Abstract
Germline mutations and copy number changes in DNA damage repair (DDR) genes such as BRCA2 are associated with aggressive forms of prostate cancer (PCa). Although the prevalence of BRCA2 variants in PCa is increasing in Japan, the genomic and biological implications in Japanese patients are unclear. An 81‐year‐old male presented with prostatic adenocarcinoma with neuroendocrine differentiation accompanied by metastatic lung nodule and brain metastases. Platinum‐based doublet chemotherapy combined with etoposide resulted in partial and complete remissions of brain and lung metastases, respectively. Next‐generation sequencing of biopsy and peripheral blood samples demonstrated a somatic BRCA2 mutation at c.7008‐2A>C and a germline mutation at p.E2877*. The patient's son had been diagnosed with breast cancer 2.5 years ago and was found to have the same germline BRCA2 mutation. BRCA2 mutation increases the risks of aggressive PCa and other cancer types in Japanese males. These forms may be highly responsive to platinum‐based chemotherapy.
Keywords: BRCA2, DNA damage repair genes, NEPC, platinum‐based chemotherapy
Abbreviations
- DDR
DNA damage repair
- HR
homologous recombination
- DSBs
double strand breaks
- NEPC
adenocarcinoma of the prostate with neuroendocrine differentiation
- PCa
prostate cancer
1. INTRODUCTION
Genome integrity is maintained by numerous DNA damage repair genes (DDRs), and germline mutations in DDRs greatly increase the risks of several cancer types, including prostate, breast, and ovarian cancer.1 Accumulating evidence also suggests that germline mutations and copy number changes in DDR‐related genes such as BRCA1, BRCA2, ATM, CHEK2, and FANCA increase the risk for aggressive forms of prostate cancer (PCa).1 Moreover, somatic events caused by DDRs mutations are associated with aggressive PCa phenotypes.2, 3 However, these associations were established in Western patient cohorts, whereas the genomic and biological implications in Japanese patients are unclear, despite the growing prevalence of PCa in Japan.
We present the case of an 81‐year‐old male with metastatic adenocarcinoma of the prostate with neuroendocrine differentiation (NEPC) accompanied by brain metastasis and a BRCA2 mutation. Moreover, he had a 51‐year‐old son with breast cancer history sharing the same mutation, implicating BRCA2 mutations in elevated cancer risk among Japanese males.
2. CASE PRESENTATION
An 81‐year‐old male presented with signs of possible lung cancer on positron emission tomography (PET) scans for follow‐up monitoring of chronic respiratory obstructive disease (Fig. 1a). A computed tomography (CT) scan also revealed an irregularly shaped prostatic grand (Fig. 1b), so he was referred to our urology department for detailed examination. Although serum prostate‐specific antigen (PSA) concentration was only 0.78 ng/mL, digital rectal examination revealed a stony hard nodule in the right lobe, suggesting PCa. On admission to the hospital for prostate biopsy, he was found to have reduced motivation. Therefore, emergent head CT and magnetic resonance imaging (MRI) were conducted that also identified an irregular ring‐like enhancing mass in the right frontal robe (Fig. 1c and d), suggesting metastatic brain cancer. Thus, emergent cerebral decompression and craniotomy biopsy were performed by a neurosurgeon, and prostate needle biopsy was performed at the same time. Bone scan also identified multiple bone metastases (Fig. 1e).
Figure 1.
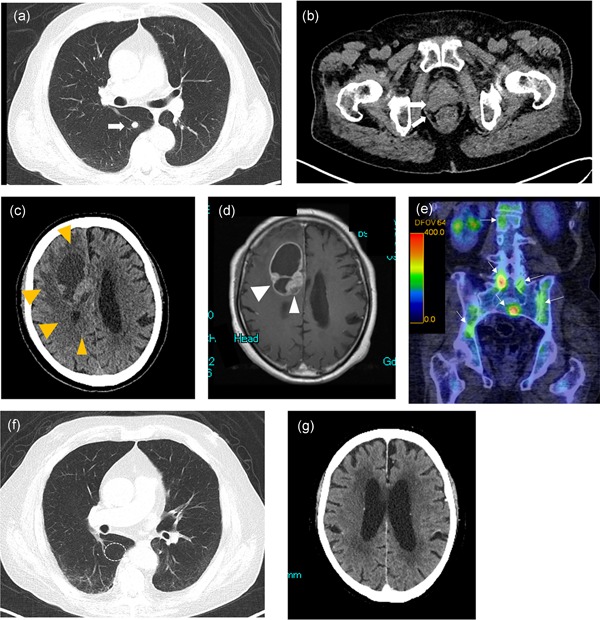
Diagnostic imaging findings on presentation. (a) PET image showing metastatic lung nodules in the right lobe. (b) CT image of the prostate showing the irregular shape. (c,d) Brain CT (c) and MRI (d) images showing signs of frontal lobe metastasis. (e) 99mTc‐HMDP bone scintigraphy revealing multiple metastases in the spinal bone. (e,f) Imaging results after treatment. Chest (e) and brain (f) CT after platinum‐based doublet chemotherapy combined with etoposide. CT shows partial remission of brain metastasis and complete remission of pulmonary metastasis. CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
3. PATHOLOGICAL FINDINGS
Pathological examination revealed that cells in prostatic adenocarcinoma exhibited solid and trabecular patterns with a partial glandular structure; thus, the patient was diagnosed with poorly differentiated adenocarcinoma, with a Gleason grade of 9 (4 + 5). According to an immunohistochemical analysis, he tested negative for PSA, NKX3.1, and p501s, the common markers of prostatic adenocarcinoma, but was tested positive for synaptophysin and chromogranin A (Fig. 2a–f). Three pathologists reached a consensus regarding the final pathological diagnosis of metastatic adenocarcinoma of NEPC. The metastatic brain tumor and prostatic adenocarcinoma had similar histological (appearance of solid and trabecular patterns) and immunohistochemical characteristics (Fig. 3a–f); therefore, the pathologist established a final diagnosis of metastatic adenocarcinoma of NEPC (Fig. 3a–f).4 Based on the results, the clinical stage was identified to be cT3bN1M1c.
Figure 2.
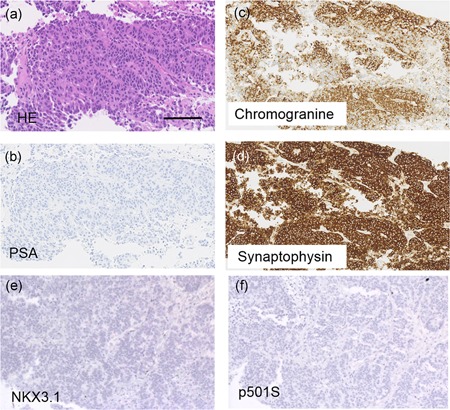
Histology and immunohistochemistry of the prostate biopsy specimen. (a) Representative images of hematoxylin and eosin (HE) staining. (b–f) Immunohistochemistry for PSA (b), chromogranine (c), synaptophysin (d), NKX3.1 (e), and p501S (f). Bars indicate 100 μm. PSA, prostate‐specific antigen.
Figure 3.
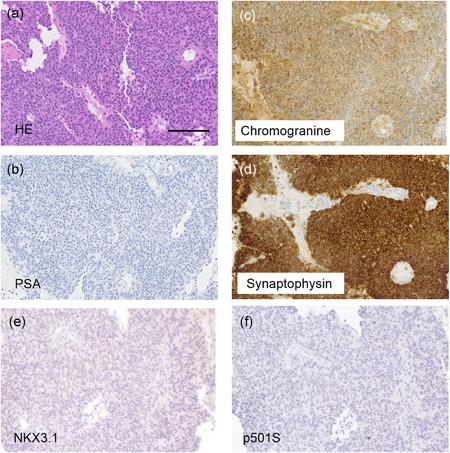
Histology and immunohistochemistry of the brain biopsy specimen. (a) Representative images of hematoxylin and eosin (HE) staining. (b–d) Immunohistochemical staining for PSA (b), chromogranine (c), and synaptophysin (d), NKX3.1 (e), and p501S (f). Bars indicate 100 μm. PSA, prostate‐specific antigen.
The patient received androgen deprivation and platinum‐based doublet chemotherapy combined with etoposide. Follow‐up CT after five cycles of chemotherapy (delivered over 5 months) revealed partial remission of brain metastasis and complete remission of pulmonary metastasis (Fig. 1f). After the sixth cycle, PSA was less than 0.01 ng/mL. Serum neuron‐specific enolase, a biomarker for NEPC, was also reduced to less than 11.8 ng/mL at 5 months.
Cancer‐related gene profiling of the formalin‐fixed paraffin‐embedded prostate tumor specimen and peripheral blood (as a control) was performed using next‐generation sequencing as previously reported5, 6 (Fig. 4a). We identified a somatic BRCA2 mutation at c.7008‐2A>C and a germline mutation at p.E2877* (p.Glu2877Ter), chr13:g.32945234G>T. The mutation at p.E2877* was observed in the ClinVar allele ID 67304 (Fig. 4b). The patient's son, who was diagnosed with breast cancer 2.5 years previously at 49 years of age, shared this germline mutation.
Figure 4.
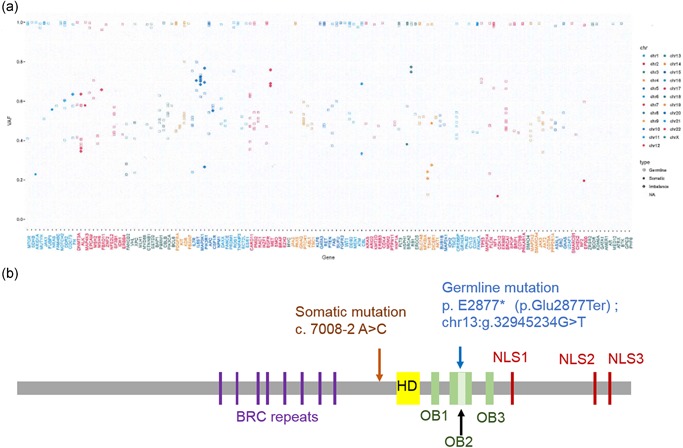
Genomic profiling of cancer‐related genes (a, b). The horizontal axis indicates the examined genes, and the vertical axis indicates the variant allele frequency (a). BRCA2 mutation sites. Profiling revealed a somatic mutation at c. c.7008‐2A>C and a germline mutation at p.E2877*(p.Glu2877Ter). The patient's son shared the germline mutation (b). Germline mutation.
4. DISCUSSION
Prostate cancer is the most frequent cancer in men and the second leading cause of male cancer death in Western countries, and incidence is rising in Asia.7 The strongest risk factor for PCa is a family history, and germline BRCA2 mutations confer the highest risk.8, 9 BRCA2 is critical for the repair of double strand breaks (DSBs) in genomic DNA by homologous recombination (HR). In response to DSBs, BRCA2 localizes RAD51 to the damaged site and mediates repair. Castration‐resistant PCa is frequently associated with genomic aberrations of DDR‐related genes, including BRCA2.3, 10, 11, 12
Prostate cancer associated with BRCA2 mutation may be particularly responsive to platinum‐based chemotherapy. Platinum agents induce intra‐strand and inter‐strand cross‐linked DNA damage, which requires HR for repair. Loss of BRCA2 function in ovarian and endometrial cancer was associated with increased sensitivity to cisplatin, so patients with DDR alterations are thought to be sensitive to platinum agents. Indeed, our case patient showed a strong response to platinum‐based chemotherapy. Brain metastasis from PCa is rare and usually associated with particularly aggressive forms with widespread metastases which has poor overall survival (3−6 months).13, 14 Resection and subsequent radiation therapy has been reported to reduce recurrence risk of brain metastasis from PCa. The brain and lung metastatic lesions in this patient responded to platinum‐based chemotherapy. For further treatment, radiation therapy can be selected after acquired resistance to platinum‐based chemotherapy.15 Moreover, some of these DDR‐related genes are actionable targets by PARP inhibitors.16, 17
Prostate cancers with neuroendocrine differentiation include adenocarcinoma of NEPC, well‐differentiated neuroendocrine tumor, small‐cell neuroendocrine carcinoma, and large‐cell neuroendocrine carcinoma.4 De novo NEPC is a rare and highly aggressive malignant cancer.2, 14, 18 Under selection pressure by modern AR‐directed therapy, AR‐positive adenocarcinoma can dedifferentiate into a small‐cell neuroendocrine‐like tumor or treatment‐emergent neuroendocrine PCa (t‐NEPC). Recently, Beltran et al. reported that DDP gene aberrations were rare in t‐NEPC. On the other hand, frequent biallelic alterations in DDR genes were identified in a minority of patients with de novo NEPC,2 suggesting sensitivity to platinum agents or PARP inhibitors.
Advances in germline genetics and targeted therapeutics have created new treatment opportunities for aggressive PCa.19 However, the choice of optimal treatment is still a challenge as there are no consensus guidelines on treatment for cases with specific mutations. In the United States, the Prostate Cancer Clinical Trials Consortium was established in 2017 to address the growing problem of translating genetic information into therapeutics.20 The consortium assessed the current status of genetic testing practices in men with PCa and suggested greater emphasis on genetic testing results for treatment decisions, testing of family members, and further research for establishing associations among germline DDR gene mutations, PCa phenotypes, and treatment responses. In our case, the patient's son had a past history of breast cancer and both patients desired genomic sequencing analysis, which revealed a shared BRCA2 mutation at p.E2877*. To our knowledge, this is the first case report in a Japanese patient with PCa exhibiting NEPC somatic and germline BRCA2 mutations who responded to platinum‐based chemotherapy.
The analysis reports were discussed and reviewed at a genome expert conference consisting of medical oncologists, molecular oncologists, pathologists, medical geneticists, clinical laboratory technicians, bioinformaticians, genetic counselors, pharmacists, and nurses. The final report including information regarding the recommended treatment based on genomic profiling was confirmed after approval at this conference, after which the report was disseminated to the physicians and patient.
We present the first Japanese case of an 81‐year‐old male with metastatic adenocarcinoma of NEPC accompanied by brain metastasis and a BRCA2 mutation. He had a 51‐year‐old son with breast cancer history sharing the same mutation, implying that BRCA2 mutations are associated with elevated cancer risk among Japanese males.
DISCLOSURE STATEMENT
None declared.
AUTHOR CONTRIBUTIONS
Conception and design of the study: TK, HN and MO. Acquisition and analysis of data: TK, HH, EA, KM, TH and HN. Drafting the manuscript and figures: TK, SM, HN and MO.
ACKNOWLEDGMENTS
We thank Yoko Suzuki, Emmy Yanagida, and Hiroshi Yamada for technical assistance. The authors would like to thank Enago (http://www.enago.com) for the English language review. This work was supported in part by a Grant‐in‐Aid for Scientific Research (#17K11158 to T.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The work was supported in part by a research grant to T. Kosaka from the Takeda Science Foundation and the SGH Foundation, Japan.
References
REFERENCES
- 1. Pritchard CC, Mateo J, Walsh MF et al Inherited DNA‐repair gene mutations in men with metastatic prostate cancer. New Engl J Med 2016; 375: 443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chedgy EC, Vandekerkhove G, Herberts C et al Biallelic tumour suppressor loss and DNA repair defects in de novo small‐cell prostate carcinoma. J Pathol 2018; 246: 244–53. [DOI] [PubMed] [Google Scholar]
- 3. Robinson D, Van Allen EM, Wu YM et al Integrative clinical genomics of advanced prostate cancer. Cell 2015; 161: 1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs‐Part B: Prostate and bladder tumours. Eur Urol 2016; 70: 106–19. [DOI] [PubMed] [Google Scholar]
- 5. Hayashi H, Tanishima S, Fujii K et al Genomic testing for pancreatic cancer in clinical practice as real‐world evidence. Pancreatology 2018; 18: 647–54. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe K, Kosaka T, Aimono E et al Japanese case of enzalutamide‐resistant prostate cancer harboring a SPOP mutation with scattered allelic imbalance: Response to platinum‐based therapy. Clin Genitourin Cancer 2019. 10.1016/j.clgc.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 7. Cancer Facts and Figures 2019. [homepage on the Internet]: American Cancer Society, 2019. (cited April 28th 2019). Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- 8. Agalliu I, Gern R, Leanza S, Burk RD. Associations of high‐grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin Cancer Res 2009; 15: 1112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallagher DJ, Gaudet MM, Pal P et al Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res 2010; 16: 2115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter HB, Helfand B, Mamawala M et al Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur Urol 2019; 75: 743–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haffner MC, Mosbruger T, Esopi DM et al Tracking the clonal origin of lethal prostate cancer. J Clin Invest 2013; 123: 4918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson PS. Beyond the androgen receptor: Targeting actionable drivers of prostate cancer. JCO Precis Oncol 2017; 1–3. [DOI] [PubMed] [Google Scholar]
- 13. Tremont‐Lukats IW, Bobustuc G, Lagos GK, Lolas K, Kyritsis AP, Puduvalli VK. Brain metastasis from prostate carcinoma: The M. D. Anderson Cancer Center experience. Cancer 2003; 98: 363–68. [DOI] [PubMed] [Google Scholar]
- 14. Spiess PE, Pettaway CA, Vakar‐Lopez F et al Treatment outcomes of small cell carcinoma of the prostate: A single‐center study. Cancer 2007; 110: 1729–37. [DOI] [PubMed] [Google Scholar]
- 15. Sita TL, Petras KG, Wafford QE, Berendsen MA, Kruser TJ. Radiotherapy for cranial and brain metastases from prostate cancer: A systematic review. J Neurooncol 2017; 133: 531–38. [DOI] [PubMed] [Google Scholar]
- 16. Mateo J, Carreira S, Sandhu S et al DNA‐repair defects and Olaparib in metastatic prostate cancer. New Engl J Med 2015; 373: 1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clarke N, Wiechno P, Alekseev B et al Olaparib combined with abiraterone in patients with metastatic castration‐resistant prostate cancer: A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Oncol 2018; 19: 975–86. [DOI] [PubMed] [Google Scholar]
- 18. Wang HT, Yao YH, Li BG, Tang Y, Chang JW, Zhang J. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: Factors associated with time to development of NEPC and survival from NEPC diagnosis – A systematic review and pooled analysis. J Clin Oncol 2014; 32: 3383–90. [DOI] [PubMed] [Google Scholar]
- 19. Beltran H, Prandi D, Mosquera JM et al Divergent clonal evolution of castration‐resistant neuroendocrine prostate cancer. Nat Med 2016; 22: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carlo MI, Giri VN, Paller CJ et al Evolving intersection between inherited cancer genetics and therapeutic clinical trials in prostate cancer: A White Paper From the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. JCO Precis Oncol 2018. 10.1200/PO.18.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]


