Abstract
The tumor microenvironment has been identified as one of the driving factors of tumor progression and invasion. Inside this microenvironment, cancer‐associated fibroblasts (CAFs), a type of perpetually activated fibroblasts, have been implicated to have a strong tumor‐modulating effect and play a key role in areas such as drug resistance. Identification of CAFs has typically been carried based on the expression of various “CAF markers”, such as fibroblast activation protein alpha (FAP) and alpha smooth muscle actin (αSMA), which separates them from the larger pool of fibroblasts present in the body. However, as outlined in this Review, the expression of various commonly used fibroblast markers is extremely heterogeneous and varies strongly between different CAF subpopulations. As such, novel selection methods based on cellular function, as well as further characterizing research, are vital for the standardization of CAF identification in order to improve the cross‐applicability of different research studies in the field. The aim of this review is to give a thorough overview of the commonly used fibroblast markers in the field and their various strengths and, more importantly, their weaknesses, as well as to highlight potential future avenues for CAF identification and targeting.
Keywords: tumor microenvironment, cancer‐associated fibroblasts, fibroblast heterogeneity, fibroblast markers
Abbreviations
- CAF
cancer‐associated fibroblasts
- TME
tumor microenvironment
- NK cells
natural killer cells
- ECM
extracellular matrix
- FAP
fibroblast activation protein α
- IKKβ
I kappa B kinase/NF‐kappa B pathway
- pERK
phosphorylated extracellular signal‐regulated kinase
- RhoK
rhodopsin kinase
- MAPK
mitogen‐activated protein kinase
- FACS
fluorescence‐activated cell sorting
- HGF
hepatocyte growth factor
- VEGFA
vascular endothelial growth factor A
- ACTA2/αSMA
alpha smooth muscle actin
- MFAP5
microfibril‐associated protein 5
- COL11A1
collagen type XI alpha 1 chain
- TNC
tenascin‐C
- PDGFRα/β
platelet derived growth factor receptor alpha/beta
- DAPI
4′,6‐diamidino‐2‐phenylindole.
- VIM
vimentin
- S100A4 (FSP1)
S100 Calcium‐Binding Protein A4
- POSTN
Periostin
- EPCAM
epithelial cell adhesion molecule
- KRT20
keratin 20
- WNT7a
Wnt family member 7A
- PDGF
platelet derived growth factor
- SHH
Sonic Hedgehog
- IL1β
interleukin 1β
- TGF‐β
transforming growth factor beta
- IL17A
interleukin 17A
- WNT10b
Wnt family member 10B
- WNT2
Wnt family member 2
- IGF2
insulin like growth factor 2
- CXCL6
C‐X‐C motif chemokine ligand 6
- CXCL12
C‐X‐C motif chemokine ligand 12
- IL11
interleukin 11
- CALD1
high molecular weight caldesmon
- SMTN
smoothelin
- PTPRC
protein tyrosine phosphatase, receptor type
- PECAM1
platelet and endothelial cell adhesion molecule 1
- MMP2
matrix metalloproteinase 2
- DCN
decorin
- COL1A2
collagen type I alpha 2 chain
- PDGFA
platelet derived growth factor subunit A
- TAGLN
transgelin
- IL6
interleukin 6
- LIF
interleukin 6 family cytokine
- CCL11
C‐C motif chemokine ligand 11
- CD29
integrin beta‐1
- CD90
Thy‐1 cell surface antigen
- CD10
neprilysin
- GPR77
G protein‐coupled receptor 77
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- NG2
neural/glial antigen 2
- PDPN
podoplanin
- ITGA11
integrin α11β1
Background
According to the Oxford Concise Medical Dictionary, cancers are malignant neoplasms (including both carcinoma and sarcoma), which arise from the abnormal and uncontrolled division of cells and which invade and destroy the surrounding tissue.1 Over the past decade, however, a new paradigm has started to arise in tumor research. One that sees cancer, not as a disease solely focused on the core population of malignant neoplastic cells, but rather a condition characterized by a fundamental misalignment of the entire cellular milieu.
This tumor‐surrounding environment, termed the tumor microenvironment (TME), has been shown to play a seemingly ever‐increasing role in tumor development, especially in relation to tumor initiation and metastasis. Numerous different types of cells and factors have been described to play a role in the TME, ranging from immune cells, such as T, B and natural killer (NK) cells,2 to wider environmental factors, such as extracellular matrix (ECM) stiffness,3 hypoxia4 and interstitial pressure.5 Amongst all these various microenvironmental players, fibroblasts have been suggested to play a key role in tumor development.6 Despite being one of the most well‐studied cell types in biology, there is still much that remains unknown about the role and behavior of fibroblasts in the tumor. Fibroblasts greatly influence the tumor environment via the secretion of cytokines and chemokines, such as vascular endothelial growth factor A (VEGFA)7 and C‐X‐C Motif Chemokine Ligand 12 (CXCL12).8 It has been hypothesized that cross‐talk between tumorigenic cells and fibroblasts (Fig. 1) may be responsible for the emergence of a subpopulation of hyper‐activated fibroblasts that are present in the TME, called cancer‐associated fibroblasts (CAFs).9 These CAFs are highly heterogeneous and have been shown to enhance cellular migration and alter metabolism of epithelial tumor cells,10, 108 display elevated pro‐angiogenic cytokine signaling,11, 12 regulate the plasticity of cancer stem cells,79 play a significant role in the development of drug resistance,89, 94 and facilitate inflammation (Fig. 1).13, 74
Figure 1.
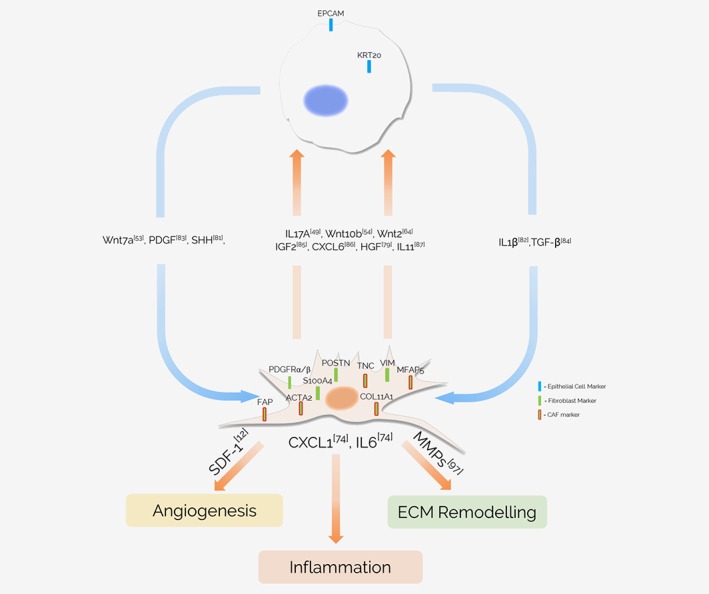
Molecular crosstalk between CAFs and tumor cells. Secretion of numerous cytokines by both epithelial tumor cells and cancer‐associated fibroblasts forms a complex network of intratumoral crosstalk between the two cell types, affecting numerous different cellular processes. The list of interactions depicted is not exhaustive. Abbreviations: FAP, fibroblast activation protein α; ACTA2(αSMA), alpha smooth muscle actin; MFAP5, microfibril‐associated protein 5; COL11A1, collagen type XI alpha 1 chain; TNC, tenascin‐C; PDGFRα/β, platelet derived growth factor receptor alpha/beta; VIM, vimentin; S100A4 (FSP1), S100 calcium‐binding protein A4; POSTN, periostin; EPCAM, epithelial cell adhesion molecule; KRT20, keratin 20; WNT7a, Wnt family member 7A; PDGF, platelet derived growth factor; SHH, sonic hedgehog; IL1β, interleukin 1β; TGF‐β, transforming growth factor beta; IL17A, interleukin 17A; WNT10b, Wnt family member 10B; WNT2, Wnt family member 2; IGF2, insulin like growth factor 2; CXCL6, C‐X‐C motif chemokine ligand 6; HGF, hepatocyte growth factor; IL11, interleukin 11; MMPs, matrix metalloproteinases; IL6, Interleukin 6; SDF‐1, stromal cell‐derived factor 1; CXCL1, chemokine (C‐X‐C motif) ligand 1. [Color figure can be viewed at http://wileyonlinelibrary.com]
The presence of CAFs is an effective predictor of tumor reoccurrence in colorectal cancer patients and has been highlighted as a significant prognostic factor in a number of tumor types.14, 15, 78 CAFs have also been suggested to potentially play a tumor‐suppressive role via the I kappa B kinase/nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) pathway, lowering hepatocyte growth factor (HGF) secretion and reducing tumor size and metastasis.16 All of this only serves to demonstrate the large number of vital roles that these cells play in the tumor microenvironment and underline the necessity of fully elucidating the function and behavior of CAFs within tumors.
However, due to their extremely heterogeneous nature and high plasticity, variation within CAF populations is extensive (Fig. 2). As such, the difference between a CAF and a normal fibroblast in the tumor microenvironment is often considered functional, rather than defined by the specific expression of a certain biological marker or easily definable feature. That is not to say that fibroblast‐ and CAF‐associated markers have not been identified (Table 1). A number of markers, such as αSMA, PDGFRα and FAP, are highly expressed in CAFs and have been widely used in order to isolate CAF populations. However, many of these markers come with their own set of downsides, such as low specificity, and questions have been raised on whether or not such markers can identify all cancer‐associated fibroblasts, or merely a specific subset of fibroblasts within the wider CAF population. This review aims to give an overview of the markers that are currently used for fibroblast and CAF identification and to discuss their various advantages and disadvantages.
Figure 2.
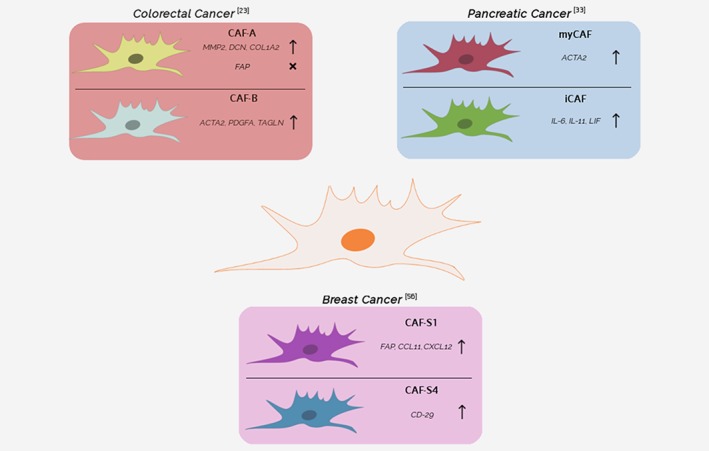
Subtypes of cancer‐associated fibroblasts. An outline of different types of CAFs found in breast, pancreatic, and colon cancer. The figure does not display an exhaustive list of all CAF subtypes and additional subtypes can be suspected to be present in the TME (and in other cancer types). Abbreviations: myCAF, myofibroblastic CAF; iCAF, inflammatory CAF; FAP, fibroblast activation protein α; ACTA2 (αSMA), alpha smooth muscle actin; MMP2, matrix metalloproteinase 2; DCN, decorin; COL1A2, collagen type I alpha 2 chain; PDGFA, platelet derived growth factor subunit A; TAGLN, transgelin; IL6, interleukin 6; IL11, Interleukin 11; LIF, interleukin 6 family cytokine; CCL11, C‐C motif chemokine ligand 11; CXCL12, C‐X‐C motif chemokine ligand 12; CD29, integrin beta‐1. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Markers used in the identification of fibroblasts and CAFs
| Positive markers | Marker comments | Surface marker |
|---|---|---|
| CAF markers | ||
| FAP | Most promising target of CAf‐based therapies. Has been shown to be mainly expressed by the non‐myofibroblast sub‐population of CAFs. Also displayed by epithelial cells undergoing EMT.23, 25, 29 | Yes |
| α‐SMA/ACTA2 | Widely considered to be the most reliable CAF‐specific marker. Not expressed by all functionally active CAFs. Downregulaled in one CAF subtype (CAF‐A).23, 28, 66 | No |
| MFAP5 | A novel marker identified by Yeung et al. Suggested to be extremely specific to CAFs. but is rarely used in existing literature. Recent studies suggest that MFAP5 expression may vary between subtypes.23, 29, 67, 68 | No |
| COL11A1 | A novel marker identified by Vazquez‐Villa et al. Suggested to be extremely specific to CAFs, but is rarely used in existing literature.69 | No |
| TN‐C | A myofibroblast‐associated marker that has been used to identify CAFs in the past. Has been shown to be an important driver of metastasis.28, 70, 71, 72 | No |
| PDPN | Often overexpressed in certain CAF subtypes. Unspecific for fibroblasts and expressed by tumor cells and macrophages.98, 99, 100 | Yes |
| ITGA11 | Shown to be upregulated in non‐small cell lung cancer CAFs Expressed by numerous tumor cell lines and shows variability based on environmental factors.101, 102, 103 | Yes |
| NG2 | A marker expressed by certain CAF sub‐populations. Not specific for fibroblasts and expressed by numerous other cells, such as myeloid and T‐cells.17, 29 | Yes |
| Fibroblast markers | ||
| PDGFRα/β | Common markers used for fibroblast identification. PDGFRα is much more widely expressed over the larger fibroblast population than more specific markers such as αSMA.29, 70, 73, 74 | Yes |
| VIM | Traditional marker for fibroblast identification. Not CAF‐specific and widely expressed by all fibroblasts.45, 46, 47 | No |
| FSP‐1/S100A4 | Common fibroblast marker. Expressed by cells of mesenchymal origins. Not expressed by all fibroblast present in a tumor. Considered to be a marker for quiescent fibroblasts, rather than CAFs.6, 25, 75 | No |
| POSTN | Not specific for cancer‐associated fibroblasts and is expressed in normal fibroblasts.41, 54 | No |
| COL1 | A histochemical marker commonly used in older lo identify fibroblast populations. Not exclusive to fibroblasts.91, 92 | No |
| Negative Markers | Marker Comments | Surface Marker |
|---|---|---|
| EPCAM | A known market for epithelial cells. Not expressed by fibroblast cells.14, 45, 56 | Yes |
| CALD1 | Negative fibroblast market. Positive for pericytes.49 | No |
| SMTN | Negative fibroblast market. Positive for smooth muscle cells.10, 49 | Yes |
| PTPRC | Negative marker used to identify leukocytes.25, 33, 53 | Yes |
| PECAM1 | Negative marker used in order to identify endothelial cell populations.14, 15, 25 | Yes |
Abbreviations: FAP, fibroblast activation protein α; ACTA2 (αSMA), alpha smooth muscle actin; MFAP5, microfibrillar‐associated protein 5; COL11A1, collagen type XI alpha 1 chain; TNC, tenascin‐C; PDPN, podoplanin; ITGA11, integrin α11β1; NG2, neural/glial antigen 2; PDGFRα/β, platelet derived growth factor receptor alpha/beta; VIM, vimentin; FSP‐1, fibroblast‐specific protein 1; POSTN, periostin; EPCAM, epithelial cell adhesion molecule; CALD1, high molecular weight caldesmon; SMTN, smoothelin; PTPRC, protein tyrosine phosphatase; receptor type C; PECAM1, platelet and endothelial cell adhesion molecule 1.
FAP
Fibroblast Activation Protein α, or FAP as it is more commonly known, is a type II integral membrane protein that belongs to the membrane‐bound serine protease family. FAP has traditionally been associated with tissue repair, fibrosis and extracellular matrix degradation by fibroblasts due to its dipeptidyl peptidase and collagenase activity,17 but has also been shown to be upregulated in fetal mesenchymal tissues and during embryogenesis.18 It is one of the most strongly expressed genes in the tumor stroma and is upregulated in over 90% of epithelial carcinomas.19
Due to its high expression in the tumor stroma, numerous studies have used FAP as a marker of activated cancer‐associated fibroblasts.14, 63, 64, 65 This has resulted in the widespread use of FAP as an identifier of potential CAF populations, typically in combination with negative epithelial markers such as epithelial cell adhesion molecule (EPCAM). FAP is also widely considered one of the most viable CAF‐markers for potential clinical application. Depletion of the FAP‐positive fibroblast population in transgenic mice led to cytokine‐mediated hypoxic necrosis of both the tumor and the stroma,20 and FAP‐based therapies, such as FAP‐inhibitors and FAP‐targeting monoclonal antibodies, have been submitted for clinical trials.21, 22 However, no proposed FAP‐based therapy has yet proven to be effective in clinical application, as both Talabostat (a small molecule FAP‐inhibitor) and Sibrotuzumab (a FAP‐targeting monoclonal antibody) were incapable of successfully passing Phase II trials, as neither therapy could demonstrate efficacy in colorectal cancer patients.21, 22 While these results were initially perplexing, other studies over the last couple of years have raised considerable questions about the viability of FAP as a clinical CAF marker. In their recent study, Li et al. used single‐cell sequencing to characterize the transcriptome of the TME and demonstrated that only a certain sub‐population of CAFs within the tumor microenvironment actually expressed FAP and that FAP‐expression was completely absent in the other identified tumor fibroblast sub‐population.23 Similar heterogeneity of FAP expression can also be observed when immunofluorescence staining of FAP is carried out on primary colon cancer fibroblasts (Fig. 3).
Figure 3.
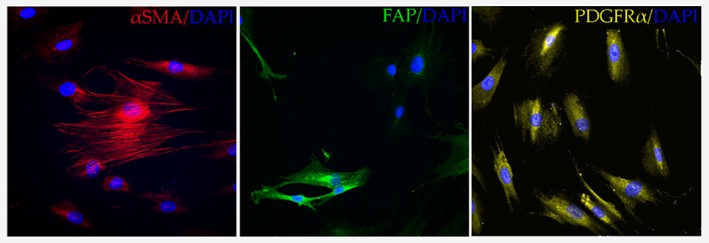
Expression of common markers in patient‐derived fibroblasts. Immunofluorescent staining of primary colon cancer fibroblasts (Neuromics, #CAF05), reveals a heterogeneous expression pattern for both αSMA/ACTA2 (abcam #ab7817, 1/200) and FAP (Santa‐Cruz Biotechnology #sc‐65,398, 1/200), while PDGFRα (abcam #ab61219, 1/200) expression in tested cells remains relatively homogenous. Nuclei of fibroblasts were stained using DAPI (DAPI Fluoromount‐G® Mounting Medium). Image is representative of three independent biological experiments. Cells were imaged using a Zeiss LSM 510 Meta laser scanning confocal microscope (Carl Zeiss, Jena, Germany) with a Plan‐Apochromat 63x/1.40 Oil DIC M27 objective (x60). Images were processed using NIS elements software (Nikon) and ImageJ 1.51 s. Abbreviations: FAP: Fibroblast Activation Protein α, αSMA: Alpha Smooth Muscle Actin, PDGFRα: Platelet Derived Growth Factor Receptor Alpha, DAPI: 4′,6‐diamidino‐2‐phenylindole.
In addition, numerous studies have shown that epithelial cells undergoing epithelial‐mesenchymal transition (EMT) also express elevated levels of FAP,24, 25, 26 raising doubts about the specificity of FAP in the tumor microenvironment. In light of these results, it would not be surprising if the lack of clinical significance shown by FAP‐targeted therapies were due to the heterogeneous expression of FAP across CAF populations and other cell types. As such, due to the existence of non‐FAP expressing CAF sub‐populations, it is unlikely that FAP is applicable as a singular marker for CAF identification in the tumor microenvironment.
αSMA
Alpha‐smooth muscle actin (αSMA), also known as smooth muscle aortic alpha‐actin (ACTA2), is a member of the actin family, a highly conserved group of proteins that play an important role in cell motility, structure and integrity. αSMA is best known for its role in wound healing, where it is one of the major causes of myofibroblast contractility, via microfilament bundle and stress fiber regulation. This αSMA‐induced mechanical stress plays a considerable role in the contraction and maturation of the granulation tissue—new connective tissue that forms on the wound surface during the injury healing process.27 As the number of myofibroblasts is much higher in the tumor microenvironment, αSMA has become one of the go‐to markers for identifying CAF populations.28, 29
In addition to its role as a marker for cancer‐associated fibroblasts, αSMA has also been identified as a prominent prognostic factor in tumor patients. αSMA expression correlates strongly with a higher risk of recurrence in colon cancer patients and higher expression of αSMA‐positive fibroblasts has been strongly linked to lower overall survival in breast30 and colon cancer.31 Myofibroblasts have also been suggested to play a role in both the secretion of cytokines, such as CXCL12 and Interleukin 6 (IL‐6), as well as the physical remodeling of the extracellular matrix, which has been shown to significantly alter patient survival rates in esophageal, colorectal and head and neck cancer.32
However, in a similar manner to FAP and other CAF‐associated markers, such as Transgelin (TAGLN), αSMA has been suggested to show variable expression between different CAF subtypes. In a paper published by Öhlund et al. in 2017, it was demonstrated that αSMA expression drops significantly in patients and murine‐derived CAFs when such cells are co‐cultivated with organoids derived from pancreatic ductal adenocarcinoma patients. This was found to be due to a transition from a more myofibroblast‐like to a more inflammatory CAF subtype, caused by paracrine factors, and resulting in a large drop in αSMA expression.33 Similar results can be seen in colon cancer, where certain subtypes have been shown to be characterized by a far lower degree of αSMA expression.23 In addition, αSMA is also not truly specific for cancer‐associated fibroblasts, as smooth muscle cells and pericytes have also been demonstrated to express significant levels of the protein.34, 35, 80 Furthermore, alpha‐smooth muscle actin, as a CAF marker, is also hampered by its intracellular localization, making it unviable for flow‐sorting CAF populations for further functional assays. All in all, it is difficult to recommend the usage of αSMA alone as a primary marker for CAF identification, mainly due to the significant heterogeneity of its expression amongst the larger CAF population. Primary selection based on αSMA expression would no doubt result in the loss of large numbers of cancer‐associated fibroblasts that do not express a myofibroblast‐like phenotype.
PDGFRα/β
Platelet‐derived growth factor receptors (PDGFRs) are tyrosine kinase receptors located on the surface of cells such as fibroblasts, astrocytes, neuroprogenitors and pericytes36 and can be divided into two main types—PDGFRα and PDGFRβ. Both are commonly used as general markers for fibroblasts, and overexpression of PDGF receptors has been observed in multiple tumor types, such as glioma, prostate and ovarian cancer.37 Expression of platelet‐derived growth factors (PDGFs), the ligands of PDGFRs, has also been heavily correlated with tumor development and CAF function. For example, expression of PDGFB has been strongly associated with tumor stroma formation in melanoma,38 while PDGFA has been shown to promote the recruitment of PDGFRα+ stromal fibroblasts to the outer rim of the tumor site in xenograft mouse models of lung carcinomas.39 Notably, elevated PDGFRβ expression, in particular, has also been shown to be associated with Tamoxifen resistance,94 as well as lower prognosis, drug resistance and higher tumor recurrence rates in both breast and prostate cancer.94, 95
In contrast to FAP and αSMA, the strength of PDGFRs lies not in their relative specificity for cancer‐associated fibroblasts, but rather in their widespread expression in the overall fibroblast population present in the tumor (Fig. 3). Neither PDGFRα nor PDGFRβ show significant upregulation in CAF populations,29 but do seem to be expressed more broadly in fibroblasts than comparative markers, such as αSMA, and in a manner that is less responsive to environmental factors such as hypoxia.40 While this means that PDGFRs are somewhat limited as primary CAF markers, they can be used as more general fibroblast markers in combination with more specific CAF markers, due to their more stable expression, especially when compared to other markers which seem to be sensitive to factors such as CAF subtype (αSMA, FAP) or hypoxia (αSMA, POSTN). Another strength of the PDGFRs is that, unlike αSMA, they are surface‐bound markers, allowing for flow cytometry‐based sorting of viable fibroblast populations for long‐term assays and cultures.
As with FAP, platelet‐derived growth factor receptors are also considered to be a potential avenue for therapeutic intervention. Crenolanib, a receptor tyrosine kinase inhibitor for PDGFRα and PDGFRβ, is currently undergoing phase III trials in advanced or metastatic gastrointestinal stromal tumor (GIST) patients.41 While this is largely driven by the common nature of PDGFRα mutations in GIST patients, which is mutated in approximately 10% of all patients,42 the heightened activity of the PDGFR pathways in CAFs make them an attractive target for potential therapeutics. Furthermore, other PDGFR inhibitors such as Dasatinib and Imatinib, have been shown to be capable of reducing the pro‐proliferative effect of CAF conditioned media and changing the microarray gene expression signature of primary cancer‐associated fibroblasts into one more closely resembling normal non‐tumorigenic fibroblasts.43
Vimentin
Vimentin is a type III intermediate filament protein, which plays an important role in the formation of the cytoskeleton network, especially in cells of mesenchymal origin. This network is key for organelle placement within cells, cellular migration and adhesion. In addition, Vimentin binds to phosphorylated extracellular signal‐regulated kinase (pERK) and rhodopsin kinase (RhoK), allowing it to alter actin organization and initiate mitogen‐activated protein kinase (MAPK) cascades.44
As fibroblasts are strongly characterized by their mesenchymal phenotype, Vimentin is highly expressed in fibroblasts of all types. This has led to the widespread use of Vimentin as a common method of visually identifying fibroblast populations through both immunofluorescent45, 46 as well as immunohistochemical staining.47 However, the effectiveness of Vimentin as a CAF‐specific marker is greatly hampered by its widespread expression throughout both the overall fibroblast population45 and numerous other cell types, such as macrophages and adipocytes.29 In addition, Vimentin is expressed by epithelial cells undergoing epithelial‐to‐mesenchymal transition (EMT), during which tumor cells display heightened expression of a wide variety of mesenchymal markers.48 As Vimentin is also present in a number of different cell types of mesenchymal origin, such as adipocytes and myocytes, its overall specificity as a marker, even for fibroblasts, is quite low. Furthermore, like αSMA, Vimentin is hampered by its intracellular localization, making it incapable of separating viable fibroblast populations via methods such as fluorescence‐activated cell sorting (FACS).
Other Markers
In addition to the four markers highlighted previously, there are other positive markers for CAFs that have been used to identify cancer‐associated fibroblasts in some capacity. However, many of them still run into similar issues to the markers described above, mainly associated with the lack of specificity, variable expression across the overall CAF population, intracellular localization, or, in some cases, simple obscurity and lack of characterization.
S100 calcium binding protein A4 (S100A4), also known as fibroblast‐specific protein 1 (FSP1), for example, is a marker that has been used in a number of publications in order to confirm the CAF phenotype of examined cells.49, 50, 51 However, recent studies have raised some suspicion on the specificity of FSP1, as it has been observed to be less reliable for fibroblast identification from primary tumor samples than FAP.25 It has also been confirmed to be expressed by metastatic prostate cancer‐derived epithelial cell lines and tissues.25 Furthermore, FSP1 expression in fibroblasts is also strongly variable between different CAF sub‐populations.52
Other putative markers, such as Transgelin (TAGLN) and Periostin (POSTN), are also highly expressed in fibroblast and CAF populations76 and have been used as secondary markers alongside the primary markers described above.40, 53 However, much like their more commonly used counterparts, specificity,54 subtype variance,23 and intracellular localization are all aspects which greatly complicate their use as CAF markers.
Podoplanin (PDPN) is another membrane‐bound marker that has been observed to be overexpressed in CAF populations. While it is not specific to fibroblasts, being also expressed in epithelial tumor cells98 and inflammatory macrophages,99 recent studies do suggest that this marker could potentially be used in order to identify pro‐tumorigenic fibroblast subpopulations, as PDPN‐positive fibroblasts have been shown to be correlated with worse outcomes across multiple different tumor types.100
In addition to the aforementioned markers, Integrin α11β1 (ITGA11) has also been highlighted as a major collagen receptor that undergoes upregulation in non‐small cell lung cancer CAFs.77, 101, 102 However, ITGA11 expression has been shown to be present in numerous different tumor cell lines,103 sensitive to environmental conditions such as hypoxia,103 and its expression has been linked to TGF‐β signaling in the past,104 which has been shown to play a role as both an inducer and antagonist of certain CAF subtypes.105 All of this suggests that further research is necessary in order to validate the subtype‐specificity of this specific integrin.
Two markers, Microfibril Associated Protein 5 (MFAP5) and Collagen Type XI Alpha I Chain (COL11A1), have also been suggested to be highly specific CAF‐markers.29, 55 Their usage is currently still limited amongst the academic community, and further characterization of these markers and their behavior and expression in different tumor types and CAF subtypes is sorely needed. Results obtained by Li et al. seem to suggest that MFAP5 expression, at the very least, is not widely conserved between different CAF populations and can significantly vary based on the subtype in question.23 However, MFAP5 has also been shown to be greatly elevated in CAF secretomes of oral tongue squamous cell carcinoma patients, where its expression was linked to the activation of various pro‐growth pathways such as MAPK.88 This suggests that while its expression may be variable in the overall CAF population, MFAP5 may still potentially play a role in identifying critical pro‐tumorogenic cancer‐driving CAF subpopulations.
Finally, there are a number of negative markers that are commonly used to help in the identification of fibroblast/CAF populations. Due to the lack of a single definitive marker of CAFs, and the lack of specificity for many of the positive markers used for CAF identification, negative selection is vital in order to exclude a number of cell types that can be typically found in tumor tissue samples. Markers such as epithelial cell adhesion molecule (EPCAM) and Smoothelin (SMTN) are widely used to discriminate against epithelial14, 45, 56 and smooth muscle cells,10, 49 respectively. Other negative markers, such as CD45, CD34 and CD11b have also been used to exclude non‐fibroblast cell populations such as leukocytes and endothelial cells.57
Heterogeneity and Plasticity: Challenges and Future Possibilities
All in all, it is clear that there are no single definitive markers that can be used in order to identify CAF populations. Indeed, considering the large number of roles that cancer‐associated fibroblasts can play in the tumor environment, including both tumor‐suppressive and tumor‐promoting activities,6 as well as the constantly increasing number of various CAF subtypes, it becomes questionable whether or not such a convenient marker even exists in the first place.
This observed heterogeneity of CAFs potentially reflects a situation similar to the one seen in cancer stem cells. Like CAFs, cancer stem cells have been shown to be highly plastic and express various markers which vary over time.93 As such, the definition of a cancer stem cell refers more to a specific cell state rather than to a distinct cell type. Indeed, flow cytometry experiments coupled to Markov model predictions have highlighted that different purified breast cancer cell populations display extensive plasticity and always return to a phenotypic proportion equilibrium over time.51 Culturing conditions, such as the presence of a 3D‐matrix, as well as numerous extrinsic factors, have also been suggested to largely influence gene expression in fibroblasts.106 Even standard cell culture passaging has been associated with changes in the gene expression profile of certain types of fibroblasts, such as rheumatoid arthritis synovial fibroblasts.107 Keeping all of this in mind, it may be that CAFs could be considered to be a dynamic state of fibroblasts, rather than a unique population. In addition, epigenetic changes could directly influence CAFs and their marker expression.6 Future studies that systematically address the expression of CAF markers, combined with genomic and transcriptomic profile analysis of single fibroblasts, could potentially help elucidate these controversies.
In light of this, functional selection, based on physical characteristics and unhampered by heterogeneous marker expression, may serve as an alternative approach for identifying CAF populations. Due to their higher contractility, contraction assays have been previously used to differentiate between CAFs and normal fibroblasts.88 As increased contractility is a characteristic that is traditionally more associated with myofibroblast subtypes, rather than secretory CAF subtypes, it could potentially serve as a novel avenue of fibroblast characterization and a method used for differentiating between CAF subtypes in the future. In addition, three‐dimensional hydrogel assays, using substances such as collagen or matrigel, are another way through which different CAF subtypes could potentially be tested and characterized in a functional manner. In fact, marker heterogeneity may even turn out to be a boon, rather than a bane, as subtype‐specific marker expression could hypothetically allow for the targeting of these populations in a relatively specific manner. A recent study by Su et.al highlights the potential of this approach, as they were able to pin down a subpopulation of Neprilysin (CD10) and G protein‐coupled receptor 77 (GPR77) positive fibroblasts that were shown to play a significant role in promoting chemoresistance and cancer stemness via persistent p65‐driven NF‐κB activation.89
Conclusion
Regardless, additional clarity is sorely needed in the field, as the inherent vagueness that surrounds the classification of cancer‐associated fibroblasts has already led to rather conflicting results. In Nature's News and Views section, E. Wagner highlighted two articles published in the Journal of Experimental Medicine where the effect of IKKβ deletion was examined in cancer‐associated fibroblasts.58 Despite looking at the same gene, the two papers came at two separate, almost contradictory, conclusions, suggesting that deletion of IKKβ results in both enhanced tumor growth16 and decreased inflammation and tumor suppression.59 While other experimental differences may play a significant role in the results observed, it should still be noted that the two papers used differing protocols and markers for fibroblast identification (COL I and COL VI) and characterization. This serves to underscore the potential difficulties that may arise when the definition of cancer‐associated fibroblasts is so extremely vague and the use of defining markers so variable between different publications.
This lack of specific definition is further confounded by the extreme heterogeneity and plasticity that can be observed within the overall CAF population (Figs. 2 and 3). As mentioned before, the marker expression within CAF subtypes can vary significantly, to the point where some of the most commonly used CAF markers, such as FAP, are simply non‐existent within certain CAF‐subtypes.23 This, in turn, casts doubt when such markers are used to produce results that are extrapolated to apply to the CAF population at large. While the usage of negative markers is relatively common, the number of positive markers used for CAF selection is still typically limited to one or two and often include markers that have been clearly demonstrated to be extremely heterogeneous (αSMA and FAP). This leaves open the possibility that the chosen markers could have selected for specific or excluded certain sub‐populations. While this is something that can be taken into account, it does potentially raise questions about a number of previously published studies that have not considered this subtype variance.
When selecting for CAF populations using antibody‐based methods such as FACS, it is essential that multiple surface markers are used in order to avoid any chance of introducing unintentional subtype bias. Other available surface markers such as PDGFRα/β work well here, as do more general fibroblast surface markers like Thy‐1 cell surface antigen (CD90), provided that the cell population is also subjected to selection with negative markers.57, 60 This is especially important, as a significant number of commonly used fibroblast markers display expression over a number of different cell types,25, 34 running the risk of inadvertent sample contamination if a proper negative selection is not carried out. Further stratification of the isolated general population could then be carried out using various more subtype‐specific markers such as FAP, PDGFRβ and GPR77/CD19.23, 89, 96
All of this serves to underline the importance of further research into the roles and characteristics of fibroblasts in the tumor microenvironment. A number of studies have already been conducted in order to identify the various CAF subtypes that reside within the overall tumor microenvironment.23, 33, 56, 89, 90, 96 These subtypes have been shown to possess extreme variability in regards to their marker expression, with certain subtypes showing almost no expression of certain markers, such as FAP23 or PDGFRβ.96 Others have highlighted CAF subpopulations with unique surface markers, such as GPR77 and CD10, which are capable of maintaining cancer stemness and promoting chemoresistance.89 Even aspects such as tumor proximity33 have been shown to play a key role in the development of different CAF subtypes with significant functional and phenotypical differences. Keeping this in mind, studies aimed at identifying and characterizing the distribution of various CAF subtypes in tumors, such as those carried out by Costa et al. in breast cancer, have recently risen to increased prominence.56 These studies are incredibly vital, as the characterization of these novel subtypes, their marker expression and discovery of new functional categories of cancer‐associated fibroblasts is essential in understanding exactly how to identify these elusive cells, as well as the role that they play within tumors.
Furthermore, despite the tumor‐promoting and tumor‐suppressing role of CAFs in the TME, it is also important to consider the opposite ‐ how various aspects of the TME change the characteristics of fibroblasts. CAF phenotype and marker expression have been suggested to vary significantly when in the presence of other members of the tumor microenvironment61, 62 and even aspects such as fibroblast proximity to tumor cells have been identified as potential drivers of fibroblast subtype differentiation.33 Additionally, further research will hopefully manage to better elucidate how tumor fibroblasts obtain and maintain a “CAF state” and dissect the various signaling pathways involved. Finally, ass most therapies targeting cancer‐associated fibroblasts have so far exhibited mixed results, future studies, using novel methods such as lineage tracing, single‐cell sequencing, or immunophenotyping, will allow us to better understand the function and behavior of tumor‐associated fibroblasts, thereby improving the capacity to identify, isolate and target these cells in a more specific and therapeutically viable manner.
Authors’ contribution
M.N. contributed to the collection of data, preparation of the figures and tables, and writing of the manuscript. P.U. contributed to data collection and preparation of Figure 1. M.N., E.L., P.U. and S.H. reviewed the manuscript. E.L. contributed to the establishment of the structure of this manuscript and acted as the main supervisor. F.R. provided assistance in carrying out immunofluorescence staining and imaging. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge Geoffroy Walbrecq for his technical assistance during confocal microscopy.
Conflict of interest: The authors declare that they have no competing interests.
References
- 1. Martin E. Concise medical dictionary. Oxford: Oxford University Press, 2015. [Google Scholar]
- 2. Giraldo N, Becht E, Remark R, et al. The immune contexture of primary and metastatic human tumours. Curr Opin Immunol 2014;27:8–15. [DOI] [PubMed] [Google Scholar]
- 3. Kalli M, Stylianopoulos T. Defining the role of solid stress and matrix stiffness in cancer cell proliferation and metastasis. Front Oncol 2018;8 10.3389/fonc.2018.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrova V, Annicchiarico‐Petruzzelli M, Melino G, et al. The hypoxic tumour microenvironment. Oncogene 2018;7 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heldin C, Rubin K, Pietras K, et al. High interstitial fluid pressure — an obstacle in cancer therapy. Nat Rev Cancer 2004;4:806–13. [DOI] [PubMed] [Google Scholar]
- 6. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–98. [DOI] [PubMed] [Google Scholar]
- 7. Guo X, Oshima H, Kitmura T, et al. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem 2008;283:19864–71. [DOI] [PubMed] [Google Scholar]
- 8. Kojima Y, Acar A, Eaton E, et al. Autocrine TGF‐ and stromal cell‐derived factor‐1 (SDF‐1) signaling drives the evolution of tumor‐promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA 2010;107:20009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moore‐Smith L, Isayeva T, Lee J, et al. Silencing of TGF‐β1 in tumor cells impacts MMP‐9 in tumor microenvironment. Sci Rep 2017;7:8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tommelein J, Verset L, Boterberg T, et al. Cancer‐associated fibroblasts connect metastasis‐promoting communication in colorectal cancer. Front Oncol 2015;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drebert Z, MacAskill M, Doughty‐Shenton D, et al. Colon cancer‐derived myofibroblasts increase endothelial cell migration by glucocorticoid‐sensitive secretion of a pro‐migratory factor. Vascul Pharmacol 2017;89:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orimo A, Gupta P, Sgroi D, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF‐1/CXCL12 secretion. Cell 2005;121:335–48. [DOI] [PubMed] [Google Scholar]
- 13. Servais C, Erez N. From sentinel cells to inflammatory culprits: cancer‐associated fibroblasts in tumour‐related inflammation. J Pathol 2012;229:198–207. [DOI] [PubMed] [Google Scholar]
- 14. Berdiel‐Acer M, Sanz‐Pamplona R, Calon A, et al. Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information. Mol Oncol 2014;8:1290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paulsson J, Micke P. Prognostic relevance of cancer‐associated fibroblasts in human cancer. Semin Cancer Biol 2014;25:61–8. [DOI] [PubMed] [Google Scholar]
- 16. Pallangyo CK, Ziegler PK, Greten FR. IKKβ acts as a tumor suppressor in cancer‐associated fibroblasts during intestinal tumorigenesis. J Exp Med 2015;212:2253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuveson D, Elyada E, Öhlund D. Fibroblast heterogeneity in the cancer wound. J Cell Biol 2014;206:1503–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brennen W, Isaacs J, Denmeade S. Rationale behind targeting fibroblast activation protein‐expressing carcinoma‐associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther 2012;11:257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huber M, Schubert R, Peter R, et al. Fibroblast activation protein: differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Investig Dermatol 2003;120:182–8. [DOI] [PubMed] [Google Scholar]
- 20. Kraman M, Bambrough P, Arnold J, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein. Science 2010;330:827–30. [DOI] [PubMed] [Google Scholar]
- 21. Narra K, Mullins S, Lee H, et al. Phase II trial of single agent Val‐boroPro (talabostat) inhibiting fibroblast activation protein in patients with metastatic colorectal cancer. Cancer Biol Ther 2007;6:1691–9. [DOI] [PubMed] [Google Scholar]
- 22. Hofheinz R, al‐Batran S, Hartmann F, et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of Sibrotuzumab in patients with metastatic colorectal cancer. Oncol Res Treat 2003;26:44–8. [DOI] [PubMed] [Google Scholar]
- 23. Li H, Courtois E, Sengupta D, et al. Reference component analysis of single‐cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet 2017;49:708–18. [DOI] [PubMed] [Google Scholar]
- 24. Iwasa S, Jin X, Okada K, et al. Increased expression of seprase, a membrane‐type serine protease, is associated with lymph node metastasis in human colorectal cancer. Cancer Lett 2003;199:91–8. [DOI] [PubMed] [Google Scholar]
- 25. Kahounová Z, Kurfürstová D, Bouchal J, et al. The fibroblast surface markers FAP, anti‐fibroblast, and FSP are expressed by cells of epithelial origin and may be altered during epithelial‐to‐mesenchymal transition. Cytometry A 2017;93:941–51. [DOI] [PubMed] [Google Scholar]
- 26. Shi M. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol 2012;18:840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Micallef L, Vedrenne N, Billet F, et al. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair 2012; 5(Supplement 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mezawa Y, Orimo A. The roles of tumor‐ and metastasis‐promoting carcinoma‐associated fibroblasts in human carcinomas. Cell Tissue Res 2016;365:675–89. [DOI] [PubMed] [Google Scholar]
- 29. Gascard P, Tlsty T. Carcinoma‐associated fibroblasts: orchestrating the composition of malignancy. Genes Dev 2016;30:1002–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Surowiak P, Murawa D, Materna V, et al. Occurrence of stromal myofibroblasts in the invasive ductal breast cancer tissue is an unfavourable prognostic factor. Anticancer Res 2007;27:2917–24. [PubMed] [Google Scholar]
- 31. Tsujino T, Seshimo I, Yamamoto H, et al. Stromal Myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res 2007;13:2082–90. [DOI] [PubMed] [Google Scholar]
- 32. Hanley C, Noble F, Ward M, et al. A subset of myofibroblastic cancer‐associated fibroblasts regulate collagen fiber elongation, which is prognostic in multiple cancers. Oncotarget 2015;7:6159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Öhlund D, Handly‐Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bergers G, Song S. The role of pericytes in blood‐vessel formation and maintenance. Neuro Oncol 2005;7:452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Latif N, Sarathchandra P, Chester A, et al. Expression of smooth muscle cell markers and co‐activators in calcified aortic valves. Eur Heart J 2014;36:1335–45. [DOI] [PubMed] [Google Scholar]
- 36. Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the Normal and diseased nervous system. J Neuroimmune Pharmacol 2013;9:168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heldin C. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 2013;11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forsberg K, Valyi‐Nagy I, Heldin C, et al. Platelet‐derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF‐BB. Proc Natl Acad Sci USA 1993;90:393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tejada M. Tumor‐driven paracrine platelet‐derived growth factor receptor signaling is a key determinant of stromal cell recruitment in a model of human lung carcinoma. Clin Cancer Res 2006;12:2676–88. [DOI] [PubMed] [Google Scholar]
- 40. Madsen C, Pedersen J, Venning F, et al. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. EMBO Rep 2015;16:1394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randomized Trial of Crenolanib in Subjects With D842V Mutated GIST ‐ Full Text View‐ http://clinicaltrials.gov [Internet]. http://clinicaltrials.gov. 2018 [cited 22 August 2018]. Available from: https://clinicaltrials.gov/ct2/show/NCT02847429
- 42. Boonstra PA, Gietema JA, Suurmeijer AJ, et al. Tyrosine kinase inhibitor sensitive PDGFRΑ mutations in GIST: two cases and review of the literature. Oncotarget 2017;8:109836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haubeiss S, Schmid J, Mürdter T, et al. Dasatinib reverses cancer‐associated fibroblasts (CAFs) from primary lung carcinomas to a phenotype comparable to that of normal fibroblasts. Mol Cancer 2010;9:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pérez‐Sala D, Oeste C, Martínez A, et al. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat Commun 2015;6:7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hsia L, Ashley N, Ouaret D, et al. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc Natl Acad Sci USA 2016;113:E2162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herrera M, Islam A, Herrera A, et al. Functional heterogeneity of cancer‐associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin Cancer Res 2013;19:5914–26. [DOI] [PubMed] [Google Scholar]
- 47. Stock K, Estrada M, Vidic S, et al. Capturing tumor complexity in vitro: comparative analysis of 2D and 3D tumor models for drug discovery. Sci Rep 2016;6:28951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalluri R, Weinberg R. The basics of epithelial‐mesenchymal transition. J Clin Investig 2010;120:1786–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lotti F, Jarrar A, Pai R, et al. Chemotherapy activates cancer‐associated fibroblasts to maintain colorectal cancer‐initiating cells by IL‐17A. J Exp Med 2013;210:2851–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ziani L, Safta‐Saadoun T, Gourbeix J, et al. Melanoma‐associated fibroblasts decrease tumor cell susceptibility to NK cell‐mediated killing through matrix‐metalloproteinases secretion. Oncotarget 2017;8:19780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herrera M, Herrera A, Domínguez G, et al. Cancer‐associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci 2013;104:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zeisberg E, Potenta S, Xie L, et al. Discovery of endothelial to mesenchymal transition as a source for carcinoma‐associated fibroblasts. Cancer Res 2007;67:10123–8. [DOI] [PubMed] [Google Scholar]
- 53. Avgustinova A, Iravani M, Robertson D, et al. Tumour cell‐derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat Commun 2016;7:10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen S, Xu X, Wang X, et al. Identification of colonic fibroblast secretomes reveals secretory factors regulating colon cancer cell proliferation. J Proteomics 2014;110:155–71. [DOI] [PubMed] [Google Scholar]
- 55. Jia D, Liu Z, Deng N, et al. A COL11A1‐correlated pan‐cancer gene signature of activated fibroblasts for the prioritization of therapeutic targets. Cancer Lett 2016;382:203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Costa A, Kieffer Y, Scholer‐Dahirel A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018;33:463–479.e10. [DOI] [PubMed] [Google Scholar]
- 57. Takahashi H, Sakakura K, Kudo T, et al. Cancer‐associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 2016;8:8633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wagner E. Fibroblasts for all seasons. Nature 2016;530:42–3. [DOI] [PubMed] [Google Scholar]
- 59. Koliaraki V, Pasparakis M, Kollias G. IKKβ in intestinal mesenchymal cells promotes initiation of colitis‐associated cancer. J Cell Biol 2015;211:2115OIA273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kisselbach L, Merges M, Bossie A, et al. CD90 expression on human primary cells and elimination of contaminating fibroblasts from cell cultures. Cytotechnology 2009;59:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ronca R, Van Ginderachter J, Turtoi A. Paracrine interactions of cancer‐associated fibroblasts, macrophages and endothelial cells. Curr Opin Oncol 2018;30:45–53. [DOI] [PubMed] [Google Scholar]
- 62. Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. J Pathol 2016;241:313–5. [DOI] [PubMed] [Google Scholar]
- 63. De Marco P, Lappano R, Francesco E, et al. GPER signalling in both cancer‐associated fibroblasts and breast cancer cells mediates a feedforward IL1β/IL1R1 response. Sci Rep 2016;6:24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kramer N, Schmöllerl J, Unger C, et al. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene 2017;36:5460–72. [DOI] [PubMed] [Google Scholar]
- 65. Isella C, Terrasi A, Bellomo S, et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet 2015;47:312–9. [DOI] [PubMed] [Google Scholar]
- 66. Mitra A, Zillhardt M, Hua Y, et al. MicroRNAs reprogram Normal fibroblasts into cancer‐associated fibroblasts in ovarian cancer. Cancer Discov 2012;2:1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yeung T, Leung C, Mok S. CAF reprogramming inhibits ovarian cancer progression. Cell Cycle 2014;13:3783–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Colangelo T, Polcaro G, Muccillo L, et al. Friend or foe? Biochim Biophys Acta 2017;1867:1–18. [DOI] [PubMed] [Google Scholar]
- 69. Vázquez‐Villa F, García‐Ocaña M, Galván J, et al. COL11A1/(pro)collagen 11A1 expression is a remarkable biomarker of human invasive carcinoma‐associated stromal cells and carcinoma progression. Tumor Biol 2015;36:2213–22. [DOI] [PubMed] [Google Scholar]
- 70. Yeo S, Ha S, Lee K, et al. Twist1 is highly expressed in cancer‐associated fibroblasts of esophageal squamous cell carcinoma with a prognostic significance. Oncotarget 2017;8:65265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. De Wever O, Nguyen Q, Van Hoorde L, et al. Tenascin‐C and SF/HGF produced by myofibroblasts in vitro provide convergent pro‐invasive signals to human colon cancer cells through RhoA and Rac. FASEB J 2004;18:1016–8. [DOI] [PubMed] [Google Scholar]
- 72. Lowy C, Oskarsson T. Tenascin C in metastasis: a view from the invasive front. Cell Adh Migr 2015;9:112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sharon Y, Alon L, Glanz S, et al. Isolation of Normal and cancer‐associated fibroblasts from fresh tissues by fluorescence activated cell sorting (FACS). J Vis Exp 2013;71:4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Erez N, Truitt M, Olson P, et al. Cancer‐associated fibroblasts are activated in incipient neoplasia to orchestrate tumor‐promoting inflammation in an NF‐κB‐dependent manner. Cancer Cell 2010;17:135–47. [DOI] [PubMed] [Google Scholar]
- 75. Osterreicher C, Penz‐Osterreicher M, Grivennikov S, et al. Fibroblast‐specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci USA 2010;108:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Planche A, Bacac M, Provero P, et al. Identification of prognostic molecular features in the reactive stroma of human breast and prostate cancer. PLoS One 2011;6:e18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Navab R, Strumpf D, Bandarchi B, et al. Prognostic gene‐expression signature of carcinoma‐associated fibroblasts in non‐small cell lung cancer. Proc Natl Acad Sci USA 2011;108:7160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ha S, Yeo S, Xuan Y, et al. The prognostic significance of cancer‐associated fibroblasts in esophageal squamous cell carcinoma. PLoS One 2014;9:e99955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lau E, Lo J, Cheng B, et al. Cancer‐associated fibroblasts regulate tumor‐initiating cell plasticity in hepatocellular carcinoma through c‐met/FRA1/HEY1 signaling. Cell Rep 2016;15:1175–89. [DOI] [PubMed] [Google Scholar]
- 80. Erkan M, Michalski C, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 2008;6:1155–61. [DOI] [PubMed] [Google Scholar]
- 81. Valenti G, Quinn H, Heynen G, et al. Cancer stem cells regulate cancer‐associated fibroblasts via activation of hedgehog signaling in mammary gland tumors. Cancer Res 2017;77:2134–47. [DOI] [PubMed] [Google Scholar]
- 82. Dudás J, Fullár A, Bitsche M, et al. Tumor‐produced, active Interleukin‐1 β regulates gene expression in carcinoma‐associated fibroblasts. Exp Cell Res 2011;317:2222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bronzert D, Pantazis P, Antoniades H, et al. Synthesis and secretion of platelet‐derived growth factor by human breast cancer cell lines. Proc Natl Acad Sci 1987;84:5763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jotzu C, Alt E, Welte G, et al. Adipose tissue derived stem cells differentiate into carcinoma‐associated fibroblast‐like cells under the influence of tumor derived factors. Cell Oncol 2011;34:55–67. [DOI] [PubMed] [Google Scholar]
- 85. Unger C, Kramer N, Unterleuthner D, et al. Stromal‐derived IGF2 promotes colon cancer progression via paracrine and autocrine mechanisms. Oncogene 2017;36:5341–55. [DOI] [PubMed] [Google Scholar]
- 86. Otomo R, Otsubo C, Matsushima‐Hibiya Y, et al. TSPAN12 is a critical factor for cancer–fibroblast cell contact‐mediated cancer invasion. Proc Natl Acad Sci 2014;111:18691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Calon A, Espinet E, Palomo‐Ponce S, et al. Dependency of colorectal cancer on a TGF‐β‐driven program in stromal cells for metastasis initiation. Cancer Cell 2012;22:571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Principe S, Mejia‐Guerrero S, Ignatchenko V, et al. Proteomic analysis of cancer‐associated fibroblasts reveals a paracrine role for MFAP5 in human Oral tongue squamous cell carcinoma. J Proteome Res 2018;17:2045–59. [DOI] [PubMed] [Google Scholar]
- 89. Su S, Chen J, Yao H, et al. CD10 + GPR77 + cancer‐associated fibroblasts promote cancer formation and Chemoresistance by sustaining cancer Stemness. Cell 2018;172:841–56.e16. [DOI] [PubMed] [Google Scholar]
- 90. Calon A, Lonardo E, Berenguer‐Llergo A, et al. Stromal gene expression defines poor‐prognosis subtypes in colorectal cancer. Nat Genet 2015;47:320–9. [DOI] [PubMed] [Google Scholar]
- 91. Sugimoto H, Mundel T, Kieran M, et al. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 2006;5:1640–6. [DOI] [PubMed] [Google Scholar]
- 92. Nishishita R, Morohashi S, Seino H, et al. Expression of cancer‐associated fibroblast markers in advanced colorectal cancer. Oncol Lett 2018;15:6195–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qureshi‐Baig K, Ullmann P, Haan S, et al. Tumor‐initiating cells: a criTICal review of isolation approaches and new challenges in targeting strategies. Mol Cancer 2017;16:7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Paulsson J, Rydén L, Strell C, et al. High expression of stromal PDGFRβ is associated with reduced benefit of tamoxifen in breast cancer. J Pathol Clin Res 2016;3:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nordby Y, Richardsen E, Rakaee M, et al. High expression of PDGFR‐β in prostate cancer stroma is independently associated with clinical and biochemical prostate cancer recurrence. Sci Rep 2017;7:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Givel A, Kieffer Y, Scholer‐Dahirel A, et al. miR200‐regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun 2018;9:43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol 2000;10:415–33. [DOI] [PubMed] [Google Scholar]
- 98. Atsumi N, Ishii G, Kojima M, et al. Podoplanin, a novel marker of tumor‐initiating cells in human squamous cell carcinoma A431. Biochem Biophys Res Commun 2008;373:36–41. [DOI] [PubMed] [Google Scholar]
- 99. Kerrigan A, Navarro‐Nuñes L, Pyz E, et al. Podoplanin‐expressing inflammatory macrophages activate murine platelets via CLEC‐2. J Thromb Haemost 2012;10:484–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu G, Wang S, Xu F, et al. Tumor‐infiltrating Podoplanin+ fibroblasts predict worse outcome in solid tumors. Cell Physiol Biochem 2018;51:1041–50. [DOI] [PubMed] [Google Scholar]
- 101. Navab R, Strumpf D, To C, et al. Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non‐small cell lung cancer. Oncogene 2015;35:1899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zeltz C, Lu N, Gullberg D. Integrin α11β1: a major collagen receptor on fibroblastic cells. Adv Exp Med Biol 2014;819:73–83. [DOI] [PubMed] [Google Scholar]
- 103. Ju J, Godet I, Ye I, et al. Hypoxia selectively enhances integrin α 5 β 1 receptor expression in breast cancer to promote metastasis. Mol Cancer Res 2017;15:723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Grella A, Kole D, Holmes W, et al. FGF2 overrides TGFβ1‐driven integrin ITGA11 expression in human dermal fibroblasts. J Cell Biochem 2015;117:1000–8. [DOI] [PubMed] [Google Scholar]
- 105. Biffi G, Oni T, Spielman B, et al. IL‐1‐induced JAK/STAT signaling is antagonized by TGF‐beta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 2018;9:282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sung K, Su X, Berthier E, et al. Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One 2013;8:e76373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Neumann E, Riepl B, Knedla A, et al. Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther 2010;12:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cruz‐Bermúdez A, Laza‐Briviesca R, Vicente‐Blanco R, et al. Cancer‐associated fibroblasts modify lung cancer metabolism involving ROS and TGF‐β signaling. Free Radic Biol Med 2018;130:163–73. [DOI] [PubMed] [Google Scholar]


