Abstract
This study evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of various doses of the anti‐CD40 monoclonal antibody bleselumab (ASKP1240) in de novo kidney transplant recipients receiving concomitant standard immunosuppression over 90 days posttransplant. Transplant recipients were randomized (1:1:1:1:1) to bleselumab 50 mg, 100 mg, 200 mg, or 500 mg, or placebo, in addition to standard maintenance immunosuppression. The primary pharmacokinetic endpoints were AUC inf, C max, and AUC last. The primary pharmacodynamic endpoint was B cell CD40 receptor occupancy over time. Overall, 50 kidney transplant recipients were randomized; 45 received their randomized treatment (bleselumab [n = 37] or placebo [n = 8]). AUC inf and AUC last demonstrated a more than dose‐proportional increase in the range of 50‐500 mg, and C max increased linearly with increasing dose. Maximal receptor occupancy for B cell CD40 was reached at all dose levels and was prolonged as dose increased. No kidney transplant recipients experienced cytokine release syndrome or a thromboembolic event. Treatment‐emergent anti‐bleselumab antibodies were found in one kidney transplant recipient in the bleselumab 50 mg group; these were detected only at Day 7. Overall, bleselumab demonstrated nonlinear pharmacokinetics and dose‐dependent prolonged B cell CD40 receptor occupancy and was well tolerated at all doses (ClinicalTrials.gov: NCT01279538).
Keywords: antibody biology, clinical research/practice, kidney transplantation/nephrology, kidney transplantation: living donor
Short abstract
In this randomized, phase 1b study in kidney transplant recipients, bleselumab, a nondepleting, fully human, anti‐CD40 monoclonal antibody, demonstrates tolerability, nonlinear pharmacokinetics, and prolonged, dose‐dependent B‐cell CD40 receptor occupancy. See the article by Harland et al on http://onlinelibrary.wiley.com/doi/10.1111/ajt.15591/abstract.
Abbreviations
- AE
adverse event
- AUC
area under the concentration–time curve
- AUCinf
area under the concentration–time curve from 0 to infinity
- AUClast
area under the concentration–time curve from time 0 to the last quantifiable concentration
- BMI
body mass index
- BPAR
biopsy‐proven acute rejection
- CI
confidence interval
- CLtot
total clearance
- Cmax
maximum concentration
- CNI
calcineurin inhibitor
- CV
coefficient of variation
- FAS
full analysis set
- GM
geometric mean
- IR‐TAC
immediate‐release tacrolimus
- MMF
mycophenolate mofetil
- PDAS
pharmacodynamic analysis set
- PKAS
pharmacokinetic analysis set
- SAF
safety analysis set
- SD
standard deviation
- t½
half‐life
- TEAE
treatment‐emergent adverse event
- tmax
time taken to reach the maximum concentration
- Vz
volume of distribution
1. INTRODUCTION
Over the past 30 years, the search for effective calcineurin inhibitor (CNI)‐free regimens has been focused on biologics that target the receptors and ligands of the costimulatory pathway. Two important factors in the pathway have emerged as critical for T cell and B cell activation. These are the CD28–CD80/CD86 receptor–ligands and the CD40–CD154 (CD40L) receptor–ligands.1, 2 Targeting these pathways in experimental models and in human kidney transplant recipients occurred simultaneously, and the first approved costimulation blocking agent for the prophylaxis of kidney transplant rejection, belatacept, targeted the CD28–CD80/CD86 pathway.3, 4, 5 The first phase 2 study in kidney transplantation to target costimulation was with a humanized CD154 antibody, hu5c8.6 The study utilized a CNI‐free regimen that had induced prolonged graft survival and even tolerance in some nonhuman primates. These exciting in vivo findings are consistent with the important role of the CD40–CD154 pathway in T cell and B cell activation. CD40 is expressed on antigen‐presenting cells, B cells, and macrophages,7, 8, 9 whereas CD154 is upregulated on activated T cells.10 CD40–CD154 participates in T cell activation by upregulating the ligands for CD28, and is critical in B cell activation and differentiation.1, 2 Hu5c8 as well as other anti‐CD154 antibodies was halted from clinical development because of the occurrence of thromboembolic complications due in part to the upregulation of CD154 on platelets.11, 12 However, interest in pursuing blockade of the CD40–CD154 pathway persisted and antibodies targeting the CD40 receptor instead emerged as an effective alternative.11, 13, 14
Bleselumab (ASKP1240) is a fully human immunoglobulin G4 anti‐CD40 monoclonal antibody that inhibits both humoral (immunoglobulin production) and cellular immune responses by blocking the interaction of CD40:CD154 between T cells, B cells, and antigen‐presenting cells.2, 11 Results from in vitro and in vivo studies have suggested a potential therapeutic role for bleselumab as an immunosuppressive therapy in transplant recipients.11 In a phase 1 study in healthy volunteers, bleselumab was well tolerated, with no evidence of cytokine release syndrome or thromboembolic events.15 Bleselumab demonstrated nonlinear pharmacokinetics in the dose range of 0.1‐10 mg/kg, with mean maximum serum concentrations (C max) and area under the serum concentration–time curves (AUC) ranging from 0.7 to 252 μg/mL and 6.5 to 55 410 μg·h/mL, respectively.15
Here we describe the results of a phase 1b, single‐dose, placebo‐controlled multicenter study that evaluated the pharmacokinetics, pharmacodynamics, safety, and tolerability of four dose levels of bleselumab administered with standard immunosuppressants to de novo kidney transplant recipients.
2. MATERIALS AND METHODS
2.1. Study design
This phase 1b, single‐dose, double‐blind, parallel‐group, placebo‐controlled multicenter study was conducted at 15 sites in the United States between November 17, 2010 and January 23, 2012 (NCT01279538). Patients were screened between 14 days pretransplant and 2 days posttransplant and were then randomized in a 1:1:1:1:1 ratio (1‐4 days posttransplant) to receive a single 30‐minute infusion of bleselumab 50 mg, 100 mg, 200 mg, or 500 mg, or placebo (Figure 1). The day of infusion was defined as Day 1, after which patients were followed for 90 days. Institutional review board/independent ethics committee approval of the protocol (protocol number 7163‐CL‐0103), informed consent, and patient information were obtained before initiation of any study‐specific procedures (see Table S1 for a list of institutional review boards).
Figure 1.
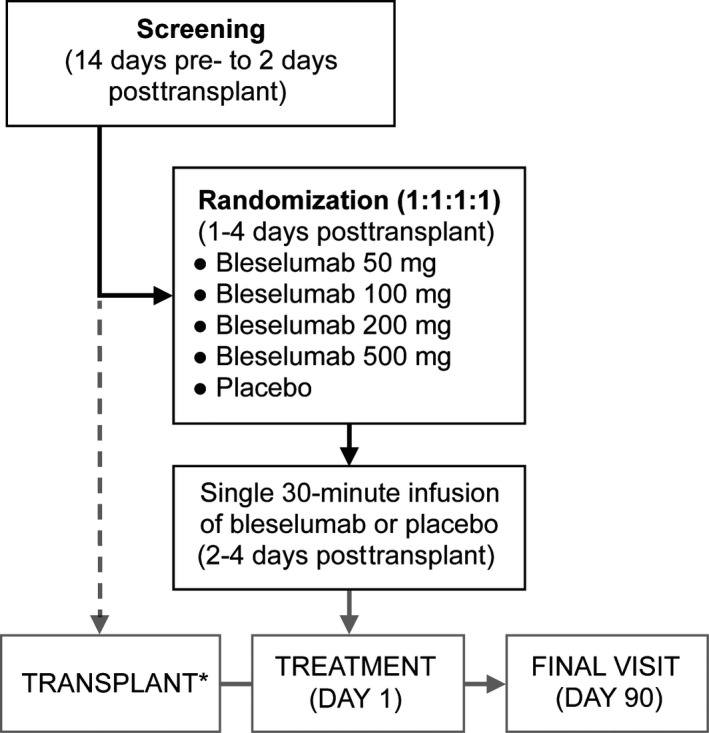
Study design. *Day 0
2.2. Transplant recipients
Adult (18‐65 years of age) de novo kidney transplant recipients who received their kidney from either a living or deceased donor were randomized. Before randomization, subjects had to have a posttransplant serum creatinine value that was ≥30% less than the pretransplant value and required no dialysis. Exclusion criteria included subjects who were receiving antibody induction therapy, and subjects who had previously received or were receiving an organ transplant other than a kidney.
2.3. Endpoints
2.3.1. Pharmacokinetics
Primary pharmacokinetic variables, through Day 90, were AUC from 0 to infinity (AUCinf), C max, and AUC from time 0 to the last quantifiable concentration (AUClast). Secondary pharmacokinetic variables, through Day 90, were time taken to reach the maximum concentration (t max), half‐life (t ½), volume of distribution (V z), and total clearance (CLtot).
Blood samples for pharmacokinetic analyses were obtained predose and 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, 72, and 144 hours postinfusion, and on Days 15, 22, 29, 43, 60, 75, and 90 (or at the time of early discontinuation). All bleselumab concentration analyses were performed by a central laboratory (APGD Bioanalysis‐US, Skokie, IL).
2.3.2. Pharmacodynamics
The primary pharmacodynamic variable was B cell CD40 receptor occupancy over time, through Day 90. Blood samples from predose and 0.5, 24, 48, 72, and 144 hours postinfusion, and on Days 15, 22, 29, 43, 60, 75, and 90 (or at the time of early discontinuation) were used for pharmacodynamic analyses. Samples were collected at the site, or, on weekends or holidays, a home health care nurse collected samples if it was not possible to collect them at the site. All pharmacodynamic evaluations were performed by a central laboratory (MedTox, St. Paul, MN). B cell CD40 receptor occupancy was calculated using the following formula: B cell CD40 receptor occupancy = (1 – B cell mean fluorescent intensity (MFI) at postdose/B cell MFI at baseline) × 100. B cell MFI was calculated using the following formula: CD40 antibody − CD40 background.
2.3.3. Exploratory efficacy
Exploratory efficacy endpoints, through Day 90, included biopsy‐proven acute rejections (BPARs; T cell– and antibody‐mediated), grade of BPAR, multiple rejection episodes, patient survival and graft survival. BPAR was determined by local review, and all biopsies of grade I (by 2007 Banff criteria16) or higher were considered as BPARs.
2.3.4. Safety and tolerability
Safety and tolerability variables, through Day 90, included adverse events (AEs) per National Cancer Institute Common Terminology Criteria for Adverse Events v4.02, antibleselumab antibodies, and new‐onset diabetes mellitus (NODAT) in at‐risk patients. Patients were considered at risk if they did not have diabetes (type I or II, or NODAT with prior transplant) present at skin closure, and did not have any pretransplant glucose value of >200 mg/dL or HbA1c value of ≥6.5%. Patients receiving antidiabetic treatment (eg, insulin or oral hypoglycemics) for ≥30 days pretransplant who did not discontinue antidiabetic treatment >7 days pretransplant were considered to have diabetes and thus were not included in the at‐risk set.
2.4. Statistical analyses
2.4.1. Analysis sets
The full analysis set (FAS) and the safety analysis set (SAF) included all randomized patients who received study drug (bleselumab or placebo). The pharmacokinetic analysis set was composed of subjects from the SAF whom the pharmacokineticist considered to have adequate pharmacokinetic data for the calculation of the primary pharmacokinetic parameters. The pharmacodynamic analysis set included subjects from the SAF whom the pharmacokineticist considered to have adequate pharmacodynamic data for assessment of B cell CD40 receptor occupancy (the primary pharmacodynamic endpoint).
2.4.2. Sample size
No statistical methods were used to determine the sample size. A planned sample size of 45 kidney transplant recipients (9 per treatment group) was consistent with other pharmacokinetic studies that were similar in design.
2.4.3. Missing data
Patients lost to follow‐up were included in the number of patients with graft loss and the number of deaths. No other imputation of missing data was done for this study. When deemed necessary, outliers for an individual's analyte concentration data were identified by pharmacokinetic plausibility and excluded from the primary analysis.
2.4.4. Pharmacokinetics and pharmacodynamics
Individual patient serum bleselumab concentrations over time were used to derive pharmacokinetic parameters using a noncompartmental method with values below the lower limit of quantification set to 0. Dose proportionality was tested for primary pharmacokinetic outcomes. A 95% confidence interval (CI) for the true slope was constructed from the slope estimate (using linear regression on loge [pharmacokinetic parameter] against loge [dose]): if the 95% CI included the value of 1, dose proportionality was concluded. B cell CD40 receptor occupancy was summarized using descriptive statistics.
3. RESULTS
3.1. Patient disposition and demographics
Overall, 50 patients were randomized to bleselumab or placebo (Figure 2). Four randomized patients withdrew before receiving study drug and one patient was excluded owing to evidence of not taking the assigned study drug. Therefore, 45 patients were included in the SAF and FAS.
Figure 2.
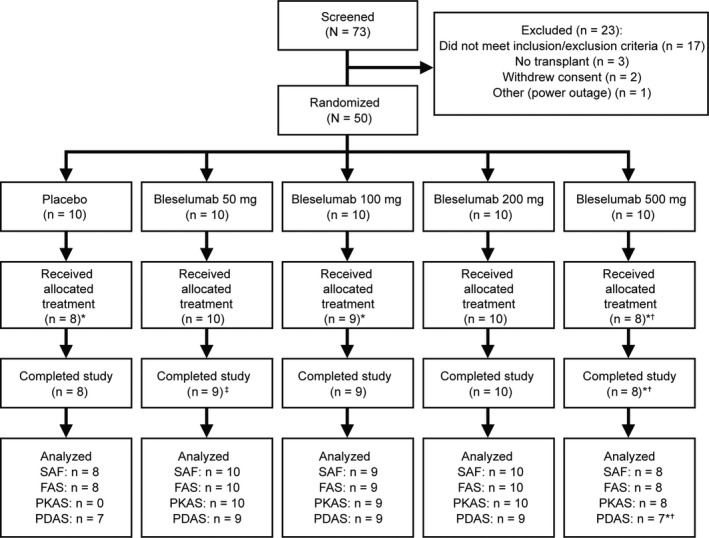
Patient disposition. *Four randomized patients withdrew before receiving study drug due to one AE during screening, one patient no longer fulfilled inclusion/exclusion criteria, two were never dispensed study drug. †one patient excluded from table summaries owing to evidence of not taking study drug. ‡The one patient who received their treatment but did not complete the study in the bleselumab 50 mg group discontinued the study owing to not returning for their final visit. AE, adverse event; FAS, full analysis set; PDAS, pharmacodynamic analysis set; PKAS, pharmacokinetic analysis set; SAF, safety analysis set
Overall, the majority of patients were male, white, and not Hispanic or Latino (Table 1). There were no females in the bleselumab 50 mg group and the majority of patients in this group were Hispanic or Latino. The mean age was highest in the placebo group (50.0 years) and lowest in the bleselumab 500 mg group (37.8 years). Mean body mass index was lowest in the placebo group (26.5 kg/m2) and highest in the bleselumab 100 mg and 500 mg groups (29.5 kg/m2). In those treated with bleselumab, 17 patients (46%) received a kidney from a living, related donor; 13 patients (35%) received a kidney from a living, unrelated donor; and 7 patients (19%) received a kidney from a deceased donor. Half of the placebo group patients received kidneys from living donors: 3 (38%) from living, related donors and 1 (13%) from a living, unrelated donor; the remaining 4 (50%) received kidneys from deceased donors.
Table 1.
Recipient and donor demographics and baseline characteristics
| Parameter | Category | Placebo (n = 8) | Bleselumab | Bleselumab total (n = 37) | |||
|---|---|---|---|---|---|---|---|
| 50 mg (n = 10) | 100 mg (n = 9) | 200 mg (n = 10) | 500 mg (n = 8)a | ||||
| Gender, n (%) | Male | 5 (62.5) | 10 (100) | 6 (66.7) | 6 (60.0) | 7 (87.5) | 29 (78.4) |
| Race, n (%) | White | 7 (87.5) | 7 (70.0) | 8 (88.9) | 10 (100) | 7 (87.5) | 32 (86.5) |
| Black or African American | 0 | 1 (10.0) | 0 | 0 | 1 (12.5) | 2 (5.4) | |
| Asian | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | |
| Other | 0 | 2 (20.0) | 1 (11.1) | 0 | 0 | 3 (8.1) | |
| Ethnicity, n (%) | Hispanic or Latino | 1 (12.5) | 6 (60.0) | 1 (11.1) | 3 (30.0) | 2 (25.0) | 12 (32.4) |
| Age, years | Mean | 50.0 | 38.2 | 48.1 | 48.2 | 37.8 | 43.2 |
| SD | 12.4 | 12.3 | 7.8 | 11.3 | 11.2 | 11.6 | |
| BMI, kg/m2 | Mean | 26.5 | 29.4 | 29.5 | 28.5 | 29.5 | 29.2 |
| SD | 4.3 | 4.5 | 4.2 | 6.3 | 5.0 | 4.9 | |
| Donor source, n (%) | Living related | 3 (37.5) | 7 (70.0) | 3 (33.3) | 5 (50.0) | 2 (25.0) | 17 (45.9) |
| Living nonrelated | 1 (12.5) | 1 (10.0) | 4 (44.4) | 4 (40.0) | 4 (50.0) | 13 (35.1) | |
| Deceased donor | 4 (50.0) | 2 (20.0) | 2 (22.2) | 1 (10.0) | 2 (25.0) | 7 (18.9) | |
BMI, body mass index; SD, standard deviation.
One patient in the bleselumab 500 mg treatment group was excluded from table summaries owing to evidence of not taking study drug.
The most frequently used concomitant immunosuppressant medications were tacrolimus, mycophenolate mofetil, and steroids. Cyclosporine was used by only two patients (one each in the bleselumab 50 mg and 500 mg groups).
3.2. Pharmacokinetics
Mean serum concentrations of bleselumab for the four active dose groups and placebo are shown in Figure 3. C max was dose proportional (slope 1.03, 95% CI 0.92‐1.13) but AUC showed a greater than proportional response: AUCinf (slope 1.74, 95% CI 1.60‐1.88); AUClast (slope 1.77, 95% CI 1.63‐1.92). As the dose increased, t ½ also increased, with a mean t½ of approximately 25‐169 hours over the bleselumab 50‐500 mg dose range (Table 2). Median t max was 1.0 hours across all groups. At the lowest dose of 50 mg, bleselumab was not detectable after Day 6. Bleselumab was not detectable after Day 15 for the bleselumab 100 mg group and after Day 29 for the bleselumab 200 mg group. At the maximum dose of 500 mg, bleselumab was not detectable after Day 60.
Figure 3.
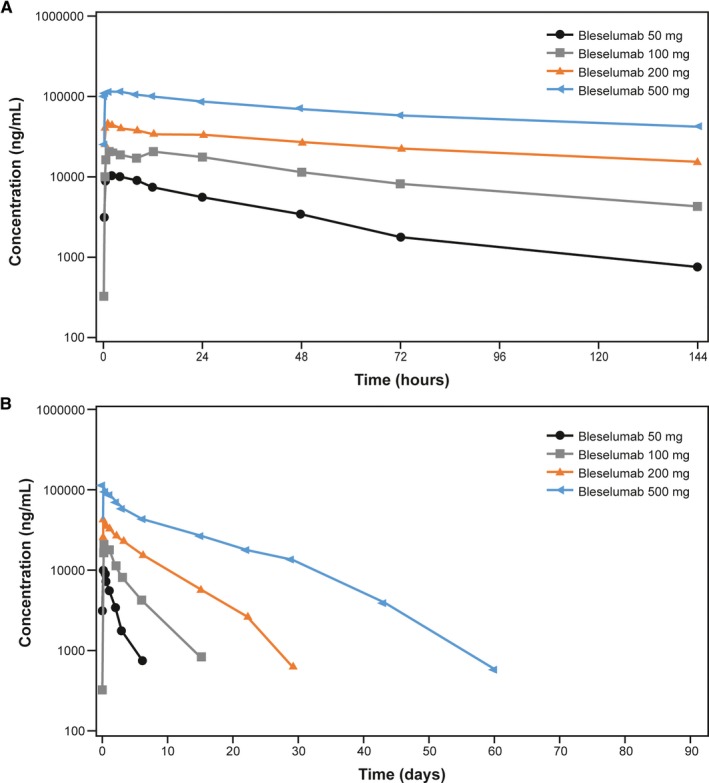
Mean serum concentration of bleselumab, from predose to (A) 144 hours and (B) 90 days
Table 2.
Bleselumab pharmacokinetic parameters
| Parameter | Category | Bleselumab | |||
|---|---|---|---|---|---|
| 50 mg (n = 10) | 100 mg (n = 9) | 200 mg (n = 10) | 500 mg (n = 8)a | ||
| AUCinf, h·ng/mL | Mean | 436 271 | 1 887 610 | 6 017 303 | 24 747 058 |
| SD | 183 380.1 | 510 897.3 | 1 620 579.6 | 9 510 021.3 | |
| AUClast, h·ng/mL | Mean | 398 469 | 1 690 095 | 5 798 516 | 24 007 991 |
| SD | 164 910.9 | 544 149.8 | 1 593 389.7 | 9 247 882.7 | |
| C max, ng/mL | Mean | 10 908 | 24 124 | 49 549 | 118 158 |
| SD | 2825.8 | 3997.0 | 17 024.6 | 34 099.1 | |
| t max, h | Median | 1.0 | 1.0 | 1.0 | 1.0 |
| Range | 0.5–8.0 | 0.5–12.0 | 0.5–4.0 | 0.5–4.0 | |
| t ½, h | Mean | 24.9 | 64.6 | 97.9 | 169.5 |
| SD | 9.0 | 11.1 | 24.2 | 53.0 | |
| V z, mL | Mean | 4320 | 5131 | 4816 | 5487 |
| SD | 1304.6 | 1141.9 | 878.0 | 2218.7 | |
| CLtot, mL/h | Mean | 128.5 | 56.0 | 35.9 | 24.2 |
| SD | 41.7 | 13.0 | 11.3 | 12.6 | |
AUCinf, area under the concentration–time curve from 0 to infinity; AUClast, area under the concentration–time curve from time 0 to the last quantifiable concentration; CLtot, total clearance; C max, maximum concentration; SD, standard deviation; t ½, half‐life; t max, time taken to reach the maximum concentration; V z, volume of distribution.
One patient in the bleselumab 500 mg treatment group was excluded from table summaries owing to evidence of not taking study drug.
3.3. Pharmacodynamics
Median B cell CD40 receptor occupancy was generally negligible in the placebo group but was demonstrated in the four active dose groups (Figure 4). In the bleselumab groups, median B cell receptor occupancy was at least 80% at 0.5 hours after dosing and remained at higher levels for longer periods of time with increasing doses. For the bleselumab 50 mg group, median B cell CD40 receptor occupancy ranged from ~89% at 0.5 hours to ~76% at 144 hours, further decreased to ~5% by Day 15 and was not detectable at Day 22. For the bleselumab 100 mg group, median B‐cell CD40 receptor occupancy ranged from ~87% at 0.5 hours to ~73% at Day 15, decreased to ~32% by Day 22, was not detectable at Day 29, and was ~5% at Day 43. For the bleselumab 200 mg group, median B cell CD40 receptor occupancy ranged from ~88% at 0.5 hours to ~79% at Day 22, decreased to ~41% on Day 29, and was not detectable at Day 43. For the bleselumab 500 mg group, median B cell CD40 receptor occupancy ranged from ~80% at 0.5 hours to ~83% on Day 29, and decreased to ~74% on Day 43, ~57% on Day 60, and was not detectable at Day 75. Table S2 provides a summary of the per‐group percentage of patients who maintained ≥80% B cell CD40 receptor occupancy over time.
Figure 4.
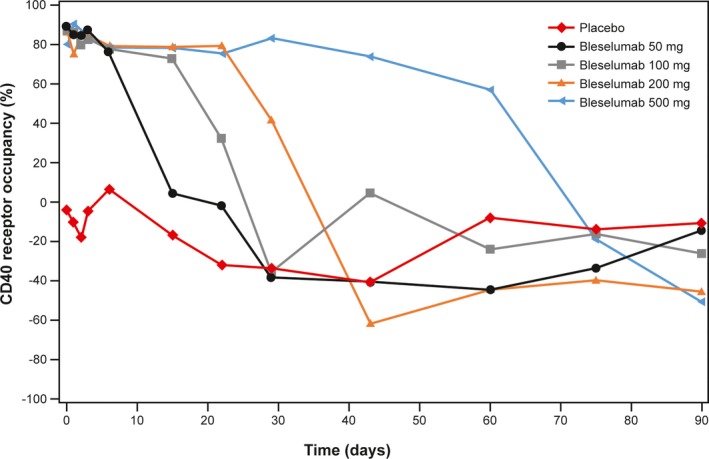
Median B cell CD40 receptor occupancy. Note that median data are shown because interpatient variability was such that mean values were not informative
3.4. Exploratory efficacy
There were no deaths or graft losses. A total of six patients had BPAR, one of whom was in the placebo group (one patient in the bleselumab 500 mg group who experienced BPAR was excluded from table summaries owing to evidence of not taking study drug; Table S3). No patient had grade III T cell‐mediated rejection or grade III antibody‐mediated rejection. However, one patient in the bleselumab 500 mg group had grade IIA antibody‐mediated rejection that started on Day 6 and ended on Day 13. No patient experienced multiple rejection episodes.
3.5. Safety and tolerability outcomes
All patients in the study experienced ≥1 treatment‐emergent AE (TEAE) during this study, most of which were grade I or II (Tables 3 and S3). Twelve patients (including three treated with placebo) experienced ≥1 serious TEAE, four of which were considered drug related (including one in the placebo group). The most commonly reported TEAEs in the active treatment groups were hypophosphatemia (54%), diarrhea (43%), hypomagnesemia (38%), tremor (30%), insomnia (30%), and edema peripheral (27%); in the placebo group, these were diarrhea (50%), vomiting (38%), tremor (38%), and headache (38%) (Table S4). BK virus infection was experienced by 1 of 8 placebo patients (13%) and 7 of 37 bleselumab patients (19%; bleselumab 50 mg [n = 4/10], 200 mg [n = 1/10], and 500 mg [n = 2/8]; Table S4). Cytomegalovirus (CMV) infection was experienced by 1 of 8 placebo patients (13%) and 1 of 37 bleselumab patients (3%; bleselumab 100 mg); one patient treated with bleselumab 500 mg (13%) experienced CMV viremia. No patients experienced Epstein–Barr virus infection during this study.
Table 3.
Summary of treatment‐emergent adverse events
| Placebo (n = 8) | Bleselumab | Bleselumab total (n = 37) | ||||
|---|---|---|---|---|---|---|
| 50 mg (n = 10) | 100 mg (n = 9) | 200 mg (n = 10) | 500 mg (n = 8)a | |||
| Adverse events, n (%) | 8 (100.0) | 10 (100.0) | 9 (100.0) | 10 (100.0) | 8 (100.0) | 37 (100.0) |
| Drug‐related adverse events, n (%) | 1 (12.5) | 5 (50.0) | 2 (22.2) | 0 | 4 (50.0) | 11 (29.7) |
| Death | 0 | 0 | 0 | 0 | 0 | 0 |
| Serious adverse events, n (%) | 3 (37.5) | 6 (60.0) | 1 (11.1) | 0 | 2 (25.0) | 9 (24.3) |
| Drug‐related serious adverse events, n (%) | 1 (12.5) | 2 (20.0) | 0 | 0 | 1 (12.5) | 3 (8.1) |
| Adverse events of interest | ||||||
| Thromboembolic event | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver function test abnormal, n (%) | 2 (25.0) | 2 (20.0) | 1 (11.1) | 0 | 1 (12.5) | 4 (10.8) |
| Kidney transplant rejection, n (%) | 1 (12.5) | 2 (20.0)b | 0 | 0 | 2 (25.0) | 4 (10.8) |
One patient in the bleselumab 500 mg treatment group was excluded from table summaries owing to evidence of not taking study drug.
One patient in the bleselumab 50 mg group had a biopsy finding of minimal positive C4d staining and was counted as a biopsy‐proven acute rejection in Table S3. This was not reported as a treatment‐emergent adverse event, so the count of kidney transplant rejection is one less in the bleselumab 50 mg group in Table 3.
NODAT was detected in n = 2 of 7 at‐risk placebo patients (29%) and 9 of 30 at‐risk bleselumab patients (30%). NODAT was determined by identifying those who had oral hypoglycemic treatment for ≥30 consecutive days (4 bleselumab patients, 13.3%); those who reported insulin use for ≥30 consecutive days (3 of 30 bleselumab patients, 10%); those with a fasting glucose ≥126 mg/dL on two occasions at least 30 days apart (2 placebo patients, 29% and 4 active treatment patients, 13%); and those with a posttransplant HgbA1C ≥6.5% (2 active treatment patients, 6.7%). Some subjects met more than one criterion.
Changes in liver function tests were observed, which were generally not clinically significant. Abnormal liver function tests were experienced by 2 of 8 placebo patients (25%) and 4 of 37 bleselumab patients (11%; bleselumab 50 mg [n = 2/10], 100 mg [n = 1/9], and 500 mg [n = 1/8]) (Table 3).
Mean alkaline phosphatase and alanine aminotransferase generally increased from baseline in all treatment groups including placebo, beginning around Day 7. Mean γ‐glutamyl transpeptidase (GGT) increased from baseline to Day 90 for all treatment groups including placebo. The greatest increase in GGT from baseline generally occurred between Days 3 and 29 in all treatment groups. With the exception of the bleselumab 50 mg group, GGT values began to normalize by Day 60. Mean indirect and total bilirubin increased from baseline during the study for all treatment groups. At Day 90, mean indirect and total bilirubin was lower than at baseline for the placebo group but higher than baseline for all the active treatment groups. A notably high mean value for indirect bilirubin at Day 75 was observed in the bleselumab 50 mg group (5.6 μmol/L at baseline; 10.5 μmol/L at Day 75). Notably high mean values for total bilirubin were observed in the bleselumab 50 mg group at Day 7 (11.3 μmol/L) and Day 75 (11.6 μmol/L); in the bleselumab 100 mg group at Day 15 (10.3 μmol/L); and in the bleselumab 500 mg group at Day 7 (10.8 μmol/L), Day 15 (10.5 μmol/L), Day 60 (12.0 μmol/L), and Day 75 (10.3 μmol/L).
Four patients (one in each of the active treatment groups) had confirmed positive test results for the presence of antibleselumab antibodies at baseline (Table S5); three of the four patients still had confirmed positive results at Day 90. A single patient given bleselumab 50 mg tested positive on Day 7 for antibleselumab antibodies and did not have any other positive tests. Specific B cell and other lymphocyte subsets were not analyzed in this study; however, only minimal differences in total lymphocyte counts were observed between any of the bleselumab treatment groups compared with placebo (Table S6). No patient experienced a thromboembolic event during this study.
4. DISCUSSION
Treatment with various doses of bleselumab was not associated with significant immediate or long‐term side effects, evidenced by a lack of cytokine release and an absence of thromboembolic complications. This is significant owing to the history of thromboembolic complications with CD154 antibodies17. This is similar to findings in healthy volunteers.15 An important practical consideration for clinical use of bleselumab is that the treatment was reasonably well tolerated in this study. Infections, including opportunistic infections, occurred in those patients given placebo or bleselumab; however, there was one bleselumab subject who had CMV viremia. A phase 2 study of bleselumab 200 mg in combination with mycophenolate mofetil (MMF) or immediate‐release tacrolimus (IR‐TAC) vs standard of care (MMF + IR‐TAC) for the prevention of BPAR in kidney transplant recipients reported a trend of higher incidences of some infectious complications, such as CMV and BK virus infections, in patients treated with bleselumab vs standard of care (reported in this issue of American Journal of Transplantation [ClinicalTrials.gov NCT01780844]). There were no cases of posttransplant lymphoproliferative disease reported in this study.
A few patients had transient but not clinically significant elevations in liver enzymes. It will be important that these elevations are carefully monitored in future studies of bleselumab.
During the period of 90 days, no unexpected increases in BPAR occurred; however, evaluating efficacy with a single dose is difficult in patients receiving standard of care immunosuppression. No death or graft loss occurred in any of the patients. The pharmacokinetic data showed that C max increased dose proportionally but AUC increased more than dose proportionally, suggesting that bleselumab showed nonlinear pharmacokinetics. The mean t ½ of the two highest bleselumab doses, 200 mg and 500 mg, were 98 and 169 hours, respectively, suggesting that either dose could be given intermittently during long‐term use to achieve prolonged drug exposure. As expected, pharmacodynamic analyses demonstrated that bleselumab was a nonagonistic blocking antibody. B cell depletion did not occur in patients treated with bleselumab, which is consistent with another study.15 The current study also demonstrated no difference in total lymphocyte counts between the bleselumab‐treated and control groups. B cell CD40 receptor occupancy of ~80% was observed with all doses, but persisted longest in the bleselumab 200 mg and 500 mg groups at ~28 and 35 days, respectively. The exact relationship between the duration of receptor occupancy and efficacy cannot be ascertained from this study but could potentially be important. The pharmacokinetic data from this single‐dose regimen are supportive of the repeat use of bleselumab 200 mg in future studies.
The safety data derived from this phase 1b study are encouraging and a larger phase 2 study to assess both safety and efficacy is reported in this issue of American Journal of Transplantation (ClinicalTrials.gov NCT01780844). In nonhuman primate kidney transplant studies, bleselumab monotherapy prolonged graft survival but was more effective in combination with immunosuppressants as part of a CNI‐free regimen.18 Ultimately, a combination of agents, perhaps including a combination of costimulatory blockade with belatacept and an anti‐CD40 monoclonal antibody, may be required to achieve efficacy and induce a tolerogenic immune environment.
DISCLOSURE
FV reports receiving grant fees from Astellas Pharma Global Development, Inc. PB, AC, and VS are employees of Astellas Pharma Global Development, Inc. JH was an employee of Astellas Pharma Global Development, Inc. during the conduct of the study. GK, HY, and VRP have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
This study was funded by Astellas Pharma Global Development, Inc. and Kyowa Hakko Kirin Co., Ltd. Medical writing support in the development of this manuscript was provided by Matthew Reynolds and Esther Race from OPEN Health Medical Communications (London, UK), funded by Astellas Pharma Global Development, Inc.
Vincenti F, Klintmalm G, Yang H, et al. A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti‐CD40 monoclonal antibody, in kidney transplantation. Am J Transplant. 2020;20:172–180. 10.1111/ajt.15560
DATA AVAILABILITY STATEMENT
Studies conducted with product indications or formulations that remain in development are assessed after study completion to determine if individual participant data can be shared. The plan to share individual participant data is based on the status of product approval or termination of the compound, in addition to other study specific criteria described on http://www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas.”
REFERENCES
- 1. Riley JL, June CH. The CD28 family: a T‐cell rheostat for therapeutic control of T‐cell activation. Blood. 2005;105(1):13‐21. [DOI] [PubMed] [Google Scholar]
- 2. Elgueta R, Benson MJ, de Vries VC, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept‐based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3):535‐546. [DOI] [PubMed] [Google Scholar]
- 4. Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT‐EXT study). Am J Transplant. 2010;10(3):547‐557. [DOI] [PubMed] [Google Scholar]
- 5. FDA . Belatacept prescribing information. https://packageinserts.bms.com/pi/pi_nulojix.pdf. Accessed December 2018.
- 6. Kirk AD, Knechtle SJ, Sollinger HW, et al. Preliminary results of the use of humanized anti‐CD54 in human renal allotransplantation. Am J Transplant. 2001;1(S1):191 (abstract 223). [Google Scholar]
- 7. Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21(5):265‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee BO, Moyron‐Quiroz J, Rangel‐Moreno J, et al. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J Immunol. 2003;171(11):5707‐5717. [DOI] [PubMed] [Google Scholar]
- 9. Dong L, Wang S, Chen M, et al. The activation of macrophage and upregulation of CD40 costimulatory molecule in lipopolysaccharide‐induced acute lung injury. J Biomed Biotechnol. 2008;2008:852571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daoussis D, Antonopoulos I, Andonopoulos AP, et al. Increased expression of CD154 (CD40L) on stimulated T‐cells from patients with psoriatic arthritis. Rheumatology (Oxford). 2007;46(2):227‐231. [DOI] [PubMed] [Google Scholar]
- 11. Okimura K, Maeta K, Kobayashi N, et al. Characterization of ASKP1240, a fully human antibody targeting human CD40 with potent immunosuppressive effects. Am J Transplant. 2014;14(6):1290‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. André P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8(3):247-252. [DOI] [PubMed] [Google Scholar]
- 13. Haanstra KG, Ringers J, Sick EA, et al. Prevention of kidney allograft rejection using anti‐CD40 and anti‐CD86 in primates. Transplantation. 2003;75(5):637‐643. [DOI] [PubMed] [Google Scholar]
- 14. Pearson TC, Trambley J, Odom K, et al. Anti‐CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74(7):933‐940. [DOI] [PubMed] [Google Scholar]
- 15. Goldwater R, Keirns J, Blahunka P, et al. A phase 1, randomized ascending single‐dose study of antagonist anti‐human CD40 ASKP1240 in healthy subjects. Am J Transplant. 2013;13(4):1040‐1046. [DOI] [PubMed] [Google Scholar]
- 16. Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753‐760. [DOI] [PubMed] [Google Scholar]
- 17. Pinelli DF, Ford ML. Novel insights into anti‐CD40/CD154 immunotherapy in transplant tolerance. Immunotherapy. 2015;7(4):399‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song L, Ma A, Dun H, et al. A novel full human anti‐CD40 monoclonal antibody, ASKP1240, mono‐ and comibnation‐therapy in prolongation of renal allograft survival in Cynomolgus monkeys. Am J Transplant. 2011;11(S2):438 (abstract 1401). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Studies conducted with product indications or formulations that remain in development are assessed after study completion to determine if individual participant data can be shared. The plan to share individual participant data is based on the status of product approval or termination of the compound, in addition to other study specific criteria described on http://www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas.”


