Abstract
Cleft lip with or without cleft palate is a congenital deformity that occurs in about 1 of 700 newborns, affecting the dentition, bone, skin, muscles and mucosa in the orofacial region. A cleft can give rise to problems with maxillofacial growth, dental development, speech, and eating, and can also cause hearing impairment. Surgical repair of the lip may lead to impaired regeneration of muscle and skin, fibrosis, and scar formation. This may result in hampered facial growth and dental development affecting oral function and lip and nose esthetics. Therefore, secondary surgery to correct the scar is often indicated. We will discuss the molecular and cellular pathways involved in facial and lip myogenesis, muscle anatomy in the normal and cleft lip, and complications following surgery. The aim of this review is to outline a novel molecular and cellular strategy to improve musculature and skin regeneration and to reduce scar formation following cleft repair. Orofacial clefting can be diagnosed in the fetus through prenatal ultrasound screening and allows planning for the harvesting of umbilical cord blood stem cells upon birth. Tissue engineering techniques using these cord blood stem cells and molecular targeting of inflammation and fibrosis during surgery may promote tissue regeneration. We expect that this novel strategy improves both muscle and skin regeneration, resulting in better function and esthetics after cleft repair.
Keywords: cleft lip and palate, oral surgery, scarring, tissue engineering, umbilical cord blood stem cells
1. INTRODUCTION
Cleft lip with (CLP) or without cleft palate (CL) occurs in about 1 to 700 newborns with ethnic and geographical variation and is one of the most common facial congenital anomalies.1, 2, 3 A CL is present in about 0.6 per 1000 live births.4 CL(P) is either uni‐ or bilateral (Figure 1) and can occur isolated or as part of a syndrome.5 Since only 30% of children born with CLP have a genetic syndrome, a combination of genetic and environmental factors is thought to play a role in the etiology of orofacial clefting.6, 7 These environmental risk factors include smoking,8 alcohol consumption,9 phenytoin exposure,10 diabetes,11 and maternal12 and paternal age.13 Other factors such as folate supplementation, zinc, and daily multivitamin intake, can reduce the risk of CL(P).14
Figure 1.
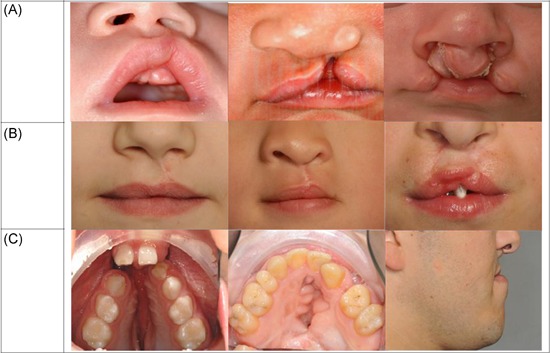
A, Unoperated orofacial clefting in mild to severe form, left to right: unilateral subepithelial cleft lip, unilateral cleft lip and palate, bilateral cleft lip alveolus. B, Lip closure with an optimal, normal and suboptimal esthetic outcome. B left: unilateral cleft lip with well‐aligned vermillion border, normal lip length, and normal shape of nostrils. B middle: unilateral cleft lip and palate with deficient lateral vermillion and white roll malalignment. B right: bilateral cleft lip and palate after surgical closing with vermillion notching, short upper lip, and high rising nostrils. C, Left: unoperated cleft lip and palate with cleft in the alveolar ridge and anterior displacement of the premaxilla. C middle: cleft palate with excessive scarring, and fistula following surgery. C right: adult patient in profile with a hypoplastic maxilla due to scarring after cleft lip and palate closure that resulted to a class III skeletal jaw relation [Color figure can be viewed at wileyonlinelibrary.com]
There is a large variation in the severity of the cleft lip, ranging from mild subepithelial to complete bilateral clefting (Figure 1). The more severe the cleft lip, the more the shape and size of the alveolar process are affected. The cleft in the alveolar process can range from a small dimple in the arch in combination with a minor cleft of the lip to a total cleft of the alveolar ridge and anterior displacement of the premaxilla (Figure 1).14 Cleft lip and primary or secondary palate clefts differ in embryonic origin and underlying fusion or differentiation defects. Fusion defects of the primary palate lead to complete clefting of the lip either or not combined with a complete or incomplete clefting of the alveolus. Differentiation defects of the primary palate give rise to incomplete, submucous or hypoplastic cleft lip and/or alveolus. Fusion defects of the secondary palate lead to complete or incomplete hard‐palate clefts that may be combined with a cleft in the soft palate and/or uvula. Differentiation defects of the secondary palate give rise to a combination of submucous, hypoplastic hard and soft palate defects. 15, 16, 17
If the cleft is not surgically corrected, patients are more prone to hearing problems due to otitis media with effusion,18 and have difficulties with speech, feeding, and abnormal dental development due to loss of lip pressure. In addition, they may encounter social isolation. Clefts can cause serious psychological problems including low self‐esteem and acceptance by peers.19, 20
Scarring following surgical correction of the cleft lip can have a major effect on subsequent anterior‐posterior and vertical growth of the maxilla, as well as on the position of the maxillary incisors.21 Excessive pressure of the repaired upper lip leads to palatal inclination of the upper front teeth and an increased overbite but decreased overjet.22 When the patient grows older a reversed overjet often develops due to growth restriction of the maxilla, especially in CLP cases. Several factors contribute to suboptimal lip muscle repair including the skills and experience of the surgeon,23 type and extension of the cleft,24 genetic factors,25 muscle fiber type distribution, and impaired muscle regeneration.26, 27 In this review, we therefore present a novel tissue engineering strategy that aims to prevent scarring and subsequent functional problems following cleft surgery by promoting muscle and skin regeneration.
2. FACIAL AND LIP MYOGENESIS
Orofacial development involves the migration, proliferation, differentiation, and apoptosis of mesenchymal and epithelial cells.28 From the 4th week of gestation cranial neural crest cells (CNCCs) migrate from the dorsal part of the neural tube to form the human face. Next, these CNCCs differentiate into the mesenchymal cells that form structures such as cartilage and bone. The cranial paraxial mesoderm (CPM) provides the precursors for the cranial muscles. Both CNCCs and CPM form the templates for the adult craniofacial structures in the branchial arches.29 In the 5th week of gestation, the CNCCs form the frontonasal prominence, the bilateral maxillary prominences and the bilateral mandibular prominences. In the 6th and 7th week the medial nasal prominences grow and fuse with each other to form an intermaxillary segment. This intermaxillary segment will later fuse with the maxillary prominences and form the upper lip. Any disruption in these well‐orchestrated processes can lead to a cleft in lip, alveolus and/or palate.28, 30, 31, 32
The muscles of the upper lip are all derived from the mesoderm of the 2nd pharyngeal arch and partly determine facial expression (Figure 2).33, 34 Mesenchymal condensations that form the individual upper lip muscles emerge sequentially, starting from the 6th week of gestation in humans.35 During the development of the facial muscles, these mesenchymal condensations differentiate and move to their definitive location where they mature.34
Figure 2.

Left: Muscle complex involved in the function of the upper lip: seven pairs of facial and lip muscles connected to the circular orbicularis oris muscle (red: superficial fibers, blue; deep fibers); buccinator (1), levator labii superioris (2), levator labii superioris alaeque nasi (3), levator anguli oris (4), zygomaticus minor (5), zygomaticus major (6), and the risorius muscle (7). Middle: unilateral cleft lip with abnormal orientation and insertion of the orbicularis oris muscle, superficial muscle fibers (red) and deep muscle fibers (blue) are deficient. Right: bilateral cleft lip with abnormal orientation and insertion of the orbicularis oris muscle [Color figure can be viewed at wileyonlinelibrary.com]
In summary, the main muscles of the upper lip originate from mesenchymal condensations in the pharyngeal arches.
3. MUSCLE ANATOMY IN THE NORMAL AND CLEFT LIP
The upper lip muscles comprise the orbicularis oris and seven pairs of other facial and lip muscles. The facial muscles are divided into a superficial (extrinsic) layer and a deep (intrinsic) layer, which work either synergistically or antagonistically in the coordination of lip movements.35 The orbicularis oris muscle is the primary muscle of the lip and therefore the most important muscle in cleft lip surgery.14 Facial expressions and lip movements during speaking are supported by the superficial part of the orbicularis oris, while mastication is mediated by the deep layer. The superficial muscle fibers end up in the philtrum ridge on one side, whereas the deep layers cross the midline to insert on the opposite side. The philtrum dimple originates from a reduced attachment of muscles to the surface (Figure 2).36
The orbicularis oris muscle together with the deep buccinator muscles compress the lips and cheeks against the teeth. The buccinator originates at the mandible to attach to the modiolus of the upper and lower lips. The superficial muscles of the lip, the levator labii superioris, the levator labii superioris alaeque nasi and the zygomaticus minor originate in the orbicularis oris and move the upper lip upwards and sideways. The zygomaticus major muscle flanks the upper lip on both sides and raises the lip. 37 The levator anguli oris is a deep muscle elevating the corners of the lips. All muscle fibers of these muscles originate and insert into the skin and the mucous membrane (Figure 2).
The orbicularis oris muscle contains both slow and fast fibers. The slow fibers are more resistant against fatigue and have a low activation threshold, whereas the fast fibers are more prone to fatigue and have a high activation threshold.38 Both the superficial and deep orbicularis oris muscle are predominantly composed of fast fibers, but there is a slight difference in fiber type composition between the superficial (98% fast) and deep muscle layers pars peripheralis (95% fast) and pars marginalis (91% fast). This indicates that these muscles should be reconstructed separately during surgery.38
In a cleft lip, all facial muscle fibers lack their insertions in the midline and therefore have an abnormal orientation on one or both sides (uni/bilateral cleft, respectively) (Figure 2). The orbicularis oris normally functions as a sphincter but, in a cleft lip, it pulls the two portions of the lip laterally leading to an imbalance between lip and tongue pressure. When a cleft lip is not surgically corrected at a young age, the anterior teeth will often become malpositioned.39
The superficial fibers of the orbicularis oris muscle are oriented parallel to the cleft, medial towards the columella and lateral to the alar base. The fibers at the medial side are deficient. These superficial fibers are more disorganized with increasing severity of the cleft. By contrast, the orientation of the deep layer of the orbicularis oris remains unchanged. This muscle runs perpendicular to the cleft and ends at the edges of the cleft (Figure 2).40, 41
In cleft lip, an increased amount of fast muscle fibers is present,42 which has also been demonstrated in the musculus levator veli palatini of the cleft soft palate.43 In vitro studies have shown that satellite cells (SCs) from fast muscle fibers proliferate less than those from slow muscle fibers.44 This, together with the reduced capillary supply and disorganization of the muscle fibers may compromise muscle regeneration in a cleft lip after surgical repair.45
Summarizing, in CL(P) the orientation, insertion, and composition of the muscles of the lip are abnormal, which hampers muscle regeneration after surgery, affecting the oral function and dental development.
4. CLEFT SURGERY CAN RESULT IN FIBROSIS AND SCAR FORMATION
The two main techniques in unilateral cleft lip surgery are the triangular flap technique (Tennison‐Randall) and the rotation advancement technique (Millard), which have similar outcomes.46, 47 Common bilateral cleft lip techniques include the Millard technique, the Manchester technique and the Tennisson technique.48 A cleft lip is often accompanied by a cleft palate and cleft alveolus,17 and is treated at different time points after birth depending on the treatment protocol.49 , 24
Surgery of the lip usually takes place in the first year after birth, often in conjunction with the repair of the nose and the soft palate.50 The hard palate is closed as late as possible, taking into account the speech development of the patient and the potential negative effects on facial growth. There is a large variation in the timing of surgical treatments between CLP surgical centers.51 However, the outcome is often suboptimal, depending on the severity of the original defect and the displacement of the cleft maxilla.52 Dehiscence of the lip or the palate shortly after surgery or scar formation with subsequent hampered growth of the maxilla are found.53 Revision surgery is therefore required in 33% of the CLP patients compared to 12% of the patients with an intact secondary palate.54
Normally, wound healing of skin and muscle is characterized by four successive and overlapping phases: hemostasis, inflammation, proliferation, and remodeling.55, 56 Disruption of blood vessels as a result of cleft surgery causes bleeding. Next, a blood clot containing fibrin will form by platelet aggregation. During hemostasis, platelets release cytokines and growth factors that attract inflammatory cells like granulocytes and macrophages.57 These inflammatory cells remove apoptotic cells, debris, and invading bacteria. Released cytokines and growth factors then stimulate keratinocyte and fibroblast proliferation, and angiogenesis to promote tissue regeneration. This proliferative phase consists of epithelial proliferation, the formation of granulation tissue and epithelialization. These processes are regulated by fibroblasts and endothelial cells.55 Fibroblasts produce collagen, glycosaminoglycans and proteoglycans, the major components of the extracellular matrix (ECM).58 Mechanical strain, transforming growth factor beta 1 (TGF‐β1) and the ECM molecule ED‐A fibronectin induce the differentiation of fibroblasts into myofibroblasts.59 Myofibroblasts contract the granulation tissue and deposit large amounts of ECM molecules. Subsequently, matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPS) control the remodeling process.60 During remodeling cross‐linking and reorientation of collagen fibers occurs, while the number of capillaries regresses returning the vascular density to normal. The immature type III collagen is modified to mature type I collagen.61 At the end of this phase, the myofibroblasts die by apoptosis leaving a rather acellular scar. However, multiple molecular and mechanical factors can affect wound healing after CL(P) surgery and promote fibrosis and scar formation resulting in major functional and aesthetic problems.62, 63, 64
In normal skin, ECM deposition and degradation are balanced. An accumulation of collagen type I and III fibers and other ECM proteins after injury results in a disorganized fiber structure and hypertrophic scar formation.65, 66 Wound tension or mechanical stress is another causative factor for scar formation.67, 68 The skin of the human face is maximally extensible perpendicular to relaxed skin tension lines, implying that tension is minimized when surgical incisions are created along these lines.67
Prolonged inflammation and oxidative stress blocks the remodeling phase and promotes excessive fibrosis and scarring.69, 70, 71 Therefore, stringent control of oxidative and inflammatory factors is important for the outcome of wound repair in skin and muscle. Several factors including the cytokines interleukin‐1 beta (IL‐1 β), interleukin‐6 (IL‐6), tumor necrosis factor alpha (TNF‐α), TGF‐ β1, monocyte chemoattractant protein‐1 (MCP1) and heme can induce fibrosis and scarring. These factors promote inflammatory cell adhesion, migration/proliferation of leukocytes and fibroblasts, and dysregulation of ECM remodeling.60, 72, 73 Thus, to prevent excessive scarring, we need to limit the inflammatory and fibrotic processes and promote regeneration.
5. STEM CELLS REPAIR WOUNDS AND FACILITATE SKIN AND MUSCLE REGENERATION
Stem cells facilitate maintenance and repair processes in skin and muscle by replenishing lost tissue and by creating a regenerative microenvironment. Stem cells have a prolonged self‐renewal capacity and are able to differentiate into various cell types, making them ideal for use in regenerative medicine. Stem cells are required at the site of injury to allow the regeneration of dermal, epidermal74 and muscle tissue.75 Moreover, they promote scarless wound healing by generating a regenerative microenvironment via the secretion of protective factors that can inhibit myofibroblasts in a paracrine fashion.76, 77, 78, 79, 80, 81
Following skin wounding, epidermal stem cells in the basal layer are activated, proliferate and migrate to the site of injury where they contribute to regeneration.82 Muscle repair following injury is facilitated by SCs. Satellite cells are muscle stem cells originating from the CPM that are responsible for postnatal muscle growth, maintenance, and repair.83, 84 They express the paired box transcription factor 7 (Pax7) and are located between the sarcolemma and basal lamina surrounding a single muscle fiber. After the injury, SCs become activated and migrate to the site of injury, proliferate, and differentiate into myoD‐positive myoblasts that fuse to form new multinucleated myosin heavy chain (MyHC)‐positive myofibers or repair damaged myofibers (Figure 3).83, 85, 86 A small portion of these SCs stay quiescent to allow future regeneration cycles. Signaling molecules like insulin‐like growth factor 1 and 2 (IGF‐1, IGF‐2), platelet‐derived growth factor (PDGF), fibroblast growth factor (FGF) and hepatocyte growth factor (HGF) derived from recruited macrophages, injured myofibers and disrupted ECM regulate this process.85, 87 HGF induces the activation of quiescent SCs.87 IGF‐1 and 2 to enhance myogenic proliferation and differentiation, and promote cell survival. PDGF regulates the proliferation and differentiation of myoblasts and supports angiogenisis. FGF is upregulated during muscle regeneration, but its exact role is unclear.
Figure 3.
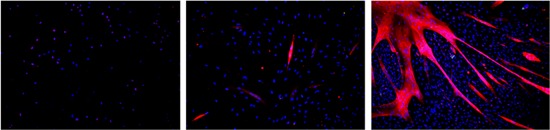
Immunofluorescence staining of muscle fiber formation from masseter satellite cells (SCs): Left: after 3 days of culture, DAPI (blue) stains nuclei, Pax 7 (red), Middle: after 7 days of culture, DAPI (blue), MyHC (red). Right: after 10 days of culture DAPI (blue), MyHC (red). Magnification ×400. DAPI, 4′,6‐diamidino‐2‐phenylindole; MyHC, myosin heavy chain; Pax 7, paired box transcription factor 7 [Color figure can be viewed at wileyonlinelibrary.com]
Muscle‐specific transcription factors like Pax7, myogenic factor 5, MyoD, myogenin, and finally structural proteins like MyHC are sequentially expressed (Figure 3).88
Most of the studies on muscle regeneration have been performed on skeletal muscles of limb and trunk. During embryonic development, the limb and trunk muscles are derived from the somites whereas lip muscles are derived from the CPM in the branchial arches.33 Head muscles also contain less SCs than limb muscles44 and injuries regenerate slower.89, 90 In addition, Pax7 and myogenic regulatory factor 4 levels in limb muscles are lower than in head muscles. SCs from the masseter muscle proliferate more and differentiate later than those from limb muscles.44 SCs from different branchiomeric muscles, however, behave in a similar way. The lower regenerative capacity of branchiomeric muscles compared to limb muscle may impair muscle healing after cleft surgery.90 The reduced blood supply and aberrant muscle orientation in a cleft lip may further impair the regeneration process.41 Thus, stem cells could provide a solution for skin repair, but also for muscle repair.
6. REGENERATIVE MEDICINE TO ATTENUATE SCARRING AND MUSCLE FIBROSIS
To overcome the functional and esthetic problems related to scarring after cleft lip surgery, we present a novel tissue engineering strategy. This involves a combination of umbilical cord blood stem cells with regenerative capacity together with anti‐inflammatory and antifibrotic molecules.
In comparison to adults, fetuses display less scarring following wound healing.91 Fetal wounds differ in several ways from adult wounds, including a reduced inflammatory response. They also have higher expression levels of MMPs compared to their inhibitors, the TIMPs.91 Further, PDGF disappears more rapidly from fetal wounds, that also show lower FGF levels and increased TGF‐β3 levels. In addition, the level of TGF‐β1/2 is lower,92 the biomechanical strain is lower and the ECM is enriched in hyaluronic acid and type III collagen.93
Tissue regeneration is often hampered by the occurrence of fibrosis. TGFβ1 is a pro‐inflammatory factor that recruits macrophages and other inflammatory cells to the wound site, thus contributing to fibrosis and excessive scarring.94 In muscles, TGF‐β1 stimulates the synthesis of collagen and other ECM components and promotes myofibroblast formation and fibrosis.95 Myostatin, also a member of the TGF‐β family inhibits muscle regeneration by reducing the proliferation of stem cells and myoblasts.96 The inhibition of myostatin and TGF‐β may enhance muscle regeneration.97 The proteoglycan decorin has a stimulatory effect on autophagy and inflammation, and an inhibitory effect on angiogenesis by inhibiting both TGF‐β1 and myostatin. This protein can be used to prevent fibrosis and enhance muscle regeneration.98 Decorin also upregulates myogenic genes and promotes skeletal muscle regeneration after injury.99 Losartan, an angiotensin II receptor antagonist, neutralizes the effect of TGF‐β1, reduces fibrosis and has already been used clinically in therapy for myocardial fibrosis.100, 101
Oxidative stress and inflammation can be counteracted by the induction of heme oxygenase (HO) by metalloporphyrins.102 The enzyme HO degrades free heme generating carbon monoxide, ferrous iron/ferritin, and biliverdin/bilirubin, which can be used to reduce inflammation. HO‐1 deficient humans and mice demonstrate chronic inflammatory stress.103, 104 HO‐1 has pro‐angiogenic effects via regulating vascular endothelial growth factor (VEGF) synthesis.105, 106 HO‐2 deficient mice have elevated levels of pro‐inflammatory chemokines including keratinocyte chemoattractant, macrophage inflammatory protein 2 (MIP‐2), and MCP‐1. These mice show delayed wound healing and an exaggerated inflammatory response after corneal epithelial wounding.107, 108
Alternatively, the systemic administration of anti‐inflammatory therapeutics during surgery may attenuate scarring. These include neuropeptide α‐melanocyte stimulating hormone,109 FGF9,110 WNT 3A,111 interleukin 10 (IL‐10),112 interleukin 6 (IL‐6),113 anti‐VEGF antibody,114 TGFβ3114 and CXC chemokine receptor type 4 antagonists.115 Il‐10 downregulates pro‐inflammatory cytokines IL‐6 and IL 8 and may thus attenuate scar formation.112
TGF‐β1 is the main target in antifibrotic therapies that include the administration of FGF basic, insulin IGF‐1, losartan, suramin, decorin, relaxin, interferon‐y, and angiotensin II receptor blockers. Antagonists of the IL‐1 receptor (anakinra; Food and Drug Administration (FDA) approved) reduces TGFβ1 expression and could be used for the reduction of scar formation.116
Over the years, various drugs have been developed that target profibrotic signaling pathways such as TGF‐β and PDGF, to reduce the progression of fibrosis. Still, hardly any drugs are available for the treatment of organ fibrosis. Currently, there are only two FDA approved drugs that are used for the treatment of idiopathic pulmonary fibrosis, namely pirfenidone and nintedanib.117
Pirfenidone, that is, pyridine(5‐methyl‐1‐phenyl‐2‐(1H)‐pyridone), is an oral agent that can elicit anti‐inflammatory, antioxidative, and antiproliferative effects.118 Even though, the exact mechanism of action is still not fully understood, the antifibrotic effect of pirfenidone has been demonstrated in several organs, for example lung, liver, and kidney.118 In human liver cells, it has been shown that it attenuates TGF‐β‐induced mRNA expression of α‐SMA and type I collagen.119 Also in human precision‐cut liver slices a reduction in the gene expression of type I collagen was observed following treatment with pirfenidone.120
Nintedanib, formerly known as BIBF 1120, is an intracellular triple angiokinase inhibitor targeting the VEGF, FGF, and PDGF receptors.121 Nintedanib competitively binds to the adenosine triphosphate binding pocket of these receptors inhibiting their activation and thereby inhibiting the fibrotic process. In addition, nintedanib has also been demonstrated to inhibit members of the proto‐oncogene tyrosine‐protein kinase (Src)‐family (Src, Lyn, and Lck), which are activated in various types of cancer and belong to the non‐receptor tyrosine kinases.122
Galunisertib, LY2157299, is an oral small molecule inhibitor of the TGF‐β receptor I kinase that specifically inhibits SMAD2 phosphorylation.123 Using human precision‐cut liver slices, it has been demonstrated that galunisertib attenuates SMAD2 phosphorylation in healthy and cirrhotic liver tissue and reduces the expression of several collagen subtypes (collagen types I, III, IV, V and VI). In addition, it reduces the expression of numerous genes associated with collagen maturation and homeostasis.124 Interestingly, there is also evidence suggesting that galunisertib can inhibit TGF‐β signaling in human oral keratinocytes.125
Overall, there are three distinct routes to target profibrotic signaling: (a) inhibiting transcription or translation of profibrotic factors, (b) blocking interleukin receptor binding and activation, and (c) interfering with down‐stream signaling events. Since the fibrotic process is highly similar in all organs, therapeutic targets identified in, for instance, the liver might also be used to reduce scar formation following cleft surgery. The three drugs described above have proven antifibrotic efficacy and are therefore ideal candidates for further study in the context of cleft repair.
7. UMBILICAL CORD: FROM WASTE MATERIAL TO SOURCE OF THERAPEUTIC STEM CELLS
Adult stem cells like mesenchymal stem/stromal cells (MSC) can be found throughout the body. Mesenchymal stem cells are currently widely used for regenerative medicine purposes. They may stimulate the regeneration of the injured skin and muscle following surgical lip closure by replenishing the required cells and by providing a microenvironment that attenuates scarring and fibrosis.126 MSCs can differentiate into several types of cells including endothelial cells, myocytes, keratinocytes, and fibroblasts.127, 128, 129 Moreover, stem cells produce several anti‐inflammatory and antifibrotic mediators, and secrete factors that create a regenerative microenvironment.130, 131, 132, 133 Cord blood stem cells embedded in a scaffold secrete for example MMP‐9, mediating collagen degradation in uterine scars, which improves the regeneration of the endometrium, myometrium and blood vessels.134 Administered cord blood stem cells also promote the recruitment of endogenous stem cells.135, 136
The isolation of MSCs from bone marrow or adipose tissue from the baby is rather invasive and can cause complications such as infection, bleeding, and chronic pain.137 Moreover, it is expensive to harvest them and to expand them by controlled cell culture.138 Therefore, there is a need for alternative sources of MSCs. Human umbilical cord blood cells are obtained by a simple, safe, and painless procedure upon birth. Mesenchymal stem cells can be easily isolated from cord blood and preserved for later.139 Since prenatal ultrasound screening allows early detection of a cleft in the 11th to 13th week of gestation,140, 141 the preservation of umbilical cord (blood) upon birth can be planned in an early stage and should be considered as a new strategy to optimize cleft surgery (Figure 4). Cord blood can be collected from the umbilical vein or from the placenta. Only the mononuclear fraction which contains the stem cells is needed for preservation. In addition, stem cells can be harvested simultaneously from the umbilical cord tissue itself. The umbilical cord mesenchymal stem cells (UCMSC) can be taken from the amniotic membrane, the cord lining, Wharton's jelly, and the perivascular region of the umbilical cord.142 The umbilical cord blood and tissue contain a heterogeneous mixture of stem and progenitor cells at different stages of differentiation.138 It is an attractive source of stem cells because it is considered biowaste accompanying the delivery of a baby. We propose here that these UCMSCs could be used during CL(P) surgeries to facilitate skin and muscle tissue regeneration (Figure 4).
Figure 4.
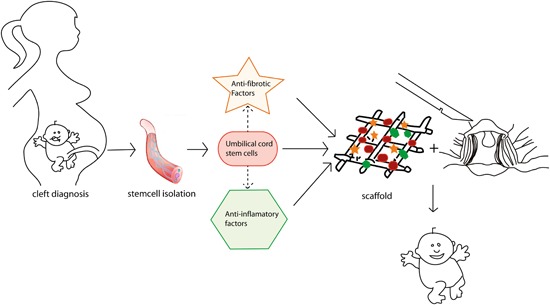
Following detection of a cleft lip and/or palate (CL/P) using prenatal ultrasound screening, cord blood stem cells are isolated upon birth. These stem cells express antifibrotic factors and anti‐inflammatory factors and can be used in tissue engineering strategies in combination with factors targeting inflammation and fibrosis (e.g. anakinra, pirfenidone and nintedanib) within a scaffold during cleft surgery to promote skin and muscle regeneration and function and to inhibit scar formation [Color figure can be viewed at wileyonlinelibrary.com]
The successful use of human umbilical cord blood and tissue mesenchymal stem cells (hUCMSC) has been shown for other fields in a variety of in vitro, animal and human studies. It has been demonstrated that umbilical cord blood cells can differentiate into epithelial cells,143 osteoblasts and adipocytes,144 and also have a myogenic potential. In a study on Duchenne Muscular Dystrophy, myoblasts from Duchenne and Becker muscular dystrophy patients were isolated from the biceps, cocultured with hUCMSCs obtained from healthy babies and analyzed by immunofluorescence microscopy. After 15 days of culturing, dystrophin‐positive myofibers were present demonstrating the differentiation of hUCMSCs into muscle cells in vitro.145 In another in vitro study, hUCMSCs—derived mononuclear cells gave rise to fibroblast‐like cells expressing mesenchymal antigens. When these cells were cultured under promyogenic conditions they expressed myogenic markers like MyoD, MyoG, and MyHC.146 This indicates that hUCMSCs can differentiate into myogenic stem cells in vitro, which could possibly facilitate regeneration of the orbicularis oris muscle after cleft lip surgery.
In vivo, the healing of deep burn wounds in adult Wistar rats was improved with intravenously injected cord blood stem cells compared to controls. The hUCMSCs migrated into the wound and decreased the quantity of inflammatory cells, while neovascularization was increased compared to the control groups.147 A biomimetic scaffold with hUCMSCs and fibrin showed an improved full‐thickness skin wound repair without scar formation in rats compared to controls without scaffold or with a scaffold without hUCMSC.148 Similarly, hUCMSCs in combination with a collagen‐fibrin double layer membrane and a single layer collagen membrane resulted in accelerated wound repair in mice when compared to mice treated without hUCMSC.149
The application of hUCMSCs within a small intestinal submucosa‐derived ECM scaffold to a full thickness excisional wound in mice also enhanced wound repair and angiogenesis at the wound site. An increase in angiogenic growth factors like HGF, VEGF, and angiopoietin was observed.150 Wharton's jelly mesenchymal stem cells also upregulated genes involved in re‐epithelialization, neovascularization, fibroblast proliferation, and migration in an excisional full thickness murine wound model.151 All these in vivo animal studies support the use of umbilical cord stem cells as a novel wound repair strategy.
In patients with Crohn's Disease, the intravenous infusion of allogeneic hUCMSCs improved disease conditions with only mild side effects like fever and respiratory tract infection.152 In atopic dermatitis, a chronic and relapsing skin disease involving pruritus, xerosis, and eczematous lesions, infusion of hUCMSCS was effective without side effects.150 Moreover, also allogeneic hUCMSCs have been successfully used in multiple sclerosis patients improving the patients' symptoms without serious adverse effects.153
Besides effectiveness, the question should also be answered whether it is safe to use hUCMSCs. In children with cerebral palsy (motor disorder in childhood), autologous hUCMSCs improved whole brain connectivity and motor function.154 Similar studies found that infusion of autologous cord blood cells in children with autism spectrum disorder improved behavior and was safe.155, 156 A 6‐year follow‐up study also demonstrated the long‐term safety profile of hUCMSCs infusion for drug‐resistant systemic lupus erythematosus patients. The treatment reduced the disease activity significantly.157 This confirms that the use of hUCMSCs is thus safe and no tumor‐related effects occur.157 A study with allogeneic hUCMSCs in an osteoarthritic patient showed regeneration of cartilage without adverse effects up to 7 years of follow up.158 Allogenic cord blood stem cell transplantation was also successfully used in babies with severe combined immunodeficiency syndrome and led to a reconstitution of hematopoietic cells.159, 160 No long‐term graft vs host disease was observed.
Summarizing, the umbilical cord tissue and umbilical cord blood is an easily accessible source of MSC for the regeneration of skin and muscle tissue. These stem cells can differentiate into the required cell types and secrete regenerative factors.143, 145, 146 hUCMSCs are save for use in children and adults.154, 155, 156, 157 while no immunogenic rejection was observed after allogeneic transplantation.152, 153 The low immunogenicity of hUCMSCs would also allow the use of hUCMSCs from a central cord blood stem cell bank when autologous stem cells are not available.
We therefore postulate that cord blood stem cells can be used as a novel adjuvant strategy in cleft surgery to prevent fibrosis and scarring to improve the outcome of the surgery. This will eventually lead to a better quality of life for patients with CL(P).
8. CLINICAL CHALLENGES FOR SCAFFOLD‐BASED CORD BLOOD STEM CELLS WITH ANTI‐FIBROTIC/ANTI‐INFLAMMATORY FACTORS
Direct injection of cord blood stem cells into a wound site is not always effective.134 In muscle tissue, isolated stem cells can be applied to the site of injury to improve regeneration.161 However, the success rate after isolation and injection of stem cells has been low. Stem cells seem to lose their migratory and regenerative potential and often die.162
Thus, stem cell therapy is complicated by the limited number of cells surviving after administration at the wound site. This is due to the harsh conditions within the wound microenvironment characterized by a wealth of pro‐oxidative and pro‐inflammatory mediators, and only limited blood flow.163, 164, 165 Stem cells inducing anti‐apoptotic, anti‐inflammatory, and anti‐oxidative genes can better withstand these insults and have a better chance of surviving.163
Cytoprotective genes include enzyme systems such as HO‐1, glutathione S‐transferase, dismutases, catalases, and peroxidases. HO‐1 induction improves stem cell survival and improves the outcome of stem cell therapy.163, 166, 167 In contrast, abrogation of HO‐activity decreases the efficacy of stem cell therapy.168, 169 Preconditioning strategies in which cytoprotective pathways (e.g. via nrf2 activation) are activated in cord blood stem cells could, therefore, promote their survival and achieve a better outcome of cleft surgery.170, 171 Alternatively, the administration of antioxidants (vitamins A, C, and E, glutathione/NAC, mannose‐specific lectins, and bilirubin) could protect stem cells from dying.172, 173, 174, 175
Anti‐inflammatory and antifibrotic factors and stem cells can be applied in a scaffold. Scaffolds are used as an artificial ECM, which supports cell attachment, proliferation, and differentiation of cells.176, 177 The scaffold guides the newly formed tissue and allows ingrowth of blood vessels and nerves crucial for cell survival. Different types of scaffolds are available prepared from a wide range of biomaterials. Skin and muscle scaffolds can be of natural origins such as fibrin, alginate or collagen, or can be synthetic such as polypropylene, polyesters, polyurethanes, and polyisocyanopeptide hydrogel.178, 179 They can be produced with a variety of methods, such as 3D printing and electro‐spinning. Synthetic biomaterials can be degradable or nondegradable. The advantages of synthetic biomaterials are their low immunogenicity and reproducible quality, while they can be custom made with the required mechanical properties and shape.180 Scaffolds can be seeded with cord blood stem cells together with the anti‐inflammatory and anti‐fibrotic factors to create a regenerative microenvironment that facilitates tissue regeneration and prevents scar formation. Tissue engineering/regenerative medicine scaffolds require the appropriate physical and cellular signals to promote tissue regeneration.181 The sum of the biochemical signals and biophysical cues from the microenvironment dictate the fate of stem cells.182 The sum of pro‐ and anti‐inflammatory and fibrotic factors, the shape of scaffolds, stiffness of the matrix, nanotopography and the presence of biofunctional groups as RGD‐sequences discriminates between scarring and regeration of tissue.183 Mechanical cues may be applied in tissue engineering by adjusting the type, stiffness and architecture of the scaffolds facilitating the differentiation of cord blood stem cells into either skin or muscle tissue.68
9. CONCLUSIONS
The functional and esthetical outcome of cleft palate repair is often hampered by fibrosis and scarring. In this review, we discuss novel tissue engineering strategies to promote muscle and skin regeneration, while preventing scarring. Umbilical cord blood stem cells are a promising, safe, and non‐invasive source of stem cells that can be combined in a scaffold together with anti‐inflammatory and anti‐fibrotic molecules. Overexpression of cytoprotective molecules in these stem cells by preconditioning before applying them to the wound area may not only increase stem cell survival but also further contribute to a regenerating microenvironment. We expect that this novel strategy improves both muscle and skin regeneration after cleft repair resulting in a better functional and esthetic outcome.
Schreurs M, Suttorp CM, Mutsaers HAM, et al. Tissue engineering strategies combining molecular targets against inflammation and fibrosis, and umbilical cord blood stem cells to improve hampered muscle and skin regeneration following cleft repair. Med Res Rev. 2020;40:9‐26. 10.1002/med.21594
References
REFERENCES
- 1. Gundlach KKH, Maus C. Epidemiological studies on the frequency of clefts in Europe and world‐wide. J. Craniomaxillofac Surg. 2006;34(Suppl 2):1‐2. [DOI] [PubMed] [Google Scholar]
- 2. Mossey PA, Little J, Munger RG, Dixon MJ, Shaw WC. Cleft lip and palate. Lancet. 2009;374(9703):1773‐1785. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka SA, Mahabir RC, Jupiter DC, Menezes JM. Updating the epidemiology of cleft lip with or without cleft palate. Plast Reconstr Surg. 2012;129(3):511e‐518e. [DOI] [PubMed] [Google Scholar]
- 4. Harville EW, Wilcox AJ, Lie RT, Vindenes H, Åbyholm F. Cleft lip and palate versus cleft lip only: Are they distinct defects? Am J Epidemiol. 2005;162(5):448‐453. [DOI] [PubMed] [Google Scholar]
- 5. Venkatesh R. Syndromes and anomalies associated with cleft. Indian J Plast Surg. 2009;42(Suppl):S51‐S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drew SJ. Clefting syndromes. Atlas Oral Maxillofac Surg Clin North Am. 2014;22(2):175‐181. [DOI] [PubMed] [Google Scholar]
- 7. Watkins SE, Meyer RE, Strauss RP, Aylsworth AS. Classification, epidemiology, and genetics of orofacial clefts. Clin Plast Surg. 2014;41(2):149‐163. [DOI] [PubMed] [Google Scholar]
- 8. Shi M, Wehby GL, Murray JC. Review on genetic variants and maternal smoking in the etiology of oral clefts and other birth defects. Birth Defects Research C Embryo Today. 2008;84(1):16‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeRoo LA, Wilcox AJ, Drevon CA, Lie RT. First‐trimester maternal alcohol consumption and the risk of infant oral clefts in Norway: a population‐based case‐control study. Am J Epidemiol. 2008;168(6):638‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webster WS, Howe AM, Abela D, Oakes DJ. The relationship between cleft lip, maxillary hypoplasia, hypoxia and phenytoin. Curr Pharm Des. 2006;12(12):1431‐1448. [DOI] [PubMed] [Google Scholar]
- 11. Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237.e231‐237.e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bille C, Skytthe A, Vach W, et al. Parent's age and the risk of oral clefts. Epidemiology. 2005;16(3):311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herkrath AP, Herkrath FJ, Rebelo MA, Vettore MV. Parental age as a risk factor for non‐syndromic oral clefts: a meta‐analysis. J Dent. 2012;40(1):3‐14. [DOI] [PubMed] [Google Scholar]
- 14. Shkoukani MA, Chen M, Vong A. Cleft lip – a comprehensive review. Front Pediatr. 2013;1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vermeij‐Keers C, Rozendaal AM, Luijsterburg AJM, et al. Subphenotyping and classification of cleft lip and alveolus in adult unoperated patients: a new embryological approach. Cleft Palate Craniofac J. 2018;55(9):1267‐1276. [DOI] [PubMed] [Google Scholar]
- 16. Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12(3):167‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asllanaj B, Kragt L, Voshol I, et al. Dentition patterns in different unilateral cleft lip subphenotypes. J Dent Res. 2017;96(13):1482‐1489. [DOI] [PubMed] [Google Scholar]
- 18. Flynn T, Moller C, Jonsson R, Lohmander A. The high prevalence of otitis media with effusion in children with cleft lip and palate as compared to children without clefts. Int J Pediatr Otorhinolaryngol. 2009;73(10):1441‐1446. [DOI] [PubMed] [Google Scholar]
- 19. Sinko K, Jagsch R, Prechtl V, Watzinger F, Hollmann K, Baumann A. Evaluation of esthetic, functional, and quality‐of‐life outcome in adult cleft lip and palate patients. Cleft Palate Craniofac J. 2005;42(4):355‐361. [DOI] [PubMed] [Google Scholar]
- 20. Raud Westberg L, Hoglund Santamarta L, Karlsson J, Nyberg J, Neovius E, Lohmander A. Speech outcome in young children born with unilateral cleft lip and palate treated with one‐ or two‐stage palatal repair and the impact of early intervention. Logoped Phoniatr Vocol. 2017:1‐9. 10.1080/14015439.2017.1390606 [DOI] [PubMed] [Google Scholar]
- 21. Shi B, Losee JE. The impact of cleft lip and palate repair on maxillofacial growth. Int J Oral Sci. 2015;7(1):14‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao YF, Mars M. Long‐term effects of clefts on craniofacial morphology in patients with unilateral cleft lip and palate. Cleft Palate Craniofac J. 2005;42(6):601‐609. [DOI] [PubMed] [Google Scholar]
- 23. Shaw W, Semb G. The Scandcleft randomised trials of primary surgery for unilateral cleft lip and palate: 11. What next? J Plast Surg Hand Surg. 2017;51(1):88‐93. [DOI] [PubMed] [Google Scholar]
- 24. Farronato G, Kairyte L, Giannini L, Galbiati G, Maspero C. How various surgical protocols of the unilateral cleft lip and palate influence the facial growth and possible orthodontic problems? Which is the best timing of lip, palate and alveolus repair? literature review. Stomatologija. 2014;16(2):53‐60. [PubMed] [Google Scholar]
- 25. Biggs LC, Naridze RL, DeMali KA, et al. Interferon regulatory factor 6 regulates keratinocyte migration. J Cell Sci. 2014;127(13):2840‐2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lazzeri D, Viacava P, Pollina LE, et al. Dystrophic‐like alterations characterize orbicularis oris and palatopharyngeal muscles in patients affected by cleft lip and palate. Cleft Palate Craniofac J. 2008;45(6):587‐591. [DOI] [PubMed] [Google Scholar]
- 27. Krey KF, Dannhauer KH, Hemprich A, et al. Cytophotometrical and immunohistochemical analysis of soft palate muscles of children with isolated cleft palate and combined cleft lip and palate. Exp Toxicol Pathol. 2002;54(1):69‐75. [DOI] [PubMed] [Google Scholar]
- 28. Rahimov F, Jugessur A, Murray JC. Genetics of nonsyndromic orofacial clefts. Cleft Palate Craniofac J. 2012;49(1):73‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rinon A, Lazar S, Marshall H, et al. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development. 2007;134(17):3065‐3075. [DOI] [PubMed] [Google Scholar]
- 30. Jugessur A, Murray JC. Orofacial clefting: recent insights into a complex trait. Curr Opin Gene Dev. 2005;15(3):270‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang R, Bush JO, Lidral AC. Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn. 2006;235(5):1152‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leslie EJ, Marazita ML. Genetics of cleft lip and cleft palate. Am J Med Genet C. 2013;163c(4):246‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Graham A, Okabe M, Quinlan R. The role of the endoderm in the development and evolution of the pharyngeal arches. J Anat. 2005;207(5):479‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tzahor E. Heart and craniofacial muscle development: a new developmental theme of distinct myogenic fields. Dev Biol. 2009;327(2):273‐279. [DOI] [PubMed] [Google Scholar]
- 35. Gasser RF. The development of the facial muscles in man. Am J Anat. 1967;120(2):357‐375. [Google Scholar]
- 36. Rogers CR, Meara JG, Mulliken JB. The philtrum in cleft lip: review of anatomy and techniques for construction. J Craniofac Surg. 2014;25(1):9‐13. [DOI] [PubMed] [Google Scholar]
- 37. Grabb WC, Rosenstein SW, Bzoch KR. Cleft Lip and Palate; Surgical, Dental, and Speech Aspects. Boston, Mass: Little, Brown and Company; 1971. [Google Scholar]
- 38. Dong C, Zheng S. Immunohistochemical analysis of orbicularis oris muscle fiber distribution at the philtrum in healthy infants. Int J Pediatr Otorhinolaryngol. 2015;79(12):2208‐2212. [DOI] [PubMed] [Google Scholar]
- 39. Spauwen PH, Hardjowasito W, Boersma J, Latief BS. Dental cast study of adult patients with untreated unilateral cleft lip or cleft lip and palate in indonesia compared with surgically treated patients in The Netherlands. Cleft Palate Craniofac J. 1993;30(3):313‐319. [DOI] [PubMed] [Google Scholar]
- 40. Gundlach KK, Pfeifer G. The arrangement of muscle fibres in cleft lips. J Maxillofac Surg. 1979;7(2):109‐116. [DOI] [PubMed] [Google Scholar]
- 41. Wijayaweera CJ, Amaratunga NA, Angunawela P. Arrangement of the orbicularis oris muscle in different types of cleft lips. J Craniofac Surg. 2000;11(3):232‐235. [DOI] [PubMed] [Google Scholar]
- 42. Wijayaweera CJ, de S, Amaratunga A, Angunawela P. Histopathology of cleft lip muscle: an enzyme histochemical and electron microscopic study. Asian J Oral Maxillofac Surg. 2002;14(4):219‐225. [Google Scholar]
- 43. Lindman R, Paulin G, Stal PS. Morphological characterization of the levator veli palatini muscle in children born with cleft palates. Cleft Palate Craniofac J. 2001;38(5):438‐448. [DOI] [PubMed] [Google Scholar]
- 44. Ono Y, Boldrin L, Knopp P, Morgan JE, Zammit PS. Muscle satellite cells are a functionally heterogeneous population in both somite‐derived and branchiomeric muscles. Dev Biol. 2010;337(1):29‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schendel SA, Pearl RM, De'Armond SJ. Pathophysiology of cleft lip muscle. Plast Reconstr Surg. 1989;83(5):777‐784. [DOI] [PubMed] [Google Scholar]
- 46. Heycock MH. A field guide to cleft‐lip repair. Br J Surg. 1971;58(8):567‐570. [DOI] [PubMed] [Google Scholar]
- 47. Adetayo AM, James O, Adeyemo WL, Ogunlewe MO, Butali A. Unilateral cleft lip repair: a comparison of treatment outcome with two surgical techniques using quantitative (anthropometry) assessment. J Korean Assoc Oral Maxillofac Surg. 2018;44(1):3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adeyemo WL, James O, Adeyemi MO, et al. An evaluation of surgical outcome of bilateral cleft lip surgery using a modified Millard's (Fork Flap) technique. Afr J Paediatr Surg. 2013;10(4):307‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Semb G, Enemark H, Friede H, et al. A Scandcleft randomised trials of primary surgery for unilateral cleft lip and palate: 1. Planning and management. J Plast Surg Hand Surg. 2017;51(1):2‐13. [DOI] [PubMed] [Google Scholar]
- 50. Hammoudeh JA, Imahiyerobo TA, Liang F, et al. Early cleft lip repair revisited: a safe and effective approach utilizing a multidisciplinary protocol. Plast Reconstr Surg Glob Open. 2017;5(6):e1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holland S, Gabbay JS, Heller JB, et al. Delayed closure of the hard palate leads to speech problems and deleterious maxillary growth. Plast Reconstr Surg. 2007;119(4):1302‐1310. [DOI] [PubMed] [Google Scholar]
- 52. Bonanthaya K, Rao DD, Shetty P, Uguru C. Correlation of vermilion symmetry to alveolar cleft defect in unilateral cleft lip repair. Int J Oral Maxillofac Surg. 2016;45(6):688‐691. [DOI] [PubMed] [Google Scholar]
- 53. Li Y, Shi B, Song QG, Zuo H, Zheng Q. Effects of lip repair on maxillary growth and facial soft tissue development in patients with a complete unilateral cleft of lip, alveolus and palate. J Craniomaxillofac Surg. 2006;34(6):355‐361. [DOI] [PubMed] [Google Scholar]
- 54. Mulliken JB, Wu JK, Padwa BL. Repair of bilateral cleft lip: review, revisions, and reflections. J Craniofac Surg. 2003;14(5):609‐620. [DOI] [PubMed] [Google Scholar]
- 55. Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4(3):119‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wagener FA, Scharstuhl A, Tyrrell RM, et al. The heme‐heme oxygenase system in wound healing; implications for scar formation. Curr Drug Targets. 2010;11(12):1571‐1585. [DOI] [PubMed] [Google Scholar]
- 57. Satish L, Kathju S. Cellular and molecular characteristics of scarless versus fibrotic wound healing. Dermatol Res Pract. 2010;2010:790234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mendonca RJ, Coutinho‐Netto J. Cellular aspects of wound healing. An Bras Dermatol. 2009;84(3):257‐262. [DOI] [PubMed] [Google Scholar]
- 59. Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500‐503. [DOI] [PubMed] [Google Scholar]
- 61. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1‐2):113‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lian N, Li T. Growth factor pathways in hypertrophic scars: molecular pathogenesis and therapeutic implications. Biomed Pharmacother. 2016;84:42‐50. [DOI] [PubMed] [Google Scholar]
- 63. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lundvig DM, Immenschuh S, Wagener FA. Heme oxygenase inflammation, and fibrosis: the good, the bad, and the ugly?. Front Pharmacol. 2012;3:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grieb G, Steffens G, Pallua N, Bernhagen J, Bucala R. Circulating fibrocytes‐‐biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol. 2011;291:1‐19. [DOI] [PubMed] [Google Scholar]
- 66. Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011;194(1):25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Son D, Harijan A. Overview of surgical scar prevention and management. J Korean Med Sci. 2014;29(6):751‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brouwer KM, Lundvig DM, Middelkoop E, Wagener FA, Von, den Hoff JW. Mechanical cues in orofacial tissue engineering and regenerative medicine. Wound Repair Regen. 2015;23(3):302‐311. [DOI] [PubMed] [Google Scholar]
- 69. Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612‐619. [DOI] [PubMed] [Google Scholar]
- 70. Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68‐69:106‐121. [DOI] [PubMed] [Google Scholar]
- 71. O'Reilly S. Toll like receptors in systemic sclerosis: an emerging target. Immunol Lett. 2018;195:2‐8. [DOI] [PubMed] [Google Scholar]
- 72. van der Veer WM, Bloemen MC, Ulrich MM, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns. 2009;35(1):15‐29. [DOI] [PubMed] [Google Scholar]
- 73. Jeney V, Balla J, Yachie A, et al. Pro‐oxidant and cytotoxic effects of circulating heme. Blood. 2002;100(3):879‐887. [DOI] [PubMed] [Google Scholar]
- 74. Chen D, Hao H, Tong C, et al. Transdifferentiation of umbilical cord‐derived mesenchymal stem cells into epidermal‐like cells by the mimicking skin microenvironment. Int J Low Extrem Wounds. 2015;14(2):136‐145. [DOI] [PubMed] [Google Scholar]
- 75. Bana N, Sanooghi D, Soleimani M, et al. A comparative study to evaluate myogenic differentiation potential of human chorion versus umbilical cord blood‐derived mesenchymal stem cells. Tissue Cell. 2017;49(4):495‐502. [DOI] [PubMed] [Google Scholar]
- 76. Li M, Luan F, Zhao Y, et al. Mesenchymal stem cell‐conditioned medium accelerates wound healing with fewer scars. Int Wound J. 2017;14(1):64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mortier L, Delesalle F, Formstecher P, Polakowska R. Human umbilical cord blood cells form epidermis in the skin equivalent model. Exp Dermatol. 2010;19(10):929‐930. [DOI] [PubMed] [Google Scholar]
- 78. Maarse W, Pistorius LR, Van Eeten WK, et al. Prenatal ultrasound screening for orofacial clefts. Ultrasound Obstet Gynecol. 2011;38(4):434‐439. [DOI] [PubMed] [Google Scholar]
- 79. Lim IJ, Phan TT. Epithelial and mesenchymal stem cells from the umbilical cord lining membrane. Cell Transplant. 2014;23(4‐5):497‐503. [DOI] [PubMed] [Google Scholar]
- 80. Zhang CP, Fu XB. Therapeutic potential of stem cells in skin repair and regeneration. Chin J Traumatol. 2008;11(4):209‐221. [DOI] [PubMed] [Google Scholar]
- 81. Kanji S, Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators Inflamm. 2017;2017:5217967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fuchs E. Epithelial skin biology: three decades of developmental biology, a hundred questions answered and a thousand new ones to address. Curr Top Dev Biol. 2016;116:357‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Carvajal Monroy PL, Grefte S, Kuijpers‐Jagtman AM, et al. A rat model for muscle regeneration in the soft palate. PLoS One. 2013;8(3):e59193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Carvajal Monroy PL, Yablonka‐Reuveni Z, Grefte S, Kuijpers‐Jagtman AM, Wagener FA, Von den Hoff JW. Isolation and characterization of satellite cells from rat head branchiomeric muscles. J Vis Exp. 2015;101:e52802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des. 2010;16(8):906‐914. [DOI] [PubMed] [Google Scholar]
- 86. Carvajal Monroy PL, Grefte S, Kuijpers‐Jagtman AM, Von den Hoff JW, Wagener FA. Neonatal satellite cells form small myotubes in vitro. J Dent Res. 2017;96(3):331‐338. [DOI] [PubMed] [Google Scholar]
- 87. Ten Broek RW, Grefte S, Von, den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224(1):7‐16. [DOI] [PubMed] [Google Scholar]
- 88. Karalaki M, Fili S, Philippou A, Koutsilieris M. Muscle regeneration: cellular and molecular events. In Vivo. 2009;23(5):779‐796. [PubMed] [Google Scholar]
- 89. Pavlath GK, Thaloor D, Rando TA, Cheong M, English AW, Zheng B. Heterogeneity among muscle precursor cells in adult skeletal muscles with differing regenerative capacities. Dev Dyn. 1998;212(4):495‐508. [DOI] [PubMed] [Google Scholar]
- 90. Carvajal Monroy PL, Grefte S, Kuijpers‐Jagtman AM, Von den Hoff JW, Wagener FA. Neonatal satellite cells form small myotubes in vitro. J Dent Res. 2016;96(3):331‐338. [DOI] [PubMed] [Google Scholar]
- 91. Dang CM, Beanes SR, Lee H, Zhang X, Soo C, Ting K. Scarless fetal wounds are associated with an increased matrix metalloproteinase‐to‐tissue‐derived inhibitor of metalloproteinase ratio. Plast Reconstr Surg. 2003;111(7):2273‐2285. [DOI] [PubMed] [Google Scholar]
- 92. Walraven M, Gouverneur M, Middelkoop E, Beelen RH, Ulrich MM. Altered TGF‐beta signaling in fetal fibroblasts: what is known about the underlying mechanisms? Wound Repair Regen. 2014;22(1):3‐13. [DOI] [PubMed] [Google Scholar]
- 93. Wang P‐H, Huang B‐S, Horng H‐C, Yeh C‐C, Chen Y‐J. Wound healing. J Chin Med Assoc. 2018;2(81):94‐101. [DOI] [PubMed] [Google Scholar]
- 94. Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF‐beta family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2(1):18‐28. [PMC free article] [PubMed] [Google Scholar]
- 95. Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286‐1292. [DOI] [PubMed] [Google Scholar]
- 96. Zhu J, Li Y, Shen W, et al. Relationships between transforming growth factor‐beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem. 2007;282(35):25852‐25863. [DOI] [PubMed] [Google Scholar]
- 97. Wagner KR. Muscle regeneration through myostatin inhibition. Curr Opin Rheumatol. 2005;17(6):720‐724. [DOI] [PubMed] [Google Scholar]
- 98. Kishioka Y, Thomas M, Wakamatsu J, et al. Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. J Cell Physiol. 2008;215(3):856‐867. [DOI] [PubMed] [Google Scholar]
- 99. Li Y, Li J, Zhu J, et al. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther. 2007;15(9):1616‐1622. [DOI] [PubMed] [Google Scholar]
- 100. Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008;36(8):1548‐1554. [DOI] [PubMed] [Google Scholar]
- 101. Shibasaki Y, Nishiue T, Masaki H, et al. Impact of the angiotensin II receptor antagonist, losartan, on myocardial fibrosis in patients with end‐stage renal disease: assessment by ultrasonic integrated backscatter and biochemical markers. Hypertens Res. 2005;28(10):787‐795. [DOI] [PubMed] [Google Scholar]
- 102. Shan Y, Pepe J, Lu TH, Elbirt KK, Lambrecht RW, Bonkovsky HL. Induction of the heme oxygenase‐1 gene by metalloporphyrins. Arch Biochem Biophys. 2000;380(2):219‐227. [DOI] [PubMed] [Google Scholar]
- 103. Yachie A, Niida Y, Wada T, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase‐1 deficiency. J Clin Invest. 1999;103(1):129‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kapturczak MH, Wasserfall C, Brusko T, et al. Heme oxygenase‐1 modulates early inflammatory responses: evidence from the heme oxygenase‐1‐deficient mouse. Am J Pathol. 2004;165(3):1045‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bussolati B, Ahmed A, Pemberton H, et al. Bifunctional role for VEGF‐induced heme oxygenase‐1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103(3):761‐766. [DOI] [PubMed] [Google Scholar]
- 106. Bussolati B, Mason JC. Dual role of VEGF‐induced heme‐oxygenase‐1 in angiogenesis. Antioxid Redox Signal. 2006;8(7‐8):1153‐1163. [DOI] [PubMed] [Google Scholar]
- 107. Seta F, Bellner L, Rezzani R, et al. Heme oxygenase‐2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol. 2006;169(5):1612‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bellner L, Wolstein J, Patil KA, Dunn MW, Laniado‐Schwartzman M. Biliverdin rescues the HO‐2 null mouse phenotype of unresolved chronic inflammation following corneal epithelial injury. Invest Ophthalmol Vis Sci. 2011;52(6):3246‐3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. de Souza KS, Cantaruti TA, Azevedo GM, Jr. , et al. Improved cutaneous wound healing after intraperitoneal injection of alpha‐melanocyte‐stimulating hormone. Exp Dermatol. 2015;24(3):198‐203. [DOI] [PubMed] [Google Scholar]
- 110. Gay D, Kwon O, Zhang Z, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19(7):916‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Whyte JL, Smith AA, Liu B, et al. Augmenting endogenous Wnt signaling improves skin wound healing. PLoS One. 2013;8(10):e76883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. King A, Balaji S, Le LD, Crombleholme TM, Keswani SG. Regenerative wound healing: the role of interleukin‐10. Adv Wound Care. 2014;3(4):315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gallucci RM, Sugawara T, Yucesoy B, et al. Interleukin‐6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001;21(8):603‐609. [DOI] [PubMed] [Google Scholar]
- 114. Moore AL, Marshall CD, Barnes LA, Murphy MP, Ransom RC, Longaker MT. Scarless wound healing: transitioning from fetal research to regenerative healing. Wiley Interdiscip Rev Dev Biol. 2018;7(2):e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ding J, Ma Z, Liu H, et al. The therapeutic potential of a C‐X‐C chemokine receptor type 4 (CXCR‐4) antagonist on hypertrophic scarring in vivo. Wound Repair Regen. 2014;22(5):622‐630. [DOI] [PubMed] [Google Scholar]
- 116. Yan C, Gao N, Sun H, et al. Targetin imbalance between IL‐1beta and IL‐1 receptor antagonist ameliorates delayed epithelium wound healing in diabetic mouse corneas. Am J Pathol. 2016;186(6):1466‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Raghu G, Selman M. Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med. 2015;191(3):252‐254. [DOI] [PubMed] [Google Scholar]
- 118. Macias‐Barragan J, Sandoval‐Rodriguez A, Navarro‐Partida J, Armendariz‐Borunda J. The multifaceted role of pirfenidone and its novel targets. Fibrogenesis Tissue Repair. 2010;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhao XY, Zeng X, Li XM, Wang TL, Wang BE. Pirfenidone inhibits carbon tetrachloride‐ and albumin complex‐induced liver fibrosis in rodents by preventing activation of hepatic stellate cells. Clin Exp Pharmacol Physiol. 2009;36(10):963‐968. [DOI] [PubMed] [Google Scholar]
- 120. Westra IM, Mutsaers HA, Luangmonkong T, et al. Human precision‐cut liver slices as a model to test antifibrotic drugs in the early onset of liver fibrosis. Toxicol In Vitro. 2016;35:77‐85. [DOI] [PubMed] [Google Scholar]
- 121. Wuyts WA, Kolb M, Stowasser S, Stansen W, Huggins JT, Raghu G. First data on efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis and forced vital capacity of </=50% of predicted value. Lung. 2016;194(5):739‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774‐4782. [DOI] [PubMed] [Google Scholar]
- 123. Herbertz S, Sawyer JS, Stauber AJ, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor‐beta signaling pathway. Drug Des Devel Ther. 2015;9:4479‐4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Luangmonkong T, Suriguga S, Bigaeva E, et al. Evaluating the antifibrotic potency of galunisertib in a human ex vivo model of liver fibrosis. Br J Pharmacol. 2017;174(18):3107‐3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang W, Xiong H, Hu Z, et al. Experimental study on TGF‐beta1‐mediated CD147 expression in oral submucous fibrosis. Oral Dis. 2018;24(6):993‐1000. [DOI] [PubMed] [Google Scholar]
- 126. Kitajima Y, Luo L, et al. Potency of umbilical cord blood‐ and Wharton's jelly‐derived mesenchymal stem cells for scarless wound healing. Sci Rep. 2016;6:18844 https://doi.org/H, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581‐2587. [DOI] [PubMed] [Google Scholar]
- 128. Nunes VA, Cavacana N, Canovas M, Strauss BE, Zatz M. Stem cells from umbilical cord blood differentiate into myotubes and express dystrophin in vitro only after exposure to in vivo muscle environment. Biol Cell. 2007;99(4):185‐196. [DOI] [PubMed] [Google Scholar]
- 129. Brzoska E, Grabowska I, Hoser G, et al. Participation of stem cells from human cord blood in skeletal muscle regeneration of SCID mice. Exp Hematol. 2006;34(9):1262‐1270. [DOI] [PubMed] [Google Scholar]
- 130. Caplan AI, Sorrell JM. The MSC curtain that stops the immune system. Immunol Lett. 2015;168(2):136‐139. [DOI] [PubMed] [Google Scholar]
- 131. Liao Y, Ivanova L, Zhu H, et al. Cord blood‐derived stem cells suppress fibrosis and may prevent malignant progression in recessive dystrophic epidermolysis bullosa. Stem Cells. 2018;36(12):1839‐1850. [DOI] [PubMed] [Google Scholar]
- 132. Moroncini G, Paolini C, Orlando F, et al. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PLoS One. 2018;13(6):e0196048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu B, Ding F, Hu D, et al. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial‐to‐mesenchymal transition via the TLR4/NF‐kappaB signaling pathway in vivo and in vitro. Stem Cell Res Ther. 2018;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Xu L, Ding L, Wang L, et al. Umbilical cord‐derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP‐9 in rat uterine scars. Stem Cell Res Ther. 2017;8(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Shin L, Peterson DA. Human mesenchymal stem cell grafts enhance normal and impaired wound healing by recruiting existing endogenous tissue stem/progenitor cells. Stem Cells Transl Med. 2013;2(1):33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kim J, Lee JH, Yeo SM, Chung HM, Chae JI. Stem cell recruitment factors secreted from cord blood‐derived stem cells that are not secreted from mature endothelial cells enhance wound healing. In Vitro Cell Dev Biol Anim. 2014;50(2):146‐154. [DOI] [PubMed] [Google Scholar]
- 137. Dandoy CE, Ardura MI, Papanicolaou GA, Auletta JJ. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis. Bone Marrow Transplant. 2017;52(8):1091‐1106. [DOI] [PubMed] [Google Scholar]
- 138. Harris DT. Stem cell banking for regenerative and personalized medicine. Biomedicines. 2014;2(1):50‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. El Omar R, Beroud J, Stoltz JF, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell‐based therapies? Tissue Eng Part B Rev. 2014;20(5):523‐544. [DOI] [PubMed] [Google Scholar]
- 140. Maarse W, Berge SJ, Pistorius L, et al. Diagnostic accuracy of transabdominal ultrasound in detecting prenatal cleft lip and palate: a systematic review. Ultrasound Obstet Gynecol. 2010;35(4):495‐502. [DOI] [PubMed] [Google Scholar]
- 141. Sepulveda W, Wong AE, Martinez‐Ten P, Perez‐Pedregosa J. Retronasal triangle: a sonographic landmark for the screening of cleft palate in the first trimester. Ultrasound Obstet Gynecol. 2010;35(1):7‐13. [DOI] [PubMed] [Google Scholar]
- 142. Araujo AB, Salton GD, Furlan JM, et al. Comparison of human mesenchymal stromal cells from four neonatal tissues: amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy. 2017;19(5):577‐585. [DOI] [PubMed] [Google Scholar]
- 143. Kamolz LP, Kolbus A, Wick N, et al. Cultured human epithelium: human umbilical cord blood stem cells differentiate into keratinocytes under in vitro conditions. Burns. 2006;32(1):16‐19. [DOI] [PubMed] [Google Scholar]
- 144. Shi S, Jia S, Liu J, Chen G. Accelerated regeneration of skin injury by co‐transplantation of mesenchymal stem cells from Wharton's jelly of the human umbilical cord mixed with microparticles. Cell Biochem Biophys. 2015;71(2):951‐956. [DOI] [PubMed] [Google Scholar]
- 145. Jazedje T, Secco M, Vieira NM, et al. Stem cells from umbilical cord blood do have myogenic potential, with and without differentiation induction in vitro. J Transl Med. 2009;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gang EJ, Jeong JA, Hong SH, et al. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22(4):617‐624. [DOI] [PubMed] [Google Scholar]
- 147. Liu L, Yu Y, Hou Y, et al. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9(2):e88348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Montanucci P, di Pasquali C, Ferri I, et al. Human umbilical cord wharton jelly‐derived adult mesenchymal stem cells, in biohybrid scaffolds, for experimental skin regeneration. Stem Cells Int. 2017;2017:1472642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nan W, Liu R, Chen H, et al. Umbilical cord mesenchymal stem cells combined with a collagenfibrin double‐layered membrane accelerates wound healing. Wounds. 2015;27(5):134‐140. [PubMed] [Google Scholar]
- 150. Kim HS, Lee JH, Roh KH, Jun HJ, Kang KS, Kim TY. Clinical trial of human umbilical cord blood‐derived stem cells for the treatment of moderate‐to‐severe atopic dermatitis: phase I/IIa studies. Stem Cells. 2017;35(1):248‐255. [DOI] [PubMed] [Google Scholar]
- 151. Arno AI, Amini‐Nik S, Blit PH, et al. Human Wharton's jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther. 2014;5(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical cord mesenchymal stem cell treatment for Crohn's disease: a randomized controlled clinical trial. Gut Liver. 2018;12(1):73‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Meng M, Liu Y, Wang W, et al. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am J Transl Res. 2018;10(1):212‐223. [PMC free article] [PubMed] [Google Scholar]
- 154. Sun JM, Song AW, Case LE, et al. Effect of autologous cord blood infusion on motor function and brain connectivity in young children with cerebral palsy: a randomized, placebo‐controlled trial. Stem Cells Transl Med. 2017;6(12):2071‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chez M, Lepage C, Parise C, Dang‐Chu A, Hankins A, Carroll M. Safety and observations from a placebo‐controlled, crossover study to assess use of autologous umbilical cord blood stem cells to improve symptoms in children with autism. Stem Cells Transl Med. 2018;7(4):333‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Dawson G, Sun JM, Davlantis KS, et al. Autologous cord blood infusions are safe and feasible in young children with autism spectrum disorder: results of a single‐center phase I open‐label trial. Stem Cells Transl Med. 2017;6(5):1332‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wang D, Niu L, Feng X, et al. Long‐term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6‐year follow‐up study. Clin Exp Med. 2017;17(3):333‐340. [DOI] [PubMed] [Google Scholar]
- 158. Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood‐derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof‐of‐concept with 7 years of extended follow‐up. Stem Cells Transl Med. 2017;6(2):613‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Schonberger S, Ott H, Gudowius S, et al. Saving the red baby: successful allogeneic cord blood transplantation in Omenn syndrome. Clin Immunol. 2009;130(3):259‐263. [DOI] [PubMed] [Google Scholar]
- 160. Fagioli F, Biasin E, Berger M, et al. Successful unrelated cord blood transplantation in two children with severe combined immunodeficiency syndrome. Bone Marrow Transplant. 2003;31(2):133‐136. [DOI] [PubMed] [Google Scholar]
- 161. Arutyunyan I, Fatkhudinov T, Elchaninov A, et al. Umbilical cord‐derived mesenchymal stromal/stem cells enhance recovery of surgically induced skeletal muscle ischemia in a rat model. Histol. Histopathol. 2018:18057 10.14670/HH-18-057 [DOI] [PubMed] [Google Scholar]
- 162. Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19(7):853‐860. [DOI] [PubMed] [Google Scholar]
- 163. Cremers NA, Lundvig DM, van Dalen SC, et al. Curcumin‐induced heme oxygenase‐1 expression prevents H2O2‐induced cell death in wild type and heme oxygenase‐2 knockout adipose‐derived mesenchymal stem cells. Int J Mol Sci. 2014;15(10):17974‐17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Mohammadzadeh M, Halabian R, Gharehbaghian A, et al. Nrf‐2 overexpression in mesenchymal stem cells reduces oxidative stress‐induced apoptosis and cytotoxicity. Cell Stress Chaperones. 2012;17(5):553‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Cerqueira MT, Pirraco RP, Marques AP. Stem cells in skin wound healing: are we there yet? Adv Wound Care. 2016;5(4):164‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Hou C, Shen L, Huang Q, et al. The effect of heme oxygenase‐1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials. 2013;34(1):112‐120. [DOI] [PubMed] [Google Scholar]
- 167. Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia‐regulated heme oxygenase‐1 vector. J Am Coll Cardiol. 2005;46(7):1339‐1350. [DOI] [PubMed] [Google Scholar]
- 168. Lin CY, Peng CY, Huang TT, et al. Exacerbation of oxidative stress‐induced cell death and differentiation in induced pluripotent stem cells lacking heme oxygenase‐1. Stem Cells Dev. 2012;21(10):1675‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hamedi‐Asl P, Halabian R, Bahmani P, et al. Adenovirus‐mediated expression of the HO‐1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperones. 2012;17(2):181‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Vijayan V, Wagener F, Immenschuh S. The macrophage heme‐heme oxygenase‐1 system and its role in inflammation. Biochem Pharmacol. 2018;153:159‐167. [DOI] [PubMed] [Google Scholar]
- 171. Wagener FA, Immenschuh S. Editorial: molecular mechanisms protecting against tissue injury. Front Pharmacol. 2016;7:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Zeng W, Xiao J, Zheng G, et al. Antioxidant treatment enhances human mesenchymal stem cell anti‐stress ability and therapeutic efficacy in an acute liver failure model. Sci Rep. 2015;5:11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hinge AS, Limaye LS, Surolia A, Kale VP. In vitro protection of umbilical cord blood‐derived primitive hematopoietic stem progenitor cell pool by mannose‐specific lectins via antioxidant mechanisms. Transfusion. 2010;50(8):1815‐1826. [DOI] [PubMed] [Google Scholar]
- 174. Drowley L, Okada M, Beckman S, et al. Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther. 2010;18(10):1865‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Yin X, Mayr M, Xiao Q, Wang W, Xu Q. Proteomic analysis reveals higher demand for antioxidant protection in embryonic stem cell‐derived smooth muscle cells. Proteomics. 2006;6(24):6437‐6446. [DOI] [PubMed] [Google Scholar]
- 176. Chaudhari AA, Vig K, Baganizi DR, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 2016;17(12):1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Roger M, Fullard N, Costello L, et al. Bioengineering the microanatomy of human skin. J Anat. 2019;234(4):438‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Op 't Veld RC, van den Boomen OI, Lundvig DMS, et al. Thermosensitive biomimetic polyisocyanopeptide hydrogels may facilitate wound repair. Biomaterials. 2018;181:392‐401. [DOI] [PubMed] [Google Scholar]
- 179. Von den Hoff JW, Carvajal Monroy PL, Ongkosuwito EM, van Kuppevelt TH, Daamen WF. Muscle fibrosis in the soft palate: delivery of cells, growth factors and anti‐fibrotics. Adv Drug Deliv Rev. 2018. 10.1016/j.addr.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 180. Jammalamadaka U, Tappa K. Recent advances in biomaterials for 3D printing and tissue engineering. J Funct Biomater. 2018;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008;213(1):66‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Kumar VS, Rao NK, Mohan KR, et al. Minimizing complications associated with coronal approach by application of various modifications in surgical technique for treating facial trauma: a prospective study. Natl J Maxillofac Surg. 2016;7(1):21‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Liu K, Mihaila SM, Rowan A, Oosterwijk E, Kouwer P. Synthetic extracellular matrices with nonlinear elasticity regulate cellular organization. Biomacromolecules. 2019;2(20):826‐834. 10.1021/acs.biomac.8b01445 [DOI] [PMC free article] [PubMed] [Google Scholar]


