Abstract
Aim
To assess local and individual factors that should be considered in the design of a pulse oximetry screening strategy in New Zealand's midwifery‐led maternity setting.
Methods
An intervention study was conducted over 2 years. Three hospitals and four primary maternity units participated in the study. Post‐ductal saturation levels were measured on well infants with a gestation of ≥35 weeks. Infant activity and age (hours) at the time of the test were recorded.
Results
Screening was performed on 16 644 of 27 172 (61%) eligible infants. The age at which the screening algorithm was initiated varied significantly among centres. The probability of achieving a pass result (saturations ≥95%) in the context of no underlying pathology ranged from .94 for an unsettled infant screened <4 hours of age to .99 (P < .001) when the test was performed after 24 hours on a settled infant. Forty‐eight (0.3%) infants failed to reach saturation targets: 37 had significant pathology of which three had cardiac disease.
Conclusion
Screening practices were influenced by the setting in which it was undertaken. Infant activity and age at the time of testing can influence saturation levels. Screening is associated with the identification of significant non‐cardiac pathology.
Keywords: congenital heart disease, midwifery‐led maternity setting, screening strategy
Key notes.
Early pulse oximetry screening identifies respiratory and other diseases in addition to cardiac anomalies.
The timing of the test as well as infant activity can influence saturation levels.
Breastfeeding at the time of testing does not result in a higher false‐positive rate compared with testing infants that are awake and settled.
1. INTRODUCTION
Early detection of disease through screening can have benefits to individuals as well as the wider community through a reduction in mortality, improvements in quality of life and a reduction in healthcare costs. The overall risk‐benefit profile of a screening programme may be dependent on the characteristics of the target population, and the design and quality of the screening programme.
Pulse oximetry has been utilised as a screening tool for the early detection of critical congenital heart disease (CCHD) for more than a decade. Various screening strategies are currently in use, and in the absence of evidence clearly identifying a superior strategy, it is likely that this heterogeneity in practice will persist.1 Superiority is commonly measured in terms of the test's sensitivity, specificity and false‐positive rate related to critical cardiac anomalies.2, 3, 4 However, in recent years the value of utilising pulse oximetry in the detection of respiratory and other diseases resulting in hypoxaemia also has been recognised.5 Regional and population‐specific factors such as the service delivery model, impact on health services and the overall health benefit are also important considerations when designing a screening strategy.
New Zealand has a widely dispersed population of 4.9 million. There is a single paediatric cardiac centre. Maternity care provision for pregnant women is universally funded. The country has a well‐developed screening programme for the antenatal detection of congenital cardiac anomalies.6 Primary maternity care is provided by a Lead Maternity Carer (LMC). The majority of LMCs are midwives7 who provide continuity of care in a partnership model from early pregnancy, through labour and birth, and up to 6 weeks post‐partum.8 Women have a choice of who provides their care (an LMC can be an obstetrician, a midwife or a General Practitioner) and where they birth. Women with low‐risk pregnancies can birth at home, in a primary maternity unit or in a secondary or tertiary facility. Following normal births in a secondary/tertiary hospital, women are often discharged to either home or primary maternity unit within a few hours of birth. The context of care is therefore different to many other developed countries. For a quality improvement initiative to be successful, it must be adaptable to local needs. In view of this, a feasibility study of pulse oximetry screening was undertaken to identify local factors that should be considered in the design of a national screening strategy for New Zealand specifically in the context of good antenatal detection rates for CCHD. In addition, there was a need to consider the optimal timing of screening dependent on birthplace and age of the infant. Strategies were explored to limit the number of low oxygen saturation readings in the absence of underlying pathology.
2. METHODS
This study was a part of an intervention study exploring the feasibility of pulse oximetry screening at hospitals and primary maternity units affiliated with three of New Zealand's 20 health districts. Screening was introduced at a metropolitan hospital and its neighbouring primary maternity unit (District A) in May 2016 followed by the introduction of screening at a regional level 1 and level 2 hospital (District B) in June 2016. Three primary maternity units from District C joined the study in November 2016. Data were collected up to 30 April 2018. District A's hospital is a quaternary referral centre where New Zealand's sole paediatric cardiac service is based. Specialist paediatric services, including echocardiography, are available at District B's level 2 hospital. District C includes a tertiary referral hospital with specialist neonatal services. This hospital did not participate in the study, but several infants born at the hospital were screened if they transferred to a participating regional primary maternity unit for post‐natal care. These units provide intrapartum and post‐natal support and care to mothers and their newborns in the first few days following the birth. The neonatal team based at this tertiary centre provide advice and support to the primary facilities.
All pathologies identified as a result of pulse oximetry screening are reported as their early detection is potentially of benefit to the newborns, their families and the healthcare sector. However, test sensitivity, specificity and positive predictive value (PPV) were calculated for critical congenital cardiac defects only. The cardiac centre's databases were interrogated to identify all infants, not identified on antenatal ultrasound screening that underwent a cardiac catheter and/or surgical intervention. Mortality data were obtained from the New Zealand Ministry of Health's Mortality Collection and the Perinatal and Maternal Mortality Review Committee. We sought to identify cardiac‐related deaths in infants born alive during the study period at any of the three participating districts. Deaths and cardiac interventions in the first 28 days after birth were identified to satisfy this definition commonly used to describe a ‘critical’ congenital cardiac defect.9
Study guidelines and resources were developed prior to the introduction of screening.10 Post‐ductal oxygen saturations were measured on well newborn infants with a gestational age of ≥35 weeks. The recommended time of entering the screening algorithm was between 2 and 24 hours after birth. Infants with a prenatal diagnosis of a congenital anomaly and other infants admitted to the Neonatal Intensive Care Unit (NICU) within 2 hours from birth were excluded from the study.
Identical hand‐held pulse oximeters (Masimo; Radical SET, version 5 with reusable sensors) with an averaging time of 8 seconds were provided to all participating centres. Infants achieving an oxygen saturation of 95% or greater passed the test and required no further evaluation provided that they remained clinically well. Results below 90% warranted a referral to the nearest paediatric service for telephonic advice and/or clinical assessment. Saturations between 90% and 94% were regarded as an inconclusive result, and therefore, repeat testing had to be performed 1‐2 hours later. Three consecutive results in the inconclusive range also warranted a paediatric referral. The relationship between oxygen saturation, infant activity and the infant's age at the time of the first screening test has been explored with the aim to assist in designing a screening strategy that will minimise saturation readings <95% in the context of no underlying pathology.
Pulse oximetry screening was performed by midwives or nurses. Screeners received education making them aware that movement and crying can affect test accuracy and that earlier testing can result in lower oxygen saturation levels. However, no recommendations were made with regard to the ideal timing to conduct the test or the infant's optimal state of activity during testing. Test results were recorded on a case report form (CRF). The following information was collected: date and time of birth; date and time of each pulse oximetry test; gestational age; ethnicity; written parental consent (as stipulated by the ethics committee); the infant's activity at the time of testing (asleep; breastfeeding; unsettled; or awake and settled); and the post‐ductal oxygen saturation level. Information was transferred to an electronic database that assigned a unique identification code to each participant.
Paediatric services completed a separate CRF for all infants that came under their care as a result of failure to reach oxygen saturation targets. This form contained three segments: (a) Clinical presentation; (b) Investigations and diagnosis; and (c) Admission summary. Clinical records and investigations were retrospectively reviewed by a neonatologist to ascertain whether the diagnosis as reported on the CRF is appropriate. This provided a detailed report of time and resources invested into transferring, investigating and treating these infants.
2.1. Statistical analysis
Median and range were used to describe continuous variables, and percentages were used for categorical variables. The chi‐square test was used for comparisons between categorical variables and the Kruskal‐Wallis test for comparisons between continuous variables. Screening strategies were compared for the prespecified outcome by using multivariable logistic regression analysis and are presented as a probability of achieving a true‐negative result. A P value <.05 was considered statistically significant. Data were analysed using statistical software (JMP, version 14.0; SAS Institute). Exclusion from analyses applied if key data fields were missing from a participant's CRF(s).
3. RESULTS
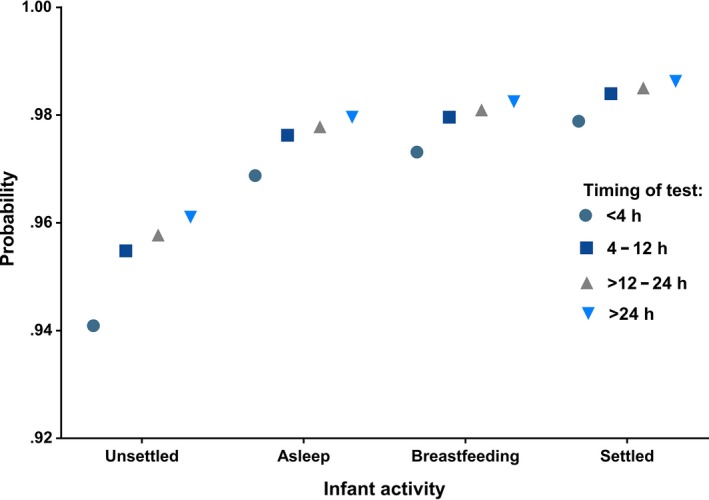
During the 24‐month study period, pulse oximetry screening was performed on 16 644 of 27 172 (61%) eligible infants with the first test conducted at a median age of 7 hours (range 1‐472). The timing of testing varied significantly among participating centres (Table 1). A higher proportion of inconclusive results requiring repeat testing were recorded for District A where infants entered the screening algorithm at a younger age (Table 1). A pulse oximetry test prior to 4 hours of age resulted in a higher proportion of infants failing to achieve a saturation level of at least 95% compared with those undergoing testing more than 24 hours after birth (2.8% vs 1.9%; P = .005, Table 2). Infants that were unsettled or asleep at the time of testing were less likely to pass compared with awake and settled infants (P < .001 and P = .002, respectively). However, breastfeeding during the recording did not result in lower oxygen saturation levels (Table 2). The probability of achieving a test result of ≥95% in the context of no underlying pathology ranged from .94 for an unsettled infant screened prior to 4 hours of age to .99 (P < .001) when the test was performed after 24 hours on a settled infant (Figure 1).
Table 1.
Timing of testing
| Total | District A | District B | District C | P value | |
|---|---|---|---|---|---|
| Total screened, n | 16 644 | 10 798 | 1739 | 4107 | |
| Age algorithm entered, median (range) | 7 h (1‐472) | 3 h (1‐292) | 15 h (1‐393) | 31 h (1‐472) | <.001 |
| Required a 2nd test, n (%) | 387 (2.3) | 292 (2.7) | 26 (1.5) | 69 (1.7) | <.001 |
| Required a 3rd test, n (%) | 83 (0.5) | 60 (0.5) | 12 (0.7) | 11 (0.3) | .05 |
Table 2.
Relationship between saturation levels, timing of 1st test and infant activity
| Total (n) | Median (range) | First saturation <95%, n (%) | Pathology (n) | No pathology, n (%) | P value | |
|---|---|---|---|---|---|---|
| Timing of testinga | ||||||
| <4 h | 6122 | 98 (77‐100) | 198 (3.2) | 25 | 173 (2.8) | .005 |
| 4‐12 h | 3092 | 99 (55‐100) | 78 (2.5) | 10 | 68 (2.2) | .4 |
| >12‐24 h | 2580 | 99 (85‐100) | 54 (2.1) | – | 54 (2.1) | .6 |
| >24 h | 3617b | 98 (78‐100) | 70 (1.9) | 1 | 69 (1.9) | d |
| Activityc | ||||||
| Asleep | 5365 | 99 (55‐100) | 144 (2.7) | 9 | 135 (2.5) | .002 |
| Breastfeeding | 2448 | 99 (77‐100) | 53 (2.2) | 4 | 49 (2.0) | .3 |
| Awake settled | 6408 | 99 (77‐100) | 122 (1.9) | 14 | 108 (1.7) | d |
| Awake unsettled | 1030 | 98 (81‐100) | 53 (5.1) | 1 | 52 (5.0) | <.001 |
Exclusions applied to 1233 due to insufficient data.
Six hundred and seventy‐four were screened >72 h after birth.
Exclusions applied to 1393 due to insufficient data. Infant activity not recorded for eight infants with pathology.
Reference for making individual comparison with other variables in relation to the proportion of readings <95% in the context of no pathology.
Figure 1.
Probability of achieving saturations ≥95% in context of no pathology
Forty‐eight infants (0.3%) ultimately did not achieve oximetry screening targets; all but 7 (15%) were admitted to a newborn unit as a result (Table 3). Eleven (23%) infants had to be transferred to a larger medical centre for assessment and investigations. The median distance travelled for an assessment was 43 km (range 1‐80).
Table 3.
Characteristics of infants that failed to reach oxygen saturation targets
| Reason failed (Saturation ± clinical signs) | Age algorithm completed (h) | Diagnosis | Transfer required for assessment | Distance transferred for assessment (km) | Hospital admission required | |
|---|---|---|---|---|---|---|
| 1. | 77% | 2 | d‐TGA | No | – | Yes |
| 2. | 3 × 90%‐94% | 6 | Birth transition | Yes | 1 | Yes |
| 3. | 3 × 90%‐94% | 7 | Birth transition | No | – | No |
| 4. | 77% | 3 | Pneumonia | No | – | Yes |
| 5. | 89% | 2 | TTN | No | – | Yes |
| 6. | 78% | 5 | Pneumonia | No | – | Yes |
| 7. | 3 × 90%‐94% | 3 | TTN | No | – | Yes |
| 8. | 85% | 2 | Pneumonia | No | – | Yes |
| 9. | 85% | 13 | Pneumonia | No | – | Yes |
| 10. | 89% | 3 | TTN | No | – | Yes |
| 11. | 81% | 2 | TTN | No | – | Yes |
| 12. | 3 × 90%‐94% | 5 | Birth transition | No | – | No |
| 13. | 93% and tachypnoeic | 3 | Meconium exposure | No | – | Yes |
| 14. | 85% | 3 | Pneumonia | No | – | Yes |
| 15. | 3 × 90%‐94% | 10 | TTN | No | – | Yes |
| 16. | 74% | 5 | PPHN | Yes | 1 | Yes |
| 17. | 90% and tachypnoeic | 3 | Pneumonia | No | – | Yes |
| 18. | 88% | 2 | Meconium exposure | No | – | Yes |
| 19. | 89% | 4 | Pneumothorax | No | – | Yes |
| 20. | 92% and tachypnoeic | 3 | Sepsis | No | – | Yes |
| 21. | 3 × 90%‐94% | 19 | Birth transition | Yes | 43 | Yes |
| 22. | 90% and marked acrocyanosis | 7 | TAPVD | Yes | 18 | Yes |
| 23. | 82% | 10 | TTN | Yes | 18 | Yes |
| 24. | 85% | 7 | Pneumonia | Yes | 43 | Yes |
| 25. | 80% | 6 | d‐TGA and CoA | Yes | 18 | Yes |
| 26. | 87% | 3 | Meconium exposure | No | – | Yes |
| 27. | 3 × 90%‐94% | 11 | Ongoing unexplained oxygen requirement | No | – | Yes |
| 28. | 55% | 6 | Pneumonia | No | – | Yes |
| 29. | 87% | 3 | Birth transition | Yes | 80 | No |
| 30. | 3 × 90%‐94% | 7 | Birth transition | Yes | 80 | No |
| 31. | 88% | 5 | PPHN | Yes | 80 | Yes |
| 32. | 98% and HR 240 bpm | 4 | Supraventricular tachycardia | No | – | Yes |
| 33. | 84% | 6 | PPHN | No | – | Yes |
| 34. | 90% and tachypnoeic | 4 | TTN | No | – | Yes |
| 35. | 88% | 3 | Sepsis | No | – | Yes |
| 36. | 3 × 90%‐94% | 19 | Pneumonia | No | – | Yes |
| 37. | 88% | 5 | Sepsis | No | – | Yes |
| 38. | 88% | 3 | Pneumonia | No | – | Yes |
| 39. | 3 × 90%‐94% | 6 | Pneumonia | No | – | Yes |
| 40. | 85% | 10 | TTN | No | – | Yes |
| 41. | 3 × 90%‐94% | 6 | Birth transition | No | – | No |
| 42. | 3 × 90%‐94% | 26 | Birth transition | No | – | Yes |
| 43. | 3 × 90%‐94% | 18 | Pneumonia | No | – | Yes |
| 44. | 83% | 3 | Birth transition | No | – | No |
| 45. | 82% | 36 | Birth transition | Yes | 43 | Yes |
| 46. | 92% and tachypnoeic | 2 | Meconium exposure | No | – | Yes |
| 47. | 88% | 2 | Pneumonia | No | – | Yes |
| 48. | 88% | 5 | Birth transition | No | – | No |
Abbreviations: CoA, coarctation of the aorta; d‐TGA, d‐loop transposition of the great arteries; PPHN, persistent pulmonary hypertension; TAPVD, total anomalous pulmonary venous drainage; TTN, transient tachypnoea of the newborn.
Critical congenital heart disease was detected in three infants. A review of cardiac surgical data revealed a further two infants with a post‐discharge diagnosis of d‐loop transposition of the great arteries and one infant with atrial and ventricular septal defects that required intervention in the first 28 days after birth. Pulse oximetry screening was, however, not performed on these infants prior to discharge—they were therefore excluded from the test sensitivity calculation for the detection of CCHD. The sensitivity of pulse oximetry screening for the detection of CCHD in this study population was therefore 100%, the specificity 99.7% and the PPV 6.25%.
A further three infants had persistent pulmonary hypertension, and one newborn was diagnosed with supraventricular tachycardia. Respiratory disease was responsible for the majority of positive screening results. There were 13 infants with congenital pneumonia, eight with transient tachypnoea of the newborn, four with meconium exposure and one with a pneumothorax. Three infants were diagnosed and treated for sepsis. One infant had an ongoing unexplained oxygen requirement (presumably related to respiratory pathology) and was discharged home on supplemental oxygen after 15 days. He remained on oxygen for a further 2 weeks after discharge (Table 3).
No pathology could be identified in a further 11 (23%) infants who failed to reach saturation targets. Four (36%) of these infants were admitted to a neonatal unit for investigation and observation. The median (range) duration of admission was 1 day (0‐2). Seven infants failed the test as a result of saturation levels persistently in the 90%‐94% range, and four had saturations below 90%. Testing was conducted at an early stage in the majority of these infants with the algorithm completed at a median age of 5 hours (range 3‐36). A summary is provided of investigations performed on all infants who failed to reach oxygen saturation targets (Table 4).
Table 4.
Investigations
|
CHD n (3) |
SVT n (1) |
PPHN n (3) |
Respiratory pathology n (27) |
Sepsis n (3) |
Slow transition/ No pathology n (11) |
Total n (48)( |
|
|---|---|---|---|---|---|---|---|
| Full blood count, n (%) | 3 (100) | 1 (100) | 3 (100) | 27 (100) | 3 (100) | 6 (55) | 43 (90) |
| Blood culture, n (%) | 2 (67) | – | 2 (67) | 26 (96) | 3 (100) | 2 (18) | 35 (73) |
| C‐reactive protein, n (%) | 2 (67) | – | 1 (33) | 13 (48) | 3 (100) | 4 (36) | 23 (48) |
| Blood gas, n (%) | 3 (100) | 1 (100) | 3 (100) | 27 (100) | 3 (100) | 5 (45) | 42 (88) |
| Chest radiograph, n (%) | 3 (100) | 1 (100) | 3 (100) | 27 (100) | 3 (100) | 7 (64) | 44 (92) |
| Electrocardiogram, n (%) | 3 (100) | 1 (100) | – | 5 (19) | 1 (33) | – | 10 (21) |
| Echocardiogram, n (%) | 3 (100) | 1 (100) | 3 (100) | 3 (11) | – | 1 (9) | 11 (23) |
Abbreviations: CHD, congenital heart disease; PPHN, persistent pulmonary hypertension; SVT, supraventricular tachycardia; n, number.
4. DISCUSSION
This study of pulse oximetry screening was implemented in a setting with a high antenatal detection rate of congenital cardiac anomalies and with a largely midwifery‐led maternity service. It demonstrated that regional‐led screening programmes may result in heterogeneous practices that can affect the standard of the service provided. A maternity system with early discharges necessitates that screening tests be conducted at an early age. This will result in a larger proportion of positive test results due to greater identification of pathology other than CCHD. In this study, several infants with significant respiratory or infectious pathology were identified prior to the onset of detectable clinical signs, likely preventing the discharge of those in need of medical intervention. The impact of positive results will be greatest in rural communities where infants have to be transferred for a specialist paediatric review. Both the timing of screening and the infant's activity during the test have an impact on oxygen saturation levels. Low oxygen saturation levels in the absence of pathology can be limited if the test is conducted after 4 hours of age on an infant that is either breastfeeding or awake and settled.
The diversity within New Zealand's maternity healthcare system is highlighted in this study. The majority of women give birth at high volume tertiary or secondary maternity facilities. It is not uncommon for post‐natal discharge home or to a primary maternity facility to occur within 2‐6 hours following an uncomplicated vaginal birth. This study's screening guidelines recommended that pulse oximetry screening should be performed prior to leaving the place of birth.10 Approximately 9% of women give birth at primary facilities11 and will often remain at the facility for several days to receive post‐natal care. Twenty‐one per cent of the study cohort were screened at an age greater than the recommended time frame of 24 hours. This is mainly because many infants born at the non‐participating tertiary centre were screened after transfer to a participating primary maternity unit. The place of birth is therefore an important factor determining the timing of testing. The feasibility of very early screening strategies has been investigated in settings with a high number of home births. The Dutch designed an algorithm where the first screening test was performed at approximately 1 hour after birth to fit in with the time midwives are routinely present. They reported a false‐positive rate of 0.6%.12 Cawsey et al13 demonstrated that early pulse oximetry screening for home births is both feasible and acceptable among midwives in Birmingham.
A meta‐analysis of pulse oximetry screening reported a sensitivity of 79.5% (95% CI 70.0‐86.6) for studies that performed screening prior to 24 hours and a sensitivity of 73.6% (95% CI 62.8‐82.1) for studies that performed screening after 24 hours.9 The advantage of early testing is that it enables the timely diagnosis of conditions for which early intervention is paramount to achieving good outcomes. The survival of infants with d‐loop transposition of the great arteries and an intact ventricular septum may depend on timely access to cardiac services capable of performing a balloon arterial septostomy.14 The infants in this study with confirmed CCHD were screened at an age of 2, 6 and 7 hours, respectively, which enabled early diagnosis and immediate intervention.
The benefits of an early screening strategy should be weighed against the harm that may come from low saturation readings in the context of transitional circulation. In comparison with late screening strategies, early screening has been shown to carry a higher false‐positive burden.15, 16 Limiting the number of positive results unrelated to cardiac disease has been a key consideration for those electing to initiate screening >24 hours after birth.17 It has been reported that 4%‐5% of infants screened on day one of life will require repeat testing in order to exclude healthy infants with transitional circulation.12, 17 In this study, the lowest rate of positive results unrelated to pathology was indeed observed after 24 hours. The yield from pulse oximetry screening does, however, appear to be inversely related to time. In this study, one pathology was identified for every 245 tests that were performed <4 hours after birth compared with one pathology for every 309 tests performed between 4 and 12 hours. One pathology was identified among the 6197 tested after 12 hours. Early pulse oximetry screening therefore improves the likelihood of identifying disease before the onset of symptoms.
The anxiety and inconvenience caused by false‐positive or inconclusive test results will likely be greatest in those based in rural areas where access to specialist services is limited. Two infants in this study found to have low saturation levels travelled 80 km for an assessment. Both babies were screened at an early age and had normal oxygen saturations by the time they reached the referral centre. It is a challenging task to design a screening algorithm that will limit the number of false‐positive results yet also enable the timely diagnosis of infants with critical cardiac disease. This is especially so for those living in remote areas where a specialised paediatric assessment is not readily available. The number of unnecessary transfers will be reduced if the screening algorithm is modified to recommend that infants with borderline low saturation recordings and a normal clinical examination should only be referred for an assessment if saturations remain <95% beyond 12 hours of age.
Avoiding the test at a time when an infant is unsettled or asleep can further reduce the proportion of measurements below 95% in healthy infants. It is generally recommended that infants should be awake and settled at the time pulse oximetry screening is performed. The recommendation to avoid testing when an infant is asleep has been based on the speculation that deep sleep can result in hypoventilation and a low saturation recording.17 However, there have been no previous reports in the literature providing the evidence that the sleep state and restlessness can result in a higher number of low saturation readings when pulse oximetry screening is performed. This study aimed to determine the feasibility of screening in a maternity setting characterised by early discharge or transfer of care following a birth. Therefore, we did not exclude unsettled infants as this would not have provided evidence to support the design of a screening strategy best suited for such an environment in which the time frame for offering screening may be very limited. It is reassuring that screening during breastfeeding was not different from screening awake and settled babies demonstrating that screening does not have to interfere with the bonding between a mother and her infant.
As single‐limb screening strategy was used in this study. The simplicity of performing the test on one limb was an important consideration as significant concerns were raised about the impact of the test on the workload of midwives. The Cochrane systematic review found no difference in sensitivity or specificity for pulse oximetry screening between post‐ductal screening alone and preductal and post‐ductal screening.9 Therefore, to optimise uptake and decrease workload, a decision was made that post‐ductal screening was most appropriate in this setting.
Several studies have reported that more than two‐thirds of positive screening results will yield a diagnosis other than cardiac disease.18, 19, 20 The broader value of this screening test is highlighted in this study where 33 out of 48 (69%) infants with a positive screening result had a respiratory or infectious disease. This is of particular significance to communities with a high antenatal detection rate of cardiac disease where the yield from pulse oximetry screening for such anomalies will be lower.
5. CONCLUSION
In a maternity setting where length of admission is often measured in hours rather than days, adopting an early pulse oximetry screening strategy is unavoidable. This is associated with low oxygen saturation recordings in association with a wide range of disease. As the term suggests, pulse oximeters identify an abnormal heart rate and low oxygen saturation levels regardless of what the underlying cause may be. The utility of the device therefore stretches beyond identifying congenital cardiac disease in the newborn population where it is challenging to detect cyanosis clinically. This study clearly demonstrates that pulse oximetry screening is a valuable test even in the context of a high antenatal detection rate for cardiac anomalies. The number of positive test results in infants with no underlying disease can be limited if the test is performed beyond 4 hours of age on an infant that is either breastfeeding or settled, but awake.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
ETHICAL APPROVAL
This population‐based audit was approved by the Health and Disability Ethics Committees of New Zealand (15/NTA/168).
ACKNOWLEDGEMENTS
We acknowledge the New Zealand Ministry of Health and the participating Health Boards for the use of their data. This work was overseen by the Pulse Oximetry Screening Steering Committee: Joshua Agnew, Jane Alsweiler, Julena Ardern, Frank Bloomfield, Elza Cloete, Sarka Davidkova, Lesley Dixon, Niki Edwards, Donna Foote, Tom Gentles, Rob Lutter, Chris McKinlay, Kelly Richards & Dianne Webster. We would like to thank the parents and infants that participated in this study, and the midwives, nurses and research assistants that made it all possible.
Cloete E, Gentles TL, Webster DR, et al. Pulse oximetry screening in a midwifery‐led maternity setting with high antenatal detection of congenital heart disease. Acta Paediatr. 2020;109:100–108. 10.1111/apa.14934
Funding information
This work is supported by awards from the Health Research Council of New Zealand, The Starship Foundation, Middlemore Foundation, Green Lane Research and Education Fund, and A+ Trust Research Fund. EC is supported by a scholarship from Gravida National Centre for Growth and Development.
REFERENCES
- 1. Narayen IC, Blom NA, Ewer AK, Vento M, Manzoni P, te Pas AB. Aspects of pulse oximetry screening for critical congenital heart defects: when, how and why? Arch Dis Child Fetal Neonatal Ed. 2016;101:F162‐F167. [DOI] [PubMed] [Google Scholar]
- 2. de Wahl GA, Mellander M, Sunnegardh J, Sandberg K, Ostman‐Smith I. Screening for duct‐dependant congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity. Acta Paediatr. 2005;94:1590‐1596. [DOI] [PubMed] [Google Scholar]
- 3. Riede FT, Worner C, Dahnert I, Mockel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine–results from a prospective multicenter study. Eur J Pediatr. 2010;169:975‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ailes EC, Gilboa SM, Honein MA, Oster ME. Estimated number of infants detected and missed by critical congenital heart defect screening. Pediatrics. 2015;135:1000‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meberg A. Newborn pulse oximetry screening is not just for heart defects. Acta Paediatr. 2015;104:856‐857. [DOI] [PubMed] [Google Scholar]
- 6. Cloete E, Bloomfield FH, Cassells SA, de Laat MWM, Sadler L, Gentles TL. Newborn pulse oximetry screening in the context of a high antenatal detection rate of critical congenital heart disease. Acta Paediatr. 2020; 109: 93–99. 10.1111/apa.14946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ministry of Health . Report on maternity 2015. 2017. https://www.health.govt.nz/publication/report-maternity-2015. Accessed April 4, 2018.
- 8. Ministry of Health . Maternity Services: Notice Pursuant to Section 88 of the New Zealand Public Health and Disability Act 2000. Wellington, New Zealand: Ministry of Health; 2007. [Google Scholar]
- 9. Plana MN, Zamora J. Suresh G, Fernandez‐Pineda L, Thangaratinam S, Ewer AK. Pulse oximetry screening for critical congenital heart defects. Cochrane Database Syst Rev. 2018;(3):CD011912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Starship Clinical Guidelines . Pulse oximetry screening in the newborn. 2016. https://www.starship.org.nz/for-health-professionals/starship-clinical-guidelines/p/pulse-oximetry-screening-in-the-newborn/. Accessed January 4, 2019
- 11. Ministry of Health . New Zealand maternity clinical indicators 2014. 2016. https://www.health.govt.nz/publication/new-zealand-maternity-clinical-indicators-2014. Accessed April 18, 2018.
- 12. Narayen IC, Blom NA, Verhart MS, et al. Adapted protocol for pulse oximetry screening for congenital heart defects in a country with homebirths. Eur J Pediatr. 2015;174:129‐132. [DOI] [PubMed] [Google Scholar]
- 13. Cawsey MJ, Noble S, Cross‐Sudworth F, Ewer AK. Feasibility of pulse oximetry screening for critical congenital heart defects in homebirths. Arch Dis Child Fetal Neonatal Ed. 2016;101:F349‐F351. [DOI] [PubMed] [Google Scholar]
- 14. Cloete E, Bloomfield FH, Sadler L, de Laat M, Finucane AK, Gentles TL. Antenatal detection of treatable critical congenital heart disease is associated with lower morbidity and mortality. J Pediatr. 2019;204:66‐70. [DOI] [PubMed] [Google Scholar]
- 15. Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta‐analysis. Lancet. 2012;379:2459‐2464. [DOI] [PubMed] [Google Scholar]
- 16. Zhao QM, Ma XJ, Ge XL, et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014;384:747‐754. [DOI] [PubMed] [Google Scholar]
- 17. Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128:e1259‐e1267. [DOI] [PubMed] [Google Scholar]
- 18. Meberg A, Brugmann‐Pieper S, Due R Jr, et al. First day of life pulse oximetry screening to detect congenital heart defects. J Pediatr. 2008;152:761‐765. [DOI] [PubMed] [Google Scholar]
- 19. de‐Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhola K, Kluckow M, Evans N. Post‐implementation review of pulse oximetry screening of well newborns in an Australian tertiary maternity hospital. J Paediatr Child Health. 2014;50:920‐925. [DOI] [PubMed] [Google Scholar]


