Abstract
Objectives
There are no published reports for anti‐interleukin‐5 therapy in children <12 years with asthma. The primary objective of this study was to characterize the pharmacokinetics and pharmacodynamics of mepolizumab following subcutaneous (SC) administration in children 6 to 11 years‐of‐age with severe eosinophilic asthma.
Hypothesis
Mepolizumab SC pharmacokinetics and pharmacodynamics in children with severe eosinophilic asthma are comparable with adults.
Study Design
Multinational, nonrandomised, open‐label (NCT02377427).
Patient Selection
Children 6 to 11 years‐of‐age with severe eosinophilic asthma (blood eosinophil count ≥150 cells/µL at screening or ≥300 cells/µL <12 months of screening) and ≥2 exacerbations in the prior year.
Methodology
Children received mepolizumab SC 40 mg (bodyweight <40 kg) or 100 mg (≥40 kg) every 4 weeks for 12 weeks.
Results
Thirty‐six children received mepolizumab (40 mg, n = 26; 100 mg, n = 10). Mepolizumab exposures were higher and apparent clearance lower than predicted based on prior existing data. Derived mepolizumab exposures normalized to mean bodyweight for the 40 mg and 100 mg dose groups were 454 μg * day/mL and 675 μg * day/mL, respectively. At week 12, blood eosinophils were reduced by 89% and 83% from baseline to 42 and 55 cells/µL, respectively. Mepolizumab was well tolerated; no new safety signals were observed compared with previous adult/adolescent studies.
Conclusion
In children 6 to 11 years‐of‐age with severe eosinophilic asthma, mepolizumab SC 40 or 100 mg provided bodyweight‐adjusted drug exposure within twofold of target adult exposure as well as marked reductions to blood eosinophil counts similar to adults, and although not designed to evaluate efficacy outcomes, demonstrated a positive clinical profile.
Keywords: asthma and early wheeze, children, severe eosinophilic asthma, subcutaneous mepolizumab
1. INTRODUCTION
Asthma is the most common chronic childhood disease worldwide.1 Approximately 2% to 5% of children with asthma have persistent symptoms, repeated hospital admissions for asthma exacerbations, school absences and poor quality of life (QoL) despite treatment with optimized therapy.2, 3, 4, 5 Compared with children with mild or moderate asthma, those with severe asthma are at increased risk of severe exacerbations and medication side effects, have impaired lung function, have significantly poorer QoL, and are greater risk of developing chronic obstructive pulmonary disease as adults.3, 6, 7 Thus, there is an unmet need for additional, effective, and well‐tolerated treatment options for severe asthma in children.8
The anti‐interleukin [IL]‐5 monoclonal antibody mepolizumab is approved for the treatment of adults and children ≥6 years of age with severe eosinophilic asthma in Europe, and for adults and adolescents ≥12 years in a number of countries, including the United States and Japan.9, 10 In the pivotal phase 3 studies, addition of mepolizumab subcutaneously (SC) to standard of care was associated with significant reductions in exacerbation rates11 and dependency on oral corticosteroid (OCS) use,12 and a significant improvement in symptom control and health‐related QoL.11, 13 In the adolescents aged 12 to 17 years included in the mepolizumab clinical development program, the exacerbation rate trended in favor of mepolizumab and the adverse event (AE) profile was generally similar to the overall population.9
Eosinophilic airway inflammation is a feature of severe asthma in some children,14, 15 and anti‐IL‐5 therapy may be an attractive treatment option. Mepolizumab, administered intravenously (IV; 0.55, 2.5, or 10 mg/kg), has been shown to markedly reduce blood eosinophil counts in children aged 2 to 17 years with eosinophilic esophagitis.16 The pharmacokinetics (PK) of mepolizumab IV in these children was similar to that observed in adults when dosed on a weight basis.16 However, mepolizumab PK, pharmacodynamics (PD) and effectiveness following SC administration have not been evaluated in children <12 years in any indication.
The primary objective of the first part of this two‐part study was to characterize the PK and PD of repeat‐dose mepolizumab following SC administration in children 6 to 11 years with severe eosinophilic asthma after 12 weeks of treatment. Secondary objectives included comparison of bodyweight‐adjusted mepo‐lizumab clearance between children aged 6 to 11 years and historic adult values, effectiveness, and tolerability. The hypothesis of the study was that subcutaneous mepolizumab pharmacokinetics and pharmacodynamics in children with severe eosinophilic asthma are comparable with adults.
2. MATERIALS AND METHODS
2.1. Study participants
Participants were children aged 6 to 11 years with a diagnosis of severe asthma, as defined by regional guidelines, and eosinophilic airway inflammation demonstrated by peripheral blood eosinophil counts ≥300 cells/μL within 12 months of screening or ≥150 cells/μL at screening. Eligible children had also experienced ≥2 exacerbations requiring treatment with systemic corticosteroids (SCS) ≤ 12 months before screening (an exacerbation in children receiving maintenance OCS must have necessitated a ≥twofold increase in their OCS dose). In the 12 months before screening, participants were receiving regular medium‐ or high‐dose ICS (>200 µg/day fluticasone propionate or equivalent) with or without maintenance OCS. They were also receiving ≥1 additional controller medication (eg, long‐acting β‐2‐agonist, leukotriene receptor antagonist, or theophylline) for ≥3 months, or had a documented failure of the additional controller medication for ≥3 successive months, in the 12 months before screening. Participants who had received omalizumab within 130 days of screening or any other biologic to treat inflammatory disease within five half‐lives of screening were not included in the study. Enrolled children had a prebronchodilator forced expiratory volume in 1 second (FEV1) < 110% predicted; or, FEV1/forced vital capacity ratio <0.8.
2.2. Study design
This was a non‐randomised, open‐label, repeat‐dose, phase 2 study conducted at 13 centers in Japan, Poland, UK, and United States (ClinicalTrials.gov number NCT02377427; GSK ID 200363). The study consisted of two parts. Part A, presented here, assessed the PK and PD of mepolizumab SC over a 12‐week treatment period and an 8‐week follow‐up (Figure 1A). Part B will assess long‐term safety and PD over a 52‐week treatment period and will be reported separately.
Figure 1.

Part A study (A) design and (B) patient flow. *Consent was withdrawn for one child during the follow‐up period after receiving all three doses of study treatment. SC, subcutaneous
Written informed consent was obtained from the parent/guardian of each child. Study protocol, amendments, and informed consent were reviewed and approved by national, regional, or investigational center ethics committees or institutional review boards, and the study was conducted according to the ethical principles outlined in the current Declaration of Helsinki (2013). Anonymised individual participant data and study documents can be requested for further research from http://www.clinicalstudydatarequest.com.
2.3. Treatments
Mepolizumab was administered SC once every 4 weeks for a total of three doses (weeks 0, 4, and 8), with the study active treatment period defined as weeks 0 to 12. Children were assigned to one of two dosing groups based on their bodyweight at baseline: mepo‐lizumab 40 mg for children <40 kg, and mepolizumab 100 mg for ≥40 kg. This dosing scheme was designed to provide similar exposure to that achieved in adults and adolescents with mepolizumab 100 mg SC.11, 12 Assigned doses remained the same irrespective of bodyweight changes during part A. Mepolizumab was added to existing stable asthma treatment, with additional controller or rescue medication permitted.
2.4. Endpoints and assessments
The primary PK endpoints were the population PK model derived estimates of mepolizumab plasma clearance, area under the plasma concentration‐time curve to infinity (AUC[0‐inf]), maximum plasma concentration (C max), and terminal phase elimination half‐life (t 1/2). The primary PD endpoint was the ratio of absolute blood eosinophil count at week 12 to baseline. Secondary endpoints included bodyweight‐adjusted plasma clearance estimates, change from baseline in Asthma Control Questionnaire 7‐item (ACQ‐7) score and Childhood Asthma Control Test (C‐ACT) score, both at weeks 4, 8, 12, 16, and 20. Mepolizumab safety and tolerability were assessed through AE reporting, immunogenicity, laboratory parameters, and vital signs.
Exploratory endpoints included asthma exacerbation frequency during the treatment period (weeks 0–12) and throughout part A (weeks 0–20), change from baseline to week 12 in FEV1 and serum total IL‐5 levels. An exacerbation was defined as worsening of asthma that required SCS treatment and/or hospitalization and/or an emergency room (ER) visit.
Blood samples for PK assessment were taken at every visit from weeks 4 to 20 (weeks 4, 8, 9, 12, 16, and 20); samples at weeks 4 and 8 were drawn before mepolizumab dosing. The ACQ‐7 and C‐ACT questionnaires were administered at baseline and every 4 weeks to week 20. Hematology, including eosinophils, and assessment of exacerbations, AEs, and vital signs were evaluated at every visit to week 20. Serum total IL‐5 levels were measured at baseline and week 12.
2.5. Sample size and statistical analysis
The sample size was determined by population PK trial simulation. A sample size of 16 to 32 children, combined with sparse PK sampling, was deemed sufficient to maintain precision of exposure estimates below 20% (compared with US Food and Drug Administration guideline recommendations of below 40% 17), provided that five PK samples (including one close to the time of C max) were collected and four model parameters were fixed to adult values in the population PK model. For blood eosinophils, assuming a similar residual variance to that observed in adults and adolescents,11 a sample size of 20 children provided sufficient precision for the 95% confidence interval (CI) of the ratio to baseline to fall within 50% of the observed geometric mean.
Mepolizumab plasma PK concentrations were analyzed by nonlinear mixed‐effect modeling methods (SAS Institute Inc, Cary, NC). A two‐compartment model with first‐order absorption and elimination was used with PK distribution parameters and bioavailability fixed to previously estimated adult values. Bodyweight was incorporated into the model using physiological allometry with allometric exponents for clearance and volumes estimated. Final model appropriateness was assessed using goodness of fit plots, simulations, and statistical tests. The ratio of blood eosinophil count at week 12 to baseline was summarized descriptively, and the ratio at each visit to baseline presented graphically. Bodyweight‐adjusted apparent clearance point estimates with 90% CIs in children 6 to 11 years were presented alongside the historical estimated adult value of 0.29 L/day (unpublished data) with a proposed 80% to 125% interval around this estimate of 0.23 to 0.36 L/day. An exploratory population PK analysis using the most recent mepolizumab population PK model with minimal estimation (absolute bioavailability, allometric exponents, and residual error) was conducted using NONMEM software (version 7.2; ICON Development Solutions, Ellicott City, MD).
3. RESULTS
3.1. Patient population
The study was conducted between 25 August 2015 and 7 December 2016. A total of 44 children were assessed for eligibility of whom seven were excluded during screening (Figure 1B). One additional patient did not meet the study continuation criteria due to an exacerbation during the run‐in phase. The remaining 36 children were assigned to study treatment; 26 children to the 40 mg dose group (weight <40 kg) and 10 to the 100 mg dose group (weight ≥40 kg). All 36 children were included in the analysis population. Part A was completed by 32 (89%) children with four children, all in the 40 mg dose group, withdrawing prematurely.
Enrolled children were predominantly male (69%), with a mean body mass index (BMI) of 16.1 kg/m2 in the 40 mg dose group (weight <40 kg) and 23.1 kg/m2 in the 100 mg dose group (weight ≥40 kg) (Table 1). In the 12 months before study entry, the median number of exacerbations requiring SCS was 3.0 (range 2‐15), and 44% (16/36) of children had required hospitalization for an exacerbation. Eight (22%) children were receiving OCS at baseline.
Table 1.
Summary of patient demographics and baseline characteristics (safety population)
| Mepolizumab 40 mg (weight <40 kg) | Mepolizumab 100 mg (weight ≥40 kg) | Total | |
|---|---|---|---|
| (N = 26) | (N = 10) | (N = 36) | |
| Derived age a , years | 8.0 (1.8) | 10.0 (1.3) | 8.6 (1.9) |
| Sex, n (%) | |||
| Female | 6 (23) | 5 (50) | 11 (31) |
| Male | 20 (77) | 5 (50) | 25 (69) |
| Weight, kg | 27.4 (4.7) | 49.5 (6.3) | 33.5 (11.2) |
| Body mass index, kg/m2 | 16.1 (1.7) | 23.1 (2.8) | 18.0 (3.7) |
| Pre‐BD lung function | |||
| FEV1 (mL) | 1407 (365) | 1940 (310) | 1555 (422) |
| Predicted normal FEV1 (%) | 89 (16.9) | 92 (6.9) | 90 (14.8) |
| FVC (mL) | 1805 (372) | 2436 (448) | 1985 (484) |
| FEV1/FVC | 0.78 (0.11) | 0.80 (0.09) | 0.79 (0.11) |
| Number of exacerbations requiring corticosteroids in prior 12 mo, median (range) | 3 (2–15) | 2 (2–10) | 3 (2–15) |
| Patients with an exacerbation requiring hospitalization in prior 12 mo, n (%) | 14 (54) | 2 (20) | 16 (44) |
| Blood eosinophils, cells/uL, geometric mean (SD logs) | 386 (0.75) | 331 (0.91) | 370 (0.78) |
| Blood eosinophil count, n (%) | |||
| ≥150 cells/μL at screening | 20 (77) | 10 (100) | 30 (83) |
| ≥300 cells/μL in 12 mo before screening | 21 (81) | 9 (90) | 30 (83) |
| ≥150 cells/μL at screening and ≥300 cells/μL in 12 mo before screening | 15 (58) | 9 (90) | 24 (67) |
| IgE U/mL, geometric mean (SD logs) | 336 (1.48) | 379 (1.09) | 348 (1.36) |
| OCS daily dose, n (%) | |||
| Any use | 6 (23) | 2 (20) | 8 (22) |
| <7.5 mg/day | 3 (12) | 0 | 3 (8) |
| ≥7.5 to <15 mg/day | 1 (4) | 0 | 1 (3) |
| ≥15 to <30 mg/day | 0 | 1 (10) | 1 (3) |
| ≥30 mg/day | 2 (8) | 1 (10) | 3 (8) |
| ICS daily dose (fluticasone propionate [DPI] equivalent b ), n (%) | |||
| Any use | 26 (100) | 10 (100) | 36 (100) |
| >200 to ≤400 µg/day | 6 (23) | 1 (10) | 7 (19) |
| >400 µg/day | 20 (77) | 9 (90) | 29 (81) |
| ACQ‐7 score | 1.99 (1.21) | 1.39 (0.96) | 1.82 (1.17) |
| C‐ACT score | 15.6 (5.7) | 20.4 (3.2) | 16.9 (5.6) |
Data are mean (standard deviation) unless stated otherwise.
Abbreviations: ACQ, Asthma Control Questionnaire; C‐ACT, Childhood Asthma Control Test; DPI, dry powder inhaler; BD, bronchodilator; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroids; IgE, immunoglobulin‐E; OCS, oral corticosteroids; SD, standard deviation.
Only year of birth was collected, with age derived using an imputed birth date of 30th June.
Data are presented as ex‐valve/metered doses.
3.2. Primary endpoints: population PK parameters
Mepolizumab population PK parameter estimates and predicted and observed mepolizumab concentration‐time plots are presented in Table 2 and Figure 2. Apparent clearance, AUC(0‐inf), C max and t ½ population PK parameter estimates are presented normalized to 27 and 50 kg (mean bodyweight for the 40 and 100 mg dose groups, respectively), and to a typical adult bodyweight (70 kg; which was not observed in the study). Derived mepolizumab exposure (AUC(0‐inf]) normalized to 27 and 50 kg were 454.4 and 675.2 μg * day/mL, respectively (Table 2). Mepolizumab estimated t ½ normalized to 27 and 50 kg was 23.6 and 21.8 days, respectively.
Table 2.
Mepolizumab population pharmacokinetic parameter estimates from the final model (primary population pharmacokinetic analysis)
| Mepolizumab population pharmacokinetic parameter estimate (95% CIs) | |||
|---|---|---|---|
| Normalized to 27 kg | Normalized to 50 kg | Normalized to 70 kg (for comparison with adults) | |
| AUC(0‐inf) (μg ⁎ day/mL) | 454.4 (422.1, 486.7) | 675.2 (602.2, 748.2) | 508.2 (423.3, 593.2) |
| C max (μg/mL) | 10.2 (9.5, 10.9) | 16.3 (15.0, 17.6) | 12.8 (11.2, 14.4) |
| C max SS (μg/mL) | 17.8 (15.3, 20.2) | 28.5 (25.0, 31.9) | 22.3 (19.2, 25.5) |
| CL/F (L/day) | 0.09 (0.08, 0.09) | 0.15 (0.13, 0.16) | 0.20 (0.16, 0.23) |
| C av (μg/mL) | 16.2 (15.1, 17.4) | 24.1 (21.5, 26.7) | 18.2 (15.1, 21.2) |
| t ½ (days) | 23.6 (21.9, 25.3) | 21.8 (19.6, 24.1) | 21.0 (17.6, 24.3) |
| KA (per day) | 0.17 (0.13, 0.20) | ||
| Between‐subject variability for CL/F (%) | 15.5 (10.6, 19.3) | ||
| CL allometric exponent | 0.84 (0.65, 1.04) | ||
| V allometric exponent | 0.72 (0.56, 0.88) | ||
| Residual error | 0.029 (0.023, 0.036) | ||
Data are normalized to 27 kg (mean in the <40 kg group receiving 40 mg mepolizumab SC), 50 kg (mean in the ≥40 kg group receiving 100 mg mepolizumab SC), and 70 kg (bodyweight for a typical adult). KA, between‐subject variability, CL, allometric exponent, and V, allometric exponent estimates apply to all subgroups.
AUC(0‐inf), area under the concentration‐time curve from time zero (predose) extrapolated to infinite time; C av, average concentration; CI, confidence interval; CL/F, apparent plasma clearance; C max, maximum plasma concentration; C max SS, maximum plasma concentration at steady state; KA, absorption rate constant; SC, subcutaneous; t ½, half‐life; V, volume of distribution.
Figure 2.
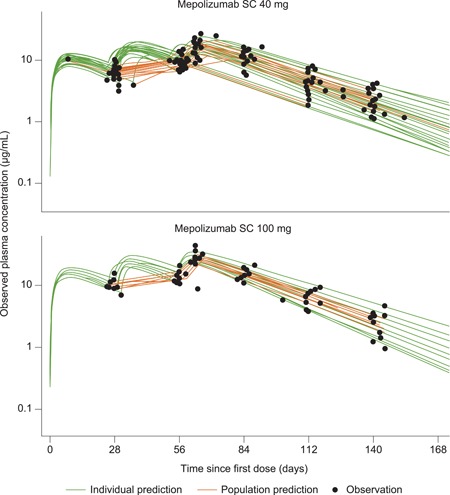
Observed and population PK model predicted mepolizumab plasma concentrations in children aged 6 to 11 years with severe eosinophilic asthma. PK, pharmacokinetic; SC, subcutaneous [Color figure can beviewed at wileyonlinelibrary.com]
3.3. Primary endpoints: blood eosinophil counts
At baseline, respective geometric mean blood eosinophil counts were 386, 331, and 370 cells/µL in the 40 mg dose group (<40 kg), 100 mg dose group (≥40 kg), and overall (Table 1). Blood eosinophil counts showed a marked reduction by the first on‐treatment assessment at week 4 (Figure 3). Blood eosinophil count reductions were of similar magnitude irrespective of dose group and were sustained throughout the treatment period. By week 12, blood eosinophil counts were reduced from baseline by 88.5% in the 40 mg dose group (to 42 cells/µL; 95% CI: 26, 67) and by 83.4% in the 100 mg dose group (to 55 cells/µL; 95% CI: 31, 97) (Figure 3 and Table 4). Once mepolizumab treatment was stopped, geometric mean blood eosinophil counts increased toward baseline values during the follow‐up period.
Figure 3.
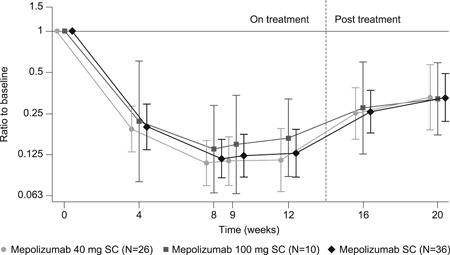
Ratio of blood eosinophil counts to baseline (geometric mean). Vertical bars represent 95% confidence intervals. SC, subcutaneous
Table 4.
Summary of secondary and other endpoints: ACQ‐7 and C‐ACT total scores and exacerbations
| Mean (95% CI) | |||
|---|---|---|---|
| Mepolizumab 40 mg SC (weight <40 kg) | Mepolizumab 100 mg SC (weight ≥40 kg) | Total | |
| (N = 26) | (N = 10) | (N = 36) | |
| Blood eosinophil counts, cells/µL | |||
| Ratio to baseline at week 4 | 0.19 (0.13, 0.28) | 0.22 (0.08, 0.61) | 0.20 (0.14, 0.29) |
| Ratio to baseline at week 8 | 0.11 (0.08, 0.16) | 0.14 (0.07, 0.29) | 0.12 (0.09, 0.16) |
| Ratio to baseline at week 12 | 0.12 (0.07, 0.20) | 0.17 (0.09, 0.32) | 0.13 (0.09, 0.19) |
| % reduction from baseline at week 12 | 88.5 | 83.4 | 87.1 |
| ACQ‐7 scorea | |||
| Change from baseline at week 4 | –0.55 (–1.01, –0.09) | –0.47 (–1.16, 0.21) | –0.53 (–0.89, –0.16) |
| Change from baseline at week 8 | –0.65 (–1.15, –1.16) | –0.30 (–1.19, 0.59) | –0.55 (–0.97, –0.14) |
| Change from baseline at week 12 | –0.41 (–0.91, 0.08) | 0.08 (–0.88, 1.04) | –0.26 (–0.69, 0.16) |
| ≥0.5 point reduction from baseline, n/N (%) | 11/23 (48) | 5/10 (50) | 16/33 (48) |
| C‐ACT scoreb | |||
| Change from baseline at week 4 | 1.8 (0.2, 3.5) | 2.4 (–0.9, 5.7) | 2.0 (0.6, 3.4) |
| Change from baseline at week 8 | 3.0 (0.7, 5.4) | 1.5 (–1.6, 4.6) | 2.6 (0.8, 4.4) |
| Change from baseline at week 12 | 2.1 (0.2, 4.1) | –0.3 (–4.0, 3.4) | 1.4 (–0.3, 3.1) |
| Prebronchodilator FEV1 (mL) | |||
| Change from baseline at week 4 | 93 (–19, 206) | 55 (–52, 162) | 83 (–1, 167) |
| Change from baseline at week 8 | 90 (–17, 198) | –63 (–314, 188) | 48 (–52, 148) |
| Change from baseline at week 12 | 72 (–37, 181) | 2 (–175, 179) | 51 (–37, 139) |
| Patients with on‐treatment exacerbations (weeks 0–12), n (%) | |||
| Any | 8 (31) | 2 (20) | 10 (28) |
| 1 exacerbation | 6 (23) | 1 (10) | 7 (19) |
| 2 exacerbations | 2 (8) | 1 (10) | 3 (8) |
Data are mean (95% CI) unless stated otherwise; adecreased scores, or bincreased scores, from baseline indicate improvement in asthma control.
Abbreviations: ACQ‐7, Asthma Control Questionnaire; C‐ACT, Childhood Asthma Control Test; CI, confidence interval; FEV1, forced expiratory volume in 1 s; SC, subcutaneous.
3.4. Secondary endpoints: bodyweight‐adjusted apparent plasma clearance
The population bodyweight‐adjusted apparent clearance (ie, CL/F at 70 kg) was 0.20 L/day (90% CI: 0.17, 0.22) for children in this study, which fell outside the prespecified 80% to 125% range around the historical adult value of 0.29 L/day (80%, 125% interval: 0.23, 0.36). This value implies a lower CL/F in children aged 6 to 11 years compared with adults (Table 3).
Table 3.
Mean bodyweight‐adjusted apparent plasma clearance
| Historic adult data | Apparent clearance (L/day) | 80% lower‐bound interval | 125% upper‐bound interval |
|---|---|---|---|
| Adult target (70 kg) a | 0.29 | 0.23 | 0.36 |
| PK/PD study of children aged 6–11 y | Apparent clearance (L/day) | Lower 90% CI | Upper 90% CI |
| Children (70 kg) b | 0.20 | 0.17 | 0.22 |
| Children (50 kg) c | 0.15 | 0.13 | 0.16 |
| Children (27 kg) d | 0.09 | 0.08 | 0.09 |
Abbreviations: CI, confidence interval; PD, pharmacodynamic; PK, pharmacokinetic; SC, subcutaneous.
Historic adult data from the MENSA trial for a 70 kg individual; data in children aged 6–11 y.
Typical adult bodyweight.
Mean bodyweight in the ≥40 kg group receiving 100 mg mepolizumab SC.
Mean bodyweight in the <40 kg group receiving 40 mg mepolizumab SC.
3.5. Exploratory population PK analysis results
An exploratory population PK analysis was performed to investigate potential explanations for the lower bodyweight‐adjusted apparent clearance in children. The analysis used the most recent mepolizumab population PK model from a comprehensive meta‐analysis of previous mepolizumab studies. All parameters were fixed except absolute bioavailability and allometric exponents for scaling of clearance and volumes by bodyweight. The estimated absolute bioavailability was 105% (95% CI: 55, 155%), and estimates for allometric exponents for clearance and volumes were 0.86 (95% CI: 0.29, 1.43) and 0.66 (95% CI: 0.11, 1.21), respectively.
3.6. Effectiveness
3.6.1. Secondary endpoints: ACQ‐7 and C‐ACT
Mepolizumab treatment was associated with a trend toward improved asthma control as indicated by numerical improvements in mean ACQ‐7 and C‐ACT total scores compared with baseline (Table 4). At week 12, a minimally clinically important improvement (≥0.5‐point reduction) in ACQ‐7 total score from baseline was reported for 48% of children, with similar response rates in the two mepolizumab dose groups (Table 4).
3.6.2. Exploratory analyses: exacerbation rate, FEV1, and serum total IL‐5 levels
Ten (28%) children reported ≥1 on‐treatment exacerbation with a total of 13 events (Table 4). Four children (all in the 40 mg dose group [<40 kg]) required an on‐treatment hospitalization or ER visit. Thirteen (36%) children reported ≥1 exacerbation over the 20‐week study period. Mean baseline prebronchodilator FEV1 values were 1407 and 1940 mL in the mepolizumab 40 (<40 kg) and 100 mg (≥40 kg) dose groups, respectively. There was no clear pattern of change in FEV1 from baseline to week 12 (Table 4). Total serum IL‐5 levels at baseline were below the assay lower limit of quantification (<7.81 ng/L) for 78% of the children (28/36). Total serum IL‐5 levels (free and mepolizumab‐bound IL‐5) had increased in all subjects at week 12 from baseline except one, to a median of 137.1 ng/L in the 40 mg dose group (<40 kg) and 96.4 ng/L in the 100 mg dose group (≥40 kg).
3.7. Safety
Overall, 72% (26/36) of children experienced an AE, with 67% (24/36) experiencing an on‐treatment AE (Table 5). Headache and injection‐site reactions were the most frequent on‐treatment AEs (both 14% [5/36] of the children). On‐treatment serious AEs (SAE) were reported for 17% (6/36) of children including two children with events considered related to the study drug by investigators. One 9‐year‐old male child in the mepolizumab 40 mg dose group (<40 kg) permanently discontinued due to a drug‐related SAE of severe asthma exacerbation 11 days after administration of the second dose. The child was hospitalized and treated with prednisolone and salbutamol; the event resolved after 10 days. A second 10‐year‐old male child experienced drug‐related SAEs of severe back pain, chest pain, dizziness, headache, nausea, and pain. There were no deaths.
Table 5.
Summary of adverse events
| Mepolizumab 40 mg SC (weight <40 kg) | Mepolizumab 100 mg SC (weight ≥40 kg) | Total | |
|---|---|---|---|
| Patients, n (%) | (N = 26) | (N = 10) | (N = 36) |
| Any AE | 20 (77) | 6 (60) | 26 (72) |
| On‐treatment a | 18 (69) | 6 (60) | 24 (67) |
| Posttreatment b | 12 (46) | 2 (20) | 14 (39) |
| Related to treatment c | 8 (31) | 3 (30) | 11 (31) |
| Leading to withdrawal d | 1 (4) | 0 | 1 (3) |
| SAEs | 6 (23) | 1 (10) | 7 (19) |
| On‐treatment a | 5 (19) | 1 (10) | 6 (17) |
| Posttreatment b | 4 (15) | 0 | 4 (11) |
| Related to treatment c | 2 (8) | 0 | 2 (6) |
| Fatal | 0 | 0 | 0 |
| Most frequent on treatment AEs (≥3 patients) | |||
| Headache | 3 (12) | 2 (20) | 5 (14) |
| Injection‐site reaction | 5 (19) | 0 | 5 (14) |
| Asthma | 4 (15) | 0 | 4 (11) |
| Nasopharyngitis | 3 (12) | 1 (10) | 4 (11) |
| Nausea | 3 (12) | 0 | 3 (8) |
| Upper respiratory tract infection | 2 (8) | 1 (10) | 3 (8) |
| Wheezing | 2 (8) | 1 (10) | 3 (8) |
| On‐treatment AEs of special interest | |||
| Anaphylaxis | 0 | 0 | 0 |
| Systemic reactions | 1 (4) | 0 | 1 (3) |
| Local injection‐site reactions | 5 (19) | 0 | 5 (14) |
| All infections | 14 (54) | 4 (40) | 18 (50) |
| Neoplasms | 0 | 0 | 0 |
| Malignancies | 0 | 0 | 0 |
| Cardiac disorders | 0 | 0 | 0 |
| Serious CVT events | 0 | 0 | 0 |
Abbreviations: AE, adverse event; CVT, cardiac, vascular, thromboembolic; SAE, serious adverse event; SC, subcutaneous.
From the first mepolizumab dose to 4 wk of the last mepolizumab dose.
≥4 wk after the last mepolizumab dose.
As assessed by the treating investigator.
A second subject in the 40 mg SC group withdrew consent from the study due to experiencing a combination of AEs throughout the study.
On‐treatment infections occurred in 50% (18/36) of children; infections reported in more than one child included nasopharyngitis (n = 4), upper respiratory tract infections (n = 3), lower respiratory tract infection (n = 2), sinusitis (n = 2), and viral upper respiratory tract infection (n = 2). Three on‐treatment infections were considered serious though not related to mepolizumab (cellulitis, n = 1; lower respiratory tract infection, n = 2); all resolved without modification to mepolizumab dosing. One 11‐year‐old child in the mepolizumab 40 mg dose group (<40 kg) had a systemic hypersensitivity reaction with symptoms of pruritus of mild intensity. The event occurred less than 24 hours after the first dose, lasted for 57 days, was considered related to treatment and resolved without mepolizumab dose modification or interruption. Injection‐site reactions were all mild in intensity and resolved without mepolizumab dose modification or discontinuation. There were no cases of anaphylaxis. No children tested positive for anti‐drug antibody (ADA) at baseline; two children had transient positive ADA results post baseline with low titers that were negative at subsequent visits. No children tested positive for neutralizing antibodies and there were no apparent drug‐related changes in clinical laboratory parameters or vital signs.
Post‐treatment, one child reported an SAE of campylobacter infection 44 days after the last mepolizumab 40 mg dose and a second child had a nonserious valvulopathy (mild mitral valve incompetence not requiring hospitalization or treatment) diagnosed 78 days after the last mepolizumab 40 mg dose. Both events were considered unrelated to study drug and were ongoing at the end of the follow‐up period.
4. DISCUSSION
This study of mepolizumab SC at bodyweight‐defined doses of 40 or 100 mg is the first reported clinical evaluation of an anti‐IL‐5 therapy in children aged 6 to 11 years with severe eosinophilic asthma. In summary, results showed that derived exposures (AUC0‐inf) normalized to the average bodyweight of each dose group were within twofold (1.32‐fold at 40 mg SC and 1.97‐fold at 100 mg SC) of the historical target adult exposure of 343 μg ⁎ day/mL (unpublished data). The mepolizumab SC half‐life of 22 to 24 days in children is, however, aligned with historical adult values of 16 to 22 days.9, 18 Mepolizumab was well tolerated with no specific AE patterns. Reported injection‐site reactions were mild in intensity and managed without mepolizumab dose modification or discontinuation. No new safety concerns were observed beyond those seen previously with mepolizumab in adults and adolescents.11, 12, 13, 19
Mepolizumab exposures were higher than predicted, and plasma apparent clearance normalized to adult bodyweight was lower than reported previously for adults (0.20 vs 0.29 L/day). An additional exploratory population PK analysis conducted to assess these discrepancies showed mepolizumab SC bioavailability to be 105% (95% CI: 55%, 155%) for children compared with the historic adult value of 76% (95% CI: 71%, 79%). This disparity may reflect differences in BMI (18 kg/m2 in this study vs ~28 kg/m2 for adults in the phase 3 trials11, 12, 13, 19). Population PK parameter estimates for the allometric exponents for clearance and volumes in children were 0.86 (95% CI: 0.29, 1.43) and 0.66 (95% CI: 0.11, 1.21), respectively, and consistent with historic adult values.
Blood eosinophil reductions were accompanied by indicators of improvement in asthma control, as reflected by ACQ‐7 and C‐ACT scores. Serum total IL‐5 levels were increased at week 12 from baseline, as anticipated since the assay measures both free and mepolizumab‐bound IL‐5. Serum IL‐5 levels confirmed antibody‐target engagement in children and were consistent with observations in adults at which saturation was observed.18 The on‐treatment incidence of asthma exacerbations (28% of children) was also similar to exacerbation rates observed in adults and adolescents over the first 12 weeks of mepolizumab treatment (approximately 12‐30%).20 However, there was no clear pattern of lung function changes in response to mepolizumab.
There continues to be scientific debate on the role of homeostatic levels of eosinophils in human health and disease based on observations in exaggerated rodent models (eg, IL‐5 or eosinophil deficient mice).21, 22, 23 There is growing evidence, suggested by murine studies, that drugs targeting the number of eosinophils in patients with elevated eosinophil levels have therapeutic benefit without adverse events.23 In line with findings in adolescents and adults,11, 12, 13, 19, 20, 24 we did not associate mepolizumab treatment with an increased incidence of opportunistic infections or neoplasms. It must however be acknowledged that the patients enrolled in the first part of this two‐part study were followed for a limited duration (20 weeks) and did not reside in countries where parasitic diseases are endemic. Limitations of the efficacy and safety data from this study include the lack of a control group as this was not a placebo‐controlled, double‐blind randomized study, the selection of the sample size to assess PK/PD rather than effectiveness, and the relatively short treatment period in this study. The use and adherence of background asthma therapies was also not systematically checked during the study; instead, it was at the investigator's discretion whether to reduce or change background therapies. This may have influenced the results to some extent, and in particular, any changes in bronchodilator therapy may have affected the results on lung function. Therefore, effectiveness and tolerability results in part A should be interpreted with caution while the long‐term safety phase (part B) will provide further insight.
5. CONCLUSION
In conclusion, mepolizumab SC, administered at bodyweight‐defined doses of 40 or 100 mg, provides higher than predicted drug exposure in children aged 6 to 11 years, but that remains within twofold of adult levels. This difference is likely due to the increased bioavailability of mepolizumab SC in children versus adults. Mepolizumab SC was associated with a similar therapeutic effect in children to adults, with marked reductions in blood eosinophil counts, a trend toward improved asthma control compared with baseline, and a favorable safety profile. The 40 and 100 mg SC dosing regimens were deemed acceptable for children aged 6 to 11 years with severe eosinophilic asthma based on mepolizumab's wide therapeutic index. Results will be used as a basis for further dosing refinement.
CONFLICT OF INTERESTS
IP, DA, RGP, RK, JS, ESB, and SWY are all GSK employees and stockholders. AG was the principal investigator for the current study at the King's College Hospital, which received grants from GSK to conduct the study. AG has also received personal fees for attendance at Advisory Boards from GSK, Boehringer Ingelheim and Novartis.
AUTHOR CONTRIBUTIONS
All authors reviewed and revised the manuscript, approved the final version, and made the decision to submit the manuscript for publication. AG contributed to the acquisition of data and analysis and interpretation of the study data. IP, DA, RGP, RK, JS, ESB, and SWY were all involved in the conception or design of the study, and in analysis and interpretation of the study data.
ACKNOWLEDGMENTS
This study was funded by GlaxoSmithKline (GSK ID 200363 ClinicalTrials.gov number NCT02377427). The sponsor did not place any restrictions on access to the data or on the statements made in the manuscript. The decision to submit for publication was that of the authors alone. The authors would like to thank the patients and caregivers who took part in this study, and Marie Duggan and Dennis Kelleher (both employees of GSK) for their roles in leading management of the study. Editorial support (in the form of writing assistance, including development of the initial draft from the study report, assembling tables and figures, collating and incorporating authors comments, grammatical editing, and referencing) was provided by Elizabeth Hutchinson PhD, CMPP, at Fishawack Indicia Ltd, UK, and was funded by GSK.
Gupta A, Pouliquen I, Austin D, et al. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatric Pulmonology. 2019;54:1957–1967. 10.1002/ppul.24508
Presented at the 37th Annual Congress of the European Academy of Allergy and Clinical Immunology, 26 to 30 May 2018, Munich, Germany.
References
REFERENCES
- 1. World Health Organization . Chronic respiratory diseases: Asthma. 2018; http://www.who.int/respiratory/asthma/en/ Last accessed February 2018.
- 2. Coverstone A, Bacharier LB, Fitzpatrick AM. Severe asthma in school‐age children: evaluation and phenotypic advances. Curr Allergy Asthma Rep. 2015;15(5):20. [DOI] [PubMed] [Google Scholar]
- 3. Lang A, Carlsen KH, Haaland G, et al. Severe asthma in childhood: assessed in 10 year olds in a birth cohort study. Allergy. 2008;63(8):1054‐1060. [DOI] [PubMed] [Google Scholar]
- 4. Nordlund B, Melén E, Schultz ES, Grönlund H, Hedlin G, Kull I. Prevalence of severe childhood asthma according to the WHO. Respir Med. 2014;108(8):1234‐1237. [DOI] [PubMed] [Google Scholar]
- 5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343‐373. [DOI] [PubMed] [Google Scholar]
- 6. Fleming L, Murray C, Bansal AT, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U‐BIOPRED cohorts. Eur Respir J. 2015;46(5):1322‐1333. [DOI] [PubMed] [Google Scholar]
- 7. Tai A, Tran H, Roberts M, Clarke N, Wilson J, Robertson CF. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. 2014;69(9):805‐810. [DOI] [PubMed] [Google Scholar]
- 8. Martin Alonso A, Saglani S. Mechanisms mediating pediatric severe asthma and potential novel therapies. Front Pediatr. 2017;5:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. GlaxoSmithKline. NUCALA (mepolizumab) prescribing information . 2017; http://www.accessdata.fda.gov/drugsatfda_docs/label/…/125526Orig1s000Lbl.pdf Last accessed February 2018.
- 10. GlaxoSmithKline. NUCALA (mepolizumab) summary of product characteristics . 2018; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003860/WC500198037.pdf Last accessed February 2018.
- 11. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198‐1207. [DOI] [PubMed] [Google Scholar]
- 12. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid‐sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189‐1197. [DOI] [PubMed] [Google Scholar]
- 13. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add‐on therapy on health‐related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390‐400. [DOI] [PubMed] [Google Scholar]
- 14. Guiddir T, Saint‐Pierre P, Purenne‐Denis E, et al. Neutrophilic steroid‐refractory recurrent wheeze and eosinophilic steroid‐refractory asthma in children. J Allergy Clin Immunol Pract. 2017;5(5):1351‐1361. e1352. [DOI] [PubMed] [Google Scholar]
- 15. Lezmi G, Deschildre A, Abou Taam R, et al. Remodelling and inflammation in preschoolers with severe recurrent wheeze and asthma outcome at school age. Clin Exp Allergy. 2018;48:806‐813. [DOI] [PubMed] [Google Scholar]
- 16. Assa'ad AH, Gupta SK, Collins MH, et al. An antibody against IL‐5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593‐1604. [DOI] [PubMed] [Google Scholar]
- 17. FDA. General clinical pharmacology considerations for pediatric studies for drugs and biological products . 2014; https://www.fda.gov/downloads/drugs/guidances/ucm425885.pdf Last accessed February 2018.
- 18. Pouliquen IJ, Kornmann O, Barton SV, Price JA, Ortega HG. Characterization of the relationship between dose and blood eosinophil response following subcutaneous administration of mepolizumab. Int J Clin Pharmacol Ther. 2015;53(12):1015‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380(9842):651‐659. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A, Steinfeld J, Price R, Azmi J, Bradford E, Yancey S. Mepolizumab for severe eosinophilic asthma: a comparison of efficacy in children, adolescents, and adults. Eur Respir J. 2018;51(Suppl):PA5447. [Google Scholar]
- 21. Gleich GJ, Klion AD, Lee JJ, Weller PF. The consequences of not having eosinophils. Allergy. 2013;68(7):829‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klion A. Recent advances in understanding eosinophil biology. F1000Research. 2017;6:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramirez GA, Yacoub M‐R, Ripa M, et al. Eosinophils from physiology to disease: a comprehensive review. BioMed Res Int. 2018;2018:1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khatri S, Moore W, Gibson PG, et al. Assessment of the long‐term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143:1742‐1751. [DOI] [PubMed] [Google Scholar]


