Abstract
Studies in IL‐32 transgenic mice and in vitro suggest that IL‐32 may have protective effects against Mycobacterium tuberculosis, but so far there are barely any studies in humans. We studied the role of IL‐32 and its splice variants in tuberculosis (TB) in vivo and in vitro. Blood transcriptional analysis showed lower total IL‐32 mRNA levels in pulmonary TB patients compared to patients with latent TB infection and healthy controls. Also, among Indonesian household contacts who were heavily exposed to an infectious TB patient, IL‐32 mRNA levels were higher among those who remained uninfected compared to those who became infected with M. tuberculosis. In peripheral blood mononuclear cells from healthy donors, we found that IL‐32γ, the most potent isoform, was down‐regulated upon M. tuberculosis stimulation. This decrease in IL‐32γ was mirrored by an increase of another splice variant, IL‐32β. Also, a higher IL‐32γ/IL‐32β ratio correlated with IFN‐γ production, whereas a lower ratio correlated with production of IL‐1Ra, IL‐6, and IL‐17. These data suggest that IL‐32 contributes to protection against M. tuberculosis infection, and that this effect may depend on the relative abundance of different IL‐32 isoforms.
Keywords: tuberculosis, Mycobacterium tuberculosis, immune response, interleukin‐32, cytokines
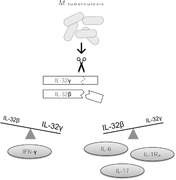
IL‐32 is associated with resistance against M. tuberculosis infection. Mycobacterium tuberculosis induces IL‐32 splicing into different isoforms, which correlates with specific cytokine profiles.
Abbreviations
- IGRA
IFN‐gamma release assay
- TB
tuberculosis
1. INTRODUCTION
Tuberculosis (TB) is an airborne infectious disease caused by Mycobacterium tuberculosis. TB remains a major public health problem, and approximately one‐fourth of the world population is latently infected with M. tuberculosis.1 Host factors may determine whether M. tuberculosis exposure results in infection, and whether infection progresses to disease. A comprehensive understanding of the immune response against M. tuberculosis is crucial for the development of preventive strategies.
Interleukin‐32 (IL‐32), which was previously called natural killer cell transcript 4, has been identified as an important player in innate and adaptive immune responses.2 Although no receptor for IL‐32 has been discovered so far, IL‐32 acts as a pro‐inflammatory cytokine3 and an intracellular regulator of cytokine production, including tumor necrosis factor α (TNF‐α).4 IL‐32 is abundantly expressed in T‐cells and NK cells, but also in the lung, and alternative splicing of IL‐32 mRNA results in at least 9 distinct isoforms,5 of which not all functions are known yet.2 The IL‐32γ isoform is the most potent pro‐inflammatory cytokine inductor,6 which can splice into the most abundant isoform IL‐32β.7, 8 Particular single nucleotide polymorphisms (SNPs) influence total IL‐32 expression in different tissue types,9 but polymorphisms influencing splicing are currently unknown. Although our knowledge of the role of IL‐32 splice variants in health and disease has expanded, many aspects regarding their mechanism of action still remain unknown.
Recent studies have identified IL‐32 as a modulating factor in the host response against M. tuberculosis. Stimulation of human PBMCs with heat‐killed mycobacteria induced a strong production of IL‐32, which is dependent on endogenous IFN‐γ.10 In human monocyte‐derived Mϕs, addition of recombinant IL‐32 increased killing of M. tuberculosis in vitro, and this effect was dependent on vitamin D.11 Addition of IL‐32γ induced caspase‐3, caspase‐1, and cathepsin‐mediated apoptosis in THP‐1 Mϕs, and reduced the intracellular burden of M. tuberculosis.12 Finally, transgenic mice expressing human IL‐32γ in type II alveolar epithelial cells showed an 85% reduction in M. tuberculosis outgrowth in the lungs compared to control mice.13 Together, these studies suggest a potential protective role for IL‐32 upon M. tuberculosis infection.
Although it has been shown that M. tuberculosis induces IL‐32 production,10 little is known about the different splice variants in the context of TB in primary cells. In addition, there are limited reports of studies performed in humans to support a role for IL‐32 in vivo. We therefore examined whole blood IL‐32 expression profiles in different TB phenotypes, and explored expression of IL‐32 isoforms in response to M. tuberculosis in vitro.
2. MATERIALS AND METHODS
Detailed material and methods can be found as Supplementary Information.
2.1. Patient whole blood gene expression
We examined previously published whole blood gene expression data from patients with pulmonary TB, individuals with latent TB infection, and healthy controls from several cohorts from Africa and Europe.14, 15, 16, 17, 18, 19 The datasets were retrieved from the Gene Expression Omnibus (GEO) using the GSE identifiers GSE83456, GSE42826, GSE19491, GSE28623, GSE37250, and GSE34608. We also examined whole blood expression of pulmonary TB patients from the United Kingdom (GSE19491) during follow‐up at 2 and 12 months into TB treatment.
In addition, we measured whole blood IL‐32 expression among household contacts of patients with active pulmonary TB. A total of 44 household contacts of TB cases in an urban setting in Indonesia were recruited within 2 weeks of the index patient starting TB treatment. Using QuantiFERON‐TB Gold, the presence of M. tuberculosis infection was tested at baseline and 3 months afterward. These IFN‐gamma release assays (IGRAs) identified persistently negative contacts, who were exposed but remained uninfected, and converters20; individuals who were positive at baseline were not included.
2.2. In vitro stimulation experiments
Clinical isolates were selected from a previous study21 for in vitro stimulation of PBMCs of 8 healthy volunteers. Details can be found in the Supplementary Information. In short, 19 clinical isolates and the laboratory strain H37Rv were used at a 3 μg/mL concentration to stimulate PBMCs (5 × 105 cells/well) in duplicate in a 96‐well plate. The plates were incubated for 24 h (for TNF‐α, IL‐1β, IL‐1Ra, IL‐10, and IL‐6 quantification) or 7 days (for IFN‐γ, IL‐17, and IL‐22 quantification) at 37°C in a 5% CO2 environment. Cytokines in the supernatants were measured using commercial ELISA kits. RNA from stimulated PBMCs was used for quantitative PCR using different IL‐32 primer sets and was corrected for expression of the housekeeping gene β2‐microglobulin (B2M).
2.3. Data analysis and statistics
All analyses were performed using GraphPad Prism version 5.3 or in R 3.2.4 using RStudio. Statistical analyses of the whole blood microarray data were performed using Kruskal–Wallis tests, including the post hoc Dunn's multiple comparison tests, and Mann–Whitney U‐tests. Differential gene expression analysis for IL‐32 in the TB contact cohort was performed using the R package DESeq2.22 Multiple univariate linear regression analyses assessed the relation between the IL‐32β/IL‐32γ ratio and cytokine levels. Cytokine levels were positively skewed and therefore log‐transformed. P‐values were corrected for multiple testing using Bonferroni correction, and P‐values of 0.05 or less were considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. IL‐32 expression levels differ between TB phenotypes
We first compared expression of total IL‐32 in pulmonary TB patients with individuals with latent TB infection and healthy controls using publicly available data. IL‐32 mRNA expression levels were lower in pulmonary TB patients compared to healthy controls and individuals with latent TB infection, and this was consistently observed in several cohorts (Fig. 1A). During the course of TB treatment, levels IL‐32 restored to normal (Fig. 1B). To examine a possible protective role of IL‐32 in primary M. tuberculosis infection, whole blood transcription levels were compared between 32 TB household contacts that remained IGRA‐negative upon heavy exposure to an infectious TB patient (so‐called “early clearers20”) and 12 household contacts who became infected with M. tuberculosis. Total IL‐32 mRNA levels were higher in the persistently IGRA‐negative group compared to those who developed latent TB infection (P = 1.8 × 10−2; Fig. 1C). The difference in IL‐32 expression was not caused by a difference in lymphocyte count: 2.64 (interquartile range [IQR], 2.08–3.06) × 109/L in individuals who remained IGRA negative vs. 2.56 (IQR 2.13–3.13) in patients who converted to a positive IGRA (P = 0.37).
Figure 1.
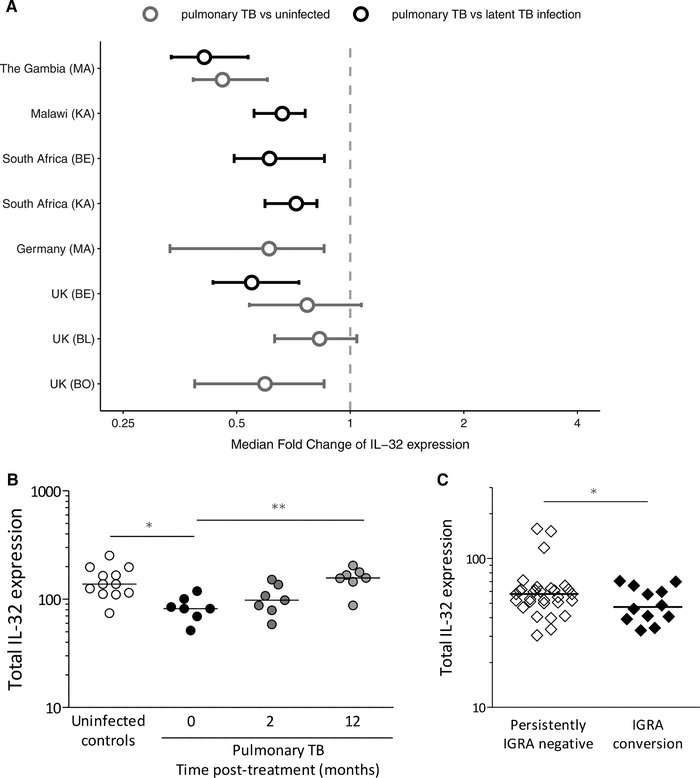
IL‐32 expression in different tuberculosis phenotypes. (A) Whole blood IL‐32 gene expression data from healthy controls, latently infected individuals, and pulmonary TB patients are presented. The data were obtained from publicly available datasets published by Maertzdorf et al. (MA), Kaforou et al. (KA), Berry et al. (BE), Bloom et al. (BO), and Blankley et al. (BL). The hollow circles represent the median fold changes of IL‐32 expression in tuberculosis patients compared to healthy controls (gray) or latently infected individuals (black), and the lines represent the 95% confidence intervals, determined by bootstrap resampling. (B) Whole blood IL‐32 expression from pulmonary TB patients (N = 7) at 0, 2, and 12 months after start of treatment is presented. Data were previously published by Berry et al., and medians are indicated. Statistical analyses of the IL‐32 mRNA levels were performed using Kruskal–Wallis tests, including the post hoc Dunn's multiple comparison tests, and Mann–Whitney U‐tests. * P < 0.05; ** P < 0.01; *** P < 0.001. (C) IL‐32 gene expression measured by RNA sequencing in whole blood of household contacts who remained uninfected (persistently IGRA negative, N = 32) and contacts who became infected after exposure (IGRA conversion, N = 12). Differential gene expression analysis of IL‐32 was performed using the R package DESeq2 with RStudio in R 3.2.4 (P = 0.018). Individual data points and medians are presented
3.2. Induction of IL‐32 isoforms after M. tuberculosis stimulation in vitro
In order to explore which IL‐32 isoforms are induced in TB, PBMCs from healthy volunteers were stimulated with 20 different M. tuberculosis clinical isolates, and after 24 h of stimulation, the mRNA levels of total IL‐32 were determined by RT‐PCR, as well as the isoforms primarily induced by M. tuberculosis 8: IL‐32β and IL‐32γ. Different clinical isolates either up‐regulated or down‐regulated the expression of total IL‐32 mRNA (Fig. 2 and Supplementary Fig. S1A). The isoform IL‐32β followed the pattern of total IL‐32 (Fig. 2 and Supplementary Fig. S1B). It has been shown before that genetically diverse mycobacterial strains vary widely in their induction of cytokines21, 23 and that these differences are observed between M. tuberculosis lineages24 and even within lineages.25 Here, we also observe differences in the expression of total IL‐32 and IL‐32β induced by different clinical isolates, suggesting that the host–microorganism relationship can determine the expression of different IL‐32 isoforms. Nevertheless, all clinical isolates induced down‐regulation of IL‐32γ (Fig. 2 and Supplementary Fig. S1C). These in vitro experiments reveal that M. tuberculosis stimulation leads to active splicing of the most potent IL‐32 isoform, IL‐32γ, into other isoforms, including IL‐32β.
Figure 2.
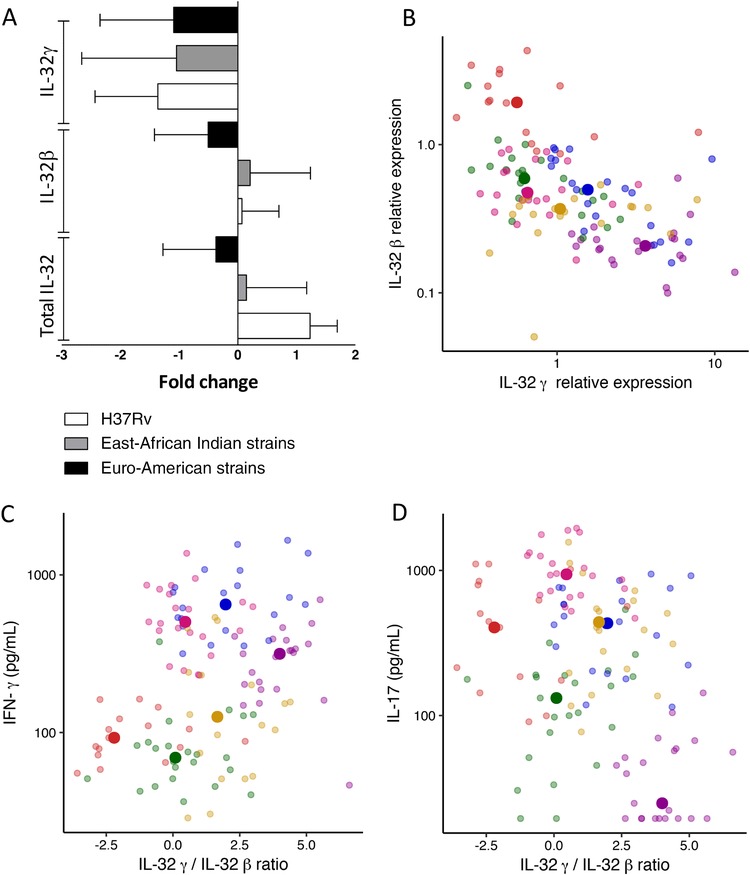
IL‐32 expression in relation to cytokine production after stimulation with M. tuberculosis. (A) Total IL‐32, IL‐32β, and IL‐32γ gene expression in PBMCs from 8 different donors stimulated with M. tuberculosis for 24 h. The East‐Asian Indian strains group shows the median of 15 strains, and the Euro‐American strains group shows the median of 4 strains compared to the unstimulated control. Data are presented as median ± interquartile range (IQR). (B–D) PBMCs from 6 different donors were stimulated with 20 M. tuberculosis isolates for 24 h or 7 days. Each color represents a donor, and each dim point shows 1 individual data point. The brighter point shows the median value of 1 donor for all 20 M. tuberculosis isolates. (B) The relative expression of IL‐32γ is plotted against the relative expression of IL‐32β in PBMCs stimulated for 24 h with M. tuberculosis isolates. The levels of IL‐32β and IL‐32γ were measured using quantitative PCR and normalized against the housekeeping gene β2‐microglobulin (B2M) and the relative expression was calculated as 2(–Δ Ct ). (C–D) Concentrations of IFN‐γ (C) and IL‐17 (D) measured by ELISA after 7 days are plotted against IL‐32γ/IL‐32β ratio
3.3. Association between IL‐32 mRNA levels and cytokine profile
In human PBMCs, IL‐32β and IL‐32γ expression showed a strong inverse relationship (P = 6.6 × 10−10) in a linear regression model corrected for stimulus, as visualized in Fig. 2B. We explored the immunological consequences by correlating IL‐32 expression with M. tuberculosis‐induced cytokines in PBMCs. The ratio between IL‐32γ and IL‐32β was predictive of cytokine production in a linear regression model with stimulus as covariate after correction for multiple testing (Table 1). The IL‐32γ/IL‐32β ratio correlated positively with IFN‐γ (Fig. 2C) and negatively with IL‐6, IL‐1Ra, and IL‐17 (Fig. 2D). Together with previously published literature, these data suggest that different IL‐32 isoforms have very distinct effects and regulation patterns. This provides a strong rationale for measuring distinct IL‐32 isoforms in future laboratory and clinical studies.
Table 1.
Multiple linear regression models assessing the variability in cytokine production explained by IL‐32 corrected for stimulus
| Ratio IL‐32γ/IL‐32β | ||
|---|---|---|
| Estimate | P‐value | |
| TNF‐α | 0.0377 | 0.1673 |
| IL‐1β | −0.0372 | 0.0629 |
| IL‐1Ra | −0.0568 | 0.0010* |
| IL‐6 | −0.0506 | 0.0018* |
| IL‐10 | 0.0285 | 0.1957 |
| IFN‐γ | 0.143 | 0.0018* |
| IL‐17 | −0.213 | 0.0003* |
| IL‐22 | 0.0614 | 0.1550 |
*
Significance at the Bonferroni‐corrected level for the number of cytokines tested (α = 0.05/8 = 0.00625).
IFN‐γ has been repeatedly shown to be crucial in the host defense against TB.26, 27 In contrast, excessive IL‐17 is thought to play a role in immunopathology through neutrophil recruitment. A balance between Th1 and Th17 responses is critical to control bacterial growth and to limit immunopathology.28 Here, the IL‐32γ/IL‐32β ratio was inversely correlated with IL‐17, and positively correlated to IFN‐γ. This suggests that splicing of IL‐32γ may influence the balance between Th1 and Th17, and therefore play a role in steering the immune response in TB. This could indicate that IL‐32γ contributes to control of M. tuberculosis infection and reduction of Th17‐mediated lung damage.
4. CONCLUDING REMARKS
Previous studies have suggested a protective effect of IL‐32 against M. tuberculosis. We analyzed multiple TB cohorts to further explore these hypotheses in vivo. We observed decreased levels of total IL‐32 mRNA in blood from pulmonary TB patients compared to individuals with latent TB infection and healthy controls. Additionally, in TB contacts in Indonesia, heavily exposed TB contacts who remained uninfected showed higher total IL‐32 expression compared to those who became latently infected, suggesting a possible role in innate protection against M. tuberculosis infection. In PBMCs from healthy individuals, a higher ratio of IL‐32γ/IL‐32β expression correlated with higher IFN‐γ and a lower ratio with higher IL‐17 cytokine responses. This is the first study to identify an association between IL‐32 and resistance against M. tuberculosis infection in an in vivo setting in humans, and of IL‐32 splice variants with specific M. tuberculosis cytokine profiles. More study is needed to unravel the exact mechanism of action of IL‐32 and its splice variants in TB. Of note, IL‐32 is also known to play a role in Leishmaniasis and viral infections, which might imply a role in host response against intracellular pathogens.29
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Supplementary Figure 1. IL‐32 expression after stimulation with M. tuberculosis. Total IL‐32 (A), IL‐32β (B), and IL‐32γ (C) gene expression in PBMCs stimulated with 20 different M. tuberculosis isolates for 24 h. The levels of total IL‐32, IL‐32β and IL‐32γ were measured using quantitative PCR and the gene expression levels were corrected for expression of the housekeeping gene β2‐microglobulin (B2M). Data are presented as median fold change (compared to the unstimulated control) ± IQR.
Supplementary MethodsS1
ACKNOWLEDGMENTS
Cohort recruitment was funded by the University of Otago and Mercy Hospital, Dunedin, New Zealand. The IGRA (QuantiFERON) was donated by Qiagen. Researchers performing this study were supported by The European Union's Seventh Framework Programme (FP7/2007–2013; grant 305279 to the TANDEM Project on Tuberculosis and Diabetes, supporting V. A. C. M. K., and R. v. C.), a New Zealand Health Research Council Clinical Training Research Fellowship to A.V, the Royal Netherlands Academy of Arts and Sciences (09‐PD‐14 to R. v. C.), the Netherlands Organisation for Scientific Research (VIDI grant 017.106.310 to R. v. C.), and Radboud University (fellowships to A. v. L. and B. A.). The authors thank Bas Heinhuis, Michelle S.M.A. Damen, and Ekta Lachmandas for their advice on the immunological experiments.
Koeken VACM, Verrall AJ, Ardiansyah E, et al. IL‐32 and its splice variants are associated with protection against Mycobacterium tuberculosis infection and skewing of Th1/Th17 cytokines. J Leukoc Biol. 2020;107:113–118. 10.1002/JLB.4AB0219-071R
The copyright line for this article was changed on 9 August 2019 after original online publication.
REFERENCES
- 1. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re‐estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH, Park MH. Interleukin 32, inflammation and cancer. Pharmacol Ther. 2017;174:127‐137. [DOI] [PubMed] [Google Scholar]
- 3. Dinarello CA, Kim SH. IL‐32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006;65(Suppl 3):iii61‐iii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin‐32: a cytokine and inducer of TNFalpha. Immunity. 2005;22(1):131‐142. [DOI] [PubMed] [Google Scholar]
- 5. Ribeiro‐Dias F, Saar Gomes R, de Lima Silva LL, Dos Santos JC, Joosten LA. Interleukin 32: a novel player in the control of infectious diseases. J Leukoc Biol. 2017;101(1):39‐52. [DOI] [PubMed] [Google Scholar]
- 6. Choi JD, Bae SY, Hong JW, et al. Identification of the most active interleukin‐32 isoform. Immunology. 2009;126(4):535‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joosten LA, Heinhuis B, Netea MG, Dinarello CA. Novel insights into the biology of interleukin‐32. Cell Mol Life Sci. 2013;70(20):3883‐3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li W, Deng W, Xie J. The biology and role of interleukin‐32 in tuberculosis. J Immunol Res. 2018;2018:1535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Consortium GT. The genotype‐tissue expression (GTEx) project. Nat Genet. 2013;45(6):580‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Netea MG, Azam T, Lewis EC, et al. Mycobacterium tuberculosis induces interleukin‐32 production through a caspase‐ 1/IL‐18/interferon‐gamma‐dependent mechanism. PLoS Med. 2006;3(8):e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montoya D, Inkeles MS, Liu PT, et al. IL‐32 is a molecular marker of a host defense network in human tuberculosis. Sci Transl Med. 2014;6(250):250ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai X, Kinney WH, Su WL, et al. Caspase‐3‐independent apoptotic pathways contribute to interleukin‐32gamma‐mediated control of Mycobacterium tuberculosis infection in THP‐1 cells. BMC Microbiol. 2015;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bai X, Shang S, Henao‐Tamayo M, et al. Human IL‐32 expression protects mice against a hypervirulent strain of Mycobacterium tuberculosis . Proc Natl Acad Sci USA. 2015;112(16):5111‐5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berry MP, Graham CM, McNab FW, et al. An interferon‐inducible neutrophil‐driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blankley S, Graham CM, Turner J, et al. The transcriptional signature of active tuberculosis reflects symptom status in extra‐pulmonary and pulmonary tuberculosis. PLoS One. 2016;11(10):e0162220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bloom CI, Graham CM, Berry MP, et al. Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PLoS One. 2013;8(8):e70630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maertzdorf J, Ota M, Repsilber D, et al. Functional correlations of pathogenesis‐driven gene expression signatures in tuberculosis. PLoS One. 2011;6(10):e26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaforou M, Wright VJ, Oni T, et al. Detection of tuberculosis in HIV‐infected and ‐uninfected African adults using whole blood RNA expression signatures: a case‐control study. PLoS Med. 2013;10(10):e1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maertzdorf J, 3rd Weiner J, Mollenkopf HJ, et al. Common patterns and disease‐related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci USA. 2012;109(20):7853‐7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verrall AJ, Netea MG, Alisjahbana B, Hill PC, van Crevel R. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology. 2014;141(4):506‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nebenzahl‐Guimaraes H, van Laarhoven A, Farhat MR, et al. Transmissible Mycobacterium tuberculosis strains share genetic markers and immune phenotypes. Am J Respir Crit Care Med. 2017;195(11):1519‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coscolla M, Gagneux S. Consequences of genomic diversity in Mycobacterium tuberculosis . Semin Immunol. 2014;26(6):431‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Portevin D, Gagneux S, Comas I, Young D. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog. 2011;7(3):e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Laarhoven A, Mandemakers JJ, Kleinnijenhuis J, et al. Low induction of proinflammatory cytokines parallels evolutionary success of modern strains within the Mycobacterium tuberculosis Beijing genotype. Infect Immun. 2013;81(10):3750‐3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type‐I cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19(11):491‐494. [DOI] [PubMed] [Google Scholar]
- 27. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249‐2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torrado E, Cooper AM. IL‐17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21(6):455‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dos Santos JC, Damen M, Joosten LAB, Ribeiro‐Dias F. Interleukin‐32: an endogenous danger signal or master regulator of intracellular pathogen infections‐Focus on leishmaniases. Semin Immunol. 2018;38:15‐23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. IL‐32 expression after stimulation with M. tuberculosis. Total IL‐32 (A), IL‐32β (B), and IL‐32γ (C) gene expression in PBMCs stimulated with 20 different M. tuberculosis isolates for 24 h. The levels of total IL‐32, IL‐32β and IL‐32γ were measured using quantitative PCR and the gene expression levels were corrected for expression of the housekeeping gene β2‐microglobulin (B2M). Data are presented as median fold change (compared to the unstimulated control) ± IQR.
Supplementary MethodsS1


