Abstract
Histopathological evaluation including subtyping and grading is the current cornerstone for endometrial cancer (EC) classification. This provides clinicians with prognostic information and input for further treatment recommendations. Nonetheless, patients with histologically similar ECs may have very different outcomes, notably in patients with high‐grade endometrial carcinomas. For endometrial cancer, four molecular subgroups have undergone extensive studies in recent years: POLE ultramutated (POLEmut), mismatch repair‐deficient (MMRd), p53 mutant (p53abn) and those EC lacking any of these alterations, referred to as NSMP (non‐specific molecular profile). Several large studies confirm the prognostic relevance of these molecular subgroups. However, this ‘histomolecular’ approach has so far not been implemented in clinical routine. The ongoing PORTEC4a trial is the first clinical setting in which the added value of integrating molecular parameters in adjuvant treatment decisions will be determined. For diagnostics, the incorporation of the molecular parameters in EC classification will add a level of objectivity which will yield biologically more homogeneous subclasses. Here we illustrate how the management of individual EC patients may be impacted when applying the molecular EC classification. We describe our current approach to the integrated diagnoses of EC with a focus on scenarios with conflicting morphological and molecular findings. We also address several pitfalls accompanying the diagnostic implementation of molecular EC classification and give practical suggestions for diagnostic scenarios.
Keywords: adjuvant treatment, endometrial carcinoma, lymphovascular space invasion, mismatch repair, molecular classification, p53, POLE, risk stratification
Introduction
In the 4th edition of the World Health Organisation (WHO) classification of tumours of female reproductive organs,1 the definition for endometrial cancer (EC) entities were mainly based on histological characteristics supplemented with immunohistochemical markers. These microscopy‐based diagnoses are the current standard and serve as important input for (adjuvant) treatment decisions. However, considerable interobserver variation, in particular in high‐grade EC, is recognised, and centralised review prior to trial inclusion has pointed out that the therapeutic consequences are not negligible.2, 3 This situation prompted research into the incorporation of robust diagnostic markers, which yields novel narrowly defined diagnostic entities.
During the last decade a paradigm shift was invoked when the results from The Cancer Genome Atlas (TCGA) project were published.4 The TCGA elegantly showed the molecular diversity of EC in which four distinct molecular subgroups were recognised based on somatic copy number alterations (SCNA) and tumour mutational burden. These four subgroups include: (i) ultra‐mutated ECs characterised by pathogenic variants in the exonuclease domain of DNA polymerase‐epsilon (POLE); (ii) hyper‐mutated ECs characterised by microsatellite instability (MSI); (iii) a copy‐number low subgroup with a low mutational burden; and (iv) a copy‐number high group with frequent TP53 mutations.4 Subsequent studies showed and validated the prognostic relevance of this molecular stratification by using surrogate markers that enable the identification of EC subgroups analogous to the four described by the TCGA.5, 6 This has formed the basis of restructuring EC classification, using only a few key molecular aberrations, into POLE ultramutated (POLEmut) EC, MMRd EC, NSMP EC and p53abn EC (Figure 1). These novel integrated ‘histomolecular’ diagnostic entities not only provide more accurate prognostic information, they also provide an opportunity to improve the current clinical management for EC patients. This novel diagnostic algorithm has shown promise in refining adjuvant treatment recommendations (radio‐ and or chemotherapy), which currently does not incorporate molecular characteristics.
Figure 1.
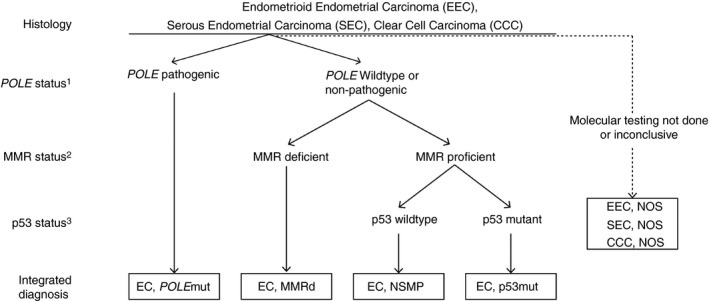
Diagnostic algorithm for a ‘histomolecular’ endometrial cancer classification. 1Pathogenic polymerase‐epsilon (POLE) variants include: P286R, V411L, S297F, A456P and S459F. 2Mismatch repair protein (MMR) deficiency is defined by the loss of one or more MMR‐proteins (MLH1, PMS2, MSH2 and MSH6). 3p53 immunohistochemistry (IHC) is an acceptable surrogate marker for TP53 mutational status in MMR‐proficient, POLE wild‐type endometrial cancer (EC).50
Adjuvant treatment is currently recommended based on a patient's individual risk (low‐, intermediate‐ and high‐risk) comprised of a combination of clinical (age) and pathological (FIGO stage, tumour type, grade and the presence of unequivocal lymphovascular space invasion (LVSI) factors.7, 8, 9, 10, 11, 12, 13, 14 How the additional molecular information should be incorporated into this risk‐based approach has still to be determined. It seems prudent, however, that treatment de‐escalation is considered in EC with favourable molecular factors (e.g. POLEmut EC) and intensified treatments are considered in the presence of unfavourable factors (e.g. p53mut EC). The ongoing PORTEC4a trial (http://ClinicalTrials.gov Identifier: NCT03469674) is the first clinical trial to prospectively investigate the incorporation of molecular EC characteristics in the context of patients who would currently be classified as high–intermediate risk based on clinicopathological factors alone. Standard postoperative vaginal brachytherapy (VBT) is being compared to adjuvant treatment based on an individual's molecular‐integrated risk profile, including no adjuvant treatment, VBT or external beam radiotherapy (EBRT). This randomised trial will provide essential information on how the ‘histomolecular’ entities may be used to personalise adjuvant treatment by decreasing both over‐ and undertreatment.
In this review, we illustrate clinical scenarios in which integration of the molecular characteristics into the current clinicopathological classification may shift the adjuvant treatment recommendations. We also discuss how the integrated molecular entities may provide clues to specific targeted treatment modalities that can be exploited in advanced‐stage or recurrent disease. For a more detailed background on the molecular EC classification, as well as assays that may be used for the diagnosis, the reader is referred to other reviews.15, 16, 17
POLEmut high‐grade endometrial carcinoma
Pathogenic POLE variants in the exonuclease domain of the POLE gene comprise approximately 10% 6of all endometrioid EC (EEC),4 and in the majority consists of one of the five hot‐spots: P286R, V411L, S297F, A456P and S459F. In the molecular EC classification these cases are referred to as ‘POLEultramutated’ or ‘POLEmut EC’. Typical POLEmut EC features include: presentation at relatively young age and early stage, high tumour grade with scattered tumour giant cells and a prominent lymphocytic infiltrate.4, 18 Although POLEmut EC are mainly microsatellite‐stable (MSS), unstable cases with MMR‐protein loss have been described.4 Importantly, the occurrence of secondary TP53 mutations have been reported in up to 42% of POLEmut EC, sometimes resulting in subclonal mutant‐like p53 immunohistochemistry.4, 19 Two examples of POLEmut EC are illustrated in Figure 2. Figure 2A,B shows an EC case which was originally diagnosed as a stage II grade 3 EEC and Figure 2C,D shows an EC case that was diagnosed as a stage IB mixed endometrioid–clear cell EC. According to the current adjuvant treatment recommendations, both these cases are considered ‘high‐risk’ and adjuvant (external beam) radiotherapy is recommended and sequential chemotherapy may be considered.20
Figure 2.
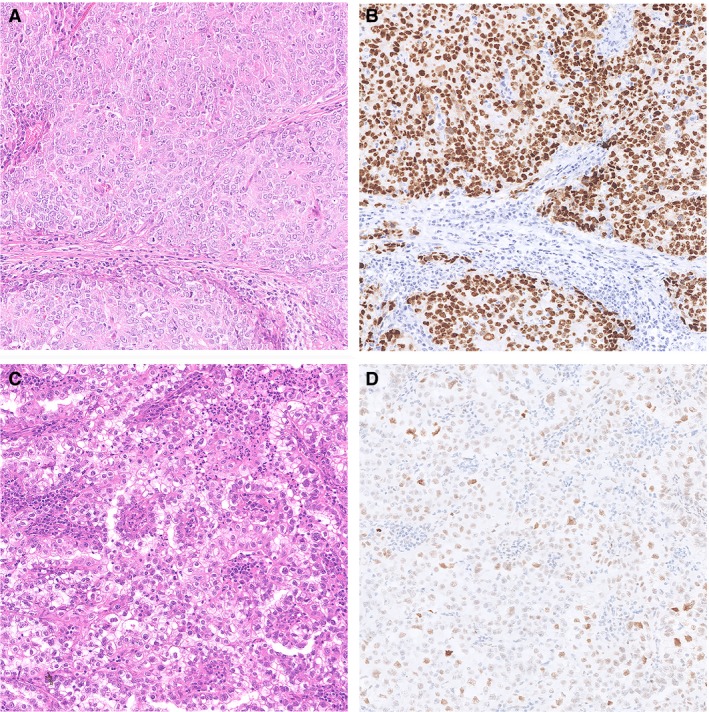
Two examples of polymerase‐epsilon mutated endometrial cancer (EC) with a pathogenic polymerase‐epsilon ultramutated (POLEmut) EC. A,B, Haematoxylin and eosin (H&E) stain of an EC diagnosed as endometrioid EC (FIGO Grade 3), based on solid growth, with aberrant mutant‐like p53 immunostaining. Molecular profiling showed the presence of a POLE P286R variant and a TP53 mutation. C,D, H&E of an EC case originally diagnosed as mixed endometroid and clear cell EC with scattered nuclear p53 immunostaining interpreted as wild‐type p53. Molecular profiling showed a POLE V411L variant and no mutations in TP53.
How May the Presence of a Pathogenic POLE Mutation Affect Adjuvant Treatment Recommendations?
Despite their association with high‐grade histology, POLEmut EC patients have an exceptionally good prognosis.5, 21, 22, 23 Most of the studies to date have analysed retrospective cohorts in which patients had received some form of adjuvant treatment, so hypersensitivity to these adjuvant treatments could, theoretically, be the basis for the favourable outcomes of POLEmut ECs in these series. Sub‐analysis of POLEmut and POLEwild‐type EC in the observation arm of the PORTEC‐1 trial (n = 245 patients with stage I intermediate risk EC), however, also showed a 10‐year recurrence‐free survival (RFS) of 100% versus 80.1%, respectively (hazard ratio = 0.143; 95% confidence interval = 0.001–0.996; P = 0.049).24 This finding suggests that POLEmut EC possess intrinsic characteristics that are beneficial for survival, independent of sensitivity to adjuvant treatment. This is supported by the lack of an increased radiation sensitivity in POLEmut embryonic stem cells.25 It has been suggested that POLEmut EC are immunogenic due to their high mutational load, which may be a more plausible explanation for the unusually low chance of recurrences observed in these patients.26 Importantly, in addition to the excellent prognosis of intermediate‐risk POLEmut EC, similar survival rates have now been reported for patients with high‐risk POLEmut EC.21, 22, 23, 27 This consistent finding of excellent clinical outcomes in POLEmut ECs raises the question of whether these patients may be unnecessarily exposed to unwanted side‐effects of radio‐ and/or chemotherapy.28 Treatment de‐escalation towards perhaps no additional treatment at all for patients with POLEmut EC has therefore been proposed.25 The results of the ongoing PORTEC4a will show if this is a valid approach for patients with intermediate‐risk POLEmut EC. For high‐risk POLEmut EC, the recently presented molecular characterisation of the PORTEC‐3 trial has been highly informative.27 Patients with POLEmut EC showed an excellent prognosis independent of the treatment arm (EBRT versus CTRT), supporting omitting the addition of chemotherapy for patients with high‐risk POLEmut EC.
Does the Co‐occurrence of TP53 Mutations Affect the Management of POLEmut EC?
The majority of EC can be classified into one of the four molecular subgroups. However, in a small subset (3–5%) of patients molecular analysis will show more than one classifying alteration (e.g. POLEmut‐MMRd EC, POLEmut‐p53abn EC, MMRd‐p53abn EC or POLEmut‐MMRd‐p53abn EC), also referred to as ‘multiple classifier’ EC.19 As there are distinct prognostic differences between the four molecular subgroups, the question arises as to which biological behaviour these multiple classifiers follow. This dilemma is most pronounced in the combination of POLEmut‐p53abn EC, in which the tumour exhibits both a favourable pathogenic mutation in the POLE exonuclease domain as well as unfavourable aberrant p53 IHC expression, such as illustrated in our case in Figure 2A,B.
In contrast to the excellent prognosis of POLEmut EC, p53abn EC are associated with poor clinical outcomes.5, 21, 23 For some time, it has been uncertain how to classify EC in which a pathogenic POLE variation and a TP53 mutation co‐occur. Molecular clustering of these ‘multiple classifier EC’ showed that POLEmut‐p53abn EC clustered together with POLEmut EC without TP53 alterations, and it was noted that p53‐IHC in these cases frequently showed ‘subclonal’ mutant‐like p53 expression.19 Subclonal expression was defined as abrupt and complete regional aberrant p53 expression, in which the subclonal region was at least 10% of the total tumour volume. This unusual p53 expression pattern can be observed in POLEmut and MMRd EC, and reflects their genetic heterogeneity. Available survival data demonstrated that POLEmut‐p53abn EC show clinical outcomes comparable to POLEmut EC without abnormal p53 expression. These findings show that TP53 mutations in these ‘multiple‐classifiers’ are probably passenger mutations not affecting the clinical behaviour, indicating that these cases should be classified and treated as POLEmut EC.
MMRd endometrioid endometrial cancer
MMR deficiency is frequent in EC (25–30%) and defined by the loss of nuclear expression of one or more MMR proteins (MLH1, PMS2, MSH6 and PMS2) by the tumour cells.4, 5, 21, 23, 29 Reflex testing for MMR status is increasingly recommended by (inter)national guidelines for the identification of patients (and families) who are at high risk of having Lynch syndrome.30 The vast majority of MMR deficiency identified by this approach, however, is due to sporadic causes (promotor hypermethylation of MLH1 or somatic MMR gene mutations), unrelated to Lynch syndrome.31 Histologically, MMRd EC show similarities to POLEmut EC in that these are also associated with higher‐grade, endometrioid‐type histology and abundance of tumour‐infiltrating lymphocytes (TILs).4, 32, 33 MMRd EC, however, have an intermediate prognosis, which significantly differs from POLEmut EC.27 Long‐term analysis of the PORTEC‐2 trial – comparing adjuvant VBT with EBRT in high–intermediate risk (HIR) EC – found that VBT was equally effective in preventing pelvic lymph node recurrences in the absence of unfavourable risk factors (p53abn, L1CAM, substantial LVSI), thus including HIR MMRd ECs having a very low absolute risk.24 In addition, for patients with HR MMRd EC, EBRT provides good pelvic control, and additional chemotherapy does not seem to improve prognosis.27 An example illustrated in Figure 3 depicts a case of a stage IB MMRd EEC with unequivocal lymphovascular space invasion (LVSI). According to the current adjuvant treatment recommendations, this patient is regarded as ‘high–intermediate risk’, and adjuvant radiotherapy is recommended.20
Figure 3.
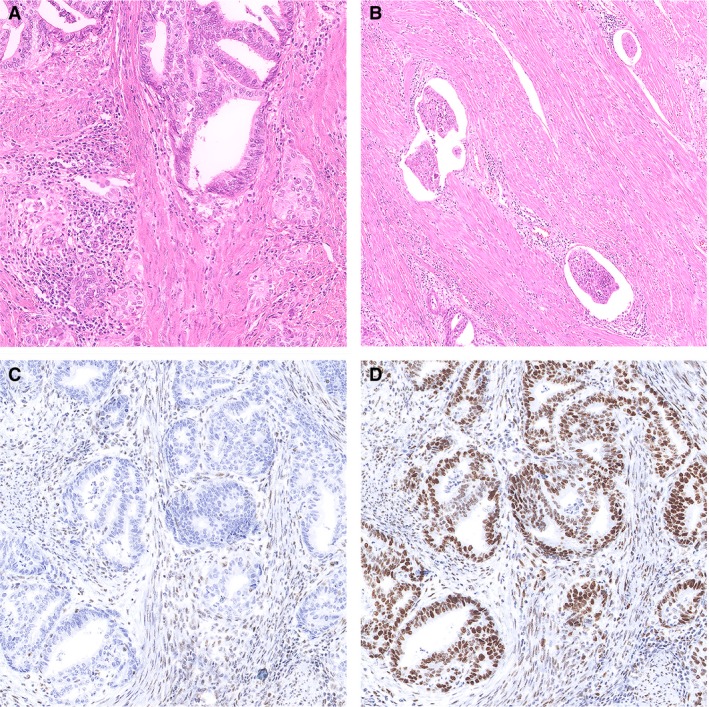
Example of a mismatch repair‐deficient (MMRd) endometrioid endometrial cancer (EEC) with lymphovascular space invasion (LVSI). A, Haematoxylin and eosin (H&E) stain of an endometrioid EC (FIGO grade 1), with prominent peritumoural lymphocytes. B, Representative image of the invasive front with foci of LVSI; the tumour contained >5 foci and was thereby reported as EC with substantial LVSI. C,D, Absence of nuclear immunoreactivity of PMS2 (C) and retained nuclear staining of MSH6 (D).
How Does the Presence of (Substantial) LVSI Relate to MMRD EC?
Histologically, LVSI is defined as the presence of tumour cells within an endothelial‐lined space that lies outside the invasive border. Interestingly, identifying one focus of LVSI (also referred to as ‘focal’) has little impact on prognosis, whereas substantial LVSI (also referred to as ‘extensive’) is associated with a significant increase in the risk of recurrence.34 Substantial LVSI is seen in a small proportion of high–intermediate risk EC (~5%).5, 24, 34 The relevance of stratifying the extent of LVSI has only recently been accepted, and will soon be adopted in pathology reporting. Although substantial LVSI has been described in all molecular EC groups, an association between the presence of substantial LVSI and MMRd has been noted, with a reported prevalence of substantial LVSI in up to 8.9% of MMRd EC (P = 0.002).5, 35 This association may explain the fact that a relatively large proportion of high‐stage EC fall within the MMRd subgroup.36 How MMRd leads to LVSI in EC is not well understood, but it may be the result of frequent epithelial‐to‐mesenchymal transition in MMRd EC.37
How Does the Presence of Substantial LVSI Impact Adjuvant Treatment in EC?
Multiple studies investigating the prognostic impact of (substantial) LVSI in EC patients have found an increased risk of lymph node metastasis and locoregional as well as distant recurrences, regardless of stage and histotype.34, 38, 39, 40, 41, 42 These studies suggest that adjuvant radio‐ or chemotherapy is recommended to reduce the risk of recurrence in these patients. This is supported by the long‐term results of the PORTEC‐2 trial – including patients with high–intermediate risk factors – that found a decreased risk of pelvic lymph node recurrence after EBRT compared to VBT and postoperative observation for HIR EC patients with substantial LVSI.24 Importantly, although LVSI is seen mainly in MMRd EC, LVSI is also an independent prognostic factor in NSMP and p53mut EC. Therefore, LVSI assessment will remain essential in risk stratification schemes, escalating adjuvant treatment regimens in those patients in whom substantial LVSI is identified. Given the lack of surrogate markers, pathologists will continue to be asked to provide this information based on H&E assessment, which has an acceptable level of interobserver agreement.43 As substantial LVSI is such a strong prognostic factor, LVSI illustrates that histological characteristics need to be integrated with the molecular classification to reach an optimal risk stratification in EC.
p53 Mutant low‐grade endometrioid endometrial carcinoma
A paradoxical scenario occurs when a morphological low‐grade endometrioid EC shows molecular characteristics associated with aggressive clinical behaviour, such as abnormal mutant‐type p53 staining. This is a rare finding in low‐grade EEC, as it is reported in 2–15% of glandular EEC with low nuclear grade,5, 6, 21, 29, 44, 45 whereas it is a more common finding (10–15%) in high‐grade EECs.36 Two examples of low‐grade EEC with TP53 mutations and abnormal p53‐IHC are shown in Figure 4. Both these tumours were diffusely positive for hormone receptors and showed loss of PTEN immunostaining, supporting the ‘endometrioid’ classification. Although the nuclear atypia in these tumours may alarm some pathologists for a ‘glandular variant’ of serous endometrial cancer, tumours such as this with smooth luminal borders continue to be difficult to distinguish from low‐grade EEC.46, 47 Both these cases were originally reported as a stage IB, low‐grade EEC and p53 was not performed. According to the current adjuvant treatment recommendations, both patients would be regarded as ‘intermediate‐risk’ and adjuvant vaginal brachytherapy is recommended.20
Figure 4.
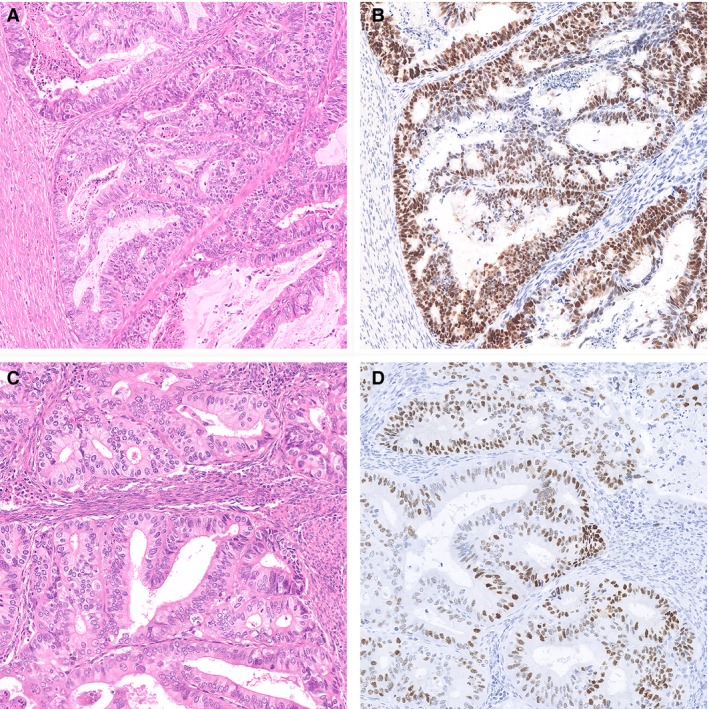
Two examples of low‐grade p53 mutation endometrioid endometrial cancer (EC). A, Representative haematoxylin and eosin (H&E) stain of an EC diagnosed as FIGO grade 2 (based on nuclear atypia) endometrioid EC. B, This case showed diffuse nuclear overexpression of p53 by IHC, interpreted as mutant‐like expression. Next‐generation sequencing (NGS) confirmed the presence of a TP53 mutation. C, Another example of an EC diagnosed as FIGO grade 1 endometrioid EC with aberrant p53 staining. NGS confirmed the presence of a pathogenic TP53 mutation. Both cases were mismatch repair protein (MMR)‐proficient and did not carry a polymerase‐epsilon (POLE) mutation.
How Does the Presence of a TP53 Mutation Impact the Adjuvant Treatment in Endometrial Cancer?
p53 IHC has proved to be a very reliable surrogate marker for detecting underlying TP53 mutations in EC, with reported sensitivity and specificity of 0.96 and 1.00, respectively.48, 49, 50 Importantly, several studies have shown that patients with p53mut EC, independent of histotype, grade or stage, show poor clinical outcomes.5, 21, 23, 29, 45 The number of low‐grade EC which fall into the p53mut EC subgroup is limited, but the available data point towards unfavourable clinical outcomes in these cases.5, 24, 45 It has been suggested that combining p53 IHC with the classical histological grading system will improve prognostic accuracy for EEC.45 However, we would prefer to see p53mut EC as a separate entity within the integrated histomolecular EC classification. The reported poor clinical outcomes have been the rationale to propose adjuvant treatment escalation from VBT to EBRT in the PORTEC‐4a trial for high–intermediate‐risk EC patients in the uncommon scenario of mutant‐like p53 immunostaining in these patients. For high‐risk patients with a p53mut EC, such as those enrolled in the PORTEC‐3 trial, further treatment escalation by additional chemotherapy significantly improves clinical outcomes (5‐year RFS with CTRT 61 versus 37% for RT).27
Potential role for molecular characteristics in the treatment of recurrent or advanced EC
To date, treatment options for patients with advanced or recurrent EC are limited. In recent years several targetable pathways in the context of molecular EC subgroups have been investigated, resulting in novel treatment strategies with potential clinical benefit. POLEmut and MMRd EC, due to their high mutational burden, obtain high levels of neoantigens and TILs, making them attractive candidates for immunotherapy such as anti‐PD1 immune check‐point blockade.32, 33, 51, 52, 53, 54, 55, 56, 57 Despite this theoretical argument, the excellent clinical outcomes for patients with POLEmut EC under current treatment regimens (independent of stage), however, argues against the rationale to the use of immunotherapy for this subpopulation. For MMRd EC, particularly when recurrent or in advanced metastatic stage (FIGO stage >III), immune check‐point inhibition may be an attractive option.53 Currently, there is no good rationale for the use of check‐point inhibition in the context of p53mut EC. As patients with p53mut EC represent the group with the worst clinical outcomes, identifying targets in p53mut EC is an urgent need. Targeting amplifications of the ERBB2 gene, encoding for human epidermal growth receptor 2 (HER2) and homologous recombination deficiency (HRD), both occurring in p53mut EC, are being explored and show some promise.58
Role of HER2 Expression in EC Treatment
Amplification of the ERBB2 gene and/or HER2 overexpression have been reported in serous EC and uterine carcinosarcomas with a serous carcinomatous component, albeit with a substantial variation in reported rates of 14–80% and 21–47%, respectively.59, 60 This variation can be explained by the lack of standardised scoring methods for HER2 immunohistochemistry in EC. Most studies have scored HER2 IHC expression using scoring guidelines designed for breast cancer; however, HER2 expression shows significant heterogeneity in EC, in contrast to HER2‐positive breast cancer, and therefore these guidelines may not be directly applicable to EC.61 An example of a serous EC with diffuse immunohistochemical overexpression of HER2 and amplification of ERBB2 by in‐situ hybridisation is shown in Figure 5. Trastuzumab is a monoclonal antibody directed against the HER2 receptor and, when combined with chemotherapy, has been shown to increase survival in HER2 overexpressing breast and gastric cancer.62, 63 Studies investigating the therapeutic potential of trastuzumab as a single agent in HER2 overexpressing EC were not able to demonstrate any prognostic benefit.64, 65 However, a recent trial including patients with stages III/IV or recurrent HER2 overexpressing serous EC found an increased progression‐free survival (PFS) from 8 to 13 months (P = 0.005) when trastuzumab was given in combination with carboplatin‐paclitaxel chemotherapy.58 These findings encourage further investigation on the efficacy of trastuzumab combined with chemotherapy to improve outcomes for patients with advanced or recurrent EC. For pathologists, the strong association between ERBB2 gene amplifications and serous histology is of interest, as it suggests that this genomic alteration may be limited to p53mut EC. It would be informative to address this association in more detail in future studies, as it would provide a rationale for focused HER2 testing in the context of p53mut EC.
Figure 5.

Example of human epidermal growth factor receptor 2 (HER2) overexpressing serous endometrial cancer (EC). A, Representative haematoxylin and eosin (H&E) stain of a serous EC with profound nuclear atypia and presence of a rugged luminal surface. B, Complete strong membranous HER2 immunohistochemical staining. In this case the ERBB2 amplification was confirmed with in‐situ hybridisation (see inset).
Role of HRD in EC Treatment
Homologous recombination repair capacity has been successfully used as a marker to identify patients that benefit from poly (ADP ribose) polymerase inhibitors (PARPi) in high‐grade serous ovarian cancer (HGSOC).66, 67 HGSOC and serous EC have very similar molecular genetic alterations4; recent data show that a subset of p53mut EC are homologous recombination‐deficient, and some of these EC can arise in the context of germline BRCA1/2 mutations.68, 69, 70, 71 The exact prevalence of HRD in p53mut EC is currently unknown; in a small and selected set of cases it was 46%.68 Together, these data build upon a rationale to target homologous recombination in p53mut EC, particularly those that are HRD. Currently, several clinical trials investigating the efficacy of different PARP‐inhibitors in recurrent or metastatic EC are either planned or recruiting; the results of these trials are eagerly awaited. In the meantime, the gold standard for testing the homologous recombination status of tumours, including EC, should be defined.
Discussion
The molecular subgrouping proposed by the TCGA has been fundamental in evolving the dualistic Bokhman model into a more refined and molecular‐based system. The subsequent development and validation of widely accessible surrogate markers for the four distinct EC subgroups has accelerated this field. This has led to a diagnostic algorithm that incorporated molecular characteristics, resulting in a novel, objective and clinically meaningful EC classification. Once regulatory authorities understand the potential clinical impact of this improved EC classification, financial aspects that come with the implementation of additional testing should be quickly resolved. Pathologists will be asked to assess the mutational status of the POLE gene and perform three immunohistochemical stains (p53, MSH6 and PMS2) in order to provide an integrated ‘histomolecular’ diagnosis. Next, the design of (adjuvant) treatment strategies incorporating this novel classification will become a priority. This shift towards a molecular driven EC classification is yet another illustration of how pathology is delivering the promise of precision medicine.
The molecular EC classification follows the subclassification proposed by the TCGA; however, further refinement of this classification is to be expected. It would be particularly attractive to further refine the largest EC subgroup, NSMP EC, which can be regarded as a heterogeneous molecular rest group mainly consisting of alterations in PI3K‐Akt and Wnt‐signalling alterations.4 NSMP EC are almost exclusively low‐grade endometrioid‐type endometrial carcinomas. One molecular alteration that stands out in terms of frequency is the presence of mutations in exon 3 of CTNNB1 (52%).4 Subsequent studies evaluating the potential prognostic significance of these CTNNB1 mutations in low‐grade early‐stage EC showed a significantly worse recurrence‐free survival.72, 73 In addition, independent from the TCGA data, CTNNB1 exon 3 mutations were shown to have prognostic significance in patients with high–intermediate‐risk NSMP EC.5 Morphologically, CTNNB1 exon 3‐mutated EC are associated with low‐grade, low abundance of TILS and with squamous differentiation.72, 74 Studies investigating both β‐catenin IHC and CTNNB1 exon 3 gene sequencing in EC have reported varying concordance rates, resulting in the conclusion that β‐catenin IHC is insufficient to act as a surrogate marker for CTNNB1mut EC.72, 74, 75 The validated prognostic impact within NSMP EC, in combination with the distinct morphological features, may qualify CTNNB1 mutant EC as the fifth molecular EC subgroup, which may be referred to as CTNNB1mut EC (after excluding MMRd and POLE mutations). It is conceivable that CTNNB1mut EC will be incorporated into the diagnostic algorithm as a separate ‘histomolecular’ entity.
Most studies that report on the impact of adjuvant treatment in the context of the molecular EC classification have included high‐intermediate risk patients. For patients who would currently be regarded as ‘low‐’ or ‘high‐risk’, the current data on the impact of adjuvant treatment are limited. Stelloo et al. showed, in an univariable analysis of 242 EEC with low‐risk features, a trend towards a higher rate of distant recurrences and lower overall survival (P = 0.061 and P = 0.058, respectively) for patients with p53mut EC.5 Despite this impact on prognosis, these data are insufficient to support adjuvant treatment recommendations based on molecular classification of low‐risk EC, and further research is needed. In addition, it would be interesting to study the effect of fertility‐sparing progestin therapy among the four molecular subgroups, as this can be informative for the management of low‐risk EC in young women of reproductive age. In contrast to low‐risk EC, it is becoming very clear that in high‐risk EC molecular subgroups not only impact prognosis, but should also influence adjuvant treatment decisions. This is exemplified by POLEmut EC in the high‐risk EC population, which show an excellent prognosis when adjuvant treatment is limited to radiotherapy, strongly suggesting that these patients do not benefit from adjuvant chemotherapy.27
This review shows that changing the EC classification to include molecular characteristics provides exciting opportunities, but will also create certain challenges with respect to testing and reporting. These challenges include the availability and choice of assays for surrogate markers, as well as the management of patients in centres that do not have access to these assays. Immunohistochemical surrogate markers, such as p53 and MMR‐protein immunohistochemistry, are readily available, but it is recognised that some centres may not have the ability to carry out POLE testing or that results from any of the required assays may not be conclusive. To molecularly classify an individual EC, following the diagnostic algorithm provided in Figure 1, POLE testing is a prerequisite. This is best illustrated by POLEmut EC that carry TP53 mutations, which would be falsely classified as p53mut EC when POLE‐testing is not performed. With this in mind, we propose that a ‘not otherwise specified’ (NOS) diagnostic designation is used when there is insufficient information to assign a specific molecular entity. This is in contrast to ‘NSMP EC’, which defines EC that have been completely profiled, but do not carry a specific classifying feature. Our proposed ‘NOS’ designation is used in combination with a specified histological subtype (e.g. EEC‐NOS, SEC‐NOS, CCC‐NOS) to ensure that the information of histological subtyping is not lost in these cases. We believe that the introduction of this NOS designation will provide necessary clarity in translational research and in reporting to clinicians.
Conclusion
The scenarios presented illustrate that the molecular EC classification provides clinically relevant and prognostic information, with the potential to influence (adjuvant) treatment strategies. When clinical trials confirm the added value of integrated molecular diagnostic entities, we envision that this will become the new standard in EC reporting. Independent of ‘histomolecular’ type, certain histopathological characteristics, such as (the extent of) LVSI and stage, do not have a molecular surrogate and will remain essential in the pathological assessment of a hysterectomy with EC. The value of histological type and FIGO grade is less certain, but it is recommended to still report on these in the years to come. The precise weight of all these intrinsic tumour variables should be evaluated to design a novel risk stratification scheme for adjuvant treatment that includes some of the most relevant molecular characteristics. Finally, the molecular classification provides a framework for subclass‐specific trial designs that should be developed over the coming years to examine the potential benefit of subclass‐specific targeted therapy.
Conflict of interest
All authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Dutch Cancer Society, Research Project Grant (TB, 11629).
Vermij L, Smit V, Nout R & Bosse T (2020) Histopathology 76, 52–63. 10.1111/his.14015 Incorporation of molecular characteristics into endometrial cancer management
References
- 1. Kurman RJ, Carcangiu ML, Herrington S, Young RH. WHO classification of tumours of female reproductive organs. 4th ed Lyon: WHO Press, 2014. [Google Scholar]
- 2. de Boer SM, Wortman BG, Bosse T et al Clinical consequences of upfront pathology review in the randomised portec‐3 trial for high‐risk endometrial cancer. Ann. Oncol. 2018; 29; 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high‐grade endometrial carcinoma. Am. J. Surg. Pathol. 2013; 37; 874–881. [DOI] [PubMed] [Google Scholar]
- 4. Cancer Genome Atlas Research N , Kandoth C, Schultz N et al Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497; 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stelloo E, Nout RA, Osse EM et al Improved risk assessment by integrating molecular and clinicopathological factors in early‐stage endometrial cancer‐combined analysis of the portec cohorts. Clin. Cancer Res. 2016; 22; 4215–4224. [DOI] [PubMed] [Google Scholar]
- 6. Talhouk A, McConechy MK, Leung S et al A clinically applicable molecular‐based classification for endometrial cancers. Br. J. Cancer 2015; 113; 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Creutzberg CL, van Putten WL, Koper PC et al Surgery and postoperative radiotherapy versus surgery alone for patients with stage‐1 endometrial carcinoma: multicentre randomised trial. Portec study group. Post operative radiation therapy in endometrial carcinoma. Lancet 2000; 355; 1404–1411. [DOI] [PubMed] [Google Scholar]
- 8. de Boer SM, Powell ME, Mileshkin L et al Adjuvant chemoradiotherapy versus radiotherapy alone for women with high‐risk endometrial cancer (portec‐3): final results of an international, open‐label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018; 19; 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nout RA, Smit VT, Putter H et al Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high‐intermediate risk (portec‐2): an open‐label, non‐inferiority, randomised trial. Lancet 2010; 375; 816–823. [DOI] [PubMed] [Google Scholar]
- 10. Keys HM, Roberts JA, Brunetto VL et al A phase iii trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic oncology group study. Gynecol. Oncol. 2004; 92; 744–751. [DOI] [PubMed] [Google Scholar]
- 11. Kong A, Simera I, Collingwood M, Williams C, Kitchener H; Cochrane Gynaecological Cancer G . Adjuvant radiotherapy for stage i endometrial cancer: systematic review and meta‐analysis. Ann. Oncol. 2007; 18; 1595–1604. [DOI] [PubMed] [Google Scholar]
- 12. ASTEC/EN.5 Study Group , Blake P, Swart AM et al Adjuvant external beam radiotherapy in the treatment of endometrial cancer (mrc astec and ncic ctg en.5 randomised trials): pooled trial results, systematic review, and meta‐analysis. Lancet 2009; 373; 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hogberg T, Signorelli M, de Oliveira CF et al Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer – results from two randomised studies. Eur. J. Cancer 2010; 46; 2422–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sorbe B, Horvath G, Andersson H, Boman K, Lundgren C, Pettersson B. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium‐risk endometrial carcinoma – a prospective randomized study. Int. J. Radiat. Oncol. Biol. Phys. 2012; 82; 1249–1255. [DOI] [PubMed] [Google Scholar]
- 15. McAlpine J, Leon‐Castillo A, Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J. Pathol. 2018; 244; 538–549. [DOI] [PubMed] [Google Scholar]
- 16. Hussein YR, Soslow RA. Molecular insights into the classification of high‐grade endometrial carcinoma. Pathology 2018; 50; 151–161. [DOI] [PubMed] [Google Scholar]
- 17. Wortman BG, Nout RA, Bosse T, Creutzberg CL. Selecting adjuvant treatment for endometrial carcinoma using molecular risk factors. Curr. Oncol. Rep. 2019; 21; 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Gool IC, Ubachs JEH, Stelloo E et al Blinded histopathological characterisation of pole exonuclease domain‐mutant endometrial cancers: sheep in wolf's clothing. Histopathology 2018; 72; 248–258. [DOI] [PubMed] [Google Scholar]
- 19. León‐Castillo A, Gilvazquez E, Nout R et al Clinicopathological and molecular characterisation of “multiple classifier” endometrial carcinomas. J Pathol 2019; In press [DOI] [PMC free article] [PubMed]
- 20. Colombo N, Creutzberg C, Amant F et al Esmo‐esgo‐estro consensus conference on endometrial cancer: diagnosis, treatment and follow‐up. Ann. Oncol. 2016; 27; 16–41. [DOI] [PubMed] [Google Scholar]
- 21. Talhouk A, McConechy MK, Leung S et al Confirmation of promise: a simple, genomics‐based clinical classifier for endometrial cancer. Cancer 2017; 123; 802–813. [DOI] [PubMed] [Google Scholar]
- 22. Church DN, Stelloo E, Nout RA et al Prognostic significance of pole proofreading mutations in endometrial cancer. J. Natl Cancer Inst. 2015; 107; 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stelloo E, Bosse T, Nout RA et al Refining prognosis and identifying targetable pathways for high‐risk endometrial cancer; a transportec initiative. Mod. Pathol. 2015; 28; 836–844. [DOI] [PubMed] [Google Scholar]
- 24. Wortman BG, Creutzberg CL, Putter H et al Ten‐year results of the portec‐2 trial for high‐intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br. J. Cancer 2018; 119; 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Gool IC, Rayner E, Osse EM et al Adjuvant treatment for pole proofreading domain‐mutant cancers: sensitivity to radiotherapy, chemotherapy, and nucleoside analogues. Clin. Cancer Res. 2018; 24; 3197–3203. [DOI] [PubMed] [Google Scholar]
- 26. van Gool IC, Eggink FA, Freeman‐Mills L et al Pole proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin. Cancer Res. 2015; 21; 3347–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Creutzberg C, Leon del Castillo A, De Boer SM et al Molecular classification of the portec‐3 trial for high‐risk endometrial cancer: impact on adjuvant therapy [accepted abstract]. ESMO Congress; 2019 27 sep – 01 oct. Barcelona, Spain, 2019.
- 28. de Boer SM, Powell ME, Mileshkin L et al Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high‐risk endometrial cancer (portec‐3): an open‐label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2016; 17; 1114–1126. [DOI] [PubMed] [Google Scholar]
- 29. Kommoss S, McConechy MK, Kommoss F et al Final validation of the promise molecular classifier for endometrial carcinoma in a large population‐based case series. Ann. Oncol. 2018; 29; 1180–1188. [DOI] [PubMed] [Google Scholar]
- 30. Ryan N, Wall J, Crosbie EJ et al Lynch syndrome screening in gynecological cancers: results of an international survey with recommendations for uniform reporting terminology for mismatch repair immunohistochemistry results. Histopathology 2019. [DOI] [PubMed] [Google Scholar]
- 31. Simpkins SB, Bocker T, Swisher EM et al Mlh1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum. Mol. Genet. 1999; 8; 661–666. [DOI] [PubMed] [Google Scholar]
- 32. Howitt BE, Shukla SA, Sholl LM et al Association of polymerase e‐mutated and microsatellite‐instable endometrial cancers with neoantigen load, number of tumor‐infiltrating lymphocytes, and expression of pd‐1 and pd‐l1. JAMA Oncol. 2015; 1; 1319–1323. [DOI] [PubMed] [Google Scholar]
- 33. Eggink FA, Van Gool IC, Leary A et al Immunological profiling of molecularly classified high‐risk endometrial cancers identifies pole‐mutant and microsatellite unstable carcinomas as candidates for checkpoint inhibition. Oncoimmunology 2017; 6; e1264565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bosse T, Peters EE, Creutzberg CL et al Substantial lymph‐vascular space invasion (lvsi) is a significant risk factor for recurrence in endometrial cancer – a pooled analysis of portec 1 and 2 trials. Eur. J. Cancer 2015; 51; 1742–1750. [DOI] [PubMed] [Google Scholar]
- 35. McMeekin DS, Tritchler DL, Cohn DE et al Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016; 34; 3062–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bosse T, Nout RA, McAlpine JN et al Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am. J. Surg. Pathol. 2018; 42; 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bosse T, Nout RA, Stelloo E et al L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: pooled portec trial results. Eur. J. Cancer 2014; 50; 2602–2610. [DOI] [PubMed] [Google Scholar]
- 38. Briet JM, Hollema H, Reesink N et al Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005; 96; 799–804. [DOI] [PubMed] [Google Scholar]
- 39. Ortoft G, Lausten‐Thomsen L, Hogdall C, Hansen ES, Dueholm M. Lymph‐vascular space invasion (lvsi) as a strong and independent predictor for non‐locoregional recurrences in endometrial cancer: a danish gynecological cancer group study. J. Gynecol. Oncol. 2019; 30; e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guntupalli SR, Zighelboim I, Kizer NT et al Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol. 2012; 124; 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koskas M, Bassot K, Graesslin O et al Impact of lymphovascular space invasion on a nomogram for predicting lymph node metastasis in endometrial cancer. Gynecol Oncol. 2013; 129; 292–297. [DOI] [PubMed] [Google Scholar]
- 42. Winer I, Ahmed QF, Mert I et al Significance of lymphovascular space invasion in uterine serous carcinoma: what matters more; extent or presence? Int. J. Gynecol. Pathol. 2015; 34; 47–56. [DOI] [PubMed] [Google Scholar]
- 43. Peters EEM, Bartosch C, McCluggage WG et al Reproducibility of lymphovascular space invasion (lvsi) assessment in endometrial cancer. Histopathology 2019; 75; 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Britton H, Huang L, Lum A et al Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol. Oncol. 2019; 153; 487–495. [DOI] [PubMed] [Google Scholar]
- 45. Yano M, Ito K, Yabuno A et al Impact of tp53 immunohistochemistry on the histological grading system for endometrial endometrioid carcinoma. Mod. Pathol. 2019; 32; 1023–1031. [DOI] [PubMed] [Google Scholar]
- 46. Darvishian F, Hummer AJ, Thaler HT et al Serous endometrial cancers that mimic endometrioid adenocarcinomas: a clinicopathologic and immunohistochemical study of a group of problematic cases. Am. J. Surg. Pathol. 2004; 28; 1568–1578. [DOI] [PubMed] [Google Scholar]
- 47. Bartosch C, Manuel Lopes J, Oliva E. Endometrial carcinomas: a review emphasizing overlapping and distinctive morphological and immunohistochemical features. Adv. Anat. Pathol. 2011; 18; 415–437. [DOI] [PubMed] [Google Scholar]
- 48. Kobel M, Piskorz AM, Lee S et al Optimized p53 immunohistochemistry is an accurate predictor of tp53 mutation in ovarian carcinoma. J. Pathol. Clin. Res. 2016; 2; 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kobel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of p53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int. J. Gynecol. Pathol. 2019; 38(Suppl 1); S123–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singh N, Piskorz A, Bosse T et al P53 immunohistochemistry is a an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J Pathol 2019; In press. [DOI] [PubMed]
- 51. Sloan EA, Ring KL, Willis BC, Modesitt SC, Mills AM. Pd‐l1 expression in mismatch repair‐deficient endometrial carcinomas, including lynch syndrome‐associated and mlh1 promoter hypermethylated tumors. Am. J. Surg. Pathol. 2017; 41; 326–333. [DOI] [PubMed] [Google Scholar]
- 52. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27; 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Le DT, Durham JN, Smith KN et al Mismatch repair deficiency predicts response of solid tumors to pd‐1 blockade. Science 2017; 357; 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mehnert JM, Panda A, Zhong H et al Immune activation and response to pembrolizumab in pole‐mutant endometrial cancer. J. Clin. Invest. 2016; 126; 2334–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ott PA, Bang YJ, Piha‐Paul SA et al T‐cell‐inflamed gene‐expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: Keynote‐028. J. Clin. Oncol. 2019; 37; 318–327. [DOI] [PubMed] [Google Scholar]
- 56. Santin AD, Bellone S, Buza N et al Regression of chemotherapy‐resistant polymerase epsilon (pole) ultra‐mutated and msh6 hyper‐mutated endometrial tumors with nivolumab. Clin. Cancer Res. 2016; 22; 5682–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Makker V, Rasco D, Vogelzang NJ et al Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open‐label, single‐arm, phase 2 trial. Lancet Oncol. 2019; 20; 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fader AN, Roque DM, Siegel E et al Randomized phase II trial of carboplatin‐paclitaxel versus carboplatin‐paclitaxel‐trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J. Clin. Oncol. 2018; 36; 2044–2051. [DOI] [PubMed] [Google Scholar]
- 59. Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4‐year experience at a large academic center and recommendations for clinical practice. Mod. Pathol. 2013; 26; 1605–1612. [DOI] [PubMed] [Google Scholar]
- 60. Rottmann D, Snir OL, Wu X et al HER2 testing of gynecologic carcinosarcomas: tumor stratification for potential targeted therapy. Mod. Pathol. 2019. 10.1038/s41379-019-0358-x [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 61. Buza N, Hui P. Marked heterogeneity of HER2/NEU gene amplification in endometrial serous carcinoma. Genes Chromosomes Cancer 2013; 52; 1178–1186. [DOI] [PubMed] [Google Scholar]
- 62. Ahmed S, Sami A, Xiang J. HER2‐directed therapy: current treatment options for HER2‐positive breast cancer. Breast Cancer 2015; 22; 101–116. [DOI] [PubMed] [Google Scholar]
- 63. Bang YJ, Van Cutsem E, Feyereislova A et al Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (toga): a phase 3, open‐label, randomised controlled trial. Lancet 2010; 376; 687–697. [DOI] [PubMed] [Google Scholar]
- 64. Buza N, Roque DM, Santin AD. HER2/NEU in endometrial cancer: a promising therapeutic target with diagnostic challenges. Arch. Pathol. Lab. Med. 2014; 138; 343–350. [DOI] [PubMed] [Google Scholar]
- 65. Fleming GF, Sill MW, Darcy KM et al Phase II trial of trastuzumab in women with advanced or recurrent, HER2‐positive endometrial carcinoma: a gynecologic oncology group study. Gynecol. Oncol. 2010; 116; 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moore K, Colombo N, Scambia G et al Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018; 379; 2495–2505. [DOI] [PubMed] [Google Scholar]
- 67. Pujade‐Lauraine E, Ledermann JA, Selle F et al Olaparib tablets as maintenance therapy in patients with platinum‐sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT‐Ov21): a double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet Oncol. 2017; 18; 1274–1284. [DOI] [PubMed] [Google Scholar]
- 68. de Jonge MM, Auguste A, van Wijk LM et al Frequent homologous recombination deficiency in high‐grade endometrial carcinomas. Clin. Cancer Res. 2019; 25; 1087–1097. [DOI] [PubMed] [Google Scholar]
- 69. de Jonge MM, Mooyaart AL, Vreeswijk MP et al Linking uterine serous carcinoma to BRCA1/2‐associated cancer syndrome: a meta‐analysis and case report. Eur. J. Cancer 2017; 72; 215–225. [DOI] [PubMed] [Google Scholar]
- 70. Shu CA, Pike MC, Jotwani AR et al Uterine cancer after risk‐reducing salpingo‐oophorectomy without hysterectomy in women with brca mutations. JAMA Oncol. 2016; 2; 1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Jonge MM, Ritterhouse LL, de Kroon CD et al Germline brca‐associated endometrial carcinoma is a distinct clinicopathologic entity. Clin. Cancer Res. 2019. 10.1158/1078-0432.CCR-19-0848 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 72. Kurnit KC, Kim GN, Fellman BM et al CTNNB1 (beta‐catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017; 30; 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu Y, Patel L, Mills GB et al Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J. Natl Cancer Inst. 2014; 106; dju245. 10.1093/jnci/dju245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Costigan DC, Dong F, Nucci MR, Howitt BE. Clinicopathologic and immunohistochemical correlates of CTNNB1 mutated endometrial endometrioid carcinoma. Int. J. Gynecol. Pathol. 2019. 10.1097/PGP.0000000000000583 [DOI] [PubMed] [Google Scholar]
- 75. Travaglino A, Raffone A, Saccone G et al Immunohistochemical nuclear expression of beta‐catenin as a surrogate of CTNNB1 exon 3 mutation in endometrial cancer. Am. J. Clin. Pathol. 2019; 151; 529–538. [DOI] [PubMed] [Google Scholar]


