Abstract
The basic components of regulatory and supporting policies for orphan drug development appear similar between the United States and Japan, but drugs designated as orphan drugs have been different between the 2 countries. The probabilities of development success (ie, marketing approval) in designated orphan drugs have also been significantly different. In this study, we analyzed recent outcomes of development for orphan drugs designated from 1993 to 2017 in Japan, considering their development and approval status in the United States. Our analysis showed that success for orphan drug development in Japan was apparently associated with prior approval status in the United States. Company size, orphan development experience, and patient enrichment were also positively associated with successful marketing approval. Although similar designations and priority review systems for orphan drugs have been enacted, economic incentives and regulatory conditions provided by the systems seem to be different between the 2 countries, which may lead to varied performance in orphan designation and approval. We need to pay close attention to the impact of industrial global development strategies when comparing the outcomes and performance of different orphan drug promotion systems.
Keywords: global development, orphan drug, patient enrichment, probability of success
There are many intractable diseases in the world for which effective treatments do not yet exist.1 For rare diseases with small numbers of patients, motivation for drug development on the part of pharmaceutical companies is not necessarily high.2 Issues related to unmet medical needs in such therapeutic areas have been discussed previously from a global perspective.3 Due to the absence of reliable data, public health officials face difficulties in estimating the prevalence of several rare diseases at a national and/or global level. Patient support groups, health care providers, and the governments worldwide implement several international collaboration programs, which help raise public awareness and encourage development and approval of novel treatments beyond the boundaries.3
The orphan drug designation program was officially introduced in the United States, Japan, and the European Union in 1982, 1993, and 2000, respectively. The programs in these jurisdictions have much in common. With the purpose of providing regulatory and economic incentives to companies developing drugs for rare diseases, these programs consist of several basic components, including a prevalence threshold as a designation requirement, financial support through public grants, preferential consultation by authorities, accelerated review, market exclusivity, and tax incentives.4, 5, 6, 7 For example, orphan drugs can obtain financial support from public grants up to 50% of the research and development cost.6, 7 Orphan drugs are treated preferentially in review time (9 months for orphan drugs, 12 months for standard review drugs). Market exclusivity for orphan drugs is longer than that of other new drugs (10 years for orphan drugs, 8 years for other new drugs).7
Although each country implements its orphan drug program with the application of common basic regulatory components, the number and type of orphan drugs actually designated for the programs differ from country to country because of companies’ strategic decisions on market entry. These decisions are based on not only orphan regulation but also various other factors.8 As a result, the historical performance of orphan drug programs measured by the number of designated drugs and probabilities of development success has been significantly different between countries. The probability that a designated orphan drug reached marketing approval in Japan was reportedly much higher than that in the United States (Japan, 73%; United States, 15%).9, 10 A study in Europe revealed that only 7.1% of the designated orphan drugs in the European Union were approved for marketing.11 These success rates cannot simply be used for comparison purposes, however, because they are based on a different set of drugs submitted to each country/region; they would instead be interpreted as a consequence of differences in orphan drugs submitted. These obvious differences suggest a couple of possible explanations, including that drug companies play a “different ballgame” in each jurisdiction, and/or that the participants in the game (ie, companies and drugs) are different.
As is clear from historically persistent launch windows in Japan and the United States, global companies can choose the most efficient (ie, profit‐maximizing) development pathway, taking advantage of differences in regulatory, medical, and economic conditions around the world.12 It is natural to suppose that drug companies behave differently for market entry in the 2 countries even in the field of orphan drug development.
Differences in specific orphan drug regulations, some of which are seemingly subtle, may make a significant difference in designation and marketing approval.13 For example, the threshold patient number in the United States is much larger than that in Japan, apparently reflecting the size of the population. In the United States, orphan status is available for indications that could affect <200,000 patients, while in Japan, it is granted to those with <50,000 patients. Considering the population of the 2 countries (Japan, 127 million; United States, 330 million), the demographic condition for granting orphan status is stricter in Japan than in the United States. In addition, the Japanese orphan drug program is unique in that it requires “development feasibility” at the time of application. Drug companies should demonstrate that an orphan drug candidate has a certain likelihood of success by providing substantial evidence such as study results obtained in previous phases and/or regulatory (approval/designation) status in other countries. Due to these requirements, the orphan drug designation is hard to obtain in Japan compared to elsewhere in the world, which probably has had a significant impact on drug companies’ decisions regarding for which drug (or indication) to apply for orphan status in Japan and the related timing.
The way orphan drugs are developed in each country thus reflects a variety of factors related to both global and local conditions, which may result in significant disparities in developmental success rates. However, mechanisms that may lead to observed disparities have not been well studied.
There are several analyses on approval probabilities of orphan drugs in the United States and Europe.11, 14, 15, 16 A study on orphan drug approval in Europe from 2000 to 2013 showed that the average approval probability of the orphan drug was lower than that of the nonorphan drugs, and that the company's size and compliance with scientific advice from the authorities were associated with positive approval decisions.14 A review showed that 74.4% of orphan drugs in Japan had already been designated as orphan drugs in the United States for more than a year.15 These studies present the real pictures of application, designation, and marketing approval of orphan drugs in certain countries, but how those differences are formed in the setting of global drug development has not been rigorously examined. Previous studies usually focused on a specific country (or region), and possible dependence on regulatory status in other countries was not explicitly presumed in analysis and discussions.
The objective of this study was to identify factors related to successful marketing approval of Japanese orphan‐designated drugs. We analyzed the designation and approval of orphan drugs in Japan using regression analysis. We explored associations between the results (success/failure) of the clinical development of orphan drugs and factors that might affect key processes and/or decisions, including corporate attributes, disease types, and drug characteristics, considering the influence of development and approval status in the United States.
Methods
We collected data for all designated orphan drugs in Japan from November 15, 1993, to August 31, 2017, from the database of the National Institutes of Biomedical Innovation, Health, and Nutrition, accessed from the organization's website. The flowchart of sample selection is shown in Figure 1. From the 401 orphan drugs designated in Japan, we excluded 3 drugs that were withdrawn because of indication expansion. As of September 7, 2018, 307 drugs were approved, 51 drugs failed to obtain approval, and for the remaining 40 drugs, the outcome had not been determined.
Figure 1.
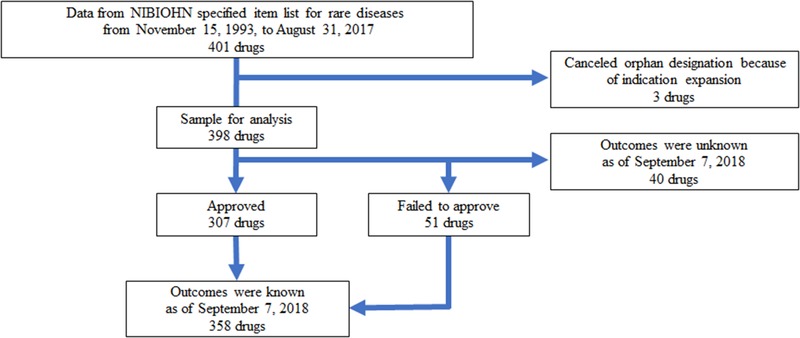
Flowchart for sample selection. NIBIOHN, National Institutes of Biomedical Innovation, Health, and Nutrition.
The main objective of this study was to identify factors related to successful marketing approval of Japanese orphan‐designated drugs. We applied 3 models to our data. Multivariate logistic regression analysis was performed with 358 orphan drugs for which official regulatory decision (ie, approved or rejected [withdrawn]) had been confirmed.17, 18 To avoid a possible impact from censored data, we also implemented 2 survival regression analyses using 398 orphan drugs, including the 40 drugs for which a regulatory decision had not been reached by the end of our observation period. In the Cox proportional hazard analysis, approval was considered the sole event, and in the competitive risk regression analysis, approval and rejection were considered the competing events. We confirmed that the assumptions of proportional hazards were not violated in the global test using the Schoenfeld residuals.
The choice of explanatory variables was based on the analysis of previous studies.17, 18, 19 The number of patients, availability of alternative treatment, company size of the applicant (3000≦ or <3000 employees), nationality of the applicant, experience with orphan drug development in Japan, new molecular entity, and Anatomical Therapeutic Chemical classification were used as explanatory variables. Drugs under the Anatomical Therapeutic Chemical classification B, C, D, G, H, M, P, R, S, and V, each of which comprised <10% of the total data set, were grouped together in logistic regression. The variable indicating that the orphan drug (and indication) was approved in the United States before Japanese approval was tested to see to what extent Japanese orphan drug designation and development were influenced by the status of foreign countries. It was also examined whether patients were “enriched” explicitly (eg, stratified by biomarkers) or implicitly (eg, subgroups of overall patient populations) in the indication.
STATA 14 (StataCorp, College Station, Texas) was used as the statistical tool.
Results
Descriptive statistics for the orphan drugs from 1993 to 2018 are shown in Table 1. Antineoplastic agents and anti‐infective agents accounted for 53% (212 of 398). For example, indications for antineoplastic agents were pancreatic carcinoma (gemcitabine hydrochloride), multiple myeloma (bortezomib), relapsed or refractory classical Hodgkin lymphoma (nivolumab). Indications for anti‐infective agents included human T‐lymphotropic virus type 1–associated myelopathy (interferon alpha), cytomegalovirus retinitis in AIDS patients (foscarnet sodium hydrate), methicillin‐cephem–resistant Staphylococcus aureus enteritis (vancomycin hydrochloride). There were 4 orphan drugs approved in the United States but rejected in Japan. Ganciclovir was withdrawn from the orphan designation at the preclinical stage. Haloantrine hydrochloride progressed to phase 2, and mefloquine hydrochloride progressed to phase 3, respectively, but the orphan designation was canceled. The reasons for these withdrawals and suspensions were not publicly available. The discontinuation of levocarnitine's development was ascribed to lack of efficacy in the phase 2 trial, where erythropoietin‐resistant renal anemia in hemodialysis patients was tested. It has been approved in the United States in 1985 indicated for the treatment of primary systemic carnitine deficiency. A total of 132 drugs were so‐called ultra‐orphan drugs with ≤1000 patients. The proportion of approved drugs was 77% (307 of 398). About 38% (152 of 398) of the drugs were approved within 24 months of orphan designation. The proportion of drugs submitted for approval within 3 months or 6 months from orphan designation acquisition was 3.3% (13 of 398) and 9% (36 of 398), respectively. Median lag time before Japanese approval for previously approved medications in the United States was 52 months. According to Figure S1, about half of the approved drugs were approved within 25 months after orphan designation, and the likelihood of success gradually declined thereafter.
Table 1.
Descriptive Statistics for Orphan‐Designated Drugs in Japan From November 15, 1993, to August 31, 2017 (as of September 7, 2018)
| Explanatory Variables | Total(N=398) | Approved(N=307) | Rejected(N=51) | Outcome Unknown(N=40) |
|---|---|---|---|---|
| Number of patients | ||||
| <100 | 61 | 52 | 6 | 3 |
| 101‐1000 | 71 | 55 | 6 | 10 |
| 1001‐10000 | 182 | 139 | 23 | 20 |
| 10001‐50000 | 84 | 61 | 16 | 7 |
| Patient enrichment in anticipated/approved indication | ||||
| Yes | 33 | 30 | 0 | 3 |
| No | 365 | 277 | 51 | 37 |
| Alternative treatment | ||||
| Not available | 152 | 116 | 24 | 12 |
| Exists (however, the disease is refractory or the treatment is insufficient) | 196 | 152 | 22 | 22 |
| Exists | 50 | 39 | 5 | 6 |
| Company size (no. of employees) | ||||
| 3000≦ | 217 | 180 | 16 | 21 |
| <3000 | 181 | 127 | 35 | 19 |
| Company capital | ||||
| Japanese capital | 185 | 136 | 30 | 19 |
| Foreign capital or foreign and Japanese capital | 213 | 171 | 21 | 21 |
| Orphan development experience in Japan | ||||
| Yes | 265 | 209 | 26 | 30 |
| No | 133 | 98 | 25 | 10 |
| Period from orphan designation to application (months) | ||||
| <12 | 92 | 88 | 1 | 3 |
| 12‐24 | 77 | 64 | 3 | 10 |
| 24‐48 | 100 | 74 | 11 | 15 |
| 48‐72 | 60 | 43 | 9 | 8 |
| 72‐96 | 35 | 23 | 11 | 1 |
| 96< | 34 | 15 | 16 | 3 |
| New molecular entity | ||||
| Yes | 247 | 192 | 30 | 25 |
| No | 151 | 115 | 21 | 15 |
| Prior approval in the United States | ||||
| Yes | 132 | 121 | 4 | 7 |
| No | 266 | 186 | 47 | 33 |
| ATC classification | ||||
| A—Alimentary tract and metabolism | 46 | 39 | 3 | 4 |
| B—Blood and blood‐forming organs | 23 | 14 | 5 | 4 |
| C—Cardiovascular system | 19 | 13 | 1 | 5 |
| D—Dermatologicals | 1 | 1 | 0 | 0 |
| G—Genitourinary system and sex hormones | 3 | 3 | 0 | 0 |
| H—Systemic hormonal preparations, excluding sex hormones and insulins | 2 | 2 | 0 | 0 |
| J—Anti‐infectives for systemic use | 75 | 63 | 9 | 3 |
| L—Antineoplastic and immunomodulating agents | 137 | 116 | 9 | 12 |
| M—Musculoskeletal system | 18 | 10 | 4 | 4 |
| N—Nervous system | 44 | 29 | 11 | 4 |
| P—Antiparasitic products, insecticides, and repellents | 4 | 2 | 1 | 1 |
| R—Respiratory system | 8 | 7 | 0 | 1 |
| S—Sensory organs | 13 | 5 | 7 | 1 |
| V—Various | 5 | 3 | 1 | 1 |
ATC, Anatomical Therapeutic Chemical classification system.
The results of the logistic regression analysis are presented in Table 2. Prior approval in the United States was associated positively with approval in Japan, a following market (β = 1.91, P = .001). Company size of the applicant was also positively correlated with approval (β = .93, P = .011). The number of patients (β = –.03, P = .038) had lower approval probabilities, other conditions being the same.
Table 2.
Results of Logistic Regression Analysis for Orphan‐Designated Drugs in Japan
| Explanatory Variables | Coefficient | SE | P Value |
|---|---|---|---|
| Number of patients (N = 1000) | –0.03 | 0.02 | .038** |
| Alternative treatment: Exist | |||
| Not available | –0.71 | 0.61 | .242 |
| Exists (however, the disease is refractory or the treatment is insufficient) | –0.41 | 0.59 | .491 |
| Company size (no. of employees): < 3000 | |||
| 3000≦ | 0.93 | 0.37 | .011** |
| Company capital: Foreign capital | |||
| Japanese capital | –0.24 | 0.36 | .512 |
| Orphan development experience in Japan: No | |||
| Yes | 0.50 | 0.34 | .141 |
| New molecular entity: No | |||
| Yes | –0.24 | 0.37 | .516 |
| Prior approval in the United States: No | |||
| Yes | 1.91 | 0.56 | .001*** |
| ATC classification: ATC V | |||
| A—Alimentary tract and metabolism | 1.05 | 1.39 | .448 |
| J—Antiinfectives for systemic use | 0.25 | 1.30 | .847 |
| L—Antineoplastic and immunomodulating agents | 0.71 | 1.31 | .587 |
| N—Nervous system | –0.76 | 1.32 | .565 |
| Others (B, C, D, G, H, M, P, R, S) | –0.43 | 1.28 | .735 |
| _cons | 1.61 | 1.32 | .222 |
ATC, Anatomical Therapeutic Chemical classification system; SE, standard error.
Baseline category is underlined.
*
P < .1; ** P < .05; *** P < .01.
Table 3 shows the results of the Cox regression and competing risk regression analyses. These models also suggested that prior approval in the United States was positively associated with success in Japan (Cox: hazard ratio [HR] = 1.85, P < .001; competing risk: subdistribution hazard ratio [SHR] = 1.98, P < .001). Companies with orphan development experience had higher probabilities of obtaining approval (Cox: HR = 1.60, P < .001; competing risk: SHR = 1.52, P = .001). Drugs with enriched indications were more likely to succeed than ones without (Cox: HR = 1.55, P = .045; competing risk: SHR = 1.62, P = .011). Drugs for the alimentary tract (Cox: HR = 1.45, P = .048; competing risk: SHR = 1.49, P = .021) and antineoplastic drugs (Cox: HR = 1.42, P = .010, competing risk: SHR = 1.41, P = .012) had a higher likelihood of success. Company size tended to present a higher likelihood of success (Cox: HR = 1.24, P = .077; competing risk: SHR = 1.23, P = .069), which was in line with the results of logistic regression analysis above. No availability of alternative treatment (Cox: HR = 1.39, P = .093) tended to have higher approval probabilities.
Table 3.
Results of Cox Regression Analysis and Competing Risk Analysis on All Orphan‐Designated Drugs in Japan
| Cox Model | Competing Risk Model | |||||
|---|---|---|---|---|---|---|
| Explanatory Variables | Hazard Ratio | SE | P Value | Sub Hazard Ratio | SE | P Value |
| Number of patients (N = 1000) | 0.99 | 0.01 | .269 | 0.99 | 0.01 | .189 |
| Patient enrichment in anticipated/approved indication: No | ||||||
| Yes | 1.55 | 0.34 | .045** | 1.62 | 0.31 | .011** |
| Alternative treatment: Exist | ||||||
| Not available | 1.39 | 0.27 | .093* | 1.18 | 0.22 | .361 |
| Exists (however, the disease is refractory or the treatment is insufficient) | 1.30 | 0.24 | .153 | 1.16 | 0.21 | .425 |
| Company size (no. of employees): < 3000 | ||||||
| 3000≦ | 1.24 | 0.15 | .077* | 1.23 | 0.14 | .069* |
| Company capital: Foreign capital | ||||||
| Japanese capital | 0.99 | 0.13 | .929 | 0.97 | 0.12 | .819 |
| Orphan development experience in Japan: No | ||||||
| Yes | 1.60 | 0.21 | <.001*** | 1.52 | 0.19 | .001*** |
| New molecular entity: No | ||||||
| Yes | 0.97 | 0.13 | .819 | 0.92 | 0.13 | .519 |
| Prior approval in the United States: No | ||||||
| Yes | 1.85 | 0.23 | <.001*** | 1.98 | 0.24 | <.001*** |
| ATC classification: ATC except A or L | ||||||
| A—Alimentary tract and metabolism | 1.45 | 0.27 | .048** | 1.49 | 0.26 | .021** |
| L—Antineoplastic and immunomodulating agents | 1.42 | 0.20 | .010** | 1.41 | 0.19 | .012** |
ATC, Anatomical Therapeutic Chemical classification system; SE, standard error.
Baseline category is underlined.
* P < .1; ** P < .05; *** P < .01.
Discussion
Our analysis of marketing approvals of Japanese orphan drugs clarified that factors that determine successful development and marketing approval are somewhat different from those observed in previous studies targeting the United States and European Union, and that we need to consider possible impacts of industrial strategic applications of orphan drug designation in response to diverse local market conditions and regulatory requirements when comparing the outcomes and performance of orphan drug programs in each jurisdiction.
As was expected, prior approval status in the United States significantly increased the likelihood of approval in Japan. This explains why approval probabilities of orphan drugs have been much higher than those in the United States and European Union.11 Many of the orphan drugs designated in Japan are already approved (or expected to be) in the United States, which inevitably leads to much higher approval rates in follow‐on countries, including Japan. This type of strategic behavior has been commonly observed not only in orphan drug development but in new drug development in general. For profit‐maximizing drug companies, entrance into smaller follow‐on markets (eg, Japan) can be compensated by higher success rates due to a follower's advantage in clinical development and approval stages.12, 20, 21, 22 It is well known that such industrial behaviors based on economic “arbitrage” have yielded historical launch delays in Japan.20
Launch delays of promising new drugs in follow‐on markets can pose a serious health hazard. It is likely that small expected sales and profits of orphan drugs exacerbate the situation. Interestingly, however, a local requirement for orphan drug designation seems to play a critical role in balancing incentives of drug companies. In Japanese regulations, drugs are given orphan status only when the applicant can provide substantial evidence on “development feasibility,” which is not included in the requirements of the United States and European Union. Approval status and/or development histories and records (eg, positive results from clinical trials) in the United States are commonly submitted as substantial evidence on “development feasibility” and enhance decisions on orphan designation in Japan. This unique requirement and the actual success probabilities that are evidently much higher than those in the United States and European Union provide drug companies with economic incentives to submit applications of orphan drugs in Japanese markets, although it is not clear whether the scheme is deliberately designed by the regulators.
Designated orphan drugs in Japan are thus different in type and number from those in the United States due to the global application strategies of drug companies and differences in local regulation. A previous survey showed that 52% of Japanese designations were also designated in the United States (191 of 364 drugs), and only 6% of US designations were designated in Japan (191 of 3390 drugs).15 This calls attention to comparing performance of different regulatory systems in different countries in this age of global drug development, because all countries/regions depend on each other in some regulatory and economic aspect.
It should be emphasized, however, that dependence between 2 countries does not impact each country equally. Previous studies examining orphan drugs in the United States and European Union show no significant correlation between approval probabilities and approval status in other countries/regions.17, 18 Because the United States and European Union are the 2 leading markets, it is unlikely that approval decisions in the 2 jurisdictions are seriously affected by the status of Japanese development and approval. To the contrary, the present results clearly suggest that the development and approval status in the United States exerts a critical impact on Japanese orphan drug development.
Although the Japanese orphan drug program has succeeded in attracting orphan drugs that have been marketed elsewhere into the Japanese market, it is difficult to discuss to what extent the program has contributed to incubate potential orphan drugs in the Japanese clinical development setting, which is also a major objective of the program. The finding that some orphan drugs approved in Japan obtained orphan status just before their new drug application shows that companies use the orphan designation only to take advantage of the new drug application review and not for clinical development. It is necessary to investigate the clinical data package for each drug, both approved and rejected, to evaluate such contributions, but it is almost impossible to obtain information from the clinical data packages submitted for rejected drugs.
Among the variables reflecting the drug companies’ attributes, the company size and orphan development experience were predictors of successful marketing approval, even when adjusting for US approval status. These results are in line with a previous study focusing on orphan drug development in the new drug application and European Union.13, 17 In general, large companies generally possess more information and “know‐how” with respect to the operation of orphan drug development than small companies. Some of the drugs developed by large companies that originate from small companies, including start‐ups and academia, may tend to have a higher probability of success.23
The number of patients was negatively associated with a likelihood of approval (Table 2); that is, designated orphan drugs with fewer patients were more likely to be approved. This may conflict with our general intuition that difficulties in clinical development increase in disease areas with a small number of patients. In orphan drug development, however, the number of (potential) patients might not be a critical barrier because the requirements for approval of standard drugs are not applicable to orphan drugs. Randomized and controlled trials are not a must for many orphan drugs, for example, and much more lenient rules are applied for approval decisions.24, 25 Orphan drugs are often approved with post hoc approval conditions, such as all‐case surveillance after marketing, which also enables regulators to apply approval requirements for orphan drugs in more flexible ways.26
There was a positive correlation between patient enrichment in indication and successful marketing approval in both the Cox proportional hazard model and the competitive risk model (Table 3). The coefficient of enrichment was not estimated in the logistic regression analysis (Table 2) because all of the 30 applications with patient enrichment were successfully approved. Enrichment in indications often occurs in antineoplastic agents. For example, rituximab (Genetical Recombination), trastuzumab (Genetical Recombination), and mogamulizumab (Genetical Recombination) have a limited patient population depending on the expression of a target antigen protein and a threshold value of the expression level. Imatinib mesilate, crizotinib, alectinib hydrochloride, and vemurafenib are patient enriched according to specific chromosomal translocations and gene mutations. Some studies have shown that setting “correct” target populations contributes to an improvement in approval probability, and that seems also true in the realm of orphan drugs.17, 27
Conclusion
In conclusion, this research showed that success for orphan drug development in Japan was associated with prior approval status in the United States, company size, orphan drug development experience in Japan, and patient enrichment; these indicate that orphan drugs designated in Japan are different from orphan drugs in the United States due to the drug industry's strategic application behaviors in follow‐on markets in this age of global development. A unique local requirement for “development feasibility” for orphan drug designation further encourages companies to submit orphan designation applications for drugs with previous development records in preceding countries, and thus increasing the high likelihood of success. It is necessary to consider relationships between countries/regions when discussing regulatory outcomes and performance of orphan drug promotion systems because it may be the case that underlying incentives and mechanisms of development, review, and approval of orphan drugs are significantly different between jurisdictions.
Supporting information
Figure 1. Cumulative approval ratio for orphan designated drugs in Japan.
Figure Caption
Funding
This study was funded by a Japanese government‐based grant‐in‐aid from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (19K07215). Kenji Harada is an employee of Kyowa Kirin Co. Ltd., Tokyo, Japan. The other authors declared no conflict of interest.
Author Contributions
KH, KT, and SO contributed to the design of the study. KH and KT collected and analyzed the data. KH and SO drafted the manuscript. All authors read and approved the final manuscript. Editage (http://www.editage.jp) was used for English language editing.
Data Sharing
Data available from the corresponding author (shun-ono@mol.f.u-tokyo.ac.jp) upon request.
[Correction added on 02 August 2019 after first online publication: The value of company size of the applicant is changed on page 3, Table 1, 2 and 3; and the last value of Period from orphan designation to application (months) is changed from ≤96 to 96< in Table 1.]
References
- 1. Melnikova I. Rare diseases and orphan drugs. Nat Rev Drug Discov. 2012;11(4):267‐268. [DOI] [PubMed] [Google Scholar]
- 2. Dear JW, Lilitkarntakul P, Webb DJ. Are rare diseases still orphans or happily adopted? The challenges of developing and using orphan medicinal products. Br J Clin Pharmacol. 2006;62(3):264‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schieppati A, Henter JI, Daina E, Aperia A. Why rare diseases are an important medical and social issue. Lancet. 2008;371(9629):2039‐2041. [DOI] [PubMed] [Google Scholar]
- 4.Electronic Code of Federal Regulations PART 316—Orphan Drugs. https://www.ecfr.gov/cgi-bin/text-idx?SID=7f77960f61d80d3fdca8f02993f23edf&mc=true&node=pt21.5.316&rgn=div5. Accessed April 21, 2019.
- 5.European Medicines Agency. Orphan designation https://www.ema.europa.eu/en/human-regulatory/overview/orphan-designation. Accessed April 21, 2019.
- 6.NIBIOHN Orphan Products Development Support Program. http://www.nibiohn.go.jp/en/activities/orphan-support.html. Accessed April 21, 2019.
- 7. Song P, Gao J, Inagaki Y, Kokudo N, Tang W. Rare diseases, orphan drugs, and their regulation in Asia: current status and future perspectives. Intractable Rare Dis Res. 2012;1(1):3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olson MK. Managing delegation in the FDA: reducing delay in new‐drug review. J Health Polit Policy Law. 2004;29(3):397‐430. [DOI] [PubMed] [Google Scholar]
- 9.NIBIOHN specified item list for rare diseases. http://www.nibiohn.go.jp/nibio/part/promote/files/h2705kisyoiyaku-hyo1_english.pdf. Accessed April 21, 2019.
- 10. FDA . Search orphan drug designations and approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/. Accessed April 21, 2019.
- 11. Joppi R, Bertele V, Garattini S. Orphan drug development is progressing too slowly. Br J Clin Pharmacol. 2006;61(3):355‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirai Y, Kinoshita H, Kusama M, Yasuda K, Sugiyama Y, Ono S. Delays in new drug applications in Japan and industrial R&D strategies. Clin Pharmacol Ther. 2010;87(2):212‐218. [DOI] [PubMed] [Google Scholar]
- 13. Heemstra HE, de Vrueh RL, van Weely S, et al. Predictors of orphan drug approval in the European Union. Eur J Clin Pharmacol. 2008;64:545‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hofer MP, Hedman H, Mavris M, et al. Marketing authorisation of orphan medicines in Europe from 2000 to 2013. Drug Discov Today. 2018;23(2):424‐433. [DOI] [PubMed] [Google Scholar]
- 15. Murakami M, Narukawa M. Matched analysis on orphan drug designations and approvals: cross regional analysis in the United States, the European Union, and Japan. Drug Discov Today. 2016;21(4):544‐549. [DOI] [PubMed] [Google Scholar]
- 16. Haffner ME, Whitley J, Moses M. Two decades of orphan product development. Nat Rev Drug Discov. 2002;1(10):821‐825. [DOI] [PubMed] [Google Scholar]
- 17. Heemstra HE, Leufkens HG, Rodgers RP, Xu K, Voordouw BC, Braun MM. Characteristics of orphan drug applications that fail to achieve marketing approval in the USA. Drug Discov Today. 2011;16(1‐2):73‐80. [DOI] [PubMed] [Google Scholar]
- 18. Putzeist M, Heemstra HE, Garcia JL, et al. Determinants for successful marketing authorisation of orphan medicinal products in the EU. Drug Discov Today. 2012;17(7‐8):352‐358. [DOI] [PubMed] [Google Scholar]
- 19. Giannuzzi V, Landi A, Bosone E, et al. Failures to further developing orphan medicinal products after designation granted in Europe: an analysis of marketing authorisation failures and abandoned drugs. BMJ Open. 2017;7(9):e017358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirai Y, Yamanaka Y, Kusama M, Ishibashi T, Sugiyama Y, Ono S. Analysis of the success rates of new drug development in Japan and the lag behind the US. Health Policy. 2012;104(3):241‐246. [DOI] [PubMed] [Google Scholar]
- 21. Danzon PM, Wang YR, Wang L. The impact of price regulation on the launch delay of new drugs‐evidence from twenty‐five major markets in the 1990s. Health Econ. 2005;14:269‐292. [DOI] [PubMed] [Google Scholar]
- 22. Kyle MK. The role of firm characteristics in pharmaceutical product launches. Rand J Econ. 2006;37:602‐618. [Google Scholar]
- 23. Takebe T, Imai R, Ono S. The current status of drug discovery and development as originated in United States academia: the influence of industrial and academic collaboration on drug discovery and development. Clin Transl Sci. 2018;11(6):597‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bell SA, Tudur Smith C. A comparison of interventional clinical trials in rare versus non‐rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis. 2014;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kesselheim AS, Myers JA, Avorn J. Characteristics of clinical trials to support approval of orphan vs nonorphan drugs for cancer. JAMA. 2011;305(22):2320‐2326. [DOI] [PubMed] [Google Scholar]
- 26. Pontes C, Fontanet JM, Vives R, et al. Evidence supporting regulatory‐decision making on orphan medicinal products authorisation in Europe: methodological uncertainties. Orphanet J Rare Dis. 2018;13(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. US Food and Drug Administration . Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products. Guidance for Industry. March 2019. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm332181.pdf. Accessed April 21, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Cumulative approval ratio for orphan designated drugs in Japan.
Figure Caption


