Abstract
Objective
Fine needle cytology (FNC) is the first‐line diagnostic method to determine the benign or malignant nature of thyroid nodules. The gray zone of cytological classifications remains, however, a crucial and challenging area for cytopathologists.
Design, patients and measurements
In the present study, 141 thyroid cytological samples, with ultrasonographic suspicious features, have been prospectively analysed. Molecular analyses were performed by an innovative technology using two multiplex PCRs for the amplification of BRAF, N‐H‐K‐RAS and RET exon genes. RNA samples were studied for RET/PTC1 and RET/PTC3 rearrangements by PCR amplification, and the conditions were set‐up to study, with a single experiment, both wild‐type PAX8 and PAX8/PPARɣ rearrangements. In total, 111 samples were examined for BRAF, N‐H‐KRAS and RET genes. An ultrasonographic, cytological and molecular correlation was also carried out in an attempt to suggest a possible way to manage the patients with thyroid nodules. Cyto‐histological correlation was available in 115 cases, and it was used to verify the global diagnostic accuracy of this combined approach.
Results
According to the histopathological diagnosis, FNC accuracy was 100% for TIR5 and metastases; 89% for TIR4; 84% for TIR3A and 58% for TIR3B. About 11% of the studied samples showed either RET‐PTC1 or RET/PTC3 chromosomal rearrangements, and only one sample simultaneously presented RET/PTC1 and RET/PTC3 rearrangements. PAX8/PPARɣ rearrangement was found in 6% of the samples.
Conclusions
A multidisciplinary approach to the thyroid is therefore necessary to develop innovative methods suitable for an improved diagnostic and prognostic definition of thyroid cancer.
Keywords: cyto‐histological correlation, fine needle cytology, indeterminate nodules, mutational analysis, papillary thyroid carcinoma, thyroid cancer treatment, ultrasound features
1. INTRODUCTION
Thyroid nodules are a common clinical problem. The estimated prevalence of palpable thyroid nodules is approximately 5% in women and 1% in men, while the prevalence of nonpalpable, ultrasonically detectable nodules is much higher.1, 2 Several classification systems of thyroid nodules have been published based on ultrasound characteristics1, 3, 4 but none of these recommendations have been validated with extensive prospective studies.
The international scoring systems have been evaluated by a meta‐analysis of 10 437 nodules,5 in which it was found that the classification proposed by Horvath and collaborators in 2009,6 known as Thyroid Imaging Reporting and Data System (TIRADS) and subsequently modified by the French Society of Endocrinology in 2011 and renamed as the European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules (EU‐TIRADS), validated by a large prospective study,7 showed a good sensitivity and specificity (79% and 71%, respectively). Based on these considerations, EU‐TIRADS has been adopted in the present case study as the ultrasound classification system.
Thyroid fine needle cytology (FNC) has proven to be an accurate, safe, efficient and cost‐effective diagnostic tool in patients with thyroid nodules.8 Published data on FNC of thyroid nodules reported a sensitivity ranging from 65% to 98%, a specificity of 72%‐100%, a positive predictive value of 34%‐100% and a negative predictive value of 83%‐100%.9
Although most thyroid lesions are classified as TIR2 (benign) according to the new Italian consensus SIAPEC‐AIT 2014 classification,10 along with the main International cytological classifications (Bethesda System and The British Thyroid Association), some cases are more challenging than others and cannot be classified as benign or malignant on cytomorphological features only, leading to an indeterminate diagnosis.
In an attempt to improve accuracy in the indeterminate FNC category, several studies have evaluated different biological markers in thyroid FNC. Early immunohistochemical markers such as galectin‐3,11 HBME‐112 and cytokeratin 1913 have yielded less than optimal results. The relative low specificity and lack of reproducibility among different laboratories make immunohistochemical studies difficult to use as a daily diagnostic tool to differentiate benign from malignant thyroid lesions.
Advancements in molecular studies have allowed the identification of genetic alterations associated with different follicular cell‐derived neoplasms in thyroid.14, 15, 16 Molecular tests on FNC samples significantly improve the accuracy of the cytological diagnosis of thyroid nodules, which could have a significant impact on clinical care.9, 10, 17
A number of genetic mutations are associated with thyroid cancer. The most common genetic mutations in papillary thyroid carcinoma (PTC) are point mutations involving BRAF and RAS genes, while the most common chromosomal rearrangement is RET/PTC, which is involved in the mitogen‐activated protein kinase (MAPK) pathway.18, 19, 20 These mutations are found in more than 70% of PTCs, and most of the times, are mutually exclusive.11, 13 RAS mutations or PAX8/PPARɣ rearrangement, which are mutually exclusive, occur in approximately 80% of follicular thyroid carcinomas (FTCs)14; 60% of poorly differentiated and anaplastic tumours are due to mutations of BRAF and RAS genes.21, 22 Medullary thyroid carcinomas (MTC) (both hereditary and sporadic types) frequently present point mutations in RET gene. In fact, MTC is transmitted in an autosomal‐dominant pattern in multiple endocrine neoplasia (MEN) syndrome (MEN 2A and MEN 2B) and familial medullary thyroid carcinoma (FMTC).15 In cases where cytological analysis of a given thyroid neoplasm is unable to formulate a definitive diagnosis, information on the presence of specific mutations related to thyroid carcinogenesis may be useful and may provide elements for a correct histological diagnosis.22, 23, 24
The American Thyroid Association Guidelines (ATA) recognize the benefits of molecular markers in the management of thyroid cancer, also by the demonstration of new molecules that have helped to highlight the pathogenetic basis of thyroid cancer, although most of them are infrequently found.1, 25 ATA guidelines recommend that molecular analyses should investigate seven genes, those mostly involved in thyroid carcinogenesis.
In the present study, by using pre‐operative cytological samples, we looked for the most frequent mutations and/or rearrangements found in thyroid lesions (BRAF, RET/PTC1‐3, RAS and PAX8/PPARɣ) in order to refine the cytopathologic diagnosis and to evaluate new possible therapeutic and prognostic targets for indeterminate, suspicious and frankly malignant thyroid nodules. DNA and/or RNA was extracted from FNC samples to identify a broader panel of genes whose mutations were related to approximately 70% of thyroid carcinomas to be studied by amplification and sequencing techniques.
2. MATERIALS AND METHODS
A prospective study on 141 patients (104 women and 37 men) with ultrasonographically and clinically suspicious thyroid nodules was carried out. Each nodule was assigned a score from 2 to 5 according to EU‐TIRADS system,6 to stratify patients into different classes of risk of malignancy.
One hundred forty‐one FNCs from thyroid nodules or secondary lesions of soft tissue and metastases of thyroid carcinoma were collected, among 2304 samples, at Istituto Nazionale dei Tumori – IRCCS – Fondazione ‘G. Pascale’ of Naples, Italy, from January 2013 to April 2018. The median patients age was 47 (15‐85 years old), with a 1:2.8 male to female ratio. Thyroid FNCs were obtained under ultrasound guidance by using 23‐25G needles, without suction, and diagnosed following the SIAPEC‐AIT 2014 classification. Direct smears were obtained which were either air‐dried and stained with Diff Quik™ for ROSE (rapid on‐site evaluation) or wet‐fixed in 95% ethanol and stained with Papanicolaou (Pap). The SIAPEC‐AIT 2014 guidelines10 consider the following categories: TIR2, which includes colloid goitre, hyperplastic nodules, autoimmune (Hashimoto's) and granulomatous (de Quervain's) thyroiditis. There are no nuclear atypias, often abundant colloid can be seen in the background. TIR3A is characterized by an increased cellularity with numerous microfollicular structures in a background containing scarce colloid; slight phenomena of anisocytosis and anisonucleosis can be observed (Figure S1A‐C).
TIR3B category is characterized by a higher cellularity, showing a monotonous and repetitive microfollicular/trabecular arrangement, with scant or absent colloid. Mild‐to‐moderate cellular atypias, transgressing capillary vessels, nuclear grooves and infrequent intranuclear inclusions may also be occasionally found in this category (Figure S1D‐F).
TIR4 category includes samples in which a definite cytological diagnosis of malignancy is strongly suspected, but cannot be established with full confidence. Cytological findings consist in a high cellularity with evident phenomena of anisocytosis and anisonucleosis, the presence of nuclear ‘grooves’ or intranuclear inclusions in only few cells, scant or absent colloid (Figure S2A‐C).
TIR5 category includes cases with a definitive cytological diagnosis of malignant neoplasm (papillary, medullary, poorly differentiated and anaplastic carcinoma, lymphoma, other nonepithelial malignancies and metastases). The cytological report should contain a cytological description and, when possible, the diagnosis of specific tumour type (Figure S2D‐F).
An aliquot of the FNC samples was directly stored into a tube containing an RLT buffer solution (QIAGEN Germantown) and beta‐mercaptoethanol and sent to Functional Genomics Unit of the Institute for DNA and/or RNA extraction for molecular biology studies.
These studies have been designed to standardize the PCR method, in order to carry out as many analyses as possible with the smallest amount of material possible. The tube was stored frozen at −80°C until DNA and RNA extraction was required. Samples were then processed for both DNA and RNA extraction using a Qiagen kit.
The DNA study was carried out by an innovative methodology that used two different multiplexes on DNA26: the first one for the simultaneous identification of seven different exons representing the most frequent point mutations of BRAF, H‐N and K‐RAS genes and the other one for the study of eight exons of RET gene, whose point mutations are associated with thyroid diseases.
RNA was reverse transcribed into cDNA as indicated in the Qiagen ‘QuantiTect reverse transcription kit’. The housekeeping gene GAPDH, uniformly expressed in all cells, was amplified by PCR to verify that the quality of the RNA samples was suitable for molecular analyses. RET‐PTC rearrangements 1 and 3 were detected by PCR amplification. A multiplex amplification of wild‐type PAX8 and PAX8/PPARɣ rearrangement was also performed.27 All DNA amplifications and only the cDNA that resulted positive for chromosomal rearrangements were purified and subjected to automatic sequencing with Sanger technique on both strands by capillary electrophoresis. This technique was carried out with a Life Technologies 3730 DNA Analyzer and, subsequently, the comparative analysis of the sequences was performed through a dedicated software, using a reference sequence without mutations (wild‐type sequence). The analyses were then repeated on a new aliquot of material for the samples carrying mutations.
3. RESULTS
3.1. Ultrasonographic features and cytological criteria of thyroid nodules
In the present case series, only mostly solitary nodules with indication for FNC according to EU‐TIRADS were included. Of the 141 cases enrolled in this study, 13 cases were lymph node metastases to which no EU‐TIRADS score was assigned. The remaining 128 cases were scored as follows: 58 as EU‐TIRADS 3, of these, 20 were TIR2 and used as control cases for subsequent molecular investigations; 56 as EU‐TIRADS 4; and 14 as EU‐TIRADS 5.
The respective PPV and NPV relative to this ultrasound classification were 80% and 43%.
The combined US and cytomorphologic evaluation predictive values were, respectively, as follows: PPV 87% and NPV 88%.
3.2. Cyto‐histological correlation
The pre‐operative cytological diagnoses of the 141 patients were as follows: 20 TIR2; 22 TIR3A; 24 TIR3B; 18 TIR4, 44 TIR5 and 13 cases were represented by infiltration/metastasis of thyroid carcinoma to soft tissues and/or lymph nodes.
Histopathological examination showed 70 primary thyroid carcinomas, 13 metastases, 26 follicular adenomas, four NIFTPs and two nodular hyperplasias or colloid goitres. A cyto‐histological correlation was available in 115 cases (Table 1). Accuracy with respect to the subsequent histological diagnosis was 100% for TIR5 and metastasis, 89% for TIR4, 84% for TIR3A and 58% for TIR3B.
Table 1.
Cyto‐histological correlation of 115 FNC samples: comparison between pre‐operative cytological diagnosis according to SIAPEC‐AIT 2014 and the corresponding subsequent histological examination
| Cytological diagnosis | Histological diagnosis | ||||
|---|---|---|---|---|---|
| Positive | Adenoma | NIFTP | Negative | TOT | |
| TIR3A | 2 | 16 | 0 | 1 | 19 |
| TIR3B | 10 | 8 | 4 | 1 | 23 |
| TIR4 | 16 | 2 | 0 | 0 | 18 |
| TIR5 | 42 | 0 | 0 | 0 | 42 |
| METASTASIS | 13 | 0 | 0 | 13 | |
| TOT | 83 | 26 | 4 | 2 | 115 |
Abbreviation: TOT, total number of tested samples.
3.3. Mutational analysis
Conditions for BRAF, N‐H‐KRAS and RET exon genes amplifications were selected in order to set‐up two multiplex PCRs: one PCR to amplify all the exons of BRAF, N‐H‐KRAS genes at the same time and another PCR to amplify eight exons of RET gene at an annealing temperature of 60°C. To set‐up multiplex method, a DNA sample from peripheral blood was used and initially amplified individual gene exons whose mutations were correlated with the disease. Primers that could work all at the same annealing temperature were selected and multiplex amplifications were set‐up using the smallest amount of DNA possible. Figure 1 shows a multiplex BRAF, N‐H‐KRAS amplification (A) and multiplex RET exons genes amplification (B) results.
Figure 1.
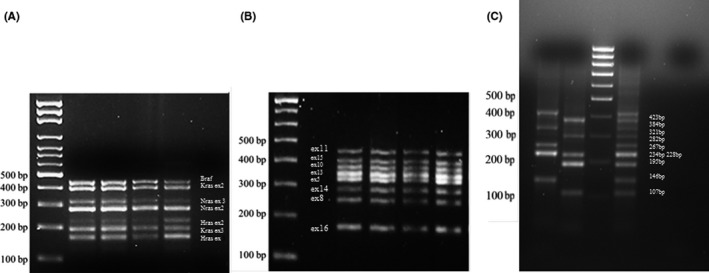
A, BRAF‐RAS multiplex amplification gel, genes are indicated. B, RET multiplex amplification gel, RET exons are reported. C, Multiplex PAX8 wild‐type and PAX8/PPARɣ rearrangement samples amplification, PAX8 wild‐type and PAX8/PPARɣ rearrangements size are reported. Amplifications of two samples (121 and 99), a positive control (Cwt + Cr) and a negative control (C−). Samples 99 was negative for PAX8 wild‐type amplification and positive for PAX8/PPARɣ rearrangement
A rapid method was developed in our laboratories, to create a positive control for the identification of PAX8/PPARɣ rearrangements, in FNC thyroid samples, by overlapping PCRs. Figure 1(C) shows the results of the procedure to study, with a single experiment, both wild‐type PAX8 and PAX8/PPARɣ rearrangement.
Sample #121 shows wild‐type PAX8 gene, and sample #99 shows PAX8/PPARɣ rearrangement. In total, 141 cases were examined and 111 DNA and 95 RNA samples were obtained; the total mutation rate was 28% (exclusive of the RET gene polymorphisms, whose presence in thyroid pathology is still studied). It is important to point out that all identified mutations concerned only BRAF exon 15 and N‐RAS exon 3; only one mutation involved K‐RAS exon 3. RET polymorphisms concerned only exons 11, 13, 14 and 15. In addition, six RET/PTC1 rearrangements (5%) and seven RET/PTC3 rearrangements (6%) were found, for a total of 11% rearrangement frequency. Besides, a sample that simultaneously presented RET/PTC1 and RET/PTC3 rearrangements was found. The median age of patients who presented these mutations was of 45 years old (ranging from 15‐ to 85‐year‐old), with a female/male ratio of 5:1. All nodules were ≥10 mm in diameter, and only two cases presented a multifocality of 35 × 25 mm and 26 × 19 mm in diameter, respectively. In these latter cases, the targeted lesion was correlated in the surgical specimen by the size of the nodule, its exact position compared to the surrounding nodules and the ultrasonographic features. Both multifocal lesions were fully sampled and, therefore, an adequate cyto‐histological correlation was possible to performed. About 80% of the patients with RET/PTC1 and/or RET/PTC3 underwent total thyroidectomy, and the remaining 20% underwent lymphadenectomy due to metastases to lateral cervical lymph nodes. Concerning the rate of RET/PTC1 and or RET/PTC 3 rearrangement in our series, it should be noticed that most of the patients in this study came from the area around Naples, notably an area with high background level of natural radiation due to volcanic activity.
PAX8/PPARɣ rearrangement was investigated by an innovative methodology, and only 6/96 rearrangements (6%) were found. The results are reported in Tables 2 and 3. The diagnostic accuracy values relative to the combination of US assessment, cytomorphological evaluation and molecular pathological analysis were, respectively, as follows: PPV 97% (if only the mutated cases that were negative on histological examination, that is nodular hyperplasia or thyroiditis, were considered as false positives) and 79% (if also the mutated cases that resulted adenomas on histology were considered as false positives); the NPV was 60%.
Table 2.
Gene alterations (BRAF, K‐RAS, N‐RAS, RET) obtained by two multiplex amplification on thyroid DNA samples
|
Analysed DNA 111 |
BRAF | K‐RAS exon3 | N‐RAS exon3 | RET | Alterations/Group | ||||
|---|---|---|---|---|---|---|---|---|---|
|
ex 11 mut691 |
ex 13 mut769 |
ex 14 mut836 |
ex 15 mut904 |
||||||
| TIR2 | 15 (20) | 2 | 11 | 1 | 14/15 | ||||
| TIR3A | 19 (22) | 4 | 5 | 12 | 2 | 7 | 31/19 | ||
| TIR3B | 21 (24) | 1 | 4 | 10 | 17 | 8 | 9 | 49/21 | |
| TIR4 | 13 (18) | 4 | 2 | 3 | 8 | 3 | 3 | 18/13 | |
| TIR5 | 33 (44) | 11 | 2 | 13 | 24 | 6 | 11 | 71/33 | |
| Others | 10 (13) | 3 | 1 | 3 | 6 | 4 | 4 | 21/10 | |
| GENE alterations | 18 | 1 | 13 | 36 | 78 | 24 | 34 | ||
| 32 | 172 | ||||||||
| Total alterations/samples | 204/111 | ||||||||
The alterations are related to the total number of tested samples for each cyto‐histological group.
Table 3.
Gene rearrangements (RET/PTC1, RET/PTC3, PAX8/PPARɣ) results by multiplex amplifications on thyroid RNA samples
|
Analysed RNA 95 |
RET/PTC1 | RET/PTC3 | ||
|---|---|---|---|---|
| TIR2 | 15 | |||
| TIR3A | 14 | |||
| TIR3B | 16 | 1 | ||
| TIR4 | 12 | 1 | ||
| TIR5 | 28 | 4 | 5 | |
| Others | 10 | 1 | 1 | |
| Gene rearrangement | 6/95 | 7/95 | ||
|
Analysed RNA 70 |
PAX8/PPARɣ | |
|---|---|---|
| TIR2 | 7 | |
| TIR3A | 12 | 3 |
| TIR3B | 13 | 2 |
| TIR4 | 11 | |
| TIR5 | 20 | |
| Others | 7 | 1 |
| Gene rearrangement | 6/70 | |
3.4. Ultrasonographic, cytological, molecular and histological correlations
Of the 141 cases in our series, it was possible to cross‐check the ultrasonographic, cytological, mutational and histological data in 103 cases. In this correlation, the cases lacking histopathological examination, the cases not suitable for molecular analyses and metastases (for which an EU‐TIRADS value is generally not assigned) were not considered. The results are shown in Figure 2. As far as the ultrasonographic data are concerned, the cases were divided into three large groups according to the assigned EU‐TIRADS.
Figure 2.
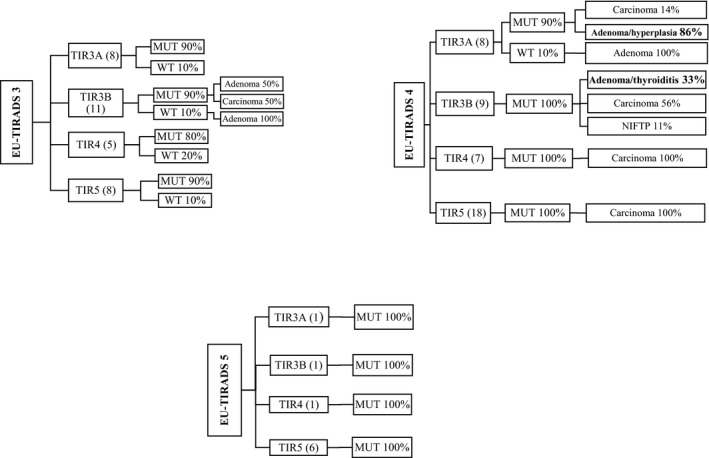
Flow chart showing the molecular status and histopathological diagnoses of cases cytologically defined as TIR3A/B, TIR4 and TIR5 on EU‐TIRADS ultrasound classification
A total of 32 cases were EU‐TIRADS 3 of which eight were TIR3A cytologically, 90% were mutated and 10% wild‐type: all resulted adenomas at the subsequent histological examination; 11 were TIR3B cytologically, 90% were mutated, which turned out to be 50% adenomas and 50% carcinomas on histological examination, and the remaining 10% were wild‐type, all resulted to be adenomas; five were TIR4 cytologically, 80% were mutated and 20% wild‐type, all resulted to be carcinomas; eight were TIR5 cytologically, 90% were mutated and 10% wild‐type, all of which turned out to be carcinomas.
A total of 42 cases were EU‐TIRADS 4:8 were TIR3A cytologically, 90% were mutated, which resulted to be adenomas/hyperplasia in 86% of the cases and carcinomas in the other 14% on subsequent histological examination – 10% were wild‐type, all resulted to be adenomas; nine were TIR3B, 100% mutated, which were found to be carcinomas in 56% of the cases, adenomas/thyroiditis in 33% of the cases and NIFTP in 11% of the cases on subsequent histological examination; seven were TIR4 cytologically, 100% mutated, all of which turned to be carcinomas; 18 were TIR5 cytologically, 100% mutated, all resulted to be carcinomas.
Nine cases were EU‐TIRADS 5:1 was TIR3A, mutated; one was TIR3B, mutated; one was TIR4 mutated and six were TIR5, mutated; all these latter cases were found to be carcinomas on subsequent histological examination.
The positive and negative predictive values increased by considering the contribution of further diagnostic methodologies as follows: PPVultrasound 80% < PPVultrasound+cytology 87% < PPVultrasound+cytology+molecular biology 97%.
Similarly, NPVultrasound 43% < NPVultrasound+cytology+molecular biology 60% < NPVultrasound+cytology 88%.
4. DISCUSSION
The first diagnostic approach of thyroid nodules is represented by ultrasound examination. The EU‐TIRADS classification seems to be a valid tool in the radiologists' hands in order to identify those nodules with low, intermediate and high‐risk ultrasound features for which FNC cytology is recommended.
Fine needle cytology is the most reliable diagnostic test for thyroid nodules, with a good accuracy and patients' compliance. Although >90% of thyroid nodules are non‐neoplastic,10 some cases are very challenging and cannot be classified as benign or malignant by the morphological features only, leading to an indeterminate diagnosis. In such cases, FNC sampling is usually repeated or sometimes surgery is performed in the attempt to establish a definitive diagnosis.
These procedures result in additional morbidity and higher health care costs. Moreover, patients with malignant tumours and indeterminate FNC cytology typically undergo limited surgery, that is lobectomy, and, once malignancy is established by pathological examination of the excised nodule, these patients undergo completion thyroidectomy, which also results in additional morbidity and costs. Moreover, 1‐3% of nodules diagnosed as benign on FNC are later found to be malignant on follow‐up8; the consequent delay in treatment places patients at risk of disease progression.
In this study, we reported the results of a prospective molecular analysis on 141 cytological samples of thyroid nodules with an indeterminate or frankly malignant cytological diagnosis to attempt a better management of the patients. Although this study concerns a small case series compared to more extensive studies,28, 29, 30 it underlines the possibility to perform molecular tests with an innovative, easy and low‐cost method.
Nikiforov and collaborators carried out a large prospective study on 967 FNC with indeterminate cytology.28 According to these authors, the mutational testing using residual material obtained during routine FNCs allows more accurate cancer risk stratification of thyroid nodules with indeterminate cytology.
Several studies31, 32, 33 show that positive testing for BRAF, RET/PTC or PAX8/PPARγ are specific for a malignant outcome in 100% of cases, whereas RAS mutations have an 84% risk of cancer and a 16% chance of benign follicular adenoma.1
The positive genetic test results for BRAF, RAS, RET/PTC, PAX8/PPARɣ could be considered as an indication for limited or total thyroidectomy in all categories of indeterminate cytology, also depending on other clinical risk factors, such as presence of bilateral suspicious areas on ultrasonography, high levels of thyroglobulin or calcitonin in conjunction with indeterminate or suggestive cytology.
In our series, three cases with indeterminate cytology presented PAX8/PPARɣ rearrangements, whose subsequent histological examination revealed two adenomas and one NIFTP.
From the 141 cases examined, 111 DNA and 95 RNA samples were obtained. The latter was mostly used for RET/PTC1‐3 amplifications, and there was no material left to analyse PAX8/PPARɣ rearrangements.
The samples were analysed for the identification of a relatively large gene panel, whose mutations were related to approximately 70% of thyroid carcinomas and were studied by amplification and sequencing techniques. Mutations are usually detected through individual amplifications of each exon with a different annealing temperature, which are costly and time‐consuming. Multiplex amplifications allow the use of small amounts of DNA and a simultaneous study of 15 exons of 5 different genes. Moreover, wild‐type PAX8 and PAX8/PPARɣ rearrangement were investigated with a single experiment. This methodology allows rapid typing and favours the usage of new therapeutic agents that have changed the treatment of thyroid cancer.
Particularly, interesting is the data related to the study of RET gene polymorphisms that present a high‐frequency value in the most aggressive forms according to Santos and collaborators.34
It is generally possible to observe a much higher frequency of RET polymorphisms in TIR3B (all samples showed RET polymorphisms) compared to TIR3A (8/22 did not show RET polymorphisms). This could be related to a different prognostic potential in these groups of lesions, but the small number of examined patients did not allow statistical evaluations. Hence, further studies could help in the discrimination of the two forms that currently represent the greatest criticality in the management of thyroid diseases. These studies could also clarify the possible correlation between the found polymorphisms and the patients' age and/or size of the neoplasm. Moreover, they could shed further light on the sequence adenoma‐carcinoma and/or the relative risk of metastasis, as already reported in the literature or in studies relatively small or not associated with all possible thyroid diseases.35 In our series, only one case showed the simultaneous presence of RET/PTC1 and RET/PTC3 rearrangements, which most likely caused the onset of a very peculiar composite malignancy.36
At present, two new generation molecular tests are used: Afirma for gene expression study and Thyroseq system that can be used solely in ‘service’ modality, which are both extremely costly solutions. Thanks to the proposed methodology many problems could be avoided, such as repeated sampling operations for diagnostic and/or nontherapeutic purposes and possible completion thyroidectomies. The proposed methodology has a low cost (about 250 euros per patient) and therefore it can be easily implemented by the National Healthcare System as well as in small laboratories, that do not have very sophisticated and expensive equipment and above all highly specialized personnel trained in complex bioinformatics systems, such as those employed in NGS technologies.
From the comparison of ultrasonographic, cytological, molecular and histological data a possible management of patients with thyroid nodules has emerged, distinguishing between candidates to surgery and patients who can simply be clinically followed.
Based on histological results, here we propose the following patient management (Figure 3): for those who are EU‐TIRADS 3 on ultrasound examination and TIR3A cytologically, with or without mutation (mutated/wild type), a clinical and instrumental follow‐up is suggested; the same applies to not mutated (wild type) TIR3B. Histologically, these cases were benign lesions. For patients who are diagnosed as TIR3B, TIR4 and TIR5 cytologically, in which a mutation is identified, surgery is suggested: they all showed a thyroid carcinoma at the subsequent histology.
Figure 3.
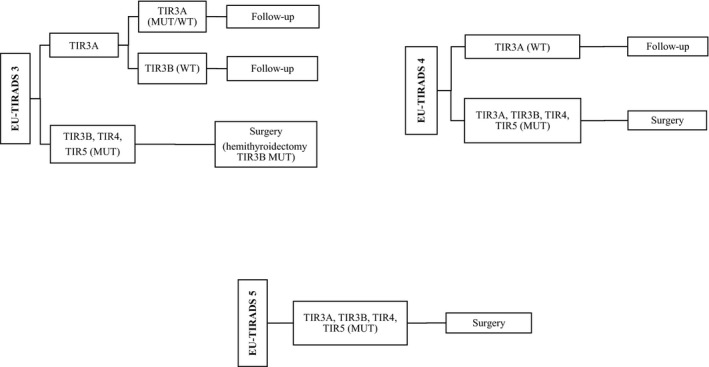
Flow chart showing the proposed treatment of thyroid nodules according to EU‐TIRADS ultrasound classification, cytological diagnosis and mutational status
For EU‐TIRADS 4 patients, cytologically TIR3A, TIR3B, TIR4, TIR5, in which a mutation is identified, surgery is suggested. They all showed a thyroid carcinoma at the subsequent histology. For patients diagnosed as TIR3A cytologically, who are wild‐type, a follow‐up is suggested because they resulted to be, in our series, benign lesions on histological examination.
For EU‐TIRADS 5 patients, who were cytologically diagnosed as TIR3A, TIR3B, TIR4, TIR5, and, in which a mutation is identified, surgery is necessary. All cases were thyroid carcinomas on histology.
Based on our results, hemithyroidectomy could be suggested only in EU‐TIRADS 3/TIR3B/mutated cases, as in 50% of cases they resulted to be adenomatous lesions, with a possible completion thyroidectomy in cases of carcinoma. For all other cases, total thyroidectomy should be recommended.
In thyroid nodules with indeterminate cytology, the refinement of the pre‐operative diagnosis is also a necessary step to optimize and economize the diagnostic and therapeutic pathway, avoiding unnecessary surgery.
A multidisciplinary approach to the thyroid nodules which comprises the surgeon, the radiologist, the pathologist and the molecular biologist is therefore necessary to develop innovative methods suitable for an improved diagnostic and prognostic definition of thyroid cancer.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Franco Fulciniti (FF), Anna Cipolletta Campanile (ACC) and Maria Gabriella Malzone (MGM) collected the data and drafted the manuscript; ACC and MGM drafted tables, flow charts and images; FF and Maria Grazia Chiofalo (MGF) collected fine needle Cytology samples; Emilia Vuttariello (EV) and Anna Capiluongo (AC) designed and carried out molecular analyses; EV, AC, Nunzia Di Maio (NDM) and Gennaro Chiappetta (GC) drafted and revised the molecular aspects of the manuscript; Fabio Sandomenico (FS) carried out the ultrasonographic scan; Luciano Pezzullo (LP) carried out the thyroid surgeries; FF and Gerardo Botti (GB) carried out the histological diagnoses; FF, GB, LP and Mario Monaco (MM) made the final revision of the manuscript. All authors agree with the contents of the manuscript.
Supporting information
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Dr Antonella Gioioso. This work was partially sponsored by an Institutional Research Project (M 34, 2016) of the Istituto Nazionale dei Tumori – IRCCS – Fondazione G. Pascale, Naples, Italy and was supported by MoH – Ministry of Health.
Fulciniti F, Cipolletta Campanile A, Malzone MG, et al. Impact of ultrasonographic features, cytomorphology and mutational testing on malignant and indeterminate thyroid nodules on diagnostic accuracy of fine needle cytology samples: A prospective analysis of 141 patients. Clin Endocrinol (Oxf). 2019;91:851–859. 10.1111/cen.14089
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, EV, upon reasonable request.
REFERENCES
- 1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roman SA. Endocrine tumors. Evaluation of thyroid nodule. Curr Opin Oncol. 2003;15(1):66‐70. [DOI] [PubMed] [Google Scholar]
- 3. Na DG, Baek JH, Sung JY, et al. Thyroid imaging reporting and data system risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid. 2016;26:562‐572. [DOI] [PubMed] [Google Scholar]
- 4. Gharib H, Papini E, Garber JR, et al. AACE/ACE/AME Task Force on Thyroid Nodules: American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocr Pract. 2016;22:622‐639. [DOI] [PubMed] [Google Scholar]
- 5. Wei X, Li Y, Zhang S, Gao M. Meta‐analysis of thyroid imaging reporting and data system in the ultrasonographic diagnosis of 10,437 thyroid nodules. Head Neck. 2016;38:309‐315. [DOI] [PubMed] [Google Scholar]
- 6. Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;90(5):1748‐1751. [DOI] [PubMed] [Google Scholar]
- 7. Russ G, Royer B, Bigorgne C, Rouxel A, Bienvenu‐Perrard M, Leenhardt L. Prospective evaluation of thyroid imaging reporting and data system on 4,550 nodules with and without elastography. Eur J Endocrinol. 2013;168:649‐655. [DOI] [PubMed] [Google Scholar]
- 8. Bukhari MH, Niazi S, Hanif G, et al. An updated audit of fine needle aspiration cytology procedure of solitary thyroid nodule. Diagn Cytopathol. 2008;36:104‐112. [DOI] [PubMed] [Google Scholar]
- 9. Cap J, Ryska A, Rehorkova P, Hovorkova E. Sensitivity and specificity of the fine needle aspiration biopsy of the thyroid: clinical point of view. Clin Endocrinol. 1999;51(4):509‐515. [DOI] [PubMed] [Google Scholar]
- 10. Nardi F, Basolo F, Crescenzi A, et al. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest. 2014;37(6):593‐599. [DOI] [PubMed] [Google Scholar]
- 11. Bartolazzi A, Orlandi F, Saggiorato E, et al. Galectin‐3‐expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine needle aspiration cytology: a prospective multicentre study. Lancet Oncol. 2008;9(6):543‐549. [DOI] [PubMed] [Google Scholar]
- 12. De Matos PS, Ferreira AP, de Oliveira FF, Assumpcao LV, Metze K, Ward LS. Usefulness of HBME‐1, cytokeratin 19 and galectin‐3 immunostaining in the diagnosis of thyroid malignancy. Histopathology. 2005;47(4):391‐401. [DOI] [PubMed] [Google Scholar]
- 13. Saggiorato E, De Pompa R, Volante M, et al. Characterization of thyroid ‘follicular neoplasms’ in fine‐needle aspiration cytological specimens using a panel of immunohistochemical markers: a proposal for clinical application. Endocr Relat Cancer. 2005;12(2):305‐317. [DOI] [PubMed] [Google Scholar]
- 14. Nikiforov YE. Molecular diagnostics of thyroid tumors. Arch Pathol Lab Med. 2011;135(5):569‐577. [DOI] [PubMed] [Google Scholar]
- 15. Ferraz C, Eszlinger M, Paschke R. Current state and future perspective of molecular diagnosis of fine‐needle aspiration biopsy of thyroid nodules. J Clin Endocrinol Metab. 2011;96(7):2016‐2026. [DOI] [PubMed] [Google Scholar]
- 16. Hysek M, Paulsson JO, Wang N, et al. TERT promoter mutational screening as a tool to predict malignant behaviour in follicular thyroid tumours—three examples from the clinical routine. Virchows Arch. 2018;473(5):639‐643. [DOI] [PubMed] [Google Scholar]
- 17. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine‐Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425‐437. [DOI] [PubMed] [Google Scholar]
- 18. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes. Cham, Switzerland: Springer International Publishing AG; 2018. [Google Scholar]
- 19. Cibas ES, Baloch ZW, Fellegara G, et al. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med. 2013;159(5):325‐332. [DOI] [PubMed] [Google Scholar]
- 20. Renshaw AA, Wang E, Haja J, et al. Fine‐needle aspiration of papillary thyroid carcinoma: distinguishing between cases that performed well and those that performed poorly in the College of American Pathologists Nongynecologic Cytology Program. Arch Pathol Lab Med. 2006;130(4):452‐455. [DOI] [PubMed] [Google Scholar]
- 21. Ciampi R, Nikiforov YE. Alterations of the BRAF gene in thyroid tumors. Endocr Pathol. 2005;16(3):163‐172. [DOI] [PubMed] [Google Scholar]
- 22. Guerra A, Di Crescenzo V, Garzi A, et al. Genetic mutations in the treatment of anaplastic thyroid cancer: a systematic review. BMC Surg. 2013;13(Suppl 2):S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nikiforov YE, Yip L, Nikiforova MN. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clin Cancer Res. 2013;19:2283‐2288. [DOI] [PubMed] [Google Scholar]
- 24. Kuo LE, Kelz RR. Management of thyroid nodular disease: current cytopathology classifications and genetic testing. Surg Oncol Clin N Am. 2016;25(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 25. Wells SA Jr, Asa SL, Dralle H, et al. American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vuttariello E, Borra M, Mauriello E, et al. Multiplex PCR approach to simultaneously identify several mutations in fine needle cytology thyroid samples. Oncotarget. 2017;8(30):49351‐49358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vuttariello E, Biffali E, Pannone R, et al. Rapid methods to create a positive control and identify the PAX8/PPARγ rearrangement in FNA thyroid samples by molecular biology. Oncotarget. 2018;9(27):19255‐19262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96(11):3390‐3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Decaussin‐Petrucci M, Descotes F, Depaepe L, et al. Molecular testing of BRAF, RAS and TERT on thyroid FNAs with indeterminate cytology improves diagnostic accuracy. Cytopathology. 2017;28(6):482‐487. [DOI] [PubMed] [Google Scholar]
- 30. Steward DL, Carty SE, Sippel RS, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol. 2019;5(2):204‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrari SM, Fallahi P, Ruffilli I, et al. Molecular testing in the diagnosis of differentiated thyroid carcinomas. Gland Surg. 2018;7(Suppl 1):S19‐S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borrelli N, Ugolini C, Giannini R, et al. Role of gene expression profiling in defining indeterminate thyroid nodules in addition to BRAF analysis. Cancer Cytopathol. 2016;124:340‐349. [DOI] [PubMed] [Google Scholar]
- 33. Ferraz C, Rehfeld C, Krogdahl A, et al. Detection of PAX8/PPARG and RET/PTC rearrangements is feasible in routine air‐dried fine needle aspiration smears. Thyroid. 2012;22:1025‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santos M, Azevedo T, Martins T, Rodrigues JF, Lemos MC. Association of RET genetic polymorphisms and haplotypes with papillary thyroid carcinoma in the Portuguese population: a case‐control study. PLoS ONE. 2014;9(10):e109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang RX, Yang F. RET polymorphisms might be the risk factors for thyroid cancer. Int J Clin Exp Pathol. 2015;8(5):5793‐5797. [PMC free article] [PubMed] [Google Scholar]
- 36. Fulciniti F, Vuttariello E, Combined CC, et al. Papillary and mucoepidermoid carcinoma of the thyroid gland: a possible collision tumor diagnosed on fine‐needle cytology. Report of a case with immunocytochemical and molecular correlations. Endocr Pathol. 2015;26:140‐144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, EV, upon reasonable request.


