controlled trial. Climacteric 2009;12:419–26. [DOI] [PubMed] [Google Scholar]
- 92. Hardy C, Griffiths A, Norton S, Hunter MS. Self‐help cognitive behavior therapy for working women with problematic hot flushes and night sweats (MENOS@Work): a multicenter randomized controlled trial. Menopause 2018;25:508–19. [DOI] [PubMed] [Google Scholar]
- 93. Hattersley G, Harris AG, Simon JA, Constantine GD. Clinical investigation of RAD1901, a novel estrogen receptor ligand, for the treatment of postmenopausal vasomotor symptoms: a phase 2 randomized, placebo‐controlled, double‐blind, dose‐ranging, proof‐of‐concept trial. Menopause 2017;24:92–9. [DOI] [PubMed] [Google Scholar]
- 94. Hedrick RE, Ackerman RT, Koltun WD, Halvorsen MB, Lambrecht LJ. Transdermal estradiol gel 0.1% for the treatment of vasomotor symptoms in postmenopausal women. Menopause 2009;16:132–40. [DOI] [PubMed] [Google Scholar]
- 95. Heger M, Ventskovskiy BM, Borzenko I, Kneis KC, Rettenberger R, Kaszkin‐Bettag M, et al. Efficacy and safety of a special extract of Rheum rhaponticum (ERr 731) in perimenopausal women with climacteric complaints: a 12‐week randomized, double‐blind, placebo‐controlled trial. [Erratum appears in Menopause 2007;14:339]. Menopause 2007;2006:744–59. [DOI] [PubMed] [Google Scholar]
- 96. Heyerick A, Vervarcke S, Depypere H, Bracke M, De Keukeleire D. A first prospective, randomized, double‐blind, placebo‐controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas 2006;54:164–75. [DOI] [PubMed] [Google Scholar]
- 97. Hidalgo LA, Chedraui PA, Morocho N, Ross S, San Miguel G. The effect of red clover isoflavones on menopausal symptoms, lipids and vaginal cytology in menopausal women: A randomized, double‐blind, placebo‐controlled study. Gynecol Endocrinol 2005;21:257–64. [DOI] [PubMed] [Google Scholar]
- 98. Hilditch JR, Lewis J, Ross AH, Peter A, van Maris B, Franssen E, et al. A comparison of the effects of oral conjugated equine estrogen and transdermal estradiol‐17 beta combined with an oral progestin on quality of life in postmenopausal women. Maturitas 1996;24:177–84. [DOI] [PubMed] [Google Scholar]
- 99. Hitchcock C, Prior J. Oral micronized progesterone for vasomotor symptoms–a placebo‐controlled randomized trial in healthy postmenopausal women. Menopause 2012;19:886–93. [DOI] [PubMed] [Google Scholar]
- 100. Holst T, Salbach B. Efficacy and tolerability of a new 7‐day transdermal estradiol patch versus placebo in hysterectomized women with postmenopausal complaints. Maturitas 2000;34:143–53. [DOI] [PubMed] [Google Scholar]
- 101. Honjo H, Taketani Y. Low‐dose estradiol for climacteric symptoms in Japanese women: a randomized, controlled trial. Climacteric 2009;12:319–28. [DOI] [PubMed] [Google Scholar]
- 102. Huang AJ, Phillips S, Schembri M, Vittinghoff E, Grady D. Device‐guided slow‐paced respiration for menopausal hot flushes: a randomized controlled trial. Obstet Gynecol 2015;125:1130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hudita D, Posea C, Ceausu I, Rusu M. Efficacy and safety of oral tibolone 1.25 or 2.5 mg/day vs. placebo in postmenopausal women. Eur Rev Med Pharmacol Sci 2003;7:117–25. [PubMed] [Google Scholar]
- 104. Jacobson JS, Troxel AB, Evans J, Klaus L, Vahdat L, Kinne D, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739–45. [DOI] [PubMed] [Google Scholar]
- 105. Jenabi E, Shobeiri F, Hazavehei SMM, Roshanaei G. The effect of Valerian on the severity and frequency of hot flashes: A triple‐blind randomized clinical trial. Women Health 2018;58:297–304. [DOI] [PubMed] [Google Scholar]
- 106. Jenks BH, Iwashita S, Nakagawa Y, Ragland K, Lee J, Carson WH, et al. A pilot study on the effects of S‐equol compared to soy isoflavones on menopausal hot flash frequency. J Women's Health. 2012;21:674–82. [DOI] [PubMed] [Google Scholar]
- 107. Joffe H, Guthrie K, LaCroix A, Reed S, Ensrud K, Manson J, et al. Low‐dose estradiol and the serotonin‐norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014;174:1058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jou HJ, Wu SC, Chang FW, Ling PY, Chu KS, Wu WH. Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. Int J Gynaecol Obstet 2008;102:44–9. [DOI] [PubMed] [Google Scholar]
- 109. Kalay AE, Demir B, Haberal A, Kalay M, Kandemir O. Efficacy of citalopram on climacteric symptoms. Menopause 2007;14:223–9. [DOI] [PubMed] [Google Scholar]
- 110. Kaszkin‐Bettag M, Ventskovskiy BM, Solskyy S, Beck S, Hasper I, Kravchenko A, et al. Confirmation of the efficacy of ERr 731 in perimenopausal women with menopausal symptoms. Altern Ther Health Med 2009;15:24–34. [PubMed] [Google Scholar]
- 111. Khaodhiar L, Ricciotti HA, Li L, Pan W, Schickel M, Zhou J, et al. Daidzein‐rich isoflavone aglycones are potentially effective in reducing hot flashes in menopausal women. Menopause 2008;15:125–32. [PMC free article] [PubMed] [Google Scholar]
- 112. Kim DI, Jeong JC, Kim KH, Rho JJ, Choi MS, Yoon SH, et al. Acupuncture for hot flushes in perimenopausal and postmenopausal women: a randomised, sham‐controlled trial. Acupuncture Med 2011;29:249–56. [DOI] [PubMed] [Google Scholar]
- 113. Kim KH, Kang KW, Kim DI, Kim HJ, Yoon HM, Lee JM, et al. Effects of acupuncture on hot flashes in perimenopausal and postmenopausal women—a multicenter randomized clinical trial. Menopause 2010;17:269–80. [DOI] [PubMed] [Google Scholar]
- 114. Kimmick GG, Lovato J, McQuellon R, Robinson E, Muss HB. Randomized, double‐blind, placebo‐controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J 2006;12:114–22. [DOI] [PubMed] [Google Scholar]
- 115. Kitanohara M, Yamamoto T, Masunaga S, Ohishi M, Komatsu Y, Nagase M. Effect of porcine placental extract on the mild menopausal symptoms of climacteric women. Climacteric 2017;20:144–50. [DOI] [PubMed] [Google Scholar]
- 116. Kroiss R, Fentiman IS, Helmond FA, Rymer J, Foidart JM, Bundred N, et al. The effect of tibolone in postmenopausal women receiving tamoxifen after surgery for breast cancer: a randomised, double‐blind, placebo‐controlled trial. BJOG 2005;112:228–33. [DOI] [PubMed] [Google Scholar]
- 117. LaCroix A, Freeman E, Larson J, Carpenter J, Joffe H, Reed S, et al. Effects of escitalopram on menopause‐specific quality of life and pain in healthy menopausal women with hot flashes: a randomized controlled trial. Maturitas 2012;73:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lambert M, Thorup A, Hansen E, Jeppesen P. Combined Red Clover isoflavones and probiotics potently reduce menopausal vasomotor symptoms. PLoS One 2017;12:e0176590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Landgren MB, Bennink HJ, Helmond FA, Engelen S. Dose‐response analysis of effects of tibolone on climacteric symptoms. BJOG 2002;109:1109–14. [DOI] [PubMed] [Google Scholar]
- 120. Lee BS, Kang BM, Yoon BK, Choi H, Park HM, Kim JG. Efficacy and tolerability of estradiol 1 mg and drospirenone 2 mg in postmenopausal Korean women: a double‐blind, randomized, placebo‐controlled, multicenter study. Maturitas 2007;57:361–9. [DOI] [PubMed] [Google Scholar]
- 121. Lesi G, Razzini G, Musti M, Stivanello E, Petrucci C, Benedetti B, et al. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer: a prospective multicenter randomized controlled trial (AcCliMaT). J Clin Oncol 2016;34:1795–802. [DOI] [PubMed] [Google Scholar]
- 122. Lewis JE, Nickell LA, Thompson LU, Szalai JP, Kiss A, Hilditch JR. A randomized controlled trial of the effect of dietary soy and flaxseed muffins on quality of life and hot flashes during menopause. Menopause 2006;13:631–42. [DOI] [PubMed] [Google Scholar]
- 123. Lin S, Sun L, Lin J, Yang X, Zhang L, Qiao J, et al. Estradiol 1 mg and drospirenone 2 mg as hormone replacement therapy in postmenopausal Chinese women. Climacteric 2011;14:472–81. [DOI] [PubMed] [Google Scholar]
- 124. Lindh‐Astrand L, Bixo M, Hirschberg AL, Sundstrom‐Poromaa I, Hammar M. A randomized controlled study of taper‐down or abrupt discontinuation of hormone therapy in women treated for vasomotor symptoms. Menopause 2010;17:72–9. [DOI] [PubMed] [Google Scholar]
- 125. Lindh‐Astrand L, Nedstrand E. Effects of applied relaxation on vasomotor symptoms in postmenopausal women: A randomized controlled trial. Menopause 2012;12. [DOI] [PubMed] [Google Scholar]
- 126. Lipovac M, Chedraui P, Gruenhut C, Gocan A, Kurz C, Neuber B, et al. The effect of red clover isoflavone supplementation over vasomotor and menopausal symptoms in postmenopausal women. Gynecol Endocrinol 2012;28:203–7. [DOI] [PubMed] [Google Scholar]
- 127. Liu JH, Reape KZ, Hait HI. Synthetic conjugated estrogens‐B and postmenopausal nocturnal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:78–84. [DOI] [PubMed] [Google Scholar]
- 128. Liu ZM, Ho SC, Woo J, Chen YM, Wong C. Randomized controlled trial of whole soy and isoflavone daidzein on menopausal symptoms in equol‐producing Chinese postmenopausal women. Menopause 2014;21:653–60. [DOI] [PubMed] [Google Scholar]
- 129. Loibl S, Schwedler K, von Minckwitz G, Strohmeier R, Mehta KM, Kaufmann M. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients—A double‐blind, randomized study. Ann Oncol 2007;18:689–93. [DOI] [PubMed] [Google Scholar]
- 130. Lopes P, Merkus H, Nauman J, Bruschi F, Foidart J, Calaf J. Randomized comparison of intranasal and transdermal estradiol. Obstet Gynecol 2000;96:906–12. [DOI] [PubMed] [Google Scholar]
- 131. Loprinzi C, Levitt R, Barton D, Sloan J, Dakhil S, Nikcevich D, et al. Phase III comparison of depomedroxyprogesterone acetate to venlafaxine for managing hot flashes: north Central Cancer Treatment Group Trial N99C7. J Clin Oncol 2006;24:1409–14. [DOI] [PubMed] [Google Scholar]
- 132. Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, LaVasseur BI, Barton DL, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: A randomised controlled trial. Lancet 2000;356:2059–63. [DOI] [PubMed] [Google Scholar]
- 133. Loprinzi CL, Qin R, Balcueva EP, Flynn KA, Rowland KM Jr, Graham DL, et al. Phase III, randomized, double‐blind, placebo‐controlled evaluation of pregabalin for alleviating hot flashes, N07C1. [Erratum appears in J Clin Oncol 2010;28:1808]. J Clin Oncol 2010;28:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Loprinzi CL, Sloan JA, Perez EA, Quella SK, Stella PJ, Mailliard JA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83. [DOI] [PubMed] [Google Scholar]
- 135. Luoto R, Moilanen J, Heinonen R, Mikkola T, Raitanen J, Tomas E, et al. Effect of aerobic training on hot flushes and quality of life–a randomized controlled trial. Ann Med 2012;44:616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. MacGregor CA, Canney PA, Patterson G, McDonald R, Paul J. A randomised double‐blind controlled trial of oral soy supplements versus placebo for treatment of menopausal symptoms in patients with early breast cancer. Eur J Cancer 2005;41:708–14. [DOI] [PubMed] [Google Scholar]
- 137. Malik S, Pannu D, Prateek S, Sinha R, Gaikwad H. Comparison of the symptomatic response in Indian menopausal women with different estrogen preparations for the treatment of menopausal symptoms: a randomized controlled trial. Arch Gynecol Obstet 2016;293:1325–33. [DOI] [PubMed] [Google Scholar]
- 138. Mann E, Smith MJ, Hellier J, Balabanovic JA, Hamed H, Grunfeld EA, et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol 2012;13:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mattsson L, Skouby S, Rees M, Heikkinen J, Kudela M, Stadnicki‐Kolendo A, et al. Efficacy and tolerability of continuous combined hormone replacement therapy in early postmenopausal women. Menopause Int 2007;13:124–31. [DOI] [PubMed] [Google Scholar]
- 140. Mattsson LA, Christiansen C, Colau JC, Palacios S, Kenemans P, Bergeron C, et al. Clinical equivalence of intranasal and oral 17beta‐estradiol for postmenopausal symptoms. Am J Obstet Gynecol 2000;182:545–52. [DOI] [PubMed] [Google Scholar]
- 141. Meng F, Duan P, Zhu J, Lou Q, Fang Z, An H, et al. Effect of Gua sha therapy on perimenopausal syndrome: a randomized controlled trial. Menopause 2017;24:299–307. [DOI] [PubMed] [Google Scholar]
- 142. Meuwissen JH, Beijers‐De Bie L, Vihtamaki T, Tuimala R, Siseles N, Magaril C, et al. A 1‐year comparison of the efficacy and clinical tolerance in postmenopausal women of two hormone replacement therapies containing estradiol in combination with either norgestrel or trimegestone. Gynecol Endocrinol 2001;15:349–58. [PubMed] [Google Scholar]
- 143. Mizunuma H. Clinical usefulness of a low‐dose maintenance therapy with transdermal estradiol gel in Japanese women with estrogen deficiency symptoms. Climacteric 2011;14:581–9. [DOI] [PubMed] [Google Scholar]
- 144. Mohammad‐Alizadeh‐Charandabi S, Shahnazi M, Nahaee J, Bayatipayan S. Efficacy of black cohosh (Cimicifuga racemosa L.) in treating early symptoms of menopause: a randomized clinical trial. Chin Med 2013;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Morais‐Socorro M, Cavalcanti MA, Martins R, Neto Francisco P, Rezende A, Azevedo G, et al. Safety and efficacy of tibolone and menopausal transition: a randomized, double‐blind placebo‐controlled trial. Gynecol Endocrinol 2012;28:483–7. [DOI] [PubMed] [Google Scholar]
- 146. Newton K, Reed S, LaCroix A, Grothaus L, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: a randomized trial. Ann Intern Med 2006;145:869–79. [DOI] [PubMed] [Google Scholar]
- 147. Newton KM, Reed SD, Guthrie KA, Sherman KJ, Booth‐LaForce C, Caan B, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause 2014;21:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Notelovitz M, Lenihan J, McDermott M, Kerber I, Nanavati N, Arce J. Initial 17beta‐estradiol dose for treating vasomotor symptoms. Obstet Gynecol 2000;95:726–31. [DOI] [PubMed] [Google Scholar]
- 149. Notelovitz M, Mattox J. Suppression of vasomotor and vulvovaginal symptoms with continuous oral 17beta‐estradiol. Menopause 2000;7:310–7. [DOI] [PubMed] [Google Scholar]
- 150. Oktem M, Eroglu D, Karahan HB, Taskintuna N, Kuscu E, Zeyneloglu HB. Black cohosh and fluoxetine in the treatment of postmenopausal symptoms: a prospective, randomized trial. Adv Ther 2007;24:448–61. [DOI] [PubMed] [Google Scholar]
- 151. Osmers R, Friede M, Liske E, Schnitker J, Freudenstein J, Henneicke‐von Zepelin HH, et al. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. [Erratum appears in Obstet Gynecol 2005;106:644]. Obstet Gynecol 2005. ;105(5 Pt 1):1074–83. [DOI] [PubMed] [Google Scholar]
- 152. Palacios S, Farias ML, Luebbert H, Gomez G, Yabur JA, Quail DC, et al. Raloxifene is not associated with biologically relevant changes in hot flushes in postmenopausal women for whom therapy is appropriate. Am J Obstet Gynecol 2004;191:121–31. [DOI] [PubMed] [Google Scholar]
- 153. Palacios S, Lilue M, Mejia A, Menendez C. Omega‐3 versus isoflavones in the control of vasomotor symptoms in postmenopausal women. Gynecol Endocrinol 2017;33:951–7. [DOI] [PubMed] [Google Scholar]
- 154. Panay N, Ylikorkala O, Archer D, Gut R, Lang E. Ultra‐low‐dose estradiol and norethisterone acetate: effective menopausal symptom relief. Climacteric 2007;10:120–31. [DOI] [PubMed] [Google Scholar]
- 155. Pandya KJ, Morrow GR, Roscoe JA, Zhao H, Hickok JT, Pajon E, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double‐blind placebo‐controlled trial. Lancet 2005;366:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Park H, Qin R, Smith TJ, Atherton PJ, Barton DL, Sturtz K, et al. North Central Cancer Treatment Group N10C2 (Alliance): a double‐blind placebo‐controlled study of magnesium supplements to reduce menopausal hot flashes. Menopause 2015;22:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Parsey K, Ellman H, Rahman M. Randomised, controlled comparison of transdermal estradiol with oral conjugated estrogens for the relief of hot flushes. Clin Drug Invest 2000;20:207–14. [Google Scholar]
- 158. Pinkerton J, Constantine G, Hwang E, Cheng R. Desvenlafaxine compared with placebo for treatment of menopausal vasomotor symptoms: a 12‐week, multicenter, parallel‐group, randomized, double‐blind, placebo‐controlled efficacy trial. Menopause 2013;20:28–37. [DOI] [PubMed] [Google Scholar]
- 159. Pinkerton J, Utian W, Constantine G, Olivier S, Pickar J. Relief of vasomotor symptoms with the tissue‐selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009;16:1116–24. [DOI] [PubMed] [Google Scholar]
- 160. Pinkerton JV, Kagan R, Portman D, Sathyanarayana R, Sweeney M, Breeze I. Phase 3 randomized controlled study of gastroretentive gabapentin for the treatment of moderate‐to‐severe hot flashes in menopause. Menopause 2014;21:567–73. [DOI] [PubMed] [Google Scholar]
- 161. Plotnikoff GA, Watanabe K, Torkelson C, La Valleur J, Radosevich DM. The TU‐025 keishibukuryogan clinical trial for hot flash management in postmenopausal women: results and lessons for future research. Menopause 2011;18:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pockaj BA, Gallagher JG, Loprinzi CL, Stella PJ, Barton DL, Sloan JA, et al. Phase III double‐blind, randomized, placebo‐controlled crossover trial of black cohosh in the management of hot flashes: NCCTG Trial N01CC1. J Clin Oncol 2006;24:2836–41. [DOI] [PubMed] [Google Scholar]
- 163. Polisseni AF, Andrade AT, Ribeiro LC, Castro IQ, Brandao M, Polisseni F, et al. Effects of a continuous‐combined regimen of low‐dose hormone therapy (oestradiol and norethindrone acetate) and tibolone on the quality of life in symptomatic postmenopausal women: a double‐blind, randomised study. Maturitas 2013;74:172–8. [DOI] [PubMed] [Google Scholar]
- 164. Pornel B. Efficacy and safety of Menorest in two positive‐controlled studies. Eur J Obstet Gynecol Reprod Biol 1996;64(Suppl):S35–7. [DOI] [PubMed] [Google Scholar]
- 165. Pornel B, Genazzani A, Costes D, Dain M, Lelann L, Vandepol C. Efficacy and tolerability of Menorest 50 compared with Estraderm TTS 50 in the treatment of postmenopausal symptoms. A randomized, multicenter, parallel group study. Maturitas 1995;22:207–18. [DOI] [PubMed] [Google Scholar]
- 166. Pornel B, Spielmann D. A study of the control of climacteric symptoms in postmenopausal women following sequential regimens of 1 mg 17beta‐estradiol and trimegestone compared with a regimen containing 1 mg estradiol valerate and norethisterone over a two‐year period. Gynecol Endocrinol 2005;21:74–81. [DOI] [PubMed] [Google Scholar]
- 167. Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double‐blind, placebo‐controlled trial. Lancet 2017;389:1809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pruthi S, Qin R, Terstreip SA, Liu H, Loprinzi CL, Shah TR, et al. A phase III, randomized, placebo‐controlled, double‐blind trial of flaxseed for the treatment of hot flashes: North Central Cancer Treatment Group N08C7. Menopause 2012;19:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Raynaud JP, Levrier M, Calaf J, Laur C, Pelissier C. Comparison of the efficacy and tolerability of a new once‐a‐week matricial estradiol transdermal system (Estrapatch 40 and Estrapatch 60) with a twice week system. J Steroid Biochem Mol Biol 2005;93:309–18. [DOI] [PubMed] [Google Scholar]
- 170. Reddy SY, Warner H, Guttuso T Jr, Messing S, DiGrazio W, Thornburg L, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol 2006;108:41–8. [DOI] [PubMed] [Google Scholar]
- 171. Rovati LC, Setnikar I, Genazzani AR. Dose‐response efficacy of a new estradiol transdermal matrix patch for 7‐day application: a randomized, double‐blind, placebo‐controlled study. Italian Menopause Research Group. Gynecol Endocrinol 2000;14:282–91. [DOI] [PubMed] [Google Scholar]
- 172. Rozenbaum H, Chevallier O, Moyal M, Durand G, Perineau M, This P. Efficacy and tolerability of pulsed estrogen therapy: A 12‐week double‐blind placebo‐controlled study in highly symptomatic postmenopausal women. Climacteric 2002;5:249–58. [PubMed] [Google Scholar]
- 173. Rozenberg S, Ylikorkala O, Arrenbrecht S. Comparison of continuous and sequential transdermal progestogen with sequential oral progestogen in postmenopausal women using continuous transdermal estrogen: vasomotor symptoms, bleeding patterns, and serum lipids. Int J Fertil Womens Med 1997;42(Suppl 2):376–87. [PubMed] [Google Scholar]
- 174. Saensak S, Vutyavanich T, Somboonporn W, Srisurapanont M. Effectiveness of a modified version of the applied relaxation technique in treatment of perimenopausal and postmenopausal symptoms. Int J Women's Health 2013;5:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Sangwan P, Kaushal J, Chauhan MB, Singhal S. A comparative study of efficacy and safety of red clover versus conjugated estrogen on vasomotor symptoms and sleep patterns in postmenopausal women. Indo Global J Pharm Sci 2015;5:233–43. [Google Scholar]
- 176. Schellenberg R, Saller R, Hess L, Melzer J, Zimmermann C, Drewe J, et al. Dose‐dependent effects of the Cimicifuga racemosa extract Ze 450 in the treatment of climacteric complaints: A randomized, placebo‐controlled study. Evid Based Complement Alternat Med 2012;2012: 260301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Schurmann R, Holler T, Benda N. Estradiol and drospirenone for climacteric symptoms in postmenopausal women: a double‐blind, randomized, placebo‐controlled study of the safety and efficacy of three dose regimens. Climacteric 2004;7:189–96. [DOI] [PubMed] [Google Scholar]
- 178. Secreto G, Chiechi L, Amadori A, Miceli R, Venturelli E, Valerio T, et al. Soy isoflavones and melatonin for the relief of climacteric symptoms: a multicenter, double‐blind, randomized study. Maturitas 2004;47:11–20. [DOI] [PubMed] [Google Scholar]
- 179. Sehhatie Shafaie F, Mirghafourvand M, Jafari M. Effect of education through support ‐group on early symptoms of menopause: a randomized controlled trial. J Caring Sci 2014;3:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Shamshad Begum S, Jayalakshmi HK, Vidyavathi HG, Gopakumar G, Abin I, Balu M, et al. A novel extract of fenugreek husk (FenuSMARTTM) alleviates postmenopausal symptoms and helps to establish the hormonal balance: a randomized, double‐blind. placebo‐controlled study. Phytother Res 2016;30:1775–84. [DOI] [PubMed] [Google Scholar]
- 181. Shobeiri F, Jenabi E, Khatiban M, Hazavehei SMM, Roshanaei G. The effect of educational program on quality of life in menopausal women: a clinical trial. J Menopausal Med 2017;23:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Shulman LP, Yankov V, Uhl K. Safety and efficacy of a continuous once‐a‐week 17beta‐estradiol/levonorgestrel transdermal system and its effects on vasomotor symptoms and endometrial safety in postmenopausal women: the results of two multicenter, double‐blind, randomized, controlled trials. [Erratum appears in Menopause 2002;9:385]. Menopause 2002;2002:195–207. [DOI] [PubMed] [Google Scholar]
- 183. Simon J. Estradiol in micellar nanoparticles: the efficacy and safety of a novel transdermal drug‐delivery technology in the management of moderate to severe vasomotor symptoms. Menopause 2006;13:222–31. [DOI] [PubMed] [Google Scholar]
- 184. Simon J, Bouchard C, Waldbaum A, Utian W, Zborowski J, Snabes M. Low dose of transdermal estradiol gel for treatment of symptomatic postmenopausal women: a randomized controlled trial. Obstet Gynecol 2007;109:588–96. [DOI] [PubMed] [Google Scholar]
- 185. Simon J, Gaines T, LaGuardia K. Extended‐release oxybutynin therapy for vasomotor symptoms in women: a randomized clinical trial. Menopause 2016;23:1214–21. [DOI] [PubMed] [Google Scholar]
- 186. Simon JA, Portman DJ, Kaunitz AM, Mekonnen H, Kazempour K, Bhaskar S, et al. Low‐dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause 2013;20:1027–35. [DOI] [PubMed] [Google Scholar]
- 187. Simon JA, Stevens RE, Ayres SA, Phelps KV. Perimenopausal women in estrogen vasomotor trials: contribution to placebo effect and efficacy outcome. Climacteric 2001;4:19–27. [PubMed] [Google Scholar]
- 188. van der Sluijs C, Bensoussan A, Chang S, Baber R. A randomized placebo‐controlled trial on the effectiveness of an herbal formula to alleviate menopausal vasomotor symptoms. Menopause 2009;16:336–44. [DOI] [PubMed] [Google Scholar]
- 189. Sood R, Sood A, Wolf SL, Linquist BM, Liu H, Sloan JA, et al. Paced breathing compared with usual breathing for hot flashes. Menopause 2013;20:179–84. [DOI] [PubMed] [Google Scholar]
- 190. Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol 2003;102:823–34. [DOI] [PubMed] [Google Scholar]
- 191. Speroff L, Gass M, Constantine G, Olivier S, Study I. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008;111:77–87. [DOI] [PubMed] [Google Scholar]
- 192. Speroff L, Haney AF, Gilbert RD, Ellman H. Estradiol Acetate Investigator G. Efficacy of a new, oral estradiol acetate formulation for relief of menopause symptoms. Menopause 2006;13:442–50. [DOI] [PubMed] [Google Scholar]
- 193. Speroff L, Symons J, Kempfert N, Rowan J. femhrt Study I. The effect of varying low‐dose combinations of norethindrone acetate and ethinyl estradiol (femhrt) on the frequency and intensity of vasomotor symptoms. Menopause 2000;7:383–90. [DOI] [PubMed] [Google Scholar]
- 194. Speroff L, Whitcomb RW, Kempfert NJ, Boyd RA, Paulissen JB, Rowan JP. Efficacy and local tolerance of a low‐dose, 7‐day matrix estradiol transdermal system in the treatment of menopausal vasomotor symptoms. Obstet Gynecol 1996;88:587–92. [DOI] [PubMed] [Google Scholar]
- 195. St Germain A, Peterson CT, Robinson JG, Alekel DL. Isoflavone‐rich or isoflavone‐poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2001;8:17–26. [DOI] [PubMed] [Google Scholar]
- 196. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003;289:2827–34. [DOI] [PubMed] [Google Scholar]
- 197. Steels E, Steele M, Harold M, Adams L, Coulson S. A double‐blind, randomized, placebo‐controlled trial evaluating safety and efficacy of an ayurvedic botanical formulation in reducing menopausal symptoms in otherwise healthy women. J Herbal Med 2018. [Google Scholar]
- 198. Steels E, Steele M, Harold M, Coulson S. Efficacy of a proprietary Trigonella foenum‐graecum L. de‐husked seed extract in reducing menopausal symptoms in otherwise healthy women: a double‐blind, randomized, placebo‐controlled study. Phyto Ther 2017;31:1316–22. [DOI] [PubMed] [Google Scholar]
- 199. Sternfeld B, Guthrie K, Ensrud K, LaCroix A, Larson J, Dunn A, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause 2014;21:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Stevenson JC, Durand G, Kahler E, Pertynski T. Oral ultra‐low dose continuous combined hormone replacement therapy with 0.5 mg 17beta‐oestradiol and 2.5 mg dydrogesterone for the treatment of vasomotor symptoms: results from a double‐blind, controlled study. Maturitas 2010;67:227–32. [DOI] [PubMed] [Google Scholar]
- 201. Stovall D, Utian W, Gass M, Qu Y, Muram D, Wong M, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause 2007;14:510–7. [DOI] [PubMed] [Google Scholar]
- 202. Studd J, McCarthy K, Zamblera D, Burger H, Silberberg S, Wren B, et al. Efficacy and tolerance of Menorest compared to Premarin in the treatment of postmenopausal women. A randomised, multicentre, double‐blind, double‐dummy study. Maturitas 1995;22:105–14. [DOI] [PubMed] [Google Scholar]
- 203. Studd J, Pornel B, Marton I, Bringer J, Varin C, Tsouderos Y, et al. Efficacy and acceptability of intranasal 17 beta‐oestradiol for menopausal symptoms: randomised dose‐response study. Aerodiol Study Group.[Erratum appears in Lancet 1999 Aug 28;354(9180):780]. Lancet 1999;353:1574–8. [DOI] [PubMed] [Google Scholar]
- 204. Sulak PJ, Caubel P, Lane R. Efficacy and safety of a constant‐estrogen, pulsed‐progestin regimen in hormone replacement therapy. Int J Fertil Womens Med 1999;44:286–96. [PubMed] [Google Scholar]
- 205. Suvanto‐Luukkonen E, Koivunen R, Sundstrom H, Bloigu R, Karjalainen E, Haiva‐Mallinen L, et al. Citalopram and fluoxetine in the treatment of postmenopausal symptoms: a prospective, randomized, 9‐month, placebo‐controlled, double‐blind study. Menopause 2005;12:18–26. [DOI] [PubMed] [Google Scholar]
- 206. Swanson SG, Drosman S, Helmond FA, Stathopoulos VM. Tibolone for the treatment of moderate to severe vasomotor symptoms and genital atrophy in postmenopausal women: a multicenter, randomized, double‐blind, placebo‐controlled study. Menopause 2006;13:917–25. [DOI] [PubMed] [Google Scholar]
- 207. Tanmahasamut P, Vichinsartvichai P, Rattanachaiyanont M, Techatraisak K, Dangrat C, Sardod P. Cimicifuga racemosa extract for relieving menopausal symptoms: a randomized controlled trial. Climacteric 2015;18:79–85. [DOI] [PubMed] [Google Scholar]
- 208. Tice JA, Ettinger B, Ensrud K, Wallace R, Blackwell T, Cummings SR. Phytoestrogen supplements for the treatment of hot flashes: the Isoflavone Clover Extract (ICE) Study: a randomized controlled trial. JAMA 2003;290:207–14. [DOI] [PubMed] [Google Scholar]
- 209. Uebelhack R, Blohmer JU, Graubaum HJ, Busch R, Gruenwald J, Wernecke KD. Black cohosh and St. John's wort for climacteric complaints: A randomized trial. Obstet Gynecol 2006;107:247–55. [DOI] [PubMed] [Google Scholar]
- 210. Utian W, Lederman S, Williams B, Vega R, Koltun W, Leonard T. Relief of hot flushes with new plant‐derived 10‐component synthetic conjugated estrogens. Obstet Gynecol 2004;103:245–53. [DOI] [PubMed] [Google Scholar]
- 211. Utian W, Speroff L, Ellman H, Dart C. Comparative controlled trial of a novel oral estrogen therapy, estradiol acetate, for relief of menopause symptoms. Menopause 2005;12:708–15. [DOI] [PubMed] [Google Scholar]
- 212. Utian WH, Burry KA, Archer DF, Gallagher JC, Boyett RL, Guy MP, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. The Esclim Study Group. Am J Obstet Gynecol 1999;181:71–9. [DOI] [PubMed] [Google Scholar]
- 213. van der Sluijs CP, Bensoussan A, Chang S, Baber R. A randomized placebo‐controlled trial on the effectiveness of an herbal formula to alleviate menopausal vasomotor symptoms. Menopause 2009;16:336–44. [DOI] [PubMed] [Google Scholar]
- 214. van Die MD, Burger HG, Bone KM, Cohen MM, Teede HJ. Hypericum perforatum with Vitex agnus‐castus in menopausal symptoms: a randomized, controlled trial. Menopause 2009;16:156–63. [DOI] [PubMed] [Google Scholar]
- 215. Van Patten CL, Olivotto IA, Chambers GK, Gelmon KA, Hislop TG, Templeton E, et al. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J Clin Oncol 2002;20:1449–55. [DOI] [PubMed] [Google Scholar]
- 216. Verhoeven MO, van der Mooren MJ, van de Weijer PH, Verdegem PJ, van der Burgt LM, Kenemans P, et al. Effect of a combination of isoflavones and Actaea racemosa Linnaeus on climacteric symptoms in healthy symptomatic perimenopausal women: a 12‐week randomized, placebo‐controlled, double‐blind study. Menopause 2005;12:412–20. [DOI] [PubMed] [Google Scholar]
- 217. Villa P, Amar ID, Bottoni C, Cipolla C, Dinoi G, Moruzzi MC, et al. The impact of combined nutraceutical supplementation on quality of life and metabolic changes during the menopausal transition: a pilot randomized trial. Arch Gynecol Obstet 2017;296:791–801. [DOI] [PubMed] [Google Scholar]
- 218. Vincent A, Barton DL, Mandrekar JN, Cha SS, Zais T, Wahner‐Roedler DL, et al. Acupuncture for hot flashes: a randomized, sham‐controlled clinical study. Menopause 2007;14:45–52. [DOI] [PubMed] [Google Scholar]
- 219. von Hagens C, Schiller P, Godbillon B, Osburg J, Klose C, Limprecht R, et al. Treating menopausal symptoms with a complex remedy or placebo: a randomized controlled trial. Climacteric 2012;15:358–67. [DOI] [PubMed] [Google Scholar]
- 220. von Holst T, Salbach B. Efficacy of a new 7‐day transdermal sequential estradiol/levonorgestrel patch in women. Maturitas 2002;41:231–42. [DOI] [PubMed] [Google Scholar]
- 221. Walker EM, Rodriguez AI, Kohn B, Ball RM, Pegg J, Pocock JR, et al. Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor‐positive breast cancer: a randomized controlled trial. J Clin Oncol 2010;28:634–40. [DOI] [PubMed] [Google Scholar]
- 222. Wang CC, Cheng KF, Lo WM, Law C, Li L, Leung PC, et al. A randomized, double‐blind, multiple‐dose escalation study of a Chinese herbal medicine preparation (Dang Gui Buxue Tang) for moderate to severe menopausal symptoms and quality of life in postmenopausal women. Menopause 2013;20:223–31. [DOI] [PubMed] [Google Scholar]
- 223. Winther K, Rein E, Hedman C. Femal, a herbal remedy made from pollen extracts, reduces hot flushes and improves quality of life in menopausal women: a randomized, placebo‐controlled, parallel study. Climacteric 2005;8:162–70. [DOI] [PubMed] [Google Scholar]
- 224. Wong C, Yip BH, Gao T, Lam KYY, Woo DMS, Yip ALK, et al. Mindfulness‐based stress reduction (MBSR) or Psychoeducation for the reduction of menopausal symptoms: a randomized. Controlled Clinical Trial. Sci Rep 2018;8:6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Wuttke W, Raus K, Gorkow C. Efficacy and tolerability of the Black cohosh (Actaea racemosa) ethanolic extract BNO 1055 on climacteric complaints: A double‐blind, placebo‐ and conjugated estrogens‐controlled study. Maturitas 2006;55:S83–S91. [Google Scholar]
- 226. Wyrwich KW, Spratt DI, Gass M, Yu H, Bobula JD. Identifying meaningful differences in vasomotor symptoms among menopausal women. Menopause 2008;15:698–705. [DOI] [PubMed] [Google Scholar]
- 227. Xi S, Mao L, Chen X, Bai W. Effect of health education combining diet and exercise supervision in Chinese women with perimenopausal symptoms: a randomized controlled trial. Climacteric 2017;20:151–6. [DOI] [PubMed] [Google Scholar]
- 228. Xia Y, Zhao Y, Ren M, Zhang J, Wang Y, Chang Y, et al. A randomized double‐blind placebo‐controlled trial of a Chinese herbal medicine preparation (Jiawei Qing'e Fang) for hot flashes and quality of life in perimenopausal women. Menopause 2012;19:234–44. [DOI] [PubMed] [Google Scholar]
- 229. Yang H, Yang J, Wen Z, Zha Q, Nie G, Huang X, et al. Effect of combining therapy with traditional chinese medicine‐based psychotherapy and herbal medicines in women with menopausal syndrome: a randomized controlled clinical trial. Evid Based Complement Alternat Med 2012;2012:354145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zhong LL, Tong Y, Tang GW, Zhang ZJ, Choi WK, Cheng KL, et al. A randomized, double‐blind, controlled trial of a Chinese herbal formula (Er‐Xian decoction) for menopausal symptoms in Hong Kong perimenopausal women. Menopause 2013;20:767–76. [DOI] [PubMed] [Google Scholar]
- 231. Hunter MS, Liao KL. A psychological analysis of menopausal hot flushes. Br J Clin Psychol 1995;34(Pt 4):589–99. [DOI] [PubMed] [Google Scholar]
- 232. Hunter M. The Women’s Health Questionnaire (WHQ): the development, standardisation and application of a measure of mid‐aged women’s emotional and physical health. Qual Life Res 2000;9:733–8. [Google Scholar]
- 233. Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage 2001;22:979–89. [DOI] [PubMed] [Google Scholar]
- 234. Carpenter JS, Bakoyannis G, Otte JL, Chen CX, Rand KL, Woods N, et al. Validity, cut‐points, and minimally important differences for two hot flash‐related daily interference scales. Menopause 2017;24:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Hoga L, Rodolpho J, Goncalves B, Quirino B. Women's experience of menopause: a systematic review of qualitative evidence. JBI Database System Rev Implement Rep 2015;13:250–337. [DOI] [PubMed] [Google Scholar]
- 236. Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int 2009;30:339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Prague JK, Dhillo WS. Neurokinin 3 receptor antagonism ‐ the magic bullet for hot flushes? Climacteric 2017;20:505–9. [DOI] [PubMed] [Google Scholar]
- 238. Nistor I, Bolignano D, Haller MC, Nagler E, van der Veer SN, Jager K, et al. Why creating standardized core outcome sets for chronic kidney disease will improve clinical practice. Nephrol Dial Transplant 2017;32:1268–73. [DOI] [PubMed] [Google Scholar]
- 239. Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a ‘Core Outcome Set’—a practical guideline. Trials 2016;17:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study characteristics and quality assessment scoring of the included studies.
Table S2. Vasomotor‐related outcomes measured by diary.
Table S3. Different definitions for frequency, severity, intensity, and vasomotor scores of vasomotor‐related outcomes.
Appendix S1. Search strategy.


