Abstract
Previous studies have shown that cognitive functioning in patients with brain tumour is associated with the functional network characteristics of specific resting‐state networks or with whole‐brain network characteristics. These studies, however, did not acknowledge the functional contribution of areas in the contralesional, non‐tumoural hemisphere, even though these healthy remote areas likely play a critical role in compensating for the loss of function in damaged tissue. In the current study, we examined whether there is an association between cognitive performance and functional network features of the contralesional hemisphere of patients with glioma. We found that local efficiency of the contralesional hemisphere was associated with performance on the reaction time domain, whereas contralesional assortativity was associated with complex attention and cognitive flexibility scores. Our results suggest that a less segregated organization of the contralesional hemisphere is associated with better reaction time scores, whereas a better spread of information over the contralesional hemisphere through mutually interconnected contralesional hubs is associated with better cognitive flexibility and better complex attention scores. These findings urge researchers to recognize the functional contribution of remote, undamaged regions and to focus more on the graph metrics of the contralesional hemisphere in the search for predictors of cognitive functioning in patients with brain tumour.
Keywords: assortativity, cognitive functioning, contralesional, local efficiency, resting state
In patients with glioma, a less segregated organization of the contralesional hemisphere (reflected by lower local efficiency) is associated with better reaction time scores. Better cognitive flexibility and better complex attention scores are associated with a better spread of information over the contralesional hemisphere through mutually interconnected hubs (reflected by higher assortativity). These results underline the importance of the contralesional hemisphere to cognitive performance.

Abbreviations
- AAL
automated anatomical labelling
- CNS VS
Central Nervous System Vital Signs
- DMN
default mode network
- Eglob
global efficiency
- Eloc
local efficiency
- EPI
echo‐planar imaging
- FDR
false discovery rate
- FWHM
full‐width at half maximum
- HGG
high‐grade glioma
- LGG
low‐grade glioma
- MNI
Montreal Neurological Institute
- ROI
region of interest
1. INTRODUCTION
A large proportion of brain tumour patients (up to 90%) show tumour‐related cognitive deficits (Gehring, Sitskoorn, Aaronson, & Taphoorn, 2008). These cognitive dysfunctions manifest themselves across multiple domains (e.g., memory, attention, information processing, executive functioning; Gehring, Roukema, & Sitskoorn, 2012) and can, therefore, be very disruptive for a person's daily functioning (Talacchi, Santini, Savazzi, & Gerosa, 2011; Taphoorn, Sizoo, & Bottomley, 2010). The wide spread of cognitive dysfunctions across multiple domains is not easily explained by local disruption of functions only in the area where the tumour is located (Devinsky & D'esposito, 2003; Heimans & Reijneveld, 2012). It rather suggests an impairment of the underlying global networks induced by the local brain tumour (Bartolomei et al., 2006; Martino et al., 2011). Recent advances in functional neuroimaging have provided new ways to measure and examine the functional interactions between brain regions. This allows the construction of global functional connectivity network maps and the investigation of the possible impairment of underlying networks induced by the local brain tumour.
Several neuroimaging studies have shown functional network differences when comparing glioma patients with healthy controls. Harris et al. (2014), for instance, showed that the integrity of the default mode network (DMN) is reduced in patients with glioma. Similarly, Mallela et al. (2016) reported reduced functional connectivity in the hand motor network in patients with glioma. The characteristics of these functional networks, as defined by graph theory metrics (Sporns, Chialvo, Kaiser, & Hilgetag, 2004), correlate with measures of cognitive functioning both in healthy subjects (for a review, see Vaidya & Gordon, 2013) and in patients with brain tumour (for reviews, see Aerts, Fias, Caeyenberghs, & Marinazzo, 2016; Derks, Reijneveld, & Douw, 2014). Rosenberg et al. (2016), for instance, showed that the connectivity strength of the functional brain networks was associated with the performance on a sustained attention task in healthy subjects. Similarly, global efficiency of functional networks was positively associated with intellectual performance. This was shown both in healthy subjects (van den Heuvel, Stam, Kahn, & Hulshoff Pol, 2009; but see Kruschwitz, Waller, Daedelow, Walter, & Veer, 2018) and in patients with brain tumour (Xu et al., 2013).
To date, all studies that examined the link between functional network characteristics and cognitive functioning in patients with brain tumour either looked at specific resting‐state networks (e.g., DMN or executive control network, Cf. Maesawa et al., 2015) or at whole‐brain functional networks (van Dellen et al., 2012), without explicitly acknowledging the functional contribution of areas in the contralesional hemisphere. However, healthy remote areas, including those in the contralesional hemisphere, likely play a critical role in compensating for the loss of function in damaged tissue. There is convincing evidence from imaging and intraoperative stimulation studies that unilateral brain lesions can lead to changes in the functional architecture of both the diseased and the intact hemisphere (Corbetta, Kincade, Lewis, Snyder, & Sapir, 2005; Voytek et al., 2010). Crucial for the current study is the finding that the severity and recovery of behavioural impairment is also determined by the functional connectivity characteristics of the contralesional hemisphere (Frost, Barbay, Friel, Plautz, & Nudo, 2003). This has been demonstrated for several functional domains, for instance movement (Calautti & Baron, 2003; Gerloff et al., 2006), language (Thiel et al., 2005; Winhuisen et al., 2005), working memory (Voytek et al., 2010) or response selection and inhibition (Kramer et al., 2013).
Functional connectivity characteristics of undamaged contralesional areas have been largely ignored in explaining cognitive impairments in patients with brain tumour. In a previous study (De Baene, Rutten, & Sitskoorn, 2017), we found that tumour growth velocity modulated the functional network topology of the hemisphere contralateral to the location of a glioma. Our results suggested that patients with a slow‐growing tumour (low‐grade glioma; LGG) differed from patients with a fast‐growing tumour (high‐grade glioma; HGG) both in the capacity for local, specialized information processing within modules and in the capacity for distributed information processing between modules in the contralesional hemisphere.
The goal of the current study was to examine whether there is an association between cognitive performance and functional network features of the contralesional (non‐tumoural) hemisphere of patients with glioma. To examine this, we used linear regression models for several graph metrics computed for the contralesional hemisphere. These graph metrics were regressed against patients’ sociodemographically corrected scores on 7 cognitive domains.
2. METHODS AND PROCEDURE
2.1. Study population
We conducted a retrospective study on the resting‐state and neuropsychological assessment data of patients recruited from the Elisabeth‐TweeSteden Hospital (Tilburg, the Netherlands) from July 2010 to March 2018. Both MRI data and neuropsychological assessment data were collected one day before surgery as part of standard clinical care. Only patients that were eligible for resective tumour surgery for a unilateral left‐hemispheric low‐grade glioma (LGG; WHO grade II) or high‐grade glioma (HGG; WHO grade IV; as demonstrated by neuropathological examination) were included in this study. Patients who were aged under 18, who had undergone a previous tumour resection, who had a history of psychiatric or neurological disorders, who had a history of cranial radiotherapy or who were unable to undergo the neuropsychological assessment were excluded from the analyses.
To classify the level of education of the patients, the Dutch Verhage scale was used (Verhage, 1964). Its seven categories were merged into the following three ordinal categories: low (Verhage 1–4), middle (Verhage 5), and high educational level (Verhage 6 and 7; Cf. Rijnen et al., 2017).
Ethical clearance to use data collected as part of standard clinical care for research purposes was obtained from the Medical Ethics Committee Brabant, The Netherlands (Reference: NW2015‐44 and File number: NL41351.008.12). All procedures were carried out with written informed consent of all subjects and in accordance with the principles of the Declaration of Helsinki.
2.2. Experimental procedure
2.2.1. Neuropsychological assessment
All patients were assessed with the official Dutch translation of the Central Nervous System Vital Signs (CNS VS; Gualtieri & Johnson, 2006). The CNS VS is a brief (30–40 min) computerized battery that includes the following subtests: Verbal Memory test, Visual Memory test, Finger Tapping task, Symbol Digit Coding task, Stroop test, Shifting Attention task and a Continuous Performance test. These 7 neuropsychological tests yield measures of performance in 11 cognitive domains. As the measures of performance for some domains are largely based on scores on the same tests, we only considered 7 cognitive domains in the analyses, in line with our previous research (De Baene et al., 2019; Rijnen et al., 2019). These domains are verbal memory, visual memory, processing speed, psychomotor speed, reaction time, complex attention, and cognitive flexibility (Table 1).
Table 1.
Description of clinical domains and cognitive tests in CNS Vital Signs
| Cognitive domain | CNS VS test(s) | Description | Domain score calculations |
|---|---|---|---|
| Verbal memory | Verbal Memory test (VBM) | Learning a list of 15 words, with a direct recognition, and after 6 more tests a delayed recognition trial | VBM direct correct hits + VBM direct correct passes + VBM delayed correct hits + VBM delayed correct passes |
| Visual memory | Visual Memory test (VIM) | Learning a list of 15 geometric figures, with a direct recognition, and after 6 more tests a delayed recognition trial | VIM direct correct hits + VIM direct correct passes + VIM delayed correct hits + VIM delayed correct passes |
| Processing speed | Symbol digit coding (SDC) | Corresponding numbers and symbols | SDC correct responses − SDC errors |
| Psychomotor speed | Finger‐tapping test (FTT) | Pressing the space bar with the right and left index finger as many times in 10 s | FTT taps right hand + FTT taps left hand + SDC correct responses |
| Symbol digit coding test (SDC) | Above‐mentioned | ||
| Reaction time | Stroop test (ST) | In part I, pressing the space bar as soon as the words RED, YELLOW, BLUE and GREEN appear. In part II, pressing the space bar when the colour of the word matches what the word says. In part III, pressing the space bar when the colour of the word does not match what the word says | (ST part II reaction time on correct responses + ST part III reaction time on correct responses)/2 |
| Complex attention | Continuous Performance test (CPT) | Responding to a target stimulus “B” but no any other letter | ST commission errors + SAT errors + CPT commission errors + CPT omission errors |
| Shifting attention test (SAT) | Shifting from one instruction to another quickly and accurately (matching geometric objects either by shape or colour) | ||
| Stroop test (ST) | Above‐mentioned | ||
| Cognitive flexibility | Shifting attention test (SAT) | Above‐mentioned | SAT correct − SAT errors − ST commission errors |
| Stroop test (ST) | Above‐mentioned |
Based on normative data from the Dutch population (Rijnen et al., 2017), the raw cognitive domain scores were transformed into sociodemographically adjusted normscores (i.e., z‐scores adjusted for effects of age, sex and educational level by regression analyses, M = 0; SD = 1).
2.2.2. MRI acquisition procedure
Subjects were positioned head first and supine in the magnetic bore. Images were collected with a 3 Tesla Philips Achieva Scanner (Philips Medical Systems) using a standard 32‐channel radio‐frequency head coil. In 31 patients, whole‐brain resting‐state fMRI data were acquired with a 3D‐PRESTO pulse sequence with parallel imaging (TR/TE = 19/27 ms, slice orientation = sagittal, flip‐angle = 10°, dynamic scan time = 1,500 ms, voxel size 4 × 4 × 4 mm, FOV = 160 × 256 × 256, reconstruction matrix = 40 × 64 × 64, number of volumes = 301). In 15 patients, whole‐brain resting‐state fMRI data were obtained using an EPI pulse sequence (TR/TE = 2,000/28 ms, slice orientation = transverse, flip‐angle = 70°, voxel size 3 × 3 × 3 mm, FOV = 240 × 240 × 111 mm, reconstruction matrix = 80 × 80 × 37, with varying number of volumes [225 in 13 patients and 220 in 2 patients]). High‐resolution whole‐brain structural scans were acquired for all patients as reference for the resting‐state maps (3D T1‐weighted sequence: TR/TE = 8.40/3.80 ms, flip angle = 8°, slice orientation = sagittal, voxel size 1 mm isotropic, with varying FOV (158 × 254 × 254 in 37 patients and 175 × 240 × 240 in 9 patients)). Subjects were instructed to close their eyes and relax, but not to sleep, in the scanner while thinking of nothing in particular.
2.3. MRI preprocessing
Scan data were analysed using SPM12 (Wellcome Trust Centre for Neuroimaging) and the CONN‐toolbox (Whitfield‐Gabrieli & Nieto‐Castanon, 2012).
Preprocessing included realignment, slice time correction (for the EPI‐data), functional outlier detection (based on scrubbing of motion‐affected functional volumes), segmentation of the structural image, spatial normalization of the structural and functional images to the template MNI brain, resampling to 2 × 2 × 2 mm cubic voxels and smoothing using a 4 mm full‐width at half maximum (FWHM) Gaussian Kernel.
Possible sources of spurious variance were regressed out from the data, including (1) the realignment and scrubbing parameters; (2) the white matter signal; and (3) the ventricular system signal. Global signal regression was not performed due to the ongoing controversy associated with this step (Caballero‐Gaudes & Reynolds, 2017; Saad et al., 2012). Finally, linear detrending and temporal band‐pass filtering (0.009–0.8 Hz) were applied to reduce the influences of low‐frequency drift and high‐frequency physiological noise.
2.4. Construction of the brain functional network
To assess the functional connectivity in each patient, preprocessed rs‐fMRI data were first parcellated into 90 regions (45 regions for each hemisphere) of interest (ROIs) from the automated anatomical labelling (AAL) atlas (Tzourio‐Mazoyer et al., 2002). Individual time‐series were averaged over the voxels in each parcel to obtain the representative time series for each ROI. A functional connectivity matrix of the contralesional hemisphere (45 × 45 nodes) was created for each patient. These functional connectivity matrices were created by correlating the time series between each pair of relevant ROIs using Pearson's correlation coefficient and applying a Fisher z‐transform (i.e., atanh(r)).
To characterize the topological properties of the brain functional networks, each individual's correlation matrix was thresholded into a weighted, undirected graph which was composed of nodes (representing brain regions) and edges (representing functional connections) between nodes. We thresholded the brain graph by identifying the top 50%–10% highest correlation coefficients (in 5% increments) resulting in nine graphs per subject in which weak or negative correlations were replaced by zeros. The topological metrics (see below) were estimated from individual graphs at each threshold value, and the resulting metrics from each threshold were then integrated into one single metric of interest.
2.5. Topologic measures
The network metrics in this study were selected based on their ability to quantify global graph characteristics and were computed with the Brain Connectivity Toolbox (Rubinov & Sporns, 2010) and are detailed below (see Figure 1).
Figure 1.
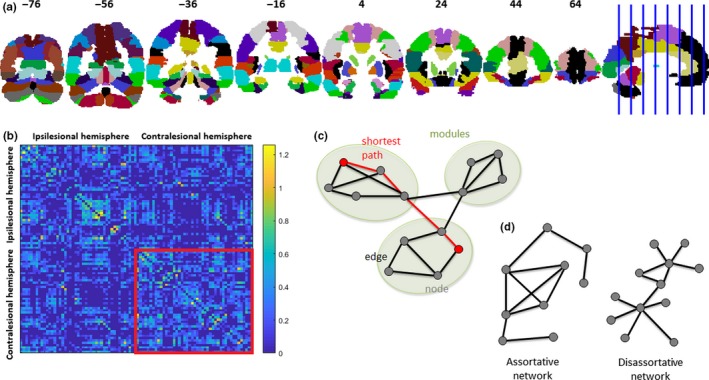
Panel a shows the 90 regions (45 regions for each hemisphere) used in this study, based on the automated anatomical labelling (AAL) atlas. MNI y coordinates of the coronal sections are given. Panel b shows the correlation matrix for one patient (thresholded in such a way that all correlation coefficients not belonging to the top 50% highest correlation coefficients were replaced by zeros). Nodes 1–45 belong to the ipsilesional hemisphere. Nodes 46–90 belong to the contralesional network. Panel c shows an example of a graph, which is a mathematical description of a network, consisting of a collection of nodes and edges. The dots represent nodes, and the lines represent edges connecting the nodes. There are three modules in the graph in which connections within modules are much denser than the connections between modules. The shortest path length describes the minimum number of connections that should be passed to travel between two nodes and is inversely related to the global efficiency. Panel d shows an example of an assortative graph to the left and a disassortative graph to the right. In the assortative graph, highly connected nodes are primarily connected to highly connected nodes and lowly connected nodes to lowly connected nodes. In the disassortative graph, the opposite holds: highly connected nodes are primarily connected to lowly connected nodes and lowly connected nodes to highly connected nodes. [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
2.5.1. Assortativity
The assortativity coefficient (r) is a measure of the correlation between the strengths (weighted degrees) of connected nodes (Leung & Chau, 2007) and reflects the tendency for nodes to be connected to other nodes of the same or similar strength. It ranges between −1 and 1. Positive assortativity indicates that nodes with high levels of connectivity (i.e., hubs) tend to be coupled with other highly connected nodes, and nodes with low levels of connectivity tend to be coupled with similarly lowly connected nodes. This is characteristic of an assortative network. A negative assortativity value implies that the hubs of the network are not connected to each other, which is characteristic of a disassortative network. An assortative network is thought to be resilient to disruption (e.g., removal of nodes), because the core of highly connected nodes provides redundancy and facilitate the spread of information over the network (Newman, 2002).
2.5.2. Global efficiency
The global efficiency (E glob) of a network is defined as the average inverse shortest path length between all nodes in a network (i.e., number of minimum connections that should be passed to join two nodes; Achard & Bullmore, 2007; Latora & Marchiori, 2001). Global efficiency is thought to represent integration of network‐wide communication.
2.5.3. Local efficiency
Contrary to global efficiency, local efficiency (E loc) is measured on a nodal basis using information about the path length between the neighbours of a single node. It assesses how well the information is communicated within the neighbours of a given node when this node is removed. High local efficiency indicates that a node is embedded in a richly connected environment. Low local efficiency, by contrast, means that the neighbours of the node are sparsely connected to one another (Power et al., 2011). The local efficiency averaged across all the nodes of a network represents the network's potential for local information transfer (Bullmore & Sporns, 2009, 2012).
To evaluate the global and local efficiency, the overall variability in overall connectivity strength across subjects needs to be accounted for (van den Heuvel et al., 2017). Therefore, these graph metrics were normalized by dividing them by the mean values from 100 random reference networks that were generated using a Markov‐chain algorithm and that match the original networks in terms of degree and strength distribution (Maslov & Sneppen, 2002). When the resulting metrics are lower than 1, global or local efficiency is lower than that of random graphs; when they exceed 1, global or local efficiency is higher than that of random graphs.
2.5.4. Modularity
Modularity quantifies the degree to which a network can be subdivided into separable, non‐overlapping sub‐networks or modules in which nodes within the same module are densely interconnected but only have sparse connections with nodes from other modules (Newman, 2006). The extent of modular organization is assessed by the weighted modularity metric Q (Newman & Girvan, 2004). A strongly modular network has a modularity value close to 1, and in a network without modular organization it will approach 0.
2.5.5. Connectivity strength
Finally, we also computed the global connectivity strength (S). A node's strength is the weighted version of the degree of a node and is defined as the sum of the weights over all connections of the node. The global connectivity strength or average weighted degree is computed as the mean of all nodal values.
2.6. Statistical analyses
To evaluate whether differences in graph metrics of the contralesional hemisphere account for a substantial proportion of individual variability in cognitive performance, we used a separate linear regression model (using the fitlm function in MATLAB R2016a (Mathworks)) for every graph metric and every cognitive domain. Separately for every cognitive domain, we first screened for possible associations between cognitive performance and several clinical and sociodemographic variables using single‐predictor models with a liberal p‐value of .20. All variables that met this screening criterion were included as predictors in the final linear regression models for that cognitive domain. The initial set of variables that we considered were age (in years), sex, educational level (low education as reference category), tumour volume (in cm3), tumour type (LGG vs. HGG), handedness, scan type (EPI with TR = 2,000 ms vs. Presto with TR = 1,500 ms), epilepsy and use of anti‐epileptic drugs.
A significance threshold of α = .05 was used. To correct for multiple testing related to the different graph metrics, we applied the false discovery rate (FDR) correction. FDR‐adjusted p‐values are reported where necessary.
3. RESULTS
3.1. Patient characteristics
From the total of 46 eligible patients, 45 patients were included in the final data analyses. One patient was excluded due to excessive head movement (as became evident from the functional outlier detection). Detailed sociodemographic and clinical information about the included patients is listed in Table 2. Twenty‐nine LGG patients and 16 HGG patients were included. Distribution of the tumours across these 45 patients is shown in Figure 2.
Table 2.
Sociodemographical and clinical characteristics
| Characteristics | All patients (n = 45) |
|---|---|
| Age in years (mean; range) | 44.80; 21–73 |
| Female, n (%) | 17 (37.78) |
| Education, n (%) | |
| Low (Verhage 1–4) | 7 (15.56) |
| Middle (Verhage 5) | 15 (33.33) |
| High (Verhage 6–7) | 23 (51.11) |
| Tumour grade (WHO), n (%) | |
| II | 29 (64.44) |
| IV | 16 (35.56) |
| Tumour volume (cm3; range) | 37.75; 7.00–104.38 |
| Epilepsy, n (%) | 29 (64.44) |
| Use of anti‐epileptic drugs, n (%) | 28 (62.22) |
Figure 2.
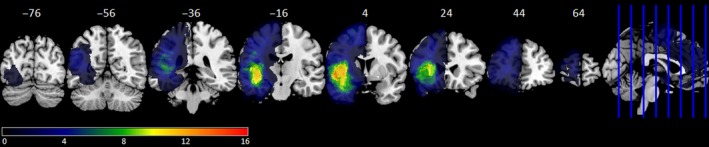
Frequency distribution of tumour (all 45 patients). The colour scale shows minimal overlap (dark blue) to maximal overlap (red). MNI y coordinates of the coronal sections are given. [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
3.2. Neuropsychological performance
The sociodemographically adjusted cognitive functioning scores for the different cognitive domains are presented in Figure 3. All patients scored within three standard deviations of the mean for verbal memory, visual memory, complex attention, and cognitive flexibility, which indicates that no outliers were detected for these cognitive domains. For the domains processing speed, psychomotor speed and reaction time, one outlier was detected and removed from further analyses.
Figure 3.
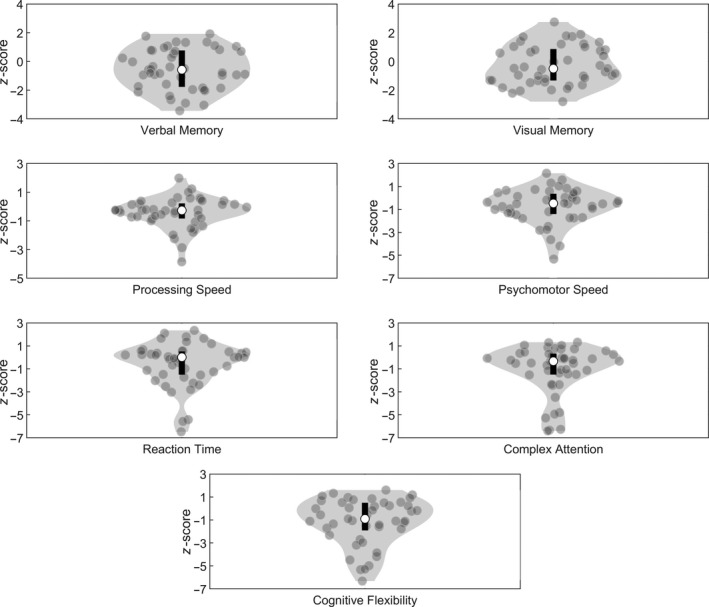
Distribution of the performance on the different cognitive domains. The contour of the violin plot represents the estimate of the density of patients with particular z‐scores, the grey filled circles represent the individual data points, the black bar at the centre of the plot represents the interval containing the central 50% of the values in the distribution and the white circle inside the bar represents the median
3.3. Relationship of performance to functional connectivity
An overview of the network metrics results for the contralesional hemisphere is presented in Figure 4.
Figure 4.
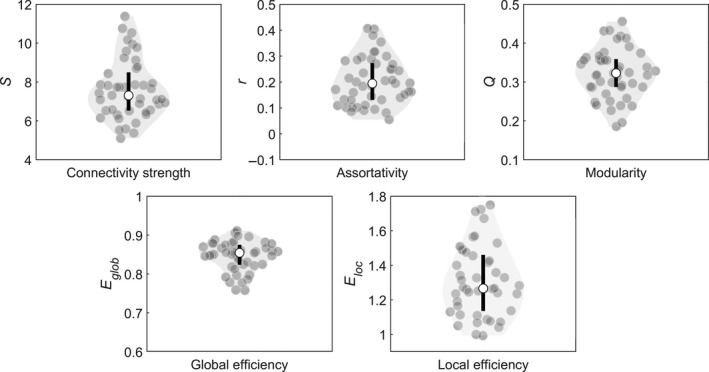
Distribution of the graph metrics for the contralesional hemisphere. The contour of the violin plot represents the estimate of the density of patients with particular graph metric values, the grey filled circles represent the individual data points, the black bar at the centre of the plot represents the interval containing the central 50% of the values in the distribution and the white circle inside the bar represents the median
Before running linear regression models (for every graph metric and every cognitive domain), we first screened for possible associations between cognitive performance and several clinical and sociodemographic variables separately for every cognitive domain. Only variables that met the screening criterion (liberal p‐value of .20) were added as predictors in the final linear regression models for that cognitive domain.
3.3.1. Verbal memory
The single‐predictor models showed that for verbal memory, only tumour type met the screening criterion. The linear regression models for verbal memory showed no significant association between one of the contralesional graph metrics and cognitive performance (all p‐values > .14, FDR corrected). Across these different models, tumour type was significantly associated with verbal memory (p < .05): having a HGG (compared with a LGG) was associated with worse cognitive performance on this domain.
3.3.2. Visual memory
For visual memory, tumour volume, educational level and epilepsy were included in the final regression models. No significant association between one of the contralesional graph metrics and visual memory performance was found (all p‐values > .46, FDR corrected). In some of these models, tumour volume was associated with visual memory (in one model: p = .077; in another model: p = .25; in all other models: p < .05): a larger tumour was associated with worse visual memory scores. In these regression models, educational level and epilepsy were not associated with visual memory scores (all p's > .057).
3.3.3. Processing speed
Tumour volume and handedness were added to the final regression models for processing speed. Again, no significant association between cognitive performance and one of the contralesional graph metrics (all p‐values > .80, FDR corrected) was found. In these linear regression models, tumour volume and handedness were not associated with processing speed (all p's > .11).
3.3.4. Psychomotor speed
Based on the results of the single‐predictor models, tumour volume and tumour type were included as predictors in the final linear regression models for psychomotor speed. No significant association between psychomotor speed scores and one of the contralesional graph metrics (all p‐values > .30, FDR corrected) was found. Across these models, tumour volume was not significantly associated with psychomotor speed scores (all p's > .057). In some of the models, tumour type was associated with psychomotor speed (p < .05 in two models; p < .074 in the other models): having a HGG (compared with a LGG) was associated with worse cognitive performance on this domain.
3.3.5. Reaction time
For reaction time, we added age, tumour type and educational level to the regression models. Reaction time scores were associated with the local efficiency of the contralesional network (p < .05; all p's > .13 for all other graph metrics; FDR corrected): lower local efficiency of the contralesional network was associated with better performance on the reaction time domain (β = −4.21, SE = 1.39; See Figure 5a). In these linear regression models, age, tumour type and educational level were not associated with reaction time (all p's > .077).
Figure 5.
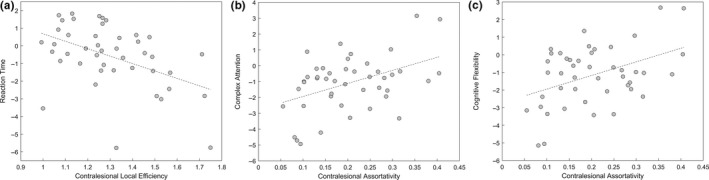
(a) Association between reaction time scores and local efficiency of the contralesional hemisphere (after adjusting for the effects of age, tumour type and educational level). (b) Association between assortativity of the contralesional hemisphere and complex attention (after adjusting for the effects of age, tumour type, epilepsy and use of anti‐epileptic drugs). (c) Association between contralesional assortativity and cognitive flexibility (after adjusting for the effects of age and tumour type)
3.3.6. Complex attention
Age, tumour type, epilepsy and the use of anti‐epileptic drugs met the screening criterion for complex attention and were added to the final model. Assortativity of the contralesional hemisphere was associated with complex attention scores (p < .05; all p's > .11 for all other graph metrics, FDR corrected): Higher contralesional assortativity was associated with higher performance on the complex attention domain (β = 8.24, SE = 2.79; See Figure 5b). Across all these models, tumour type was associated with complex attention (p < .01): having a HGG (compared to a LGG) was associated with worse complex attention scores. Age, epilepsy and the use of anti‐epileptic drugs were not associated with cognitive functioning in this domain (all p's > .30).
3.3.7. Cognitive flexibility
For the domain of cognitive flexibility, age and tumour type met the screening criterion. Cognitive flexibility was associated with contralesional assortativity (p < .05; all other p's > .09, FDR corrected): Higher contralesional assortativity was associated with higher performance on the cognitive flexibility domain (β = 7.73, SE = 2.67; See Figure 5c). Across all these models, tumour type was associated with cognitive flexibility (p < .01): having a HGG (compared to a LGG) was associated with worse cognitive flexibility scores. Age was not associated with cognitive flexibility (all p's > .21).
4. DISCUSSION
Previous studies in patients with brain tumour have shown that functional network characteristics are associated with cognitive functioning (for reviews, see Aerts et al., 2016; Derks et al., 2014). This relationship has been examined for specific resting‐state networks and for whole‐brain connectivity measures. However, previous studies did not acknowledge the functional contribution of areas in the contralesional hemisphere (Frost et al., 2003; Riecker et al., 2010). We found in our current study that local efficiency of the contralesional hemisphere is associated with reaction time scores, whereas contralesional assortativity is associated with scores on the complex attention and cognitive flexibility domain.
Local efficiency indicates how efficiently information is integrated between the immediate neighbours of a given network node (Bullmore & Sporns, 2009, 2012). It thus reflects “segregation”, or the ability for specialized processing within functionally related brain regions arranged in modules. Higher local efficiency of the contralesional hemisphere suggests thus that the contralesional network organization is more segregated (Latora & Marchiori, 2001). Consequently, our results show that a more segregated organization of the contralesional hemisphere, reflected in higher local efficiency, is associated with worse reaction time scores. This finding concurs with previous studies that showed a negative effect of local efficiency measured at the whole‐brain level on cognitive performance in healthy populations (Kawagoe, Onoda, & Yamaguchi, 2017; Stanley et al., 2015). This negative association might especially be true for cognitive functions that rely on co‐operated processing of multiple modules (Cf. Cohen & D'Esposito, 2016), which might be impaired when there is a higher dependence on the specialized processing of specific modules.
Both complex attention and cognitive flexibility performances are associated with contralesional assortativity. Assortativity reflects the extent to which highly connected nodes (i.e., hubs) are coupled to other highly connected nodes and lowly connected nodes are linked to nodes with low levels of connectivity. Higher assortativity of the contralesional hemisphere suggests thus that the contralesional network organization has more mutually interconnected hubs. Hubs connected to one another facilitate the spread of information over the network (Newman, 2002). Consequently, our results suggest that a better spread of information over the network through mutually interconnected contralesional hubs (reflected in higher assortativity) is associated with better cognitive flexibility and better complex attention.
One additional predictor for cognitive performance on the complex attention and cognitive flexibility domain, besides contralesional assortativity, was tumour type, whereby worse cognitive performance is associated with having a high‐grade glioma. This is in line with previous studies showing that cognitive impairments are more common and more severe in HGG patients compared with LGG patients (for a review, see van Kessel, Baumfalk, van Zandvoort, Robe, & Snijders, 2017). Low‐grade gliomas tend to grow more slowly and less aggressively with lower degrees of cell infiltration and proliferation than high‐grade gliomas. In contrast, HGG and in particular grade IV glioblastomas grow much faster (circa 10‐fold; Swanson, Bridge, Murray, & Alvord, 2003). This difference in growth velocity could lead to more extensive plastic effects in LGG compared with HGG patients (Esposito et al., 2012; Kong, Gibb, & Tate, 2016) which are thought to underlie the better neurocognitive functioning in LGG patients (Hom & Reitan, 1984; Miotto et al., 2011; Noll, Sullaway, Ziu, Weinberg, & Wefel, 2015).
In the present study, no associations were found between contralesional graph metrics and performance on the domains verbal and visual memory, psychomotor speed and processing speed. One possible cause for this might be the small variation in the scores on these cognitive domains compared with the scores on the reaction time, complex attention and cognitive flexibility domain. Due to this too small range, the analyses might have been not sensitive enough to produce statistical associations.
In the quest for potential predictors of cognitive functioning, graph theoretical metrics have been proposed for specific clinical populations (Caeyenberghs, Verhelst, Clemente, & Wilson, 2017; e.g., Fornito, Zalesky, & Breakspear, 2015). For patients with glioma, several graph metrics have been proposed to be predictive for cognitive functioning (Carbo et al., 2017; Douw et al., 2011). The current result, together with earlier findings (De Baene et al., 2017), however, underlines the importance of taken the graph metrics of the contralesional hemisphere into account when searching for predictors of cognitive functioning in patients with brain tumour. Considering the association between the contralesional local efficiency and assortativity and, respectively, patients’ reaction time scores and patient's complex attention and cognitive flexibility scores found in this current study, we believe that these contralesional graph metrics carry the potential to serve as predictors for patients’ cognitive functioning. Additionally, the contralesional graph theoretical information can guide the potential enrolment of patients into cognitive intervention programs upfront (Gehring et al., 2009). However, extensive additional validation is necessary.
A limitation of the current study is that the exact location of the tumour is not taken into account. Cognitive functions rely on the dynamic interactions between distributed brain areas that operate in large‐scale functional networks (Bressler & Menon, 2010). For instance, cognitive flexibility relies on a bilateral (although somewhat left‐lateralized) fronto‐parietal network (Brass & Von Cramon, 2002; De Baene, Albers, & Brass, 2012; De Baene & Brass, 2013; Dreher & Berman, 2002). Consequently, given that only patients with unilateral tumours in the left hemisphere were included, the tumour overlapped with the fronto‐parietal network underlying cognitive flexibility in some of the patients. The tumour overlap with regions relevant for a specific cognitive function might also be an additional predictor for performance on that cognitive domain.
Furthermore, before computing the different graph metrics, each individual's structural brain image was first registered to the standard MNI space by applying a normalization procedure. In patients with brain tumour, however, there is a lack of perfect correspondence between the patient's brain image and the MNI template due to the mass effect and the deformation of the brain. Therefore, the spatial normalization process might not have been perfect. However, given that we carefully selected the patients to include in this study to only have apparent tumour tissue in the left hemisphere, we are convinced that normalization issues that might have occurred were mainly restricted to that hemisphere and should not greatly have distorted the normalization of the contralesional hemisphere, which was the main focus of our study.
Additionally, the graph metrics in the current study were computed based on the regions defined in the AAL atlas (Tzourio‐Mazoyer et al., 2002). However, observable anatomical landmarks do not necessarily correspond to functional units (Smith et al., 2013). This anatomically based parcellation scheme does not capture variation between individuals in regional function boundaries and assumes that a common parcellation is representative of all individuals. Recently, information from multiple modalities has been combined to define the parcellations (Glasser et al., 2016) but it is unclear how these parcellations would translate to our individual patients with brain tumour.
A critical question arising from our results is whether the differences between patients in functional network features of the contralesional hemisphere reflect lesion‐induced functional changes, compensatory changes, individual differences unrelated to the tumour or a combination of these. Future studies should include longitudinal measures and a healthy control group to distinguish between these possibilities. Additionally, in the current study, we showed an association between contralesional graph metrics and cognitive performance measured prior to surgery. Future studies are needed to examine whether this link also holds for cognitive performance after tumour resection and at the long term.
Furthermore, cognitive performance has not only been related to the graph metrics of functional networks but also to the graph metrics of the structural networks, both in healthy people (Li et al., 2009), in patients with brain tumour (Kesler, Noll, Cahill, Rao, & Wefel, 2017) and in traumatic brain injury patients (Caeyenberghs et al., 2014; Fagerholm, Hellyer, Scott, Leech, & Sharp, 2015). Despite the fact that the exact relationship between the structural and functional networks remains unclear (Honey, Thivierge, & Sporns, 2010; Park & Friston, 2013), the functional network might be based on the structural network (Meier et al., 2016). Future studies should therefore also examine the predictive power of the structural network characteristics of the contralesional hemisphere for cognitive functioning.
5. CONCLUSION
In the current study, we examined whether there is an association between cognitive performance and functional network features of the contralesional hemisphere of patients with glioma. We found that local efficiency of the contralesional hemisphere is predictive of performance on the reaction time domain, suggesting that better reaction time scores will be achieved when the contralesional hemisphere has a less segregated organization. Furthermore, we found that contralesional assortativity, in combination with tumour type, is predictive of complex attention and cognitive flexibility scores. This suggests that better complex attention and cognitive flexibility performance will be achieved with a better spread of information over the contralesional hemisphere through mutually interconnected contralesional hubs. We conclude that the functional connectivity characteristics of the contralesional hemisphere play a role in determining the severity of behavioural impairment. We therefore urge researchers to fully appreciate the functional contribution of the remote, undamaged regions and to focus more on the graph metrics of the contralesional hemisphere in the search for predictors of cognitive functioning in patients with brain tumour.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
DATA AVAILABILITY STATEMENT
The Data Files and code have been made publicly available via Figshare (https://doi.org/10.6084/m9.figshare.c.4586678.v1).
AUTHOR CONTRIBUTIONS
All authors designed the study, revised and approved the final version. W De Baene performed the analyses and interpretation of the data.
ACKNOWLEDGEMENTS
The authors thank the Department of Radiology of the Elisabeth‐TweeSteden Hospital (Tilburg, the Netherlands) for collecting the data as part of the clinical care of the patients with glioma.
De Baene W, Rutten G‐JM, Sitskoorn MM. Cognitive functioning in glioma patients is related to functional connectivity measures of the non‐tumoural hemisphere. Eur J Neurosci. 2019;50:3921–3933. 10.1111/ejn.14535
Edited by Ali Mazaheri.
The peer review history for this article is available at https://publons.com/publon/10.1111/EJN.14535
REFERENCES
- Achard, S. , & Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Computational Biology, 3(2), e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts, H. , Fias, W. , Caeyenberghs, K. , & Marinazzo, D. (2016). Brain networks under attack: Robustness properties and the impact of lesions. Brain, 139(Pt 12), 3063–3083. [DOI] [PubMed] [Google Scholar]
- Bartolomei, F. , Bosma, I. , Klein, M. , Baayen, J. C. , Reijneveld, J. C. , Postma, T. J. , … Stam, C. J. (2006). Disturbed functional connectivity in brain tumour patients: Evaluation by graph analysis of synchronization matrices. Clinical Neurophysiology, 117(9), 2039–2049. [DOI] [PubMed] [Google Scholar]
- Brass, M. , & Von Cramon, D. Y. (2002). The role of the frontal cortex in task preparation. Cerebral Cortex, 12, 908–914. [DOI] [PubMed] [Google Scholar]
- Bressler, S. L. , & Menon, V. (2010). Large‐scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. [DOI] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3), 186–198. [DOI] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2012). The economy of brain network organization. Nature Reviews Neuroscience, 13(5), 336–349. [DOI] [PubMed] [Google Scholar]
- Caballero‐Gaudes, C. , & Reynolds, R. C. (2017). Methods for cleaning the BOLD fMRI signal. NeuroImage, 154, 128–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs, K. , Leemans, A. , Leunissen, I. , Gooijers, J. , Michiels, K. , Sunaert, S. , & Swinnen, S. (2014). Altered structural networks and executive deficits in traumatic brain injury patients. Brain Structure and Function, 219(1), 193–209. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs, K. , Verhelst, H. , Clemente, A. , & Wilson, P. H. (2017). Mapping the functional connectome in traumatic brain injury: What can graph metrics tell us? NeuroImage, 160, 113–123. [DOI] [PubMed] [Google Scholar]
- Calautti, C. , & Baron, J.‐C. (2003). Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke, 34(6), 1553–1566. [DOI] [PubMed] [Google Scholar]
- Carbo, E. W. , Hillebrand, A. , van Dellen, E. , Tewarie, P. , de Witt Hamer, P. C. , Baayen, J. C. , … Douw, L. (2017). Dynamic hub load predicts cognitive decline after resective neurosurgery. Scientific Reports, 7, 42117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. R. , & D'Esposito, M. (2016). The segregation and integration of distinct brain networks and their relationship to cognition. Journal of Neuroscience, 36(48), 12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , Kincade, M. J. , Lewis, C. , Snyder, A. Z. , & Sapir, A. (2005). Neural basis and recovery of spatial attention deficits in spatial neglect. Nature Neuroscience, 8(11), 1603–1610. [DOI] [PubMed] [Google Scholar]
- De Baene, W. , Albers, A. M. , & Brass, M. (2012). The what and how components of cognitive control. NeuroImage, 63(1), 203–211. [DOI] [PubMed] [Google Scholar]
- De Baene, W. , & Brass, M. (2013). Switch probability context (in)sensitivity within the cognitive control network. NeuroImage, 77, 207–214. [DOI] [PubMed] [Google Scholar]
- De Baene, W. , Rijnen, S. J. M. , Gehring, K. , Meskal, I. , Rutten, G. M. , & Sitskoorn, M. M. (2019). Lesion symptom mapping at the regional level in patients with a meningioma. Neuropsychology, 33(1), 103–110. [DOI] [PubMed] [Google Scholar]
- De Baene, W. , Rutten, G. J. M. , & Sitskoorn, M. M. (2017). The temporal pattern of a lesion modulates the functional network topology of remote brain regions. Neural Plasticity, 2017, 3530723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks, J. , Reijneveld, J. C. , & Douw, L. (2014). Neural network alterations underlie cognitive deficits in brain tumor patients. Current Opinion in Oncology, 26(6), 627–633. [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , & D'esposito, M. (2003). Neurology of cognitive and behavioral disorders. Oxford, UK: Oxford University Press. [Google Scholar]
- Douw, L. , Schoonheim, M. M. , Landi, D. , van der Meer, M. L. , Geurts, J. J. , Reijneveld, J. C. , … Stam, C. J. (2011). Cognition is related to resting‐state small‐world network topology: An magnetoencephalographic study. Neuroscience, 175, 169–177. [DOI] [PubMed] [Google Scholar]
- Dreher, J. C. , & Berman, K. F. (2002). Fractionating the neural substrate of cognitive control processes. Proceedings of the National Academy of Sciences of the United States of America, 99(22), 14595–14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito, R. , Mattei, P. A. , Briganti, C. , Romani, G. L. , Tartaro, A. , & Caulo, M. (2012). Modifications of default‐mode network connectivity in patients with cerebral glioma. PLoS ONE, 7(7), e40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm, E. D. , Hellyer, P. J. , Scott, G. , Leech, R. , & Sharp, D. J. (2015). Disconnection of network hubs and cognitive impairment after traumatic brain injury. Brain, 138(Pt 6), 1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito, A. , Zalesky, A. , & Breakspear, M. (2015). The connectomics of brain disorders. Nature Reviews Neuroscience, 16(3), 159–172. [DOI] [PubMed] [Google Scholar]
- Frost, S. B. , Barbay, S. , Friel, K. M. , Plautz, E. J. , & Nudo, R. J. (2003). Reorganization of remote cortical regions after ischemic brain injury: A potential substrate for stroke recovery. Journal of Neurophysiology, 89, 3205–3214. [DOI] [PubMed] [Google Scholar]
- Gehring, K. , Roukema, J. A. , & Sitskoorn, M. M. (2012). Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Review of Anticancer Therapy, 12(2), 255–269. [DOI] [PubMed] [Google Scholar]
- Gehring, K. , Sitskoorn, M. M. , Aaronson, N. K. , & Taphoorn, M. J. B. (2008). Interventions for cognitive deficits in adults with brain tumours. The Lancet Neurology, 7(6), 548–560. [DOI] [PubMed] [Google Scholar]
- Gehring, K. , Sitskoorn, M. M. , Gundy, C. M. , Sikkes, S. A. , Klein, M. , Postma, T. J. , … Aaronson, N. K. (2009). Cognitive rehabilitation in patients with gliomas: A randomized, controlled trial. Journal of Clinical Oncology, 27(22), 3712–3722. [DOI] [PubMed] [Google Scholar]
- Gerloff, C. , Bushara, K. , Sailer, A. , Wassermann, E. M. , Chen, R. , Matsuoka, T. , … Hallett, M. (2006). Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain, 129(Pt 3), 791–808. [DOI] [PubMed] [Google Scholar]
- Glasser, M. F. , Coalson, T. S. , Robinson, E. C. , Hacker, C. D. , Harwell, J. , Yacoub, E. , … Van Essen, D. C. (2016). A multi‐modal parcellation of human cerebral cortex. Nature, 536(7615), 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri, C. T. , & Johnson, L. G. (2006). Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Archives of Clinical Neuropsychology, 21(7), 623–643. [DOI] [PubMed] [Google Scholar]
- Harris, R. J. , Bookheimer, S. Y. , Cloughesy, T. F. , Kim, H. J. , Pope, W. B. , Lai, A. , … Ellingson, B. M. (2014). Altered functional connectivity of the default mode network in diffuse gliomas measured with pseudo‐resting state fMRI. Journal of Neuro‐oncology, 116(2), 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimans, J. J. , & Reijneveld, J. C. (2012). Factors affecting the cerebral network in brain tumor patients. Journal of Neuro‐oncology, 108(2), 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom, J. , & Reitan, R. M. (1984). Neuropsychological correlates of rapidly vs. slowly growing intrinsic cerebral neoplasms. Journal of Clinical Neuropsychology, 6(3), 309–324. [DOI] [PubMed] [Google Scholar]
- Honey, C. J. , Thivierge, J. P. , & Sporns, O. (2010). Can structure predict function in the human brain? NeuroImage, 52(3), 766–776. [DOI] [PubMed] [Google Scholar]
- Kawagoe, T. , Onoda, K. , & Yamaguchi, S. (2017). Associations among executive function, cardiorespiratory fitness, and brain network properties in older adults. Scientific Reports, 7, 40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler, S. R. , Noll, K. , Cahill, D. P. , Rao, G. , & Wefel, J. S. (2017). The effect of IDH1 mutation on the structural connectome in malignant astrocytoma. Journal of Neuro‐Oncology, 131(3), 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, N. W. , Gibb, W. R. , & Tate, M. C. (2016). Neuroplasticity: Insights from patients harboring gliomas. Neural Plasticity, 2016, 2365063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, U. M. , Solbakk, A. K. , Funderud, I. , Lovstad, M. , Endestad, T. , & Knight, R. T. (2013). The role of the lateral prefrontal cortex in inhibitory motor control. Cortex, 49(3), 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschwitz, J. D. , Waller, L. , Daedelow, L. S. , Walter, H. , & Veer, I. M. (2018). General, crystallized and fluid intelligence are not associated with functional global network efficiency: A replication study with the human connectome project 1200 data set. NeuroImage, 171, 323–331. [DOI] [PubMed] [Google Scholar]
- Latora, V. , & Marchiori, M. (2001). Efficient behavior of small‐world networks. Physical Review Letters, 87(19), 198701. [DOI] [PubMed] [Google Scholar]
- Leung, C. C. , & Chau, H. F. (2007). Weighted assortative and disassortative networks model. Physica A: Statistical Mechanics and its Applications, 378(2), 591–602. [Google Scholar]
- Li, Y. , Liu, Y. , Li, J. , Qin, W. , Li, K. , Yu, C. , & Jiang, T. (2009). Brain anatomical network and intelligence. PLoS Computational Biology, 5(5), e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesawa, S. , Bagarinao, E. , Fujii, M. , Futamura, M. , Motomura, K. , Watanabe, H. , … Wakabayashi, T. (2015). Evaluation of resting state networks in patients with gliomas: Connectivity changes in the unaffected side and its relation to cognitive function. PLoS ONE, 10(2), e0118072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallela, A. N. , Peck, K. K. , Petrovich‐Brennan, N. M. , Zhang, Z. , Lou, W. , & Holodny, A. I. (2016). Altered resting‐state functional connectivity in the hand motor network in glioma patients. Brain Connectivity, 6, 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, J. , Honma, S. M. , Findlay, A. M. , Guggisberg, A. G. , Owen, J. P. , Kirsch, H. E. , … Nagarajan, S. S. (2011). Resting functional connectivity in patients with brain tumors in eloquent areas. Annals of Neurology, 69(3), 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov, S. , & Sneppen, K. (2002). Specificity and stability in topology of protein networks. Science, 296(5569), 910–913. [DOI] [PubMed] [Google Scholar]
- Meier, J. , Tewarie, P. , Hillebrand, A. , Douw, L. , van Dijk, B. W. , Stufflebeam, S. M. , & Van Mieghem, P. (2016). A mapping between structural and functional brain networks. Brain Connectivity, 6(4), 298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto, E. C. , Silva Junior, A. , Silva, C. C. , Cabrera, H. N. , Machado, M. A. , Benute, G. R. , … Teixeira, M. J. (2011). Cognitive impairments in patients with low grade gliomas and high grade gliomas. Arquivos de Neuro‐Psiquiatria, 69(4), 596–601. [DOI] [PubMed] [Google Scholar]
- Newman, M. E. (2002). Assortative mixing in networks. Physical Review Letters, 89(20), 208701. [DOI] [PubMed] [Google Scholar]
- Newman, M. E. (2006). Modularity and community structure in networks. Proceedings of the National Academy of Sciences of the United States of America, 103(23), 8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, M. E. , & Girvan, M. (2004). Finding and evaluating community structure in networks. Physical Review E, 69(2), 026113. [DOI] [PubMed] [Google Scholar]
- Noll, K. R. , Sullaway, C. , Ziu, M. , Weinberg, J. S. , & Wefel, J. S. (2015). Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro‐Oncology, 17(4), 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. J. , & Friston, K. (2013). Structural and functional brain networks: From connections to cognition. Science, 342(6158), 1238411. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Cohen, A. L. , Nelson, S. M. , Wig, G. S. , Barnes, K. A. , Church, J. A. , … Petersen, S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker, A. , Groschel, K. , Ackermann, H. , Schnaudigel, S. , Kassubek, J. , & Kastrup, A. (2010). The role of the unaffected hemisphere in motor recovery after stroke. Human Brain Mapping, 31(7), 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnen, S. J. , Meskal, I. , Bakker, M. , De Baene, W. , Rutten, G. J. M. , Gehring, K. , & Sitskoorn, M. M. (2019). Cognitive outcomes in meningioma patients undergoing surgery: Individual changes over time and predictors of late cognitive functioning. Neuro‐Oncology, 21, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnen, S. J. M. , Meskal, I. , Emons, W. H. M. , Campman, C. A. M. , van der Linden, S. D. , Gehring, K. , & Sitskoorn, M. M. (2017). Evaluation of normative data of a widely used computerized neuropsychological battery: Applicability and effects of sociodemographic variables in a Dutch sample. Assessment, 1073191117727346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, M. D. , Finn, E. S. , Scheinost, D. , Papademetris, X. , Shen, X. , Constable, R. T. , & Chun, M. M. (2016). A neuromarker of sustained attention from whole‐brain functional connectivity. Nature Neuroscience, 19(1), 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. [DOI] [PubMed] [Google Scholar]
- Saad, Z. S. , Gotts, S. J. , Murphy, K. , Chen, G. , Jo, H. J. , Martin, A. , & Cox, R. W. (2012). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2(1), 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Vidaurre, D. , Beckmann, C. F. , Glasser, M. F. , Jenkinson, M. , Miller, K. L. , … Van Essen, D. C. (2013). Functional connectomics from resting‐state fMRI. Trends in Cognitive Sciences, 17(12), 666–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. , Chialvo, D. R. , Kaiser, M. , & Hilgetag, C. C. (2004). Organization, development and function of complex brain networks. Trends in Cognitive Sciences, 8(9), 418–425. [DOI] [PubMed] [Google Scholar]
- Stanley, M. L. , Simpson, S. L. , Dagenbach, D. , Lyday, R. G. , Burdette, J. H. , & Laurienti, P. J. (2015). Changes in brain network efficiency and working memory performance in aging. PLoS ONE, 10(4), e0123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, K. R. , Bridge, C. , Murray, J. D. , & Alvord, E. C. (2003). Virtual and real brain tumors: Using mathematical modeling to quantify glioma growth and invasion. Journal of the Neurological Sciences, 216(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Talacchi, A. , Santini, B. , Savazzi, S. , & Gerosa, M. (2011). Cognitive effects of tumour and surgical treatment in glioma patients. Journal of Neuro‐oncology, 103(3), 541–549. [DOI] [PubMed] [Google Scholar]
- Taphoorn, M. J. , Sizoo, E. M. , & Bottomley, A. (2010). Review on quality of life issues in patients with primary brain tumors. Oncologist, 15(6), 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel, A. , Habedank, B. , Winhuisen, L. , Herholz, K. , Kessler, J. , Haupt, W. F. , & Heiss, W. D. (2005). Essential language function of the right hemisphere in brain tumor patients. Annals of Neurology, 57(1), 128–131. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Vaidya, C. J. , & Gordon, E. M. (2013). Phenotypic variability in resting‐state functional connectivity: Current status. Brain Connectivity, 3(2), 99–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dellen, E. , Douw, L. , Hillebrand, A. , Ris‐Hilgersom, I. H. , Schoonheim, M. M. , Baayen, J. C. , … Reijneveld, J. C. (2012). MEG network differences between low‐ and high‐grade glioma related to epilepsy and cognition. PLoS ONE, 7(11), e50122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , de Lange, S. C. , Zalesky, A. , Seguin, C. , Yeo, B. T. T. , & Schmidt, R. (2017). Proportional thresholding in resting‐state fMRI functional connectivity networks and consequences for patient‐control connectome studies: Issues and recommendations. NeuroImage, 152, 437–449. [DOI] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , Stam, C. J. , Kahn, R. S. , & Hulshoff Pol, H. E. (2009). Efficiency of functional brain networks and intellectual performance. Journal of Neuroscience, 29(23), 7619–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel, E. , Baumfalk, A. E. , van Zandvoort, M. J. E. , Robe, P. A. , & Snijders, T. J. (2017). Tumor‐related neurocognitive dysfunction in patients with diffuse glioma: A systematic review of neurocognitive functioning prior to anti‐tumor treatment. Journal of Neuro‐oncology, 134(1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage, F. (1964). Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar. Assen, the Netherlands: Van Gorcum. [Google Scholar]
- Voytek, B. , Davis, M. , Yago, E. , Barcelo, F. , Vogel, E. K. , & Knight, R. T. (2010). Dynamic neuroplasticity after human prefrontal cortex damage. Neuron, 68(3), 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- Winhuisen, L. , Thiel, A. , Schumacher, B. , Kessler, J. , Rudolf, J. , Haupt, W. F. , & Heiss, W. D. (2005). Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: A combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke, 36(8), 1759–1763. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Ding, S. , Hu, X. , Yang, K. , Xiao, C. , Zou, Y. , … Qian, Z. (2013). Reduced efficiency of functional brain network underlying intellectual decline in patients with low‐grade glioma. Neuroscience Letters, 543, 27–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Data Files and code have been made publicly available via Figshare (https://doi.org/10.6084/m9.figshare.c.4586678.v1).


