Abstract
Developing efficacious vaccines for human malaria caused by Plasmodium falciparum is a major global health priority, although this has proven to be immensely challenging over the decades. One major hindrance is the incomplete understanding of specific immune responses that confer protection against disease and/or infection. While antibodies to play a crucial role in malaria immunity, the functional mechanisms of these antibodies remain unclear as most research has primarily focused on the direct inhibitory or neutralizing activity of antibodies. Recently, there is a growing body of evidence that antibodies can also mediate effector functions through activating the complement system against multiple developmental stages of the parasite life cycle. These antibody‐complement interactions can have detrimental consequences to parasite function and viability, and have been significantly associated with protection against clinical malaria in naturally acquired immunity, and emerging findings suggest these mechanisms could contribute to vaccine‐induced immunity. In order to develop highly efficacious vaccines, strategies are needed that prioritize the induction of antibodies with enhanced functional activity, including the ability to activate complement. Here we review the role of complement in acquired immunity to malaria, and provide insights into how this knowledge could be used to harness complement in malaria vaccine development.
Keywords: antibodies, complement, immunity, malaria, vaccines
1. INTRODUCTION
Despite major successes in reducing malaria burden over the past decade, malaria remains a substantial cause of morbidity and mortality, with over 200 million clinical cases and almost half a million deaths estimated to have occurred in 2017 alone.1 Malaria is caused by several Plasmodium spp. with P. falciparum accounting for the majority of cases and P. vivax being a second major cause.1 Other species, P. ovale, P. malariae, and P. knowlesi represent a small burden globally, but are important in some populations. Malaria control and elimination efforts have been spearheaded by currently available tools for surveillance, vector control, and drug treatment, but one intervention strategy lagging behind is the development of a highly efficacious malaria vaccine. Vaccine development has been immensely challenging, partly due to the complex biology and life cycle of Plasmodium spp. Briefly, infected mosquitoes deposit sporozoites into the skin of a human host prior to taking a blood meal. These sporozoites progress to establish infection in the liver, where they develop into merozoite forms that enter the blood. Merozoites invade red blood cells (RBCs) and undergo asexual replication, releasing newly produced merozoites that continue this cycle. Some parasites will develop into sexual‐stage forms known as gametocytes, which can be transmitted to mosquitoes, where they develop into infectious sporozoites. The long and complex life cycle of the parasite provides many opportunities for targeting by malaria vaccines (Figure 1). Over the past several decades, multiple approaches and vaccines have been developed and evaluated, mainly for P. falciparum, including subunit and whole parasite vaccines.2 However, very few have demonstrated significant reproducible efficacy in different populations and achieving high‐level efficacy in endemic populations has proved elusive, suggesting that new strategies and approaches in vaccine development may be needed.
Figure 1.
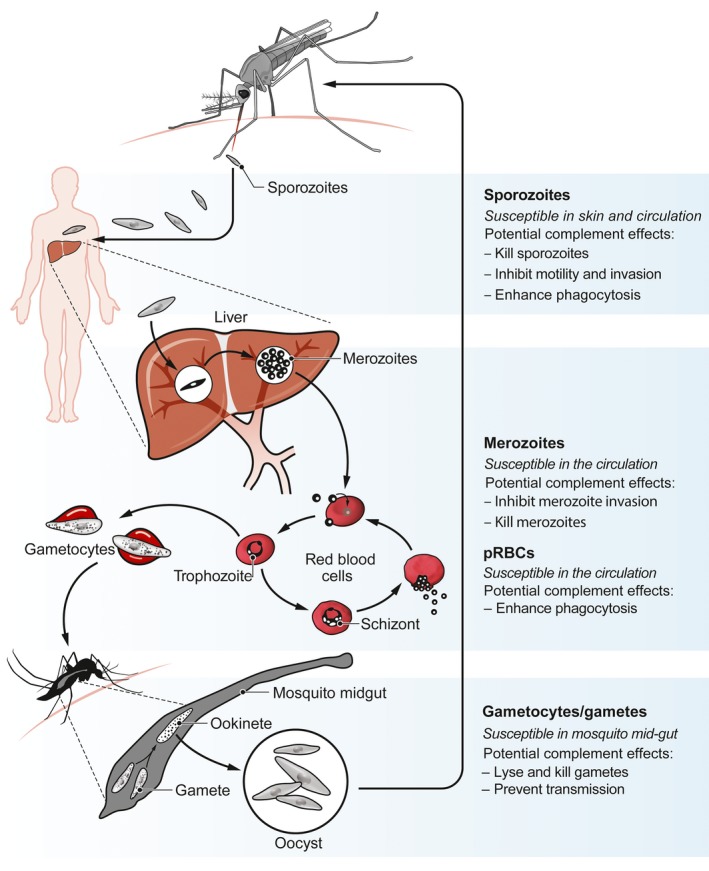
Plasmodium life cycle and opportunities for antibody‐complement attack. Plasmodium spp. has a complex life cycle within the human host, whereby several developmental stages of the parasite are known to be susceptible to antibody recognition, and subsequently complement fixation and activation. These include sporozoites, merozoites, parasitized red blood cells (pRBCs) and sexual‐stage parasites (gametocytes/gametes). Figure adapted from Beeson et al, 20192 (Copyright © 2019 the Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science)
A further challenge to developing efficacious malaria vaccines is that we have a limited understanding of the immune mechanisms that confer protection against infection and/or disease, and therefore, how to induce high levels of protective immunity through vaccination. There is a substantial body of evidence that antibodies targeting multiple developmental stages are important for immunity. Seminal studies performed in the 1960s demonstrated that transferring antibodies isolated from semi‐immune adults to clinically ill children could reduce parasitemia and malaria symptoms.3 Since this observation, we have identified that antibodies specifically targeting blood‐stage antigens are important for anti‐disease immunity, which prevents high density parasitemia and clinical illness, but does not necessarily prevent infection per se.4, 5 Individuals also naturally acquire antibodies to antigens expressed by sporozoites, although these are generally at lower levels, and it is unclear what role they play in malaria immunity.6, 7 However, murine studies have demonstrated an important proof‐of‐concept that naive animals administered with sporozoite‐specific antibodies can be completely protected against infection via mosquito bite challenge, and do not develop blood‐stage parasitemia.8 Collectively, these studies demonstrate that antibodies are important in malaria immunity. However, a common observation is that high concentrations of antibodies were required to confer protection in these experimental models.
A striking example of how this relates to vaccination strategies is the RTS,S malaria vaccine. RTS,S is based on the major sporozoite surface antigen, circumsporozoite protein (CSP) expressed as a virus‐like particle, and is co‐administered with the potent AS01 adjuvant. Children who receive the standard 3‐dose RTS,S vaccine regimen initially acquire very high antibody concentrations, although vaccine efficacy was only modest against clinical malaria.9 Considering RTS,S already induces maximal antibody titers, it seems unlikely that next‐generation vaccines could induce substantially higher antibody levels. Therefore, an alternative approach to enhancing vaccine efficacy is to focus on improving antibody functional activity rather than antibody titer.2, 10
One avenue for enhancing antibody function and protective efficacy is to harness Fc‐mediated functions, such as interactions with complement and Fcγ‐receptors on immune cells. Antibody‐dependent complement activation has been identified as an important mechanism against other pathogens, resulting in neutralization and lysis of the target cell.11, 12, 13, 14 There are several examples from our research and others that demonstrate a role for antibody‐complement interactions in immunity to malaria, which we will review here, and highlight potential opportunities to harness this knowledge for developing vaccines that induce greater protective efficacy.
2. COMPLEMENT FUNCTIONS AND ROLES AGAINST INFECTION
2.1. Complement activation pathways
The complement system is a large collection of serum proteins that act in a complex cascade of events, which results in various immunological functions (Figure 2). Complement can be initiated by three distinct pathways termed the classical, mannose‐binding lectin (MBL) and alternative pathways.15 Briefly, the classical pathway requires complement component 1 (C1) that is a complex containing the C1r and C1s serine proteases, and C1q that extends as a bouquet‐like arrangement with six globular head domains. Binding of C1q to immune complexes is referred to as C1q fixation, which activates the associated serine proteases, and initiates the classical activation pathway. Therefore, complement is considered both an adaptive and innate immune response that can act in an antibody‐dependent manner via C1q, or directly against pathogens via the MBL and alternative pathways as follows. The MBL pathway requires the MBL protein that also acts as a ligand recognition molecule and can bind to sugar molecules commonly expressed on pathogens, including bacteria and viruses. These interactions activate the MBL‐associated serine proteases to initiate the MBL pathway. Activation of the alternative pathway differs in that it does not involve a recognition molecule, but instead occurs by spontaneous hydrolysis of C3 and generation of the alternative C3 convertase molecule. Although all three complement pathways are initiated differently, they converge at the point of generating C3 convertase, which enables downstream complement activation events to occur.
Figure 2.
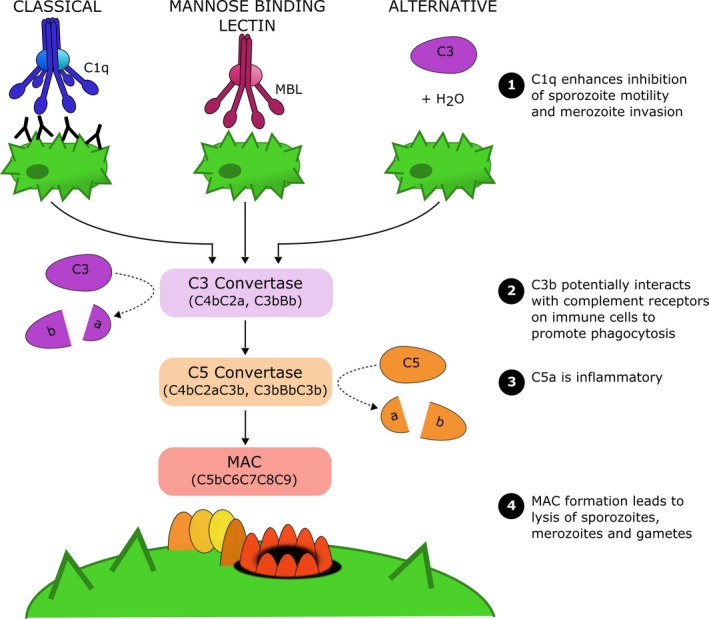
Complement activation pathways. The complement system can be activated by three distinct pathways termed the classical, mannose‐binding lectin and alternative pathways. Complement activation via any pathway leads to formation of C3 convertase, which cleaves C3 into the active C3a and C3b fragments. Subsequently, the C5 convertase molecule is generated, which cleaves C5 into the activate C5a and C5b fragments. C5b, together with C6, C7, C8 and multiple C9 monomers create the membrane attack complex (MAC) that deposits in the target cell membrane and causes cell lysis. Several complement proteins mediate anti‐malarial immunity, including: (1) C1q enhances antibody‐mediated neutralization of sporozoites and merozoites, (2) C3b can mediate opsonic phagocytosis and is a potential anti‐malarial immune mechanism, (3) C5a is pro‐inflammatory, and (4) MAC formation can lyse sporozoites, merozoites and gametes
The classical and MBL pathways both activate serine proteases, which cleave C4 and C2 into fragments that associate into the C4bC2a molecule also known as C3 convertase. The alternative pathway instead involves spontaneous C3 hydrolysis that associates with Factor B, which is then cleaved by Factor D to generate the alternative C3 convertase composed of C3bBb. Regardless of activation pathway, these C3 convertase molecules all function to cleave C3 into the activate C3a and C3b fragments. Interestingly, cleaved C3b can directly adhere to the target cell surface, and associate with Factor B to generate more alternative C3 convertase molecules, overall leading to increased cleavage of C3 and surface bound C3b fragments. These C3b fragments can also interact with the C3 convertase to generate a new complex known as C5 convertase (C4bC2aC3b or C3bBbC3b). The C5 convertase functions to cleave C5 into C5a and C5b, which is essential for the terminal phase of complement and formation of the membrane attack complex (MAC). Firstly, C5b will form a complex with C6 and C7 proteins, and adhere to the target cell. C5b‐C7 then interacts with C8, and multiple C9 monomers that polymerize and create a ring‐like structure that inserts into the cell membrane. The resulting MAC (C5b‐C9) creates a pore in the membrane, disrupting its integrity and leading to the death of the target cell.16
Overall, fixation and activation of complement can function against infecting pathogens in several ways. These include fixation of C1q that has been shown to enhance virus neutralization by antibodies,17 formation of the active C3b fragment on the pathogen surface that can promote complement‐mediated opsonic phagocytosis by monocytes and neutrophils, and formation of the MAC on the pathogen surface, leading to cell death and lysis.15 Due to the potent nature of complement, it is also important for excessive complement activation to be tightly controlled, and this is achieved by a series of membrane‐bound and soluble regulatory proteins. Furthermore, these regulatory proteins are also important to protect host cells against direct complement attack.
2.2. Complement effector functions against other pathogens
Complement plays a vital role in the immune response to multiple pathogens, particularly for bacteria and viruses.11, 12, 13, 14 Complement proteins can bind to viruses directly or via antigen‐specific antibodies, resulting in various consequences. These include neutralization and inhibition of viral infectivity (eg influenza,18 cytomegalovirus virus,19, 20 vaccinia virus21, 22), formation of the MAC and lysis (eg HIV‐1,23 influenza,24 herpes simplex virus,25 measles,26 mumps,27, 28 vaccinia virus21, 22), interactions with complement receptors on immune cells resulting in opsonic phagocytosis (eg herpes simplex virus29), and the formation of viral aggregates (eg influenza,30, 31 paramyxoviruses,28 polyoma32) that limit the number of viral units available for infection and can also promote phagocytosis. Even in the absence of MAC formation and lysis, complement can also directly enhance the neutralizing activity of anti‐viral antibodies by reducing the amount of virus binding antibodies required to inhibit infectivity.17, 33, 34
Similar to viruses, complement activation also leads to formation of the MAC and lysis of bacteria. For example, protective antibodies against non‐typhoidal salmonella in Malawian children have been associated with bacterial killing activity.35, 36 Additionally, formation of the active C3b fragment on bacterial surfaces can also enhance uptake by phagocytic cells,36, 37 and promote oxidative burst activity by monocytes and neutrophils.36
3. EVASION OF INNATE COMPLEMENT ACTIVATION BY MALARIA
3.1. Merozoites evade direct complement activation in the blood
Host cells use complement regulatory proteins for protection against complement attack; however, several pathogens, including Plasmodium spp. have also evolved mechanisms to recruit human regulatory proteins and evade the complement system.
Upon egress from schizonts, merozoites are released into the blood‐stream prior to invading a new RBC, and therefore exposed to blood components such as the complement system. Complement acts as a front‐line defense system against pathogens, and so evasion of direct complement attack is critical for merozoite survival in the blood. Merozoites can successfully invade RBCs in the presence of serum complement,38 and are able to evade complement activation via the recruitment of several host regulatory proteins to their surface.39, 40, 41 These include Factor H (FH) and Factor H‐like protein 1 (FHL‐1), which are key regulators of the alternative pathway and positive feedback loop. Upon binding to the merozoite surface, FH and FHL‐1 retain cofactor activity and can downregulate the alternative pathway of complement, thereby reducing complement lysis of merozoites.40 Using a panel of recombinant FH fragments, flow cytometry and immunofluorescence assays showed binding of complement control protein modules (CCPs) 5 and 6 of FH is key to the interaction with merozoites. The merozoite ligand responsible for FH and FHL‐1 recruitment is the merozoite surface protein Pf92, a member of the six‐cysteine protein family.40
C1 esterase inhibitor (C1‐INH) is a soluble complement regulator that regulates the classical and lectin pathways of complement activation. C1‐INH has also been shown to bind to the merozoite surface where it forms a complex with C1s, and the MBL‐associated serine proteases.42 C1‐INH interacts with the merozoite via another surface protein (MSP3), which alters complement fixation and subsequently enhances merozoite invasion into RBCs. The ability of P. falciparum to control alternative complement activation by recruiting human regulators is an important element of its immune evasion repertoire. New interventions to disrupt these parasite strategies could increase the efficacy of the human immune response.
The use of genetically modified merozoites that lack specific surface proteins is a powerful approach to define the direct effects of complement and the roles of specific merozoite proteins. Using this approach, we demonstrated the important role of merozoite surface protein Pf92 in complement regulation and evasion. These approaches can be applied using modified growth assays conducted over multiple invasion cycles or with invasion assays using isolated viable merozoites, both performed in the presence and absence of active human complement.38, 40 In particular, the latter method may be advantageous as isolated merozoites are directly exposed to complement without any temporal delay present in growth assays.
Further studies are needed to precisely define the real‐time kinetics of complement activation on merozoites and the impact on parasite invasion or lysis, which will require flow cytometric and live cell imaging techniques. In cancer research, complement activity is monitored using antibodies that recognize activated C3b as well as a cell viability dye to track cell lysis.43 Similar methods and reagents could be adapted to flow cytometric‐based assays using isolated merozoites, to determine the kinetics of complement fixation and activation, and cell lysis. Furthermore, the use of a fluorescent calcium sensor was recently described to image the rapid dynamics of calcium signaling during merozoites.44 We propose that a combination of these methods could be the best way to examine the complex interplay between complement fixation, merozoite viability, and merozoite invasion (as measured by calcium signaling). This would also allow direct observation and quantitation of whether particular evasion strategies allow successful RBC invasion and how death occurs when evasion fails.
3.2. Gametocytes evade human complement proteins in the mosquito midgut
During a blood meal, various host components are consumed by the mosquito including healthy uninfected and gametocyte‐infected RBCs, and serum proteins such as complement. These host serum proteins can remain stable in the mosquito midgut for some time. As the parasite develops, it is no longer shielded by the human RBC, and free gametes are now susceptible to human complement proteins in the mosquito midgut. However, gametes have adapted to bind human FH, to prevent activation of the alternative pathway and complement‐mediated lysis.41 This is an important mechanism of immune evasion, because when FH binding is disrupted, human complement significantly impairs parasite transmission to the mosquito.41
Notably, intracellular parasites such as gametocyte‐infected RBCs and parasitized red blood cells (pRBCs) are largely resistant to direct complement attack because of host regulatory proteins expressed on the RBC surface. This includes the membrane‐bound protein, CD59, which prevents MAC formation on the RBC surface and therefore prevents complement‐mediated killing.45 Interestingly, pRBCs have been shown to express higher levels of complement regulatory proteins overall, potentially to remain even more resistant against the direct effects of serum complement.46
4. MECHANISMS OF ANTIBODY‐COMPLEMENT INTERACTIONS AGAINST MALARIA
4.1. Sporozoites
Our group has demonstrated that immune antibodies fix and activate complement against P. falciparum sporozoites. Sporozoites incubated with immune antibodies (from residents of malaria‐endemic regions) had substantially increased amounts of C1q and C3 deposited, compared to that of sporozoites incubated with control non‐immune antibodies.7 Sporozoites rely on motility to successfully establish infection in the liver, including the traversal form of motility that enables active migration through impeding host cells. It is well established that antibodies can inhibit sporozoite traversal of hepatocytes, and we found inhibitory activity to be greatly enhanced by active human complement, especially when antibodies were tested at low concentrations (Figure 3A).7, 47 It is possible that C1q binding alone enhanced antibody‐mediated neutralization, or that additional downstream complement proteins were also involved. Notably, sporozoite lysis occurred in the presence of immune antibodies and complement, which was dependent on C5 and presumably formation of the MAC.7, 47 Another study suggested that immune IgM could mediate complement activity against sporozoites. However, complement activity was only measured as increased levels of soluble C5a (indicative of C5 cleavage), and it was not directly shown that complement bound to the parasite, and the functional effects of complement activation were not assessed.48 A previous study also suggested that sporozoites were susceptible to complement‐mediated lysis in the presence of antibodies, although lysis was indirectly measured as bleb formation using microscopy.49 Furthermore, in that study murine antibodies and murine complement were used, which have considerable differences to their human counterparts as discussed later in this review. Other studies have reported that antibodies can directly mediate cytotoxic effects against sporozoites independent of Fc‐dependent effector function, using monoclonal antibodies (MAbs) in a mouse model.50 However, the potential enhancing effects of complement were not assessed because the MAb used was a non‐complement fixing subclass (murine IgG1).
Figure 3.
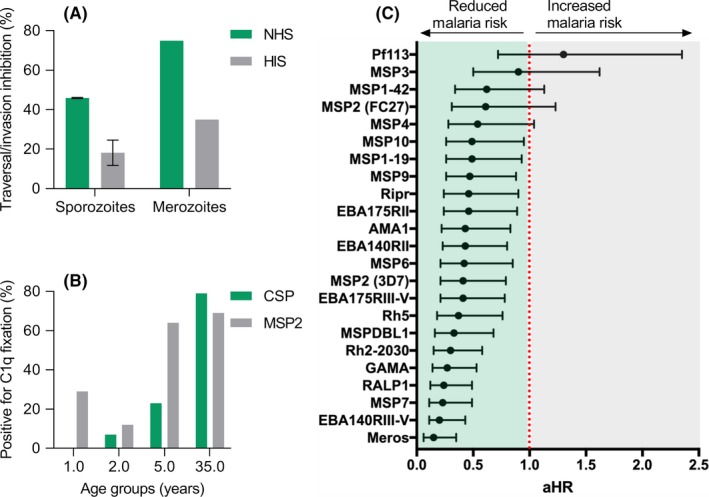
Naturally acquired human antibodies can fix and activate complement against P. falciparum sporozoites and merozoites, and are associated with protection. A, Sporozoites and merozoites were treated with immune antibodies from malaria‐exposed populations, and tested for sporozoite traversal of hepatocytes (HC‐04 cell line) or merozoite invasion of red blood cells in the presence of active (NHS) or inactive (HIS) human complement. Traversal/invasion inhibition by antibodies was greatly enhanced by active complement compared to inactive complement (mean and range is shown). Data were originally presented in Kurtovic et al, 2018 and Boyle et al, 2015.7, 38 B, Natural acquisition of C1q fixing antibodies to sporozoite and merozoite surface antigens (CSP and MSP2, respectively) among children and adults resident in Kenya, n = 64 (percentage positive is shown). Data were originally presented in Kurtovic et al, 2018.7 C, High levels of C1q fixing antibodies (compared to low levels of C1q fixing antibodies) to selected merozoite antigens are significantly associated with protection against clinical malaria in a longitudinal cohort study of Papua New Guinean children, n = 206 (adjusted hazard ratio is shown). Data were originally presented in Reiling et al, 201965
While our studies have only tested a limited number of antibody samples, we established an important proof‐of‐concept that P. falciparum sporozoites are susceptible to antibody‐mediated complement fixation and activation. Furthermore, we confirmed that the major sporozoite surface antigen, CSP, is an important target of complement fixation, demonstrated using multiple cohort studies of malaria‐endemic populations.7 There is also evidence that non‐CSP antibodies play a role, but the specific antigenic targets are yet to be identified.47 Previous studies of the murine and avian Plasmodium spp. suggested that sporozoites are resistant against lysis by host complement, but susceptible to non‐host complement activation via the alternative pathway.51, 52 Our studies similarly found that P. falciparum sporozoites were poorly susceptible to host (human) complement, but susceptibility increased in the presence of immune antibodies and activation of the classical pathway.7, 47 A similar effect has also been observed for the rodent pathogen P. berghei, whereby glycan‐specific antibodies, including IgG and IgM, recognized the sporozoite surface and protected mice against infection. These antibodies could activate host (murine) complement, which inhibited parasite motility and had cytotoxic effects, and protection appeared to be complement‐dependent.53 Therefore, it is likely that sporozoites have some mechanism to evade host complement attack, but it appears that antibodies of the right specificity and properties can overcome immune evasion through activation of the classical pathway.
4.2. Merozoites
Our studies established that antibodies targeting P. falciparum merozoites can activate the classical complement pathway via binding to C1q, which consequently inhibits merozoite invasion, and results in formation of the MAC and merozoite lysis.38 Human antibodies to merozoite antigens are predominantly comprised of IgG1 and IgG3, which are the most potent subclasses for complement activation. We found that the majority of antibody samples tested from malaria‐exposed subjects either required complement for effective inhibition of invasion in vitro, or had enhanced inhibitory activity in the presence of complement (Figure 3A). In the presence of merozoite specific antibodies, complement‐mediated lysis occurs rapidly, reaching maximum lysis in approximately 4 minutes.38 Kinetics studies suggest that 80% of invasion events by merozoites occur in the first 10 minutes,54, 55 indicating that there is sufficient time for complement to act against merozoites. Lysis appears to be even more rapid in the presence of immune IgM rather than IgG antibodies, suggesting that lysis may be a key mechanism of IgM mediated immunity.56 Some merozoite lysis does occur slowly over time in the presence of active complement alone and in the absence of antibodies.38 While formation of the MAC leads to lysis and killing of merozoites, we also found that fixation of C1q in the absence of other complement components could enhance the invasion‐inhibitory activity of antibodies.38 Viral pathogens can also be effectively neutralized by antibodies and C1q fixation, which was hypothesized to be mediated in part by steric hindrance.17, 33 Indeed, in the absence of complement, we and others have shown that some non‐neutralizing antibodies can be internalized into the RBC during merozoite invasion.57, 58, 59, 60 However, this has not been reported in the presence of active complement or C1q, supporting the conclusion that complement may prevent antibody internalization due to steric hindrance. In further support of the role of C1q and the classical complement pathway in malaria immunity, P. chabaudi models have demonstrated that C1q‐deficient mice were significantly more susceptible to secondary challenge with the same parasite strain.61 This may have been due to the loss of C1q fixation, or possibly downstream effects of complement activation.
Complement fixation at the merozoite surface may also have other implications, such as enhanced activation of phagocytic cells which express multiple complement receptors able to bind fixed complement proteins. Indeed, complement in the absence of antibodies is able to promote phagocytosis of merozoites by neutrophils,62 or THP‐1 monocyte cell line.63 However, neutrophil respiratory burst induced by immune antibodies and merozoites was reportedly not enhanced by complement,64 and further study is needed to understand whether immune antibodies to merozoites interact with complement to promote activation of immune cell responses.
Multiple P. falciparum merozoite antigens have been identified as targets of complement fixing antibodies,38, 65 and functional antibodies appear to be acquired with age (Figure 3B).7 These include merozoite surface antigens such as MSP1, MSP2, MSP6, MSP7, and MSP‐DBL,38 but also apical organelle proteins including vaccine candidates PfRH5 and AMA1, as well as EBA ligands, PfRH2, RALP1, and GAMA.65 Interestingly, antibodies to some antigens, and antibodies among some individuals, did not effectively fix and activate complement. This may reflect epitope specificity of antibodies, such that the density or spatial orientation of antibody binding was insufficient for complement fixation. Antibodies to some merozoite surface antigens (eg MSP2, MSP1‐block‐2) do not have substantial inhibitory activity on their own, and were only able to effectively inhibit invasion in the presence of active complement,38 suggesting that complement is essential for some antibodies to mediate functional activity. The rate of acquisition of antibodies to different merozoite antigens is known to vary,66 and this may impact on the acquisition of complement fixing antibodies and their role in immunity.
Little is known about the role of antibody‐complement interactions in immunity to P. vivax, or other species, but we recently identified that P. vivax merozoite surface protein, PvMSP3α, is also a target of complement fixing antibodies, indicating that antibody‐complement interactions are likely important across human infecting Plasmodium species.46, 67 However, because of key differences in merozoite proteins between species,68 we should be cautious about extrapolating findings from P. falciparum to P. vivax. Further studies are needed to define the key targets of complement fixing antibodies and the importance of complement for inhibition of invasion.
4.3. Parasitized red blood cells (pRBCs)
To support intra‐erythrocytic development, Plasmodium spp. modify the RBC membrane and express parasite‐derived proteins on the surface of pRBCs.69 Some of these proteins are significant targets of acquired antibodies that contribute to protective immunity.69 Only a handful of studies have explored the interactions between trophozoite stage pRBCs, anti‐malarial antibodies and complement. Early work showed that human immune serum (from malaria‐exposed donors) could opsonize pRBCs, and activate the classical complement pathway in a dose‐dependent manner.45, 70 More recently, our laboratory has also confirmed complement fixation on the surface of pRBCs in the presence of immune antibodies from malaria‐exposed populations, as detected using immunoblot and flow cytometric approaches (Opi et al, ASTMH 66th annual meeting, 2017, Baltimore). Interestingly, while complement activation occurs to some extent, complement failed to result in lysis of the target cell, most likely due to the expression of MAC‐inhibitory protein CD59 on the RBC surface45, 70 (Opi et al, ASTMH 66th annual meeting, 2017, Baltimore). However, antibody‐mediated complement fixation appears important to opsonize pRBCs for phagocytic uptake and degradation by monocytes.71
PfEMP1 is a major parasite‐derived antigen expressed on the surface of pRBCs, and is also an important target of naturally acquired immunity.69 We have demonstrated that PfEMP1 is the major target of complement fixing antibodies using transgenic P. falciparum parasites with impaired PfEMP1 expression72 (Opi et al, ASTMH 66th annual meeting, 2017, Baltimore). Furthermore, we confirmed that antibodies fixed complement directly to recombinant PfEMP1 using plate‐based detection approaches (Opi et al, ASTMH 66th annual meeting, 2017, Baltimore). On the contrary, a recent study demonstrated that immune antibodies mediated complement fixation on recombinant PfEMP1, but showed no evidence of complement fixation on native PfEMP1 expressed on the pRBC surface, suggesting that P. falciparum might have developed mechanisms to evade complement fixation.73 However, only one MAb and a polyclonal antibody pool targeting PfEMP1 was used in that study. It is possible that only antibodies of certain specificities and properties effectively fix and activate complement. Therefore, this functional activity may be uncommon in some populations or subgroups. Additionally, PfEMP1 binding to non‐immune IgM, a common phenotype of severe malaria isolates, was shown to target the same binding site as C1q, possibly to limit C1q fixation and downstream complement attack.74
4.4. Gametocytes
After invading RBCs, a small portion of merozoites undergo commitment to develop into sexual‐stage gametocytes, which reside within the RBC until maturity. These gametocyte‐infected RBCs are generally poorly recognized by naturally acquired antibodies in humans and they do not express PfEMP1 on the RBC surface.75 However, recent studies have identified several antigens on the surface of gametocyte‐infected RBCs that could be targeted by immunity.76 During a blood meal, mosquitoes take up gametocyte‐infected RBCs along with other serum components such as antibodies and complement proteins. It is within the mosquito midgut that gametocytes emerge from the RBC to form gametes, and become directly exposed and susceptible to recognition by human immune components. These antibody responses can be measured using standard membrane feeding assays (SMFA), where mature gametocyte‐infected RBCs are incubated with immune antibodies, and then fed to mosquitoes through an artificial membrane feeder. Mosquito midguts are dissected a week later and the number of oocysts per midgut is enumerated to determine transmission‐blocking activity. Early studies reported that the transmission‐blocking activity of antibodies was greatly enhanced by human complement, for P. falciparum and P. vivax.77, 78, 79 This enhancement was likely the result of complement activation and subsequent complement‐mediated lysis, which has been observed against both gametes and zygotes using microscopy‐based approaches.77, 80, 81, 82
The leading antigenic targets for transmission‐blocking vaccines are Pfs230 and Pfs48/45, which are expressed on the surface of P. falciparum gametocytes and gametes. Pfs230 appears to be an important target of complement fixing antibodies, and to date, all studies of Pfs230 MAbs report a crucial role for complement in blocking parasite transmission.78, 81 This has been demonstrated using murine Pfs230 MAbs of different IgG subclass, whereby only the functional subclass in combination with complement was able to block parasite transmission in SMFA, and the non‐functional subclass (murine IgG1) demonstrated no inhibitory activity.
4.5. Antibody factors that influence complement fixation and activation
There are several antibody properties that can influence the ability to fix C1q and activate complement. Notably there are six C1q globular head domains in the C1 complex, and multiple subunits must be engaged to initiate the classical complement pathway. Therefore IgM can potentially activate complement as it naturally occurs in pentameric or hexameric forms, and so only one IgM molecule is needed to bind multiple C1q subunits and activate complement. These C1q binding sites are located within the Cμ3 domain of the IgM Fc region,83 but are only accessible upon antibody stabilization in antigen‐antibody complexes.15 Our recent studies showed that naturally acquired IgM against merozoites was substantially more potent at activating complement fixation than IgG.56
Complement can also be activated by the IgG isotype, and the C1q binding site is always accessible regardless of antigen binding.15 However, the affinity of C1q to monomeric IgG is weak compared to immune complexes, and so multiple IgG molecules are required to activate C1.84, 85 The cytophilic IgG subclasses, IgG1 and IgG3, can potently activate complement (IgG3 has greater activity than IgG1), whereas IgG2 has lower activity, and IgG4 does not activate complement.86 This variability can be explained by differences in the C1q binding site located within the CH2 domain of the IgG Fc region,87 whereby specific mutations within this region can dramatically alter the ability to activate complement.88, 89, 90, 91, 92 Another important property is the hinge region, which provides a flexible spacer between the Fc region and antigen binding fragment (Fab).93 Of the IgG subclasses, IgG3 has the largest hinge region and is considered the most potent at activating complement.94 While increased flexibility does not correlate with complement activation per se,95 it is likely advantageous in accommodating the binding of multiple IgG3 molecules to multiple C1q subunits, which is required for C1 activation. Interactions between neighboring IgG molecules also appears favorable, and formation of these hexamer‐like structures results in increased complement activity.85 Specific point mutations in the Fc region can promote hexamer formation of IgG molecules, and these antibodies have enhanced complement activity and are being explored as cancer therapeutics.96
Apart from antibody isotype and IgG subclass, there are additional properties that can influence antibody function. These include antibody polymorphisms that are referred to as allotypes; IgG1 and IgG2 have several allotypes, IgG3 has 13 allotypes and IgG4 has none. IgG allotypes can differ between populations, and are likely to alter antibody property and functionality. One striking example is that a minor change in the amino acid sequence of IgG3 dramatically affects antibody half‐life,97 and other studies have reported differences in Fc‐dependent effector functions between the IgG1 allotypes in vitro.98, 99 Direct comparisons between all allotypes has not been extensively studied, but this would be valuable in future studies, particularly because allotype can influence susceptibility to malaria.100 IgG molecules also contain a conserved glycan and there are various glycoforms that have differing abilities to activate complement.101, 102, 103 Interestingly, the epitope specificity of antibodies can also greatly affect function. Studies comparing MAbs targeting CD20 showed varying abilities to activate complement, and this was attributed to differences in the specific epitope that was recognized.104 It has also been demonstrated that antigen distance from the cell membrane plays a role, and in particular, antigens presented closer to the cell membrane had greater complement activation by antigen‐specific antibodies.105
The importance of these antibody properties for complement fixation in malaria immunity remains largely undefined. IgG1 and IgG3 comprise the majority of IgG responses to malaria68 and these broadly correlate with complement fixation against merozoites and sporozoites.7, 38, 65 Initial studies found no relationship between differences in antibody avidity and complement fixing activity for merozoite responses among individuals, possibly because antibody avidity was already above the threshold required for effective complement fixation.65
5. CONTRIBUTION OF ANTIBODY‐MEDIATED COMPLEMENT ACTIVITY IN IMMUNITY
5.1. Pre‐erythrocytic stage immunity
Individuals are known to acquire antibodies to sporozoites following natural malaria exposure,106 but there is no strong relationship between acquired antibodies and clinical outcome. Indeed, we and others have not found significant associations between antibodies targeting sporozoite surface antigens (including CSP) quantified using ELISA, and protection against malaria in naturally exposed cohort studies.6, 7, 107, 108 However, we found that some children resident in Papua New Guinea had naturally acquired functional anti‐CSP antibodies that could fix complement proteins. While this response was uncommon, the children who had acquired high levels of C1q fixing antibodies had a significantly reduced risk of malaria during the 6 months follow‐up compared to those with no detectable C1q fixing antibodies.7 This was the first identification of a functional antibody response to CSP that demonstrated some association with protection against malaria in naturally exposed populations. It is possible that functional antibodies may reduce the number of sporozoites that successfully invade the liver and therefore reduce parasite burden and disease, especially in combination with responses to blood‐stages.
5.2. Blood‐stage immunity
Antibodies play a key role in naturally acquired immunity against the blood‐stages of infection, and there is a growing understanding of the functional effector mechanisms involved.68, 109 While direct inhibition of RBC invasion has been demonstrated for both naturally acquired and vaccine‐induced antibodies, there has not been a consistent relationship with protection from infection or clinical disease among published data for this functional activity.65, 110, 111, 112, 113, 114 More recently, evidence has started to emerge that antibody interactions with complement and Fcγ‐receptors are likely to play a role in acquired immunity against merozoites.38, 64, 65, 115 A higher magnitude of complement fixing antibodies against whole merozoites among children in a malaria‐endemic region were strongly associated with protection against clinical malaria, and with a lower incidence of high density parasitemia.38 Of note, complement fixing antibodies were more strongly associated with protection than total IgG or IgM levels, and more strongly than functional activity quantified in growth‐inhibition assays, which is the current reference assay for evaluating merozoite antigen vaccines.56, 65 These findings suggest that complement fixing antibodies should be evaluated in merozoite vaccine development.
Merozoite antigens including surface antigens and apical organelle proteins were identified as targets of complement fixing antibodies, and the relationships between these functional antibodies and acquired protective immunity were investigated (Figure 3C).65 In a longitudinal cohort study of children, complement fixing antibodies to several antigens were strongly associated with protection. In some cases (eg EBA140RIII‐V, EBA175RII, MSP2, MSP4, MSP7) the protective association was stronger for complement fixing antibodies than observed for standard IgG reactivity using ELISA.108 Notably, total IgG measured using standard ELISA only reflects the presence of antibodies and does not discriminate antibodies by their effector functions. While high levels of antibodies can correlate with protection, we identified that for some antigens, the measure of functional complement fixing antibodies was more strongly associated with protection against malaria. Our findings highlighted several less‐studied merozoite antigens as important targets that warrant further evaluation as vaccine candidates. Overall, there were good correlations between the presence of antibodies and complement fixation, but complement fixation was very low for some antigens.65 This emphasizes that the presence of IgG does not necessarily mean we will also observe complement fixation, and so it is important to specifically measure antibody effector functions such as complement fixation. In our studies, we observed a strong correlation between C1q fixation by antibodies and the formation of the MAC, which indicated that complement‐induced lysis was likely to be a contributing effector mechanism. We also found that combining complement fixing antibody responses against as few as three specific antigens gave strong protective associations (>95% predicted protective efficacy), supporting the concept of a multi‐antigen vaccine approach. Antigens that were prominent in the most protective combinations included MSP7, EBA140, RALP1, PfRipr, PfRH5, and PfRH2. Antibodies also contribute to parasite clearance after drug treatment,116 and high levels of complement fixing antibodies have been associated with faster parasite clearance.117
While our studies and others of human immunity support a role for complement fixation against merozoites in mediating protection from malaria, this relationship is less clear in murine models. A study with P. chabaudi and P. yoelii mouse infection models and cobra venom factor to deplete complement activity suggested that antibody‐mediated immunity was not dependent on complement in these models.118 However, this approach would not have excluded potential activity of C1q, which we have found can alone inhibit the invasion of P. falciparum merozoites to RBCs using in vitro cultures.38 As further discussed in the following section, murine models have significant limitations when studying complement because of their much lower complement activity, and key differences in the biology and infection kinetics of rodent malaria species used. Others reported that a mouse MAb to MSP1 (19kDa C‐terminal domain) could enhance invasion in the presence of complement, although the absolute effect size was generally small.119, 120 This might suggest that some specific antibodies can interact with complement to enhance invasion.
There are very little data on antibody‐complement interactions targeting pRBCs and immunity to malaria. Our initial studies on malaria in pregnancy showed that complement fixing antibodies to placental‐binding PfEMP1 on pRBCs were associated with reduced placental parasitemia (Opi et al, ASTMH 66th annual meeting, 2017, Baltimore). Further studies are needed to understand whether this is an important mechanism in malaria immunity. Antibodies to PfEMP1 expressed on pRBCs are associated with immunity to severe malaria,121 and complement interactions could potentially play a role in contributing to immunity. However, excessive and widespread complement activation, via multiple activation pathways, has also been associated with pathogenesis of severe malaria.122 Therefore, understanding the balance between complement activation targeted specifically to pRBC surface antigens and system complement activation will be important to completely understand immunity and pathogenesis of severe malaria.
5.3. Sexual‐stage immunity
Many individuals living in malaria‐endemic countries develop antibodies against antigens expressed during the sexual developmental stages of P. falciparum. However, data on the importance of antibody‐complement interactions in providing transmission‐blocking immunity in population studies are currently limited. Naturally acquired antibodies have been shown to recognize Pfs230,123 which is a known target of antibody‐complement interactions that appears important in blocking parasite transmission to the mosquito.78, 81 Furthermore, acquired antibodies to Pfs230 are mostly of IgG1 and IgG3 subclass,80 which have strong potential to fix complement, although this has not been investigated in detail. Interestingly, human serum depleted of antibodies to Pfs230, and another important target Pfs48/45, still demonstrated transmission‐blocking activity using SMFA, suggesting that antibodies to other antigens were also involved.124 Indeed, antibodies are also naturally acquired to other antigens including members of the 6‐cysteine‐domain family Pf12, Pf38 and Pf41,66, 108 although complement fixation by antibodies to these antigens appeared limited in preliminary studies.65 Studies of P. vivax transmission‐blocking immunity have been more limited, but suggest that a high proportion of exposed individuals develop transmission‐blocking antibodies, and activity is highly dependent on complement.79
5.4. Challenges with animal models to study complement‐mediated immunity
Our group has primarily investigated the functional effects of antibody‐complement activity against P. falciparum in vitro using a combination of human and animal antibodies, and human complement proteins or fresh human serum.7, 38, 47, 117 We have developed reproducible methods that measure the effects of antibody‐complement interactions on P. falciparum viability and parasite function (such as sporozoite traversal and merozoite invasion). An additional approach to define the roles of antibody‐complement interactions is using animal models of malaria. However, there are important differences in complement activity and immune function between humans and experimental animal models, and between Plasmodium spp. causing human malaria and those used in animal models. These differences limit our ability to extrapolate findings from animal models to understanding human immunity.
Mice are the most widely used model in malaria research, typically with rodent malaria species such as P. berghei, P. chabaudi, or P. yoelii. Murine antibodies include the IgG1, IgG2a, IgG2b, and IgG3 subclasses, which differ substantially in function from human IgG subclasses (IgG1‐4). While IgG1 is the most prevalent subclass in both mice and humans, and IgG1 is typically the most prominent response to malaria infection and vaccines in mice, murine IgG1 does not activate complement. Instead, complement fixation and activation is primarily mediated by murine IgG3, as well as IgG2a and IgG2b depending on antigen specificity.125 This contrasts the human immune response, which is dominated by IgG1 and IgG3 that are potent complement activators. This is particularly important for studies that use MAbs of a murine IgG1 backbone (the most commonly used subclass of murine antibodies), because these antibodies have no complement activity, and therefore the potential role of complement cannot be appropriately evaluated. An additional limitation is that complement activity of laboratory mice used in these models is very low compared to human complement (<5% of human activity), and so the potential importance of complement is unlikely to be observed in these models.126 This is also an issue in other infectious diseases. For example, Salmonella is highly susceptible to antibodies and complement‐mediated killing in humans, whereas these bactericidal effects are poorly observed in the presence of mouse complement.127 For Salmonella infections, murine models are poorly indicative of immune mechanisms that are known to be important in humans.
The ability of complement to lyse opsonized target cells has been directly compared using serum from various species.128 Murine complement demonstrated almost no cytotoxic activity, even when target cells were optimized with either human or murine (murine IgG2a) antibodies. Notably, complement‐mediated lysis was strongly observed by human serum, but also serum from guinea pigs and rats.128 Since several rodent Plasmodium spp. can infect rats,129 we propose that further investigation of this model is warranted to aid the evaluation of malaria vaccines and the potential role of antibody‐complement interactions.
A further issue is that Plasmodium spp. that are typically used in animal models have significant differences in antigens expressed, biological processes, and the kinetics and time‐course of infection and disease compared to human malaria. Nearly all malaria vaccine candidate antigens differ substantially in sequence, and often structure, in species used in animal models, and many lead antigens for human malaria vaccines are not present in rodent and monkey malarias. For example, P. falciparum merozoite vaccine antigens PfRH5, PfRipr, MSP2, and MSP3 are not present in rodent and monkey Plasmodium spp. The major antibody epitope (NANP‐repeat) of P. falciparum CSP, the most advanced vaccine candidate antigen, is not present in other malaria species. To get around this, models using rodent Plasmodium spp. that are genetically modified to express P. falciparum antigens or epitopes have been developed, as have immunodeficient mice engrafted with human cells. However, such models still have significant limitations in recapitulating human immunity. Therefore, careful linkage between human studies and animal models is required.
6. COMPLEMENT‐MEDIATED MECHANISMS IN VACCINE DEVELOPMENT
6.1. Malaria vaccines and complement
Currently there are no malaria vaccines available for population‐level vaccination programs, although several promising candidates are in development. The leading candidate, RTS,S, showed significant, but modest, efficacy in a large phase III clinical trial9 and has now commenced pilot implementation in three African countries.130 Developing highly efficacious malaria vaccines has been challenging, partly because we do not completely understand the specific targets and mechanisms of immunity that are essential for protection against infection and disease.2 There has been a major focus on the direct role of antibodies in vaccine development, but little investigation into other functional antibody mechanisms, including the ability to activate complement. As reviewed here, there is a growing body of evidence that antibodies targeting various stages of the Plasmodium life cycle can activate complement, and these functional antibodies play a role in protective immunity against malaria.
The RTS,S vaccine is based on a truncated form of CSP, including only the central repeat and C‐terminal regions of the protein. RTS,S has consistently demonstrated modest efficacy against clinical malaria in field evaluations of children and infants (approximately 30%‐50%), which is largely attributed to the induction of anti‐CSP antibodies.131 However, protective efficacy and antibody titer rapidly wane after vaccination. Efforts to improve RTS,S vaccine design, delivery, or formulation will be important to enhance vaccine efficacy and longevity. Our group recently identified that RTS,S‐induced antibodies in children were capable of fixing and activating human complement, and antibodies targeting both regions of CSP included in the RTS,S construct appeared favorable for complement fixation.132 We also evaluated the longevity of functional complement fixing antibodies, and found a rapid decay after the final vaccine dose, which was significantly associated with the waning of anti‐CSP IgG1 and IgG3 antibodies (Figure 4).132 Therefore we speculate that improving the induction and longevity of these cytophilic antibodies, particularly IgG3, may enhance complement fixing activity and vaccine durability overall. Interestingly, in a study of naturally acquired immunity in children, we found that complement fixing antibodies to merozoites were well maintained over time,133 suggesting that achieving sustained functional antibodies is possible.
Figure 4.
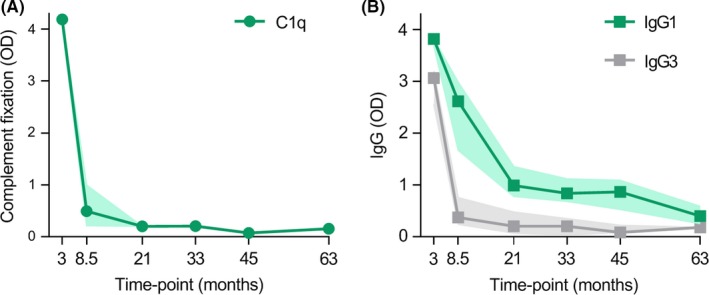
The RTS,S malaria vaccine induces complement fixing antibodies that rapidly wane over time. Children resident in Mozambique were vaccinated with RTS,S, and antibody responses were evaluated 30 d after the third and final vaccine dose (month 3) and at later time‐points, n = 30. Decay of (A) functional anti‐CSP antibodies that fix C1q, and the decay of (B) anti‐CSP antibodies of the IgG1 and IgG3 subclass (median and 95% CI of the median is shown). Data were originally presented in Kurtovic et al, 2019132
The dynamic relationship between the different IgG subclasses is particularly interesting, and often overlooked in vaccine studies. RTS,S primarily induces IgG1, moderate IgG2 and IgG3 and little IgG4 in field studies of children,132 and a higher ratio of cytophilic (IgG1 and IgG3) to non‐cytophilic (IgG2 and IgG4) antibodies has been associated with protection.134 This was possibly due to IgG1 and IgG3 having enhanced functional activities such as activating complement, or because there was less IgG2 and IgG4 that may be inhibitory to complement activation. This concept was demonstrated using murine subclass‐switched MAbs to non‐malaria antigens, whereby functional murine antibodies (IgG2a, IgG2b, and IgG3) had reduced complement activity in the presence of non‐functional murine antibodies (IgG1).135 These MAbs all bound the same epitope, and so it was suggested that reduced complement activity may have been due to steric interference between the subclasses. Therefore, a greater induction of IgG1 and IgG3 by RTS,S may be favorable, but also a lower induction of IgG2 and IgG4 that potentially inhibit or reduce functional responses.
Vaccination with whole sporozoites can confer a very high level of protection (>90%) against experimental infection challenge via infectious mosquito bite using the same strain as the vaccine strain. This has been achieved through two approaches: (a) participants are inoculated with viable sporozoites in combination with chemo‐prophylaxis (CPS) that treats any blood‐stage parasitemia, and so only the asymptomatic, pre‐erythrocytic stage of infection can occur,136 or (b) repeated vaccination using radiation‐attenuated sporozoites that infect hepatocytes but cannot develop into blood‐stage infection.137 CPS vaccination is highly efficacious against experimental infection with the homologous P. falciparum strain, and provides a valuable vaccine model to study malaria immunity in humans.136 Indeed, CPS vaccination strongly induces antibodies to CSP and other antigens, which can fix and activate complement against sporozoites.47 Furthermore, these antibody‐complement interactions greatly inhibited sporozoite traversal, and also led to sporozoite death in vivo.47 However, the role of antibody‐complement interactions in protection remains unclear in this model and will need to be investigated in a larger trial.
There have been limited studies on the induction of complement fixing antibodies by blood‐stage vaccines in humans. However, we demonstrated that vaccination with P. falciparum MSP2 can induce complement fixing antibodies in humans, which inhibited RBC invasion in a complement‐dependent manner.38, 138 For other P. falciparum blood‐stage vaccines that have progressed to clinical trials, such as AMA1, PfRh5, and MSP1, studies have primarily focused on direct inhibitory antibodies, and for MSP3 and GLURP functional assays focussed on antibody‐monocyte mediated immunity.4
While some studies have shown in animal models that passive transfer of MAbs lacking complement fixing activity (through mutation of the Fc region) can protect against experimental malaria infections,139 it is difficult to extrapolate the potential role for vaccine‐induced antibody‐complement immunity from such studies. Vaccination generates polyclonal antibodies to multiple epitopes that may better fix and activate complement than a single MAb. Furthermore, a polyclonal response in humans will target multiple epitopes, and the epitope specificity of responses can vary substantially between individuals.138, 140, 141 Therefore, the specificity and function of a single MAb is likely to only represent a small proportion of the human vaccine response. Careful analysis of the function and specificity of MAbs and human polyclonal antibody responses will be important for defining mechanisms and targets of immunity to inform optimal vaccine design.
6.2. Malaria immunity by passive immunization using recombinant antibodies
Protective immunity against malaria could also be achieved by passive immunization strategies rather than active immunization strategies. One example is to passively transfer an antibody‐based therapeutic that would confer protection for a limited period of time. Antibody‐based therapeutics have been primarily investigated against cancer and autoimmune diseases, but are also becoming of interest for use against infectious diseases such as influenza, HIV, and more recently for malaria.10, 86, 142 Antibody half‐life is regulated by interactions with the neonatal Fc receptor (FcRn), and based on these interactions, antibodies can be engineered to be longer‐lived and persist for several months.143 Therefore, antibodies could potentially be used to confer protection for the duration of peak malaria seasons in endemic populations where malaria is highly seasonal.
There has been a large body of research investigating passive antibody transfer and protection against malaria in animal models, using antibodies specific to sporozoite and merozoite targets (including CSP and MSP1).144, 145 Recently, the protective effect of a PfRh5 MAb engineered (by Fc region mutations) to not engage complement or mediate other Fc‐dependent effector functions, was investigated in non‐human primates.139 The passive transfer of these mutant antibodies was protective against malaria challenge, demonstrating that neutralizing antibodies can be protective in the absence of Fc‐dependent effector functions.139 However, very high concentrations of antibodies were required for protection, and the authors did not compare the protective effects of the non‐functional mutant antibody with the functional wildtype antibody. It is possible that antibody‐mediated protection may have been enhanced by active complement, especially if polyclonal antibodies are used that target multiple epitopes. Indeed, PfRH5 is a target of naturally acquired IgG1 and IgG3 antibodies with complement fixing activity,65 and complement fixation was associated with protection from malaria in children.65, 146
Of note, antibody‐based therapeutics against some forms of cancer identified an important role for complement‐mediated cytotoxicity, and therapeutic antibodies can be engineered to enhance interactions with neighboring IgG molecules and the formation of hexameric structures.85 These hexamer antibodies have potent complement activity in vitro, and may be an enhanced therapeutic in humans.96
6.3. Strategies for enhancing complement activity
There is a large body of evidence that antibodies can directly inhibit parasite function and afford some level of protection against malaria. However, we and others have demonstrated that antibody inhibitory activity against malaria sporozoites, merozoites, and transmission stages in humans can be greatly enhanced by complement. Many antigens expressed during multiple stages of the life cycle have been identified as targets of antibodies with strong complement fixing activity (Table 1). Therefore, a potential approach for malaria vaccine development is to enhance the induction of functional antibodies that activate complement to improve vaccine efficacy.
Table 1.
Antigenic targets of complement fixing antibodies for P. falciparum
| Life cycle stage | Target antigena | Naturally acquired | Vaccine‐inducedb |
|---|---|---|---|
| Sporozoites | CSP | Y | Y |
| Merozoites | MSP1‐19 | Y | |
| MSP1‐42 | Y | ||
| MSP2 (3D7) | Y | Y | |
| MSP2 (FC27) | Y | Y | |
| MSP3 | Y | ||
| MSP4 | Y | ||
| MSP6 | Y | ||
| MSP7 | Y | ||
| MSP9 | Y | ||
| MSP10 | Y | ||
| MSP‐DBL1 | Y | ||
| Ripr | Y | ||
| GAMA | Y | ||
| RALP1 | Y | ||
| AMA1c | Y | ||
| EBA140 R2 | Y | ||
| EBA140 R3‐5 | Y | ||
| EBA175 R3‐5 | Y | ||
| EBA175 R2 | Y | ||
| Rh2‐2030 | Y | ||
| PfRh5 | Y | ||
| Pf113 | N (poorly acquired) | ||
| pRBC | PfEMP1 | Y | |
| Gametocyte/gamete | Pfs230 | Y |
Abbreviations: CSP, circumsporozoite protein; pRBC, parasitized red blood cells.
Only antigens that have undergone substantial investigation as targets of complement fixing antibodies are listed here. Many antigens have not yet been evaluated.
At the time of writing, published data on vaccine‐induced complement fixing antibodies were only available for CSP and MSP2. Other antigens have not yet been evaluated.
AMA1 is also expressed by sporozoites and could, therefore, be a target of complement fixing antibodies to sporozoites.
We have already discussed various factors that can influence complement activity to be considered in developing potent malaria vaccines. These include selection of specific epitopes or combinations of epitopes in the vaccine design to most effectively induce antibodies that fix and activate complement. Further, a strong understanding of the IgG subclass response induced by selected vaccine adjuvants and constructs is required since manipulating this response can enhance complement activity, such as promoting IgG3 responses. Importantly, the induction of functional and non‐functional antibodies needs consideration, as high levels of IgG2 and IgG4 antibodies may negatively influence complement activity by having a potential competing effect with IgG1 and IgG3. On a final note, individual differences in host complement proteins could also be important, and identifying common complement deficiencies or polymorphisms in certain populations may also contribute to understanding the efficacy of a complement‐dependent vaccine. Recent studies have revealed a role for IgM in complement‐mediated activity against merozoites and sporozoites.47, 56 Whether IgM induction can be exploited to enhance complement‐mediated immunity remains unknown, but warrants further investigation.
6.4. Lessons learned from other vaccines
Some studies have reported an important role for complement in vaccine‐induced immunity of non‐malaria pathogens, and understanding these mechanisms may provide insights into developing effective malaria vaccines. Indeed, antibody‐complement interactions have been associated with protection in a number of studies for both licensed vaccines and promising candidates against infectious diseases including meningococcal and HIV‐1 infection. Antibody‐mediated complement activation and killing is an important protective mechanism against bacteria,147 and measuring this response using serum bactericidal assays (SBAs) is routinely used as a gold standard correlate of protection in naturally acquired immunity and vaccine studies against multiple bacterial isolates (eg Neisseria meningitides,148, 149, 150 Haemophilus influenza,151, 152 Salmonella spp,153 Shigella,154 and meningococcal infections148, 149, 150). Identifying this functional correlate of protection has proven to be powerful, as the development of the currently licensed vaccines against N. meningitidis serogroups B and C were developed based on complement‐mediated killing activity measured via SBAs, rather than performing clinical trials to evaluate protective efficacy.147, 155 Similar to Plasmodium spp., N. meningitidis can also evade the alternative activation pathway of complement. N. meningitidis expresses several surface proteins that can recruit FH to the bacterial surface to downregulate alternative complement activation and therefore evade complement‐mediated killing.156, 157, 158, 159, 160, 161 These FH‐binding proteins can be targeted by antibodies to inhibit FH recruitment,156 and are the basis of several meningococcal vaccines.162, 163 This includes the 4CMenB vaccine, which is part of the routine infant immunization program in a number of European countries, and confers 83% efficacy over one year after two doses, and might offer cross‐protection to other isolates.164 Considering P. falciparum merozoites and gametes have also been shown to bind FH suggests that a similar strategy could be applied to malaria vaccines and potentially overcome complement evasion.39, 40, 41
The RV144 trial is the only HIV vaccine study to demonstrate protection in humans, although efficacy was only modest at 31%.165 In this trial, a reduced risk of infection was correlated with IgG to the variable regions (V1V2) of the HIV‐1 gp120 glycoprotein.166, 167 Compared to other HIV vaccine trials that were not efficacious (eg VAX003 and VAX004), the RV144 vaccine‐induced significantly higher levels anti‐V1V2 antibodies of the IgG3 subclass, which was also associated with increased complement activation.168, 169, 170, 171, 172 Notably, enhanced IgG to V1V2 and enhanced complement activation were both associated with a reduced risk of infection the RV144 trial.172
7. FUTURE DIRECTIONS AND CONCLUSIONS
While major gains have been made recently in understanding complement‐mediated mechanisms of immunity in malaria, there are many gaps in our knowledge, which are priorities for future research (Table 2). These include studies to better define and understand immune mechanisms involved in protection from malaria infection and disease, and the specific targets of antibodies that most effectively harness complement functions. Including complement‐mediated mechanisms in vaccine design, and evaluating the induction of these mechanisms by existing vaccines, may substantially facilitate the development of efficacious vaccines. Finally, most available data come from studies of P. falciparum, and studies of P. vivax immunity are also needed to help advance vaccines that will contribute to malaria elimination.
Table 2.
Future directions and research priorities
| Immune mechanisms | Define antibody properties that maximize complement fixation and activation, and whether non‐cytophilic antibodies are potentially inhibitory |
| Define the role of complement fixation in promoting opsonic phagocytosis by monocytes, neutrophils and other cell types | |
| Determine whether complement in the skin plays a role against sporozoites | |
| Determine the role of antibody‐complement interactions in immunity to parasitized red blood cells | |
| Determine whether sporozoites can evade direct complement activation via the alternative pathway | |
| Determine the importance of antibody‐complement interactions for P. vivax | |
| Immune targets | Identify major targets of complement‐fixing antibodies that inhibit blood‐stage replication |
| Identify major antigen and epitope targets of complement‐fixing antibodies to sporozoites | |
| Understand how epitope specificity of antibodies influences complement fixation and activation for lead vaccine antigens | |
| Vaccines | Evaluate complement‐fixing antibodies in vaccine development and clinical trials of merozoite vaccines |
| Determine whether antibody‐complement interactions contribute to the protective immunity of RTS,S vaccine | |
| Identify vaccine strategies that maximally induce complement‐fixing antibodies | |
| Determine whether combinations of antigens or specific epitopes can more effectively induce complement‐fixing antibodies | |
| Development of a suitable animal model to study antibody‐complement mechanisms in vaccine development | |
| Incorporate the use of high‐throughput cell‐free assays to assess functional complement activity in vaccine trials |
There is great potential to exploit antibody‐complement interactions in malaria vaccine development, further supported by the demonstrated importance of these mechanisms in anti‐viral and anti‐bacterial immunity, including some licensed vaccines. In this review, we have summarized the growing knowledge of the role of antibody‐complement interactions in human immunity to malaria, and highlighted the potential importance of these mechanisms in malaria vaccines, and how they could be harnessed to achieve vaccines with greater efficacy.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge support from the following: National Health and Medical Research Council (NHMRC) of Australia (Senior Research Fellowship, 1077636; Program Grant, 1092789; and Project Grant, 1141278 to JGB), Australian Government Research Training Program Scholarship to LK, and LK, MJB, DHO, LR, JAC and JGB are supported by the Centre for Research Excellence in Malaria Elimination (NHMRC, 1134989). The Burnet Institute is supported by a Victorian State Government Operational Infrastructure Support grant, and the NHMRC Independent Research Institutes Infrastructure Support Scheme.
Kurtovic L, Boyle MJ, Opi DH, et al. Complement in malaria immunity and vaccines. Immunol Rev. 2020;293:38–56. 10.1111/imr.12802
This article is part of a series of reviews covering Immunity to Malaria appearing in Volume 293 of Immunological Reviews.
REFERENCES
- 1. World Health Organization . World Malaria Report 2018. Geneva: World Health Organisation; 2018. [Google Scholar]
- 2. Beeson JG, Kurtovic L, Dobaño C, et al. Challenges and strategies for developing efficacious and long‐lasting malaria vaccines. Sci Transl Med. 2019;11:eaau1458. [DOI] [PubMed] [Google Scholar]
- 3. Cohen S, McGregor IA, Carrington S. Gamma‐globulin and acquired immunity to human malaria. Nature. 1961;192:733‐737. [DOI] [PubMed] [Google Scholar]
- 4. Richards JS, Beeson JG. The future for blood‐stage vaccines against malaria. Immunol Cell Biol. 2009;87:377‐390. [DOI] [PubMed] [Google Scholar]
- 5. Stanisic DI, Richards JS, McCallum FJ, et al. Immunoglobulin G subclass‐specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffman S, Oster C, Plowe C, et al. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987;237:639‐642. [DOI] [PubMed] [Google Scholar]
- 7. Kurtovic L, Behet MC, Feng G, et al. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med. 2018;16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sack BK, Miller JL, Vaughan AM, et al. Model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infect Immun. 2014;82:808‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. RTSS Clinical Trial Partnerships . Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cockburn IA, Seder RA. Malaria prevention: from immunological concepts to effective vaccines and protective antibodies. Nature Immunol. 2018;19:1199. [DOI] [PubMed] [Google Scholar]
- 11. Heesterbeek DA, Angelier ML, Harrison RA, Rooijakkers SH. Complement and bacterial infections: from molecular mechanisms to therapeutic applications. J Innate Immun. 2018;10:455‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stoermer KA, Morrison TE. Complement and viral pathogenesis. Virology. 2011;411:362‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berends ET, Kuipers A, Ravesloot MM, Urbanus RT, Rooijakkers SH. Bacteria under stress by complement and coagulation. FEMS Microbiol Rev. 2014;38:1146‐1171. [DOI] [PubMed] [Google Scholar]
- 14. Lachmann PJ, Davies A. Complement and immunity to viruses. Immunol Rev. 1997;159:69‐77. [DOI] [PubMed] [Google Scholar]
- 15. Merle NS, Church SE, Fremeaux‐Bacchi V, Roumenina LT. Complement system part I ‐ molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morgan BP, Walters D, Serna M, Bubeck D. Terminal complexes of the complement system: new structural insights and their relevance to function. Immunol Rev. 2016;274:141‐151. [DOI] [PubMed] [Google Scholar]
- 17. Mehlhop E, Nelson S, Jost CA, et al. Complement protein C1q reduces the stoichiometric threshold for antibody‐mediated neutralization of West Nile virus. Cell Host Microbe. 2009;6:381‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beebe D, Schreiber R, Cooper N. Neutralization of influenza virus by normal human sera: mechanisms involving antibody and complement. J Immunol. 1983;130:1317‐1322. [PubMed] [Google Scholar]
- 19. Britt W, Vugler L, Stephens E. Induction of complement‐dependent and‐independent neutralizing antibodies by recombinant‐derived human cytomegalovirus gp55‐116 (gB). J Virol. 1988;62:3309‐3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rasmussen L, Mullenax J, Nelson R, Merigan TC. Viral polypeptides detected by a complement‐dependent neutralizing murine monoclonal antibody to human cytomegalovirus. J Virol. 1985;55:274‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benhnia MR‐E‐I, McCausland MM, Laudenslager J, et al. Heavily isotype‐dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5. J Virol. 2009;83:12355‐12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benhnia MR‐E‐I, McCausland MM, Moyron J, et al. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J Virol. 2009;83:1201‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spear GT, Sullivan BL, Landay AL, Lint TF. Neutralization of human immunodeficiency virus type 1 by complement occurs by viral lysis. J Virol. 1990;64:5869‐5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verbonitz M, Ennis F, Hicks J, Albrecht P. Hemagglutinin‐specific complement‐dependent antibody response to influenza infection. J Exp Med. 1978;147:265‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cromeans TL, Shore SL. Lysis of herpes simplex virus‐infected cells early in the infectious cycle by human antiviral antibody and complement. Infect Immun. 1981;31:1054‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sissons JP, Schreiber RD, Perrin LH, et al. Lysis of measles virus‐infected cells by the purified cytolytic alternative complement pathway and antibody. J Exp Med. 1979;150:445‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirsch R, Wolinsky J, Winkelstein J. Activation of the alternative complement pathway by mumps infected cells: relationship to viral neuraminidase activity. Arch Virol. 1986;87:181‐190. [DOI] [PubMed] [Google Scholar]
- 28. Johnson JB, Capraro GA, Parks GD. Differential mechanisms of complement‐mediated neutralization of the closely related paramyxoviruses simian virus 5 and mumps virus. Virology. 2008;376:112‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Strijp J, Van Kessel K, Van der Tol M, Verhoef J. Complement‐mediated phagocytosis of herpes simplex virus by granulocytes. Binding or ingestion. J Clin Invest. 1989;84:107‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hartshorn KL, White MR, Shepherd V, Reid K, Jensenius JC, Crouch EC. Mechanisms of anti‐influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol Lung Cell Mol Physiol. 1997;273:L1156‐L1166. [DOI] [PubMed] [Google Scholar]
- 31. Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487‐3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oldstone MB, Cooper NR, Larson DL. Formation and biologic role of polyoma virus‐antibody complexes: a critical role for complement. J Exp Med. 1974;140:549‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng JQ, Mozdzanowska K, Gerhard W. Complement component C1q enhances the biological activity of influenza virus hemagglutinin‐specific antibodies depending on their fine antigen specificity and heavy‐chain isotype. J Virol. 2002;76:1369‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus‐specific antibodies by serum components. Virology. 2006;352:418‐426. [DOI] [PubMed] [Google Scholar]
- 35. MacLennan CA, Gondwe EN, Msefula CL, et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest. 2008;118:1553‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gondwe EN, Molyneux ME, Goodall M, et al. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci USA. 2010;107:3070‐3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horwitz MA, Silverstein SC. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980;65:82‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boyle MJ, Reiling L, Feng G, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against Malaria. Immunity. 2015;42:580‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosa TF, Flammersfeld A, Ngwa CJ, et al. The Plasmodium falciparum blood stages acquire factor H family proteins to evade destruction by human complement. Cell Microbiol. 2016;18:573‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kennedy AT, Schmidt CQ, Thompson JK, et al. Recruitment of factor H as a novel complement evasion strategy for blood‐stage Plasmodium falciparum infection. J Immunol. 2016;196:1239‐1248. [DOI] [PubMed] [Google Scholar]
- 41. Simon N, Lasonder E, Scheuermayer M, et al. Malaria parasites co‐opt human factor H to prevent complement‐mediated lysis in the mosquito midgut. Cell Host Microbe. 2013;13:29‐41. [DOI] [PubMed] [Google Scholar]
- 42. Kennedy AT, Wijeyewickrema LC, Huglo A, et al. Recruitment of human C1 esterase inhibitor controls complement activation on blood stage Plasmodium falciparum merozoites. J Immunol. 2017;198:4728‐4737. [DOI] [PubMed] [Google Scholar]
- 43. Lindorfer MA, Cook EM, Tupitza JC, et al. Real‐time analysis of the detailed sequence of cellular events in mAb‐mediated complement‐dependent cytotoxicity of B‐cell lines and of chronic lymphocytic leukemia B‐cells. Mol Immunol. 2016;70:13‐23. [DOI] [PubMed] [Google Scholar]
- 44. Singh S, Alam MM, Pal‐Bhowmick I, Brzostowski JA, Chitnis CE. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010;6:e1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiesner J, Jomaa H, Wilhelm M, et al. Host cell factor CD59 restricts complement lysis of Plasmodium falciparum‐infected erythrocytes. Eur J Immunol. 1997;27:2708‐2713. [DOI] [PubMed] [Google Scholar]
- 46. Oyong DA, Kenangalem E, Poespoprodjo JR, et al. Loss of complement regulatory proteins on uninfected erythrocytes in vivax and falciparum malaria anemia. JCI insight. 2018;3:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Behet M, Kurtovic L, van Gemert GJ, et al. The complement system contributes to functional antibody‐mediated responses induced by immunization with Plasmodium falciparum malaria sporozoites. Infect Immun. 2018;86(7):e00920-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zenklusen I, Jongo S, Abdulla S, et al. Immunization of malaria‐preexposed volunteers with PfSPZ vaccine elicits long‐lived IgM invasion‐inhibitory and complement‐fixing antibodies. J Infect Dis. 2018;217:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCoy ME, Golden HE, Doll TA, et al. Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein‐based, self‐assembling protein nanoparticle vaccine. Malar J. 2013;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aliprandini E, Tavares J, Panatieri RH, et al. Cytotoxic anti‐circumsporozoite antibodies target malaria sporozoites in the host skin. Nat Microbiol. 2018;3:1224‐1233. [DOI] [PubMed] [Google Scholar]
- 51. Kawamoto Y, Winger LA, Hong K, et al. Plasmodium berghei: sporozoites are sensitive to human serum but not susceptible host serum. Exp Parisitol. 1992;75:361‐368. [DOI] [PubMed] [Google Scholar]
- 52. Touray MG, Seeley D, Miller LH Plasmodium gallinaceum: differential lysis of two developmental stages of malaria sporozoites by the alternative pathway of complement. Exp Parisitol. 1994;78:294‐301. [DOI] [PubMed] [Google Scholar]
- 53. Yilmaz B, Portugal S, Tran T, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014;159:1277‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boyle MJ, Wilson DW, Richards JS, et al. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc Natl Acad Sci USA. 2010;107:14378‐14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boyle MJ, Wilson DW, Beeson JG. New approaches to studying Plasmodium falciparum merozoite invasion and insights into invasion biology. Int J Parasitol. 2013;43:1‐10. [DOI] [PubMed] [Google Scholar]
- 56. Boyle MJ, Chan J‐A, Handayuni I, et al. IgM in human immunity to Plasmodium falciparum malaria. Sci Adv. 2019; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dluzewski AR, Ling IT, Hopkins JM, et al. Formation of the food vacuole in Plasmodium falciparum: a potential role for the 19 kDa fragment of merozoite surface protein 1 (MSP119). PLoS ONE. 2008;3:e3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blackman MJ, Scott‐Finnigan TJ, Shai S, Holder AA. Antibodies inhibit the protease‐mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moss DK, Remarque EJ, Faber BW, et al. Plasmodium falciparum 19‐kilodalton merozoite surface protein 1 (MSP1)‐specific antibodies that interfere with parasite growth in vitro can inhibit MSP1 processing, merozoite invasion, and intracellular parasite development. Infect Immun. 2012;80:1280‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boyle MJ, Langer C, Chan J‐A, et al. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum . Infect Immun. 2014;82:924‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taylor PR, Seixas E, Walport MJ, Langhorne J, Botto M. Complement contributes to protective immunity against reinfection by Plasmodium chabaudi chabaudi parasites. Infect Immun. 2001;69:3853‐3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kumaratilake L, Ferrante A, Jaeger T, Rzepczyk C. Effects of cytokines, complement, and antibody on the neutrophil respiratory burst and phagocytic response to Plasmodium falciparum merozoites. Infect Immun. 1992;60:3731‐3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kumaratilake L, Ferrante A, Jaeger T, Morris‐Jones S. The role of complement, antibody, and tumor necrosis factor alpha in the killing of Plasmodium falciparum by the monocytic cell line THP‐1. Infect Immun. 1997;65:5342‐5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Joos C, Marrama L, Polson H, et al. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS ONE. 2010;5:e9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reiling L, Boyle MJ, White MT, et al. Targets of complement‐fixing antibodies in protective immunity against malaria in children. Nat Commun. 2019;10:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McCallum FJ, Persson K, Fowkes F, et al. Differing rates of antibody acquisition to merozoite antigens in malaria: implications for immunity and surveillance. J Leukoc Biol. 2017;101:913‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oyong DA, Wilson DW, Barber BE, et al. Induction and kinetics of complement‐fixing antibodies against Plasmodium vivax MSP3α and relationship with IgG subclasses and IgM. J Infect Dis. 2019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes F, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev. 2016;40:343‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chan JA, Fowkes FJ, Beeson JG. Surface antigens of Plasmodium falciparum‐infected erythrocytes as immune targets and malaria vaccine candidates. Cell Mol Life Sci. 2014;71:3633‐3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stanley HA, Mayes JT, Cooper NR, Reese RT. Complement activation by the surface of Plasmodium falciparum infected erythrocytes. Mol Immunol. 1984;21:145‐150. [DOI] [PubMed] [Google Scholar]
- 71. Zhou J, Feng G, Beeson J, et al. CD14(hi)CD16+ monocytes phagocytose antibody‐opsonised Plasmodium falciparum infected erythrocytes more efficiently than other monocyte subsets, and require CD16 and complement to do so. BMC Med. 2015;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maier AG, Rug M, O'Neill MT, et al. Skeleton‐binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum–infected erythrocyte surface. Blood. 2007;109:1289‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Larsen MD, Quintana M, Ditlev SB, et al. Evasion of classical complement pathway activation on Plasmodium falciparum‐infected erythrocytes opsonized by PfEMP1‐specific IgG. Front Immunol. 2018;9:3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Akhouri RR, Goel S, Furusho H, Skoglund U, Wahlgren M. Architecture of human IgM in complex with P falciparum erythrocyte membrane protein 1. Cell Rep. 2016;14:723‐736. [DOI] [PubMed] [Google Scholar]
- 75. Chan J‐A, Drew DR, Reiling L, et al. Low levels of human antibodies to gametocyte‐infected erythrocytes contrasts the PfEMP1‐dominant response to asexual stages in P falciparum malaria. Front Immunol. 2018;9:3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dantzler KW, Ma S, Ngotho P, et al. Naturally acquired immunity against immature Plasmodium falciparum gametocytes. Sci Transl Med. 2019;11:eaav3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Quakyi I, Carter R, Rener J, et al. The 230‐kDa gamete surface protein of Plasmodium falciparum is also a target for transmission‐blocking antibodies. J Immunol. 1987;139:4213‐4217. [PubMed] [Google Scholar]
- 78. Roeffen W, Beckers P, Teelen K, et al. Plasmodium falciparum: a comparison of the activity of Pfs230‐specific antibodies in an assay of transmission‐blocking immunity and specific competition ELISAs. Exp Parisitol. 1995;80:15‐26. [DOI] [PubMed] [Google Scholar]
- 79. Mendis K, Munesinghe Y, De Silva Y, Keragalla I, Carter R. Malaria transmission‐blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect Immun. 1987;55:369‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Healer J, McGuinness D, Hopcroft P, et al. Complement‐mediated lysis of Plasmodium falciparum gametes by malaria‐immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun. 1997;65:3017‐3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Read D, Lensen A, Begarnie S, Haley S, Raza A, Carter R. Transmission‐blocking antibodies against multiple, non‐variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement‐fixing. Parasite Immunol. 1994;16:511‐519. [DOI] [PubMed] [Google Scholar]
- 82. Williamson KC, Keister DB, Muratova O, Kaslow DC. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol Biochem Parasit. 1995;75:33‐42. [DOI] [PubMed] [Google Scholar]
- 83. Wright JF, Shulman M, Isenman DE, Painter R. C1 binding by murine IgM. The effect of a Pro‐to‐Ser exchange at residue 436 of the mu‐chain. J Biol Chem. 1988;263:11221‐11226. [PubMed] [Google Scholar]
- 84. Hughes‐Jones N, Gardner B. The reaction between the complement subcomponent C1q. IgG complexes and polyionic molecules. Immunology. 1978;34:459. [PMC free article] [PubMed] [Google Scholar]
- 85. Diebolder CA, Beurskens FJ, de Jong RN, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. 2015;67:171‐182. [DOI] [PubMed] [Google Scholar]
- 87. Idusogie EE, Presta LG, Gazzano‐Santoro H, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178‐4184. [DOI] [PubMed] [Google Scholar]
- 88. Xu Y, Oomen R, Klein MH. Residue at position 331 in the IgG1 and IgG4 CH2 domains contributes to their differential ability to bind and activate complement. J Biol Chem. 1994;269:3469‐3474. [PubMed] [Google Scholar]
- 89. Morgan A, Jones N, Nesbitt A, et al. The N‐terminal end of the CH2 domain of chimeric human IgG1 anti‐HLA‐DR is necessary for C1q, Fc gamma RI and Fc gamma RIII binding. Immunology. 1995;86:319. [PMC free article] [PubMed] [Google Scholar]
- 90. Sensel MG, Kane LM, Morrison SL. Amino acid differences in the N‐terminus of CH2 influence the relative abilities of IgG2 and IgG3 to activate complement. Mol Immunol. 1997;34:1019‐1029. [DOI] [PubMed] [Google Scholar]
- 91. Tao M‐H, Canfield SM, Morrison SL. The differential ability of human IgG1 and IgG4 to activate complement is determined by the COOH‐terminal sequence of the CH2 domain. J Exp Med. 1991;173:1025‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Idusogie EE, Wong PY, Presta LG, et al. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166:2571‐2575. [DOI] [PubMed] [Google Scholar]
- 93. Coloma MJ, Trinh KR, Wims LA, Morrison SL. The hinge as a spacer contributes to covalent assembly and is required for function of IgG. J Immunol. 1997;158:733‐740. [PubMed] [Google Scholar]
- 94. Huck S, Fort P, Crawford D, Lefranc M‐P, Lefranc G. Sequence of a human immunoglobulin gamma 3 heavy chain constant region gene: comparison with the other human C γ genes. Nucleic Acids Res. 1986;14:1779‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tan LK, Shopes RJ, Oi VT, Morrison SL. Influence of the hinge region on complement activation, C1q binding, and segmental flexibility in chimeric human immunoglobulins. Proc Natl Acad Sci USA. 1990;87:162‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cook EM, Lindorfer MA, van der Horst H, et al. Antibodies that efficiently form hexamers upon antigen binding can induce complement‐dependent cytotoxicity under complement‐limiting conditions. J Immunol. 2016;197:1762‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stapleton NM, Andersen JT, Stemerding AM, et al. Competition for FcRn‐mediated transport gives rise to short half‐life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pandey JP, Namboodiri AM. Genetic variants of IgG1 antibodies and FcγRIIIa receptors influence the magnitude of antibody‐dependent cell‐mediated cytotoxicity against prostate cancer cells. Oncoimmunology. 2014;3:e27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Redpath S, Michaelsen T, Sandlie I, Clark M. Activation of complement by human IgG1 and human IgG3 antibodies against the human leucocyte antigen CD52. Immunology. 1998;93:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Migot‐Nabias F, Noukpo JM, Guitard E, et al. Imbalanced distribution of GM immunoglobulin allotypes according to the clinical presentation of Plasmodium falciparum malaria in Beninese children. J Infect Dis. 2008;198:1892‐1895. [DOI] [PubMed] [Google Scholar]
- 101. Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7:1401‐1413. [DOI] [PubMed] [Google Scholar]
- 102. Quast I, Keller CW, Maurer MA, et al. Sialylation of IgG Fc domain impairs complement‐dependent cytotoxicity. J Clin Invest. 2015;125:4160‐4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sudo M, Yamaguchi Y, Späth PJ, et al. Different IVIG glycoforms affect in vitro inhibition of anti‐ganglioside antibody‐mediated complement deposition. PLoS ONE. 2014;9:e107772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Teeling JL, Mackus W, Wiegman L, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362‐371. [DOI] [PubMed] [Google Scholar]
- 105. Cleary KL, Chan HC, James S, Glennie MJ, Cragg MS. Antibody distance from the cell membrane regulates antibody effector mechanisms. J Immunol. 2017;198(10):3999‐4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nardin EH, Nussenzweig RS, McGregor IA, Bryan JH. Antibodies to sporozoites: their frequent occurrence in individuals living in an area of hyperendemic malaria. Science. 1979;206:597‐599. [DOI] [PubMed] [Google Scholar]
- 107. Webster H, Brown A, Chuenchitra C, Permpanich B, Pipithkul J. Characterization of antibodies to sporozoites in Plasmodium falciparum malaria and correlation with protection. J Clin Microbiol. 1988;26:923‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Richards JS, Arumugam TU, Reiling L, et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol. 2013;191(2):795‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Teo A, Feng G, Brown GV, Beeson JG, Rogerson SJ. Functional antibodies and protection against blood‐stage malaria. Trends Parasitol. 2016;32:887‐898. [DOI] [PubMed] [Google Scholar]
- 110. McCallum FJ, Persson K, Mugyenyi CK, et al. Acquisition of growth‐inhibitory antibodies against blood‐stage Plasmodium falciparum . PLoS ONE. 2008;3:e3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dent AE, Bergmann‐Leitner ES, Wilson DW, et al. Antibody‐mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS ONE. 2008;3:e3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Perraut R, Marrama L, Diouf B, et al. Antibodies to the conserved C‐terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J Infect Dis. 2005;191:264‐271. [DOI] [PubMed] [Google Scholar]
- 113. Marsh K, Otoo L, Hayes R, Carson D, Greenwood B. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293‐303. [DOI] [PubMed] [Google Scholar]
- 114. Duncan CJ, Hill AV, Ellis RD. Can growth inhibition assays (GIA) predict blood‐stage malaria vaccine efficacy? Hum Vaccin Immunother. 2012;8:706‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Osier F, Feng G, Boyle MJ, et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med. 2014;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ataide R, Ashley EA, Powell R, et al. Host immunity to Plasmodium falciparum and the assessment of emerging artemisinin resistance in a multinational cohort. Proc Natl Acad Sci USA. 2017;114:3515‐3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. O'Flaherty K, Ataíde R, Zaloumis SG, et al. The contribution of functional antimalarial immunity to measures of parasite clearance in therapeutic efficacy studies of artemisinin derivatives. J Infect Dis. 2019;220(7):1178‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Akter J, Khoury DS, Aogo R, et al. Plasmodium‐specific antibodies block in vivo parasite growth without clearing infected red blood cells. PLoS Pathog. 2019;15:e1007599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Biryukov S, Angov E, Landmesser ME, Spring MD, Ockenhouse CF, Stoute JA. Complement and antibody‐mediated enhancement of red blood cell invasion and growth of malaria parasites. EBioMedicine. 2016;9:207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Boyle M, Reiling L, Beeson J. Evaluating complement‐mediated humoral immunity to P falciparum blood stages. EBioMedicine. 2016;14:9‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chan J‐A, Boyle MJ, Moore KA, et al. Antibody targets on the surface of Plasmodium falciparum–infected erythrocytes that are associated with immunity to severe malaria in young children. J Infect Dis. 2018;219:819‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Silver KL, Higgins SJ, McDonald CR, Kain KC. Complement driven innate immune response to malaria: fuelling severe malarial diseases. Cell Microbiol. 2010;12:1036‐1045. [DOI] [PubMed] [Google Scholar]
- 123. Ouédraogo AL, Roeffen W, Luty A, et al. Naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs48/45 and Pfs230 in an area of seasonal transmission. Infect Immun. 2011;79:4957‐4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Stone W, Campo JJ, Ouédraogo AL, et al. Unravelling the immune signature of Plasmodium falciparum transmission‐reducing immunity. Nat Commun. 2018;9:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mershon KL, Morrison SL. Antibody‐complement Interaction. Therapeutic Monoclonal Antibodies: From Bench to Clinic. Hoboken, NJ: Wiley; 2009:371‐383. [Google Scholar]
- 126. Ong GL, Mattes MJ. Mouse strains with typical mammalian levels of complement activity. J Immunol Methods. 1989;125:147‐158. [DOI] [PubMed] [Google Scholar]
- 127. Siggins MK, Cunningham AF, Marshall JL, et al. Absent bactericidal activity of mouse serum against invasive African nontyphoidal Salmonella results from impaired complement function but not a lack of antibody. J Immunol. 2011;186:2365‐2371. [DOI] [PubMed] [Google Scholar]
- 128. Ratelade J, Verkman A. Inhibitor (s) of the classical complement pathway in mouse serum limit the utility of mice as experimental models of neuromyelitis optica. Mol Immunol. 2014;62:104‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Conteh S, Anderson C, Lambert L, et al. Grammomys surdaster, the natural host for Plasmodium berghei parasites, as a model to study whole‐organism vaccines against malaria. Am J Trop Med Hyg. 2017;96:835‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Malaria Policy Advisory Committee Meeting . Update on RTS, S malaria vaccine implementation programme. 2018. https://www.who.int/malaria/mpac/mpac-april2019-session3-rtss-mvip-update.pdf?ua=1. Accessed August 20, 2019.
- 131. White MT, Bejon P, Olotu A, et al. A combined analysis of immunogenicity, antibody kinetics and vaccine efficacy from phase 2 trials of the RTS,S malaria vaccine . BMC Med. 2014;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kurtovic L, Agius PA, Feng G, et al. Induction and decay of functional complement‐fixing antibodies by the RTS,S malaria vaccine in children, and a negative impact of malaria exposure. BMC Med. 2019;17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Mugyenyi CK, Elliott SR, Yap XZ, et al. Declining malaria transmission differentially impacts the maintenance of humoral immunity to Plasmodium falciparum in children. J Infect Dis. 2017;216:887‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ubillos I, Ayestaran A, Nhabomba AJ, et al. Baseline exposure, antibody subclass, and hepatitis B response differentially affect malaria protective immunity following RTS,S/AS01E vaccination in African children. BMC Med. 2018;16:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lilienthal G‐M, Rahmöller J, Petry J, Bartsch YC, Leliavski A, Ehlers M. Potential of murine IgG1 and human IgG4 to inhibit the classical complement and Fcγ receptor activation pathways. Front Immunol. 2018;9:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468‐477. [DOI] [PubMed] [Google Scholar]
- 137. Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation‐attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155‐1164. [DOI] [PubMed] [Google Scholar]
- 138. Feng G, Boyle MJ, Cross N, et al. Human immunization with a polymorphic malaria vaccine candidate induced antibodies to conserved epitopes that promote functional antibodies to multiple parasite strains. J Infect Dis. 2018;218:35‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Douglas AD, Baldeviano GC, Jin J, et al. A defined mechanistic correlate of protection against Plasmodium falciparum malaria in non‐human primates. Nat Commun. 2019;10:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mugyenyi CK, Elliott SR, McCallum FJ, Anders RF, Marsh K, Beeson JG. Antibodies to polymorphic invasion‐inhibitory and non‐inhibitory epitopes of Plasmodium falciparum apical membrane antigen 1 in human malaria. PLoS ONE. 2013;8:e68304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Drew DR, Wilson DW, Elliott SR, et al. A novel approach to identifying patterns of human invasion‐inhibitory antibodies guides the design of malaria vaccines incorporating polymorphic antigens. BMC Med. 2016;14:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sparrow E, Friede M, Sheikh M, Torvaldsen S. Therapeutic antibodies for infectious diseases. Bull World Health Organ. 2017;95(3):235‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ward ES, Ober RJ. Targeting FcRn to generate antibody‐based therapeutics. Trends Pharmacol Sci. 2018;39(10):892‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kisalu NK, Idris AH, Weidle C, et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med. 2018;24:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wood J, Holland C, Kester KE, et al. Passive transfer of growth‐inhibitory antibodies raised against yeast‐expressed recombinant Plasmodium falciparum merozoite surface protein‐1 (19). Am J Trop Med Hyg. 1998;59:991‐997. [DOI] [PubMed] [Google Scholar]
- 146. Weaver R, Reiling L, Feng G, et al. The association between naturally acquired IgG subclass specific antibodies to the PfRH5 invasion complex and protection from Plasmodium falciparum malaria. Sci Rep. 2016;6:33094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus: I. The role of humoral antibodies. J Exp Med. 1969;129:1307‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Maslanka SE, Gheesling LL, Libutti DE, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4:156‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001;69:1568‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Borrow R, Carlone GM, Rosenstein N, et al. Neisseria meningitidis group B correlates of protection and assay standardization–international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006;24:5093. [DOI] [PubMed] [Google Scholar]
- 151. Romero‐Steiner S, Fernandez J, Biltoft C, et al. Functional antibody activity elicited by fractional doses of haemophilus influenzae type b conjugate vaccine (polyribosylribitol phosphate‐tetanus toxoid conjugate). Clin Diagn Lab Immunol. 2001;8:1115‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Green BA, Quinn‐Dey T, Zlotnick GW. Biologic activities of antibody to a peptidoglycan‐associated lipoprotein of Haemophilus influenzae against multiple clinical isolates of H influenzae type b. Infect Immun. 1987;55:2878‐2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Boyd MA, Tennant SM, Saague VA, et al. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin Vaccine Immunol. 2014;21:712‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Shimanovich AA, Buskirk AD, Heine SJ, et al. Functional and antigen‐specific serum antibody levels as correlates of protection against shigellosis in a controlled human challenge study. Clin Vaccine Immunol. 2017;24:e00412-e00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. McIntosh E, Bröker M, Wassil J, Welsch J, Borrow R. Serum bactericidal antibody assays–the role of complement in infection and immunity. Vaccine. 2015;33:4414‐4421. [DOI] [PubMed] [Google Scholar]
- 156. Madico G, Welsch JA, Lewis LA, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Schneider MC, Exley RM, Chan H, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566‐7575. [DOI] [PubMed] [Google Scholar]
- 158. Masaninga F, Chanda E, Chanda‐Kapata P, et al. Review of the malaria epidemiology and trends in Zambia. Asian Pac J Trop Biomed. 2013;3:89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fletcher LD, Bernfield L, Barniak V, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72:2088‐2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. Factor H‐dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis. MBio. 2013;4:e00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lewis LA, Ngampasutadol J, Wallace R, Reid J, Vogel U, Ram S. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 2010;6:e1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Giuliani MM, Adu‐Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci USA. 2006;103:10834‐10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Jiang H‐Q, Hoiseth SK, Harris SL, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28:6086‐6093. [DOI] [PubMed] [Google Scholar]
- 164. Rappuoli R, Pizza M, Masignani V, Vadivelu K. Meningococcal B vaccine (4CMenB): the journey from research to real world experience. Expert Rev Vaccines. 2018;17:1111‐1121. [DOI] [PubMed] [Google Scholar]
- 165. Rerks‐Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV‐1 infection in Thailand. N Engl J Med. 2009;361:2209‐2220. [DOI] [PubMed] [Google Scholar]
- 166. Haynes BF, Gilbert PB, McElrath MJ, et al. Immune‐correlates analysis of an HIV‐1 vaccine efficacy trial. N Engl J Med. 2012;366:1275‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zolla‐Pazner S, deCamp A, Gilbert PB, et al. Vaccine‐induced IgG antibodies to V1V2 regions of multiple HIV‐1 subtypes correlate with decreased risk of HIV‐1 infection. PLoS ONE. 2014;9:e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double‐blind, placebo‐controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV‐1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661‐1671. [DOI] [PubMed] [Google Scholar]
- 169. Chung AW, Ghebremichael M, Robinson H, et al. Polyfunctional Fc‐effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6:228ra238. [DOI] [PubMed] [Google Scholar]
- 170. Yates NL, Liao H‐X, Fong Y, et al. Vaccine‐induced Env V1–V2 IgG3 correlates with lower HIV‐1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6:228ra239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Group rHVS . Placebo‐controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV‐1 infection. J Infect Dis. 2005;191:654‐665. [DOI] [PubMed] [Google Scholar]
- 172. Perez LG, Martinez DR, deCamp AC, et al. V1V2‐specific complement activating serum IgG as a correlate of reduced HIV‐1 infection risk in RV144. PLoS ONE. 2017;12:e0180720. [DOI] [PMC free article] [PubMed] [Google Scholar]


