Abstract
Understanding local viral hepatitis and HIV epidemiology is essential if WHO elimination targets are to be achieved. We demonstrate a consistently high prevalence of undiagnosed active infection in urban emergency department attendees in England, with variations in local risk groups crucial to informing targeted testing initiatives.
Keywords: emergency department, hepatitis B virus, hepatitis C virus, high prevalence, screening
Abbreviations
- BBV
blood‐borne virus
- CI
95% confidence interval
- CXH
Charing Cross Hospital
- ED
emergency department
- EDs
emergency departments
- EPR
electronic patient record
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- RNA
ribonucleic acid
- RR
risk ratio
- SMH
St Mary's Hospital
1. INTRODUCTION
Innovative care pathways including case finding and linkage to care are crucial to achieve the World Health Organization targets for the elimination of viral hepatitis.1 In England, there were over 23.4 million attendances at Emergency departments (EDs) in 2016‐2017 representing a significant opportunity to engage for case finding.2 EDs may be the only healthcare access point for some marginalized groups including recent migrants, homeless or people who inject drugs. Seroprevalence studies have been used in the USA to guide public health interventions where large scale, integrated ED testing and linkage programs are increasingly common.3 Since 2008, routine opt‐out testing for HIV in UK ED settings has been recommended for those in high prevalence areas (>0.2%). However, similar recommendations for hepatitis B virus (HBV) and hepatitis C virus (HCV) have not been produced given a lack of UK evidence to support routine testing in this setting.4
The aim of this study was to estimate undiagnosed BBV prevalence in three urban EDs in different areas of England to inform testing strategies.
2. METHODS
Standardized unlinked anonymous BBV prevalence surveys were conducted using residual biochemistry blood from ED attendees at three sites in England: two London hospitals in the southeast of England (Charing Cross (CXH) and St Mary's (SMH)) and one city in the northwest of England (Liverpool). Unselected deduplicated lists of ED U&E blood samples for inclusion in the study were generated using laboratory information systems. Inclusion criteria were (a) age 16‐65 years and (b) ≥0.75 mL surplus serum in the primary blood tube. Previously diagnosed BBV prevalence in this cohort was estimated through interrogation of local health care IT records to report the aggregate number of diagnosed infections. Individual routine pathology results were not retained. Samples were then irreversibly anonymized except for sex, age and ethnicity prior to screening for hepatitis B surface antigen (HBsAg), HCV antibody and HIV antigen/antibody. Positive results were confirmed using neutralization, RNA and lineblot assays. Active infection was defined as HBsAg that was either neutralizable or confirmed on a second assay, HCV RNA ≥15 IU/mL, HIV antigen/antibody confirmed with two different immunoassays or HIV‐1 RNA. All testing was undertaken in accredited NHS laboratories in accordance with ISO 15189. Proportions were calculated amongst all samples with known results. Undiagnosed BBV was estimated by subtracting previously diagnosed from survey‐diagnosed prevalence. Crude risk ratios for overall infection (as defined by positive study result) were calculated using Stata 15 for each BBV by sex, age and ethnicity. The study was approved by the NHS Research Ethics Service (reference 17/LO/0255, 17/SW/0096).
3. RESULTS
4574 samples from unique ED attendees between May 2017 and August 2017 were tested: CXH n = 1500; SMH n = 1500; Liverpool n = 1574. 46.3% of samples were from male patients. Median age was 41 (IQR 29‐53) years. Ethnicity was recorded for 3541 (77.4%) attendees, of whom 1680 (47.4%) self‐identified as white British. The study population is described in greater detail in the supporting information (Table S1).
Overall active BBV infection prevalence in the total study population was 3.3% (95% confidence interval [CI] 2.8%‐3.8%). Of these, 101/150 (67.3%) were undiagnosed according to local databases giving an undiagnosed BBV prevalence of 2.2% (range across the three sites 1.1%‐3.3%).
Active infection prevalence for each virus was HCV 1.5% (range 0.7%‐2.7%), HBV 1.1% (range 0.4%‐2.0%) and HIV 0.8% (range 0.1%‐1.5%), with corresponding undiagnosed prevalence of HCV 0.7% (0.5%‐1.0%), HBV 0.8% (0.1‐1.7%) and HIV 0.8% (0.1%‐1.3%). There were 7 co‐infections (0.1%). Whilst overall active BBV infection prevalence was broadly similar across sites (CXH 2.7% [CI 1.9%‐3.6%], SMH 4.0% [CI 3.1%‐5.1%], Liverpool 3.2% [CI 2.4%‐4.2%]), variation was seen in local BBV prevalence patterns (Figure 1). For example, active HCV infection accounted for 86% of local BBV infections in Liverpool (HCV prevalence 2.7% [CI 2.0%‐3.7%]), compared with 25% in London (HCV prevalence 1.0% [CI 0.6%‐1.6%] and 0.7% [CI 0.3%‐1.2%] at CXH and SMH, respectively). Conversely, HBV and HIV infections were more common in London: HBV 0.9% (CI 0.5%‐1.6%), HIV 1.0% (CI 0.6%‐1.7%) at CXH; HBV 2.0% (CI 1.4%‐2.8%), HIV 1.5% (CI 0.9%‐2.2%) at SMH compared with HBV 0.4% (CI 0.2%‐0.9%), HIV 0.1% (CI 0.0%‐0.4%) in Liverpool.
Figure 1.
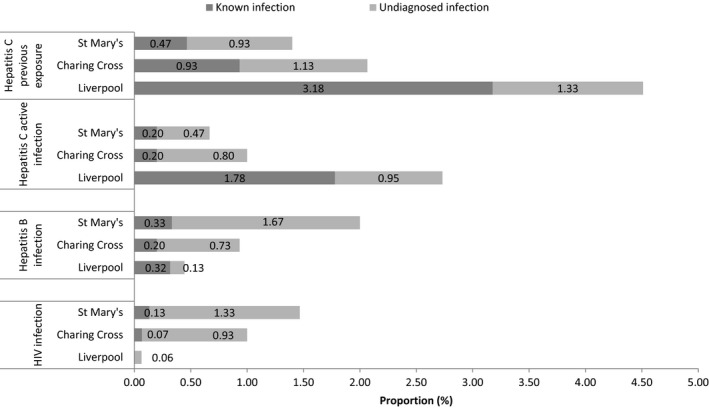
Prevalence of previously known and undiagnosed active blood‐borne virus infection in unselected ED attendees in England stratified by infection and location. A total of 4574 unique patient samples were tested at three sites: two London hospitals (Charing Cross; n = 1500 and St Mary's; n = 1500) in the southeast of England and one city in the northwest of England (Liverpool; n = 1574). Undiagnosed prevalence calculated using local health records. Previous exposure to HCV infection defined by detection of HCV antibody. HCV active infection defined by detection of HCV RNA. BBV, blood‐borne virus; ED, emergency department
All infections were more common in males than females (5.3% vs 1.5%). This increased risk was greatest for HIV (risk ratio [RR] of 5.1 [CI 2.3‐11.7], P < .001), followed by HCV (RR 4.5 [CI 2.5‐8.0], P < .001)), but males also had twice the risk of HBV (RR 2.1 [CI1.2‐3.7], P = .008). Highest prevalence was consistently in the 36‐45 age group (HCV 3.0%, HBV 1.9%, HIV 1.6%) with risk ratios of 2.2 [CI1.2‐4.1] for HCV P = .013, 2.8 [CI1.0‐7.6] for HBV P = .045, 5.9 [CI 1.3‐26.0] for HIV P < .019 compared with the risk in those aged 16‐25.
A disproportionately high prevalence of all BBV infection was found in people of black ethnicities in ethnically diverse London; 6.1% of 115 Black Caribbean attendees had a BBV infection (4.4% HCV, 0.9% HBV, 0.9% HIV) and 9.6% of 136 Black African attendees (0.0% HCV, 6.6% HBV, 3.7% HIV). In contrast, most infections (0.3% HBV, 2.3% HCV, 0.0% HIV) in Liverpool were amongst white British.
4. DISCUSSION
To our knowledge, this study is the largest multicentre anonymous BBV data set from UK ED attendees. We demonstrate a consistently high active BBV prevalence at all urban ED sites (3.3% [range 2.7%‐4.0%]), and the results confirm those of an earlier HCV study adding weight to the case for systematic BBV testing in this population.5 Of particular importance given existing public health objectives was that two thirds of active infections were estimated to be locally undiagnosed.
Our overall HIV prevalence findings are in line with those reported in previous UK studies, although the proportion of previously undiagnosed infections was higher (0.8% vs 0.3%).6 This may reflect the limitation of using local databases for prior diagnosis but also local demographic variation or chance.
National BBV surveillance is coordinated through Public Health England with ED data accounting for a small proportion of this surveillance. Estimated prevalence figures in 2015 were HCV antibody 2.2%, HBsAg 1.2% and HIV Ag/Ab 1.4%. The 2015 surveillance does not report if these are previously diagnosed and/or engaged with care. Using this data and modelling estimates the general population prevalence of active HCV infection in England is estimated at 0.4%.7 In contrast to this, we demonstrated nearly fourfold higher HCV RNA prevalence in ED attendees overall, increasing to sevenfold in Liverpool, and estimated the proportion of new cases without previous diagnosis.
Also our estimate of undiagnosed HBV infection (0.8%) suggests inclusion of EDs within HBV elimination strategies will allow case finding in populations not covered by targeted testing initiatives or existing HBV screening such as the infectious diseases in pregnancy. Commissioners are encouraged to develop locally enhanced services for hepatitis B and C in areas where there is a higher than average number of people at increased risk; however, developing policies beyond risk‐based testing in ED is hampered by a lack of robust local epidemiological data.4 There is significant opportunity within EDs to identify new cases of viral hepatitis through increased testing with subsequent linkage to treatment and immunization initiatives: the EDs involved in this study engaged with 352 618 attendees in 2016‐2017, of which half can be expected to have bloods taken, but as yet none of these EDs currently employ systematic BBV testing.8 Our results support the case to implement systematic BBV testing in urban ED attendees triaged for a blood draw, although we recommend other regions undertake prior assessment of local prevalence to inform if a selective testing policy may be appropriate.
Laboratory BBV test tariffs vary considerably between providers in the UK. When including resolution of false positives and using averaged standard tariffs, laboratory costs per active diagnosis in our study were £663 HCV, £563 HBV and £1277 HIV (Table S2). Laboratory assay cost will have a key impact on high‐volume ED testing. However, savings are likely achievable through price‐volume and test bundle agreements. Further work is underway to estimate cost‐effectiveness of systematic testing in the English EDs.9 Testing in this area is not without challenge given the high pressure on staff time and potential costs involved. However, there is substantial US and emerging UK evidence of the effectiveness of electronic patient record (EPR) triggered ED BBV screening programmes and that subsequent engagement with the care cascade can be effective and sustainable.3, 8 Molecular platforms now facilitate near‐patient nucleic acid testing, and results can be available within NHS ED waiting time targets.10 Coupled with improved therapy options, the environment now exists for new pathways to be considered for increased diagnosis and rapid linkage to care.
Our study has a number of limitations. Our study used routinely collected ED data and was not designed to collect clinical data or other risk factors such as homelessness or injecting drug use. Further, our data pertain to populations triaged for a blood draw which may differ from low‐risk patients attending for minor injuries.10 Conversely, the requirement for surplus serum might add a bias against patients with poor venous access such as PWID, although this would have led to our results representing an underestimate. The anonymous study model relied on local healthcare databases for known prevalence estimates, so prior diagnosis in another healthcare service using different laboratories cannot be excluded. However, we feel this would apply less to the undiagnosed prevalence in the more unitary healthcare economies outside London. Importantly, prior diagnosis does not equate to engagement with care which is especially relevant for HCV and marginalized populations in general. Testing in the ED provides an opportunity to re‐diagnose and re‐engage for treatment. Whilst our aim was primarily to inform viral hepatitis ED epidemiology, and our site sample size therefore sufficiently powered for HCV and HBV, we acknowledge it was likely underpowered to precisely estimate HIV prevalence.
Our multicentre results support emerging evidence that urban ED BBV testing initiatives could provide a valuable contribution to improve diagnosis and linkage to care pathways in England. We recommend that testing strategies are informed by local prevalence data if WHO targets are to be achieved.
CONFLICT OF INTERESTS
MJH has received speaker honoraria from Hologic. ZM is the beneficiary of a Gilead HIV/Hepatitis Fellowship. RV is involved in a project to evaluate the impact of rotavirus vaccination funded by GSK. ASB has received research funding from, acted as an investigator for, sat on advisory boards for, and/or received speaker honoraria from the following: Abbvie, Gilead, MSD. MR is an employee of Gilead Sciences. ST, MB, CA, D.M, AC report no potential conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
This project has been supported through an Association of the British Pharmaceutical Industry (ABPI) ‘Joint Working' initiative with Gilead Sciences. Gilead provided technical expertize, project management and funding for BBV testing. National Health Service (NHS) and Public Health England (PHE) partners contributed independent expertize and skills in their own professional capacity without additional financial support. Data generation and analysis were done independently by NHS and PHE partners. Content, conclusions and recommendations were agreed by consensus by the authors. These are the authors own agreed views and do not necessarily reflect those of their employers.
Hopkins MJ, Todd S, Beadsworth M, et al. Consistent high prevalence of undiagnosed blood‐borne virus infection in patients attending large urban emergency departments in England. J Viral Hepat. 2020;27:88–91. 10.1111/jvh.13197
REFERENCES
- 1. World Health Organization . Global health sector strategy on viral hepatitis 2016–2021. http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/. Accessed February 24, 2019.
- 2. National Health Service Digital . Hospital accident and emergency activity 2016–17. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-accident-emergency-activity/2016-17. Accessed February 24, 2019 NHS.
- 3. Anderson ES, Galbraith JW, Deering LJ, et al. Continuum of care for hepatitis C virus among patients diagnosed in the emergency department setting. Clin Infect Dis. 2017;64(11):1540‐1546. [DOI] [PubMed] [Google Scholar]
- 4. National Insitute for Health and Care Excellence . Surveillance report 2017 – Hepatitis B and C testing: people at risk of infection; 2012. NICE guideline PH43. https://www.nice.org.uk/guidance/ph43/resources/surveillance-report-2017-hepatitis-b-and-c-testing-people-at-risk-of-infection-2012-nice-guideline-ph43-4666262221/chapter/How-we-made-the-decision?tab=evidence. Accessed August 22, 2018.
- 5. Orkin C, Leach E, Flanagan S, et al. High prevalence of hepatitis C (HCV) in the emergency department (ED) of a London hospital: should we be screening for HCV in ED attendees? Epidemiol Infect. 2015;143(13):2837‐2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orkin C, Flanagan S, Wallis E, et al. Incorporating HIV/hepatitis B virus/hepatitis C virus combined testing into routine blood tests in nine UK Emergency Departments: the "Going Viral" campaign. HIV Med. 2016;17(3):222‐230. [DOI] [PubMed] [Google Scholar]
- 7. Public Health England . Hepatitis C in the UK 2015 Report. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/448710/NEW_FINAL_HCV_2015_IN_THE_UK_REPORT_28072015_v2.pdf. Accessed August 22, 2018.
- 8. Flower B, Harrison J, Evans H, et al. VirA&EmiC Project: Universal hepatitis C & B screening with integrated linkage to care in an Urban London Emergency Department ‐ Interim Results. Hepatology. 2017;66(S1):524A. [Google Scholar]
- 9. Williams J, Vickerman P, Douthwaite S, et al. A threshold analysis for the cost‐effectiveness of hepatitis B and hepatitis C testing in emergency departments in the UK. International Liver Conference 2019. J Hepatol. 2019; 70(S1):e348 THU‐435. [Google Scholar]
- 10. Geretti AM, Austin H, Villa G, et al. Point‐of‐care screening for a current hepatitis C virus infection: influence on uptake of a concomitant offer of HIV screening. Sci Rep. 2018;8(1):15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


