Purpose and Sample Types
This 14‐color, 16‐antibody OMIP was designed for enumeration of leukocyte responses in pediatric samples, where sample volumes and cell numbers can be very low. Leukocytes identified by this panel include all major members of the innate lymphoid cell (ILC) family (ILC1s, ILC2s, and ILC3s), natural killer cells (NK cells), granulocytes (neutrophils and eosinophils), T‐cells (CD4+ and CD8+), mucosal‐associated invariant T cells (MAIT cells) and NKT‐like cells. The protocol was optimized using small volumes of peripheral blood and validated in airway samples obtained from children (< 2 years of age) admitted to a pediatric intensive care unit (PICU). Given this backdrop, this OMIP is widely applicable to clinical research using low volume or paucicellular samples, such as studies of innate and adaptive immune responses in infants and children, with potential clinical application in diagnostics and monitoring of patients by pediatricians.
Background
The immaturity of the immune system in early life renders infants vulnerable to infectious diseases, particularly those of the gastrointestinal and respiratory tracts. According to the World Health Organization, acute lower respiratory tract infections are the leading cause of death and hospitalization in children globally 1. How the immune system matures in early life is not fully understood. A better understanding of the nature of the immune and inflammatory response to infection in infants and why some infants develop disease could help to identify new clinical biomarkers, treatments, and prophylactics.
One of the major hurdles in the field of neonatal and pediatric immunology is obtaining relevant samples, which is limited by ethical restrictions on the volume and number of samples that can be taken. As a result, much of our understanding of cellular immunity in infants comes from studies of umbilical cord blood. Less is known about immunity in more mature infants, and less still is known about immunity to infection at mucosal sites, such as the lung 2, 3, 4. Moreover, the use of invasive procedures to obtain, for example, lower airway samples cannot be justified in healthy infants. It is important, therefore, that techniques are developed that facilitate the characterization of the immune response in small‐volume and paucicellular samples, particularly from sites of infection where sampling is limited. In designing this OMIP 5 we focused on developing a panel to enumerate the innate lymphoid cell (ILC), granulocyte and T‐cell responses in infants, particularly those with a lung infection (Table 1). To reflect the challenging situation of limited amounts of blood and airway samples from infants we optimized this panel using a small volume (300 μl) of adult peripheral blood. Pitoiset et al. (2018) were able to detect regulatory T cells (Tregs) in as little as 60 μl of whole blood from children 6 and others have demonstrated the feasibility of detecting T cell subsets and Type 2 ILCs within pediatric airway samples 7, 8.
Table 1.
Summary table
| Purpose | Myeloid and innate/adaptive lymphoid comprehensive immunophenotyping |
|---|---|
| Cell types | Whole peripheral blood, cord blood, PBMCs, nasal aspirate, and tracheal aspirate samples |
| Species | Human |
| Cross‐reference | OMIP‐55, OMIP‐007, OMIP‐27, OMIP‐029, OMIP‐038, OMIP‐039 |
The recently discovered ILCs have been implicated in playing a pivotal role in immune responses to viral and bacterial pathogens, and they are particularly abundant at mucosal sites 9, 10, 11. There are three main subsets of ILC; ILC1s, ILC2s, and ILC3s, which may mirror CD4+ T helper 1 (Th1), Th2, and Th17 subsets, whereas NK cells may complement the cytokine profile and function of CD8+ T cells 12. ILCs comprise only 0.1 to 0.01% of lymphocytes in adult blood 13 and most published methods for detection of ILCs are optimized on relatively large volumes of peripheral blood, however, since ILCs are relatively abundant in children we anticipated relatively high frequencies in small volume pediatric samples 8, 14, 15. Immunophenotyping ILCs can be challenging as they are defined as lineage negative lymphocytes, which lack specific markers, including CD3 (T cells), CD19 (B cells), CD34 (progenitor cells), FcεR1α (mast cells) and CD1a, CD123, BDCA2 (plasmacytoid dendritic cells, pDC) 15, 16. Accordingly, while designing this panel we made sure that a very stringent gating strategy was utilized to obtain a pure ILC subset. We used biaxial gating to delineate ILCs as lineage negative, CD127 (IL‐7 receptor‐α) positive, live lymphocytes. We distinguished ILC subsets using surface expression of prostaglandin D2 receptor chemoattractant receptor‐homologous molecule expressed on Th2 cells (CRTH2) and CD117 (c‐Kit) 8, 10, 17. In designing this OMIP other components of the cellular immune response were included, which gives this panel broad applicability to other pediatric clinical studies. These were myeloid cells (neutrophils, eosinophils), invariant T cells (mucosal invariant T cells (MAITs) and NKT‐like cells) and adaptive T cells (CD4+ and CD8+). By immunophenotyping whole blood, rather than peripheral blood mononuclear cells (PBMC), we reduced the processing time and retained granulocyte populations. All fluorochrome‐conjugated antibodies were titrated during panel optimization and are listed in Table 2.
Table 2.
Antibodies used in the optimized multicolor immunofluorescence panel (OMIP‐062)
| Antibody | Fluorochrome | Ab Clone | Purpose |
|---|---|---|---|
| CD127 (IL‐7Rα) | BV421 | A019D5 | ILCs |
| CD14a | BV510 | 63D3 | Lineage |
| CD19a | BV510 | HIB19 | Lineage |
| FcεRIαa | BV510 | AER‐37 (CRA‐1) | Lineage |
| CD123a | BV510 | 6H6 | Lineage |
| CD4 | BV605 | RPA‐T4 | CD4+ T cells |
| CD16 | BV650 | 3G8 | NK cells/neutrophils |
| CD8 | BV711 | SK1 | CD8+ T cells |
| TCR Vα7.2 | BV785 | 3C10 | MAIT cells |
| CD45 | FITC | HI30 | Leukocytes |
| CD117 (c‐kit) | PerCP‐Cy5.5 | A3C6E2 | ILC3 |
| CD3b | PE | OKT3 | T cells |
| CD161 | PE‐Dazzle | HP‐3G10 | MAIT cells |
| CD56 (NCAM)b | PE‐Cy7 | 5.1H11 | NK/NKT‐like cells |
| CD294 (CRTH2) | AF647 | BM16 | ILC2/Th2/Tc2 subsets |
| CD66b | AF700 | G10F5 | Eosinophils |
| Live/Dead | Near IR‐fluorescent reactive dye | n/a | Viability |
Lineage cocktail includes the following antibodies: CD14, CD19, CD123, FcεR1α.
Antibodies used to define the lineage negative population, but not part of the lineage cocktail.
Cells were delineated using the following gating strategy: FSC/SSC, single cells, live cells. Granulocytes, monocytes, and lymphocytes were gated using FSC and the CD45 cell marker (Fig. 1A). Within the granulocyte population, neutrophils were defined as CD16HCD66b+/− and eosinophils as CD16+/‐CD66b+ (Fig. 1B). Within the lymphocyte gate, the markers CD56 and CD3 were used to identify NK cells (CD56+CD3−), conventional T lymphocytes (CD3+CD56−) and NKT‐like cells (CD56+CD3+) (Fig. 1D) 18, 19, 20. NK cells were further subdivided into CD56HCD16L and CD56LCD16H populations according to the CD56 and CD16 density (Fig. 1E). T lymphocytes (CD3+CD56−) were segregated into CD4+ or CD8+ T cells (Fig. 1H). Th2 and Tc2 cells were defined within the CD4+ and CD8+ T cell subsets, respectively, using the CRTH2 surface marker, as these Type‐2 cytokine‐producing cells express high levels of CRTH2 8, 21 (Fig. 1F,G). Within CD8+ T cells, we also identified MAIT cells. MAIT cells are invariant T lymphocytes which respond to riboflavin metabolites in the context of the MHC class I related molecule MR1 22, 23. MAIT cells were identified within the CD3+CD56−CD8+ gate as CD161HVα7.2+cells (Fig. 1I). NKT cells are conventionally described as CD3+CD56+ lymphocytes 24, 25, however, the use of these markers alone defines a heterogeneous population of lymphocytes, which we designate here as “NKT‐like” cells. CD56 can be expressed by many CD3+ lymphocyte subpopulations, including invariant NKT cells (iNKT cells), and some γδ T cells and MAIT cells (which have previously been described as mucosal NKT cells) 26, 27. Strategies to define these subpopulations are discussed further in the Supporting Information (Part B). Finally, ILCs were defined using the following gating strategy: live, single, CD45+ lymphocytes, lineage (CD3, CD56, CD14, CD19, CD123, FcεR1α) negative, and CD127+ cells (Fig. 1C); ILC1s were defined as CD117−CRTH2−, ILC2s as CRTH2+ CD117int, and ILC3s as CD117+CRTH2− 15, 28.
Figure 1.
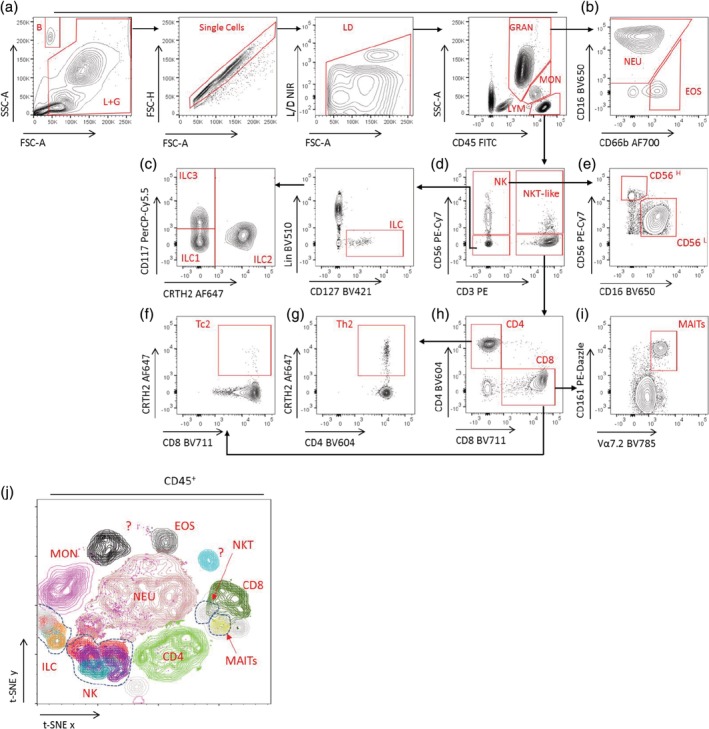
Example gating strategy and t‐SNE analysis for visualization of ILCs (including NK cells), granulocytes, MAIT, NKT‐like cells, and other T cell populations in pediatric clinical samples. Abbreviations: B, counting beads; L + G, lymphocyte and granulocyte gate; LD, live/dead; GRAN, granulocytes; MON, monocytes; LYM, lymphocytes; NEU, neutrophils; EOS, eosinophils; ILC, innate lymphoid cells; MAIT, mucosal‐associated invariant T cells; NK, natural killer cells; NKT‐like, natural killer T like cells; ?, Unidentified populations. [Color figure can be viewed at http://wileyonlinelibrary.com]
To further analyze different populations of cells in this OMIP, unbiased high dimensional stoichiometry (t‐SNE) analysis was performed on live, single, CD45+ cells incorporating the lymphocyte, granulocyte, and monocyte gates (Fig. 1J). In this example, t‐SNE not only revealed that all the populations of cells segregated according to the gating strategy illustrated in Figure 1A–I but additionally discriminated distinct or poorly defined populations. Therefore, this 14‐color flow panel can be used to delineate other unknown populations using high‐dimensional data visualization techniques 29.
Following optimization on adult peripheral blood, we confirmed that the panel was suitable for staining umbilical cord blood, and peripheral blood, tracheal aspirate, and nasal aspirate samples from infants. The inclusion of CD45 in the panel allowed us to separate leukocytes from structural cells, such as epithelial cells, found in airway samples. Concentrations of antibodies were kept the same for analysis of different samples as they gave the same optimal separation between positive and negative populations; however, the protocol for staining airway samples was more methodically complicated and included the use of Fc block. Counting beads were included to allow absolute counts of cell numbers. Detailed information on the panel development and optimization can be found in the Supporting Information (Part B).
Human Samples
All blood and airway samples were collected from subjects after gaining written consent. Some samples were collected as a part of the Early Life Lung Infection (ELLI) and RSV‐SAM studies, at St. Mary's Hospital, London. The use of all human tissue samples was approved by the Health Research Authority (HRA) and Health and Care Research Wales (HCRW) Ethics Committee (REC numbers 18/LO/1570; 15/WM/0343 and 13/LO/1712) and in accordance with the Declaration of Helsinki 1964.
Similarity to Other OMIPs
OMIP‐55 cross‐references to the ILC immunophenotyping; OMIP‐007, OMIP‐027, and OMIP‐029, OMIP‐38 and OMIP‐039 include phenotypic analysis of NK cells.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
Research Support: Asthma UK Centre in Allergic Mechanisms of Asthma, Imperial College London; Grant number: AUK‐BC‐2015‐01; National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London; Grant numbers: RDA02 and P82570, The Wellcome Trust; Grant number: 109008/Z/15/A.
Supporting information
Appendix S1: Supporting Information
MIFlowCyt: MIFlowCyt‐Compliant Items
Acknowledgments
We thank the St. Mary's Flow Cytometry Core Facility for support and instrumentation during the design of this panel. We are particularly thankful to Prof Beate Kampmann, Dr Beth Holder, Dr Tom Rice, and Sara Barnett for providing cord blood samples. We would like to thank Prof Trevor Hansel for valuable discussions. Finally, we are grateful to research nurses Sarah Darnell, Sobia Mustafa, Ladan Ali, Amina Abdulla, and Suzanne Laing for collecting pediatric samples. This article is independent research funded by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Literature Cited
- 1. WHO . WHO Consultation on respiratory syncytial virus (RSV) vaccine development 2015.
- 2. Adkins B, Leclerc C, Marshall‐Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004;4:553–564. [DOI] [PubMed] [Google Scholar]
- 3. Lambert L, Sagfors AM, Openshaw PJM, Culley FJ. Immunity to RSV in early life. Front Immunol 2014;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu JC, Khodadadi H, Malik A, Davidson B, Salles ÉDSL, Bhatia J, Hale VL, Baban B. Innate immunity of neonates and infants. Front Immunol 2018;9:1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahnke Y, Chattopadhyay P, Roederer M. Publication of optimized multicolor immunofluorescence panels. Cytom Part A 2010;77:814–818. [DOI] [PubMed] [Google Scholar]
- 6. Pitoiset F, Barbié M, Monneret G, Braudeau C, Pochard P, Pellegrin I, Trauet J, Labalette M, Klatzmann D, Rosenzwajg M. A standardized flow cytometry procedure for the monitoring of regulatory T cells in clinical trials. Cytom Part B 2018;94:777–782. [DOI] [PubMed] [Google Scholar]
- 7. Connors TJ, Baird JS, Yopes MC, Zens KD, Pethe K, Ravindranath TM, et al. Developmental regulation of effector and resident memory T cell generation during pediatric viral respiratory tract infection. J Immunol 2018;201(2):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol 2016;137:624–626. [DOI] [PubMed] [Google Scholar]
- 9. Bernink JH, Peters CP, Munneke M, Te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 2013;14:221–229. [DOI] [PubMed] [Google Scholar]
- 10. Dhariwal J, Cameron A, Trujillo‐Torralbo M‐B, del Rosario A, Bakhsoliani E, Paulsen M, Jackson DJ, Edwards MR, BMJ R, Cousins DJ, et al. Mucosal type 2 innate lymphoid cells are a key component of the allergic response to aeroallergens. Am J Respir Crit Care Med 2017;195:1586–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elemam NM, Hannawi S, Maghazachi AA. Innate lymphoid cells (ILCs) as mediators of inflammation, release of cytokines and lytic molecules. Toxins (Basel) 2017;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebbo M, Crinier A, Vély F, Vivier E. Innate lymphoid cells: Major players in inflammatory diseases. Nat Rev Immunol 2017;17:665–678. [DOI] [PubMed] [Google Scholar]
- 13. Hazenberg MD, Spits H. Human innate lymphoid cells. Blood 2014;124:700–710. [DOI] [PubMed] [Google Scholar]
- 14. Björklund ÅK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D, Sandberg R, Mjösberg J. The heterogeneity of human CD127+ innate lymphoid cells revealed by single‐cell RNA sequencing. Nat Immunol 2016;17:451–460. [DOI] [PubMed] [Google Scholar]
- 15. Vély F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Ebbo M, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol 2016;17:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simoni Y, Fehlings M, Kløverpris HN, Tan IB, Ginhoux F, Newell EW. Human innate lymphoid cell subsets possess human innate lymphoid cell subsets possess tissue‐type based heterogeneity in phenotype and frequency. Immunity 2017;46:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour R‐EE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, et al. The neuropeptide NMU amplifies ILC2‐driven allergic lung inflammation. Nature 2017;549:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costanzo MC, Creegan M, Lal KG, Eller MA. OMIP‐027: Functional analysis of human natural killer cells. Cytom Part A 2015;87:803–805. [DOI] [PubMed] [Google Scholar]
- 19. Mahnke YD, Beddall MH, Roederer M. OMIP‐029: Human NK‐cell phenotypization. Cytom Part A 2015;87:986–988. [DOI] [PubMed] [Google Scholar]
- 20. Eller MA, Currier JR. OMIP‐007: Phenotypic analysis of human natural killer cells. Cytom Part A 2012;81A:447–449. [DOI] [PubMed] [Google Scholar]
- 21. Hilvering B, Hinks TSC, Stöger L, Marchi E, Salimi M, Shrimanker R, Liu W, Chen W, Luo J, Go S, et al. Synergistic activation of pro‐inflammatory type‐2 CD8+ T lymphocytes by lipid mediators in severe eosinophilic asthma. Mucosal Immunol 2018;11:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang H, Hogquist KA. How MAIT cells get their start. Nat Immunol 2016;17:1238–1240. [DOI] [PubMed] [Google Scholar]
- 23. Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat Immunol 2019;20:1110–1128. [DOI] [PubMed] [Google Scholar]
- 24. Peng L, Mao F, Zhao Y, Wang T, Chen N, Zhang J, Cheng P, Li WH, Lv YP, Teng YS, et al. Altered phenotypic and functional characteristics of CD3+CD56+ NKT‐like cells in human gastric cancer. Oncotarget 2016;7:55222–55230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammer Q, Romagnani C. OMIP‐039: Detection and analysis of human adaptive NKG2C + natural killer cells. Cytom Part A 2017;91:997–1000. [DOI] [PubMed] [Google Scholar]
- 26. Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the immune system: More than a marker for cytotoxicity? Front Immunol 2017;8:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wingender G, Kronenberg M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am J Physiol Liver Physiol 2007;294:G1–G8. [DOI] [PubMed] [Google Scholar]
- 28. Bianca Bennstein S, Riccarda Manser A, Weinhold S, Scherenschlich N, Uhrberg M. OMIP‐055: Characterization of human innate lymphoid cells from neonatal and peripheral blood. Cytom Part A 2019;95:427–430. [DOI] [PubMed] [Google Scholar]
- 29. Van der Maaten L, Hinton G. Visualizing Data using t‐SNE. J Mach Learn Res 2008;9:2579–2605. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
MIFlowCyt: MIFlowCyt‐Compliant Items


