Abstract
Background
Magnetic sphincter augmentation (MSA) is reported to be an innovative alternative to antireflux surgery for patients with gastro‐oesophageal reflux disease. Although used in practice, little is known about how it has been evaluated. This study aimed to systematically summarize and appraise the reporting of MSA and its introduction into clinical practice, in the context of guidelines (such as IDEAL) for evaluating innovative surgical devices.
Methods
Systematic searches were used to identify all published studies reporting MSA insertion. Data collected included patient selection, governance arrangements, surgeon expertise, technique description and outcome reporting.
Results
Searches identified 587 abstracts; 39 full‐text papers were included (1 RCT 5 cohort, 3 case–control, 25 case series, 5 case reports). Twenty‐one followed US Food and Drug Administration eligibility criteria for MSA insertion. Twenty‐six documented that ethical approval was obtained. Two reported that participating surgeons received training in MSA; 18 provided information about how MSA insertion was performed, although techniques varied between studies. Follow‐up ranged from 4 weeks to 5 years; in 14 studies, it was less than 1 year.
Conclusion
Most studies on MSA lacked information about patient selection, governance, expertise, techniques and outcomes, or varied between studies. Currently, MSA is being used despite a lack of robust evidence for its effectiveness.
This systematic review summarizes published evidence for a novel procedure, magnetic sphincter augmentation of the oesophagus, a treatment for gastro‐oesophageal reflux. It found limited governance arrangements, a lack of long‐term follow‐up and varied methods of device insertion. There is a need for improvement in the rigour with which innovative surgical procedures are evaluated. Ideally, this would comprise high‐quality RCTs and long‐term registries, to allow safe adoption of the device into clinical practice.
Innovation in need for IDEAL investigation
Antecedentes
El aumento de esfínter con un dispositivo magnético (magnetic sphincter augmentation, MSA) se ha descrito como una alternativa innovadora a la cirugía antirreflujo para pacientes con enfermedad por reflujo gastroesofágico. Aunque este procedimiento se utiliza en la práctica, se sabe poco acerca de cómo ha sido evaluado. Este estudio se propuso resumir sistemáticamente y evaluar los trabajos sobre MSA y su introducción en la práctica clínica, en el contexto de las guías (como IDEAL) para la evaluación de dispositivos quirúrgicos innovadores.
Métodos
Se identificaron todos los estudios publicados que describían la colocación de MSA efectuando búsquedas sistemáticas. Los datos recogidos incluían la selección de los pacientes, disposiciones de gobernanza, experiencia del cirujano, descripción técnica, y descripción de resultados.
Resultados
Las búsquedas identificaron 587 resúmenes, incluyéndose 39 artículos completos (5 estudios de cohortes, 3 estudios de casos y controles, 26 series de casos, 5 casos clínicos). En 21 estudios se siguieron los criterios de elegibilidad de la FDA para la colocación de MSA. En 26 estudios se confirmaba que se había obtenido la aprobación ética. Dos estudios describieron que los cirujanos participantes habían recibido formación en MSA; 18 proporcionaron información sobre cómo se realizó la colocación de MSA, aunque las técnicas variaron entre los estudios. El seguimiento oscilaba entre 4 semanas y 5 años; en 14 estudios fue inferior a un año.
Conclusión
La mayoría de los estudios sobre MSA fueron casos aislados y series de casos, sin un incremento apreciable en la calidad de la evidencia sobre MSA. La información sobre la selección de los pacientes, gobernanza, experiencia, técnicas, y resultados estaba ausente o variaba entre los estudios, haciendo difíciles las comparaciones. En la actualidad, MSA se utiliza a pesar de la falta de evidencia robusta sobre su efectividad.
Introduction
Gastro‐oesophageal reflux disease (GORD) is a common condition, affecting 10–20 per cent of the Western population1. It is associated with sequelae including Barrett's metaplasia and oesophageal adenocarcinoma, and can have a detrimental effect on quality of life1. The primary treatment is proton pump inhibitors (PPIs), which are generally well tolerated1, 2. Some patients, however, have recalcitrant symptoms despite treatment and others cannot tolerate, or do not wish to take, long‐term medication2, 3. In these scenarios, antireflux surgery may be offered2, 3. The most common surgical option is fundoplication, which can be associated with short‐ and long‐term complications. There is also a risk of recurrent reflux; as many as 30 per cent of patients resume PPIs within 5 years of surgery4. Innovative approaches to the management of refractive or recurrent reflux have therefore been sought, and one recent development is magnetic sphincter augmentation (MSA) of the lower oesophagus5.
First described in 2008, MSA comprises an expandable chain of magnetic titanium beads designed to augment the lower oesophageal sphincter and prevent inappropriate relaxation5. MSA is reported to be a safe, effective and relatively straightforward alternative to antireflux surgery6, 7, 8, 9. The LINX™ system (Torax Medical, North Shoreview, Minnesota, USA; bought by Ethicon, Johnson & Johnson, Somerville, New Jersey, USA, in March 2017) is currently the only device marketed, receiving Conformité Européenne (CE) marking in 2008, and Food and Drug Administration (FDA) approval in 2012 for use in patients fulfilling specific criteria10. The National Institute for Health and Care Excellence (NICE) issued guidance in September 2012 permitting the use of MSA solely under ‘special arrangements for clinical governance, consent and audit or research’ owing to ‘limited evidence of the safety and efficacy’11. Unlike pharmaceuticals, there is no requirement for novel devices such as MSA to be evaluated within the context of RCTs before they are marketed12, meaning that it can be difficult to monitor outcomes and risking delayed detection of long‐term adverse events.
A change in UK law around consent stipulates that, when patients are offered new procedures or devices, there is a requirement to provide information about the proposed treatment and all potential alternatives so they can make informed decisions13. This information should be based on the best available evidence, ideally from well designed and conducted RCTs. However, if devices are marketed on a background of poor‐quality evidence about the potential benefits and risks, without consistent and transparent reporting of outcomes, it may be difficult for surgeons to provide information, decide whether to offer the treatment to patients and, crucially, to obtain fully informed consent. This raises important issues about how best to evaluate surgical innovation.
The IDEAL (Idea, Development, Exploration, Assessment, Long‐term follow‐up) framework aims to overcome this problem using a stepwise means of introducing and evaluating innovative surgical procedures and devices (IDEAL‐D), with the objective of improving transparency, evaluation and reporting of innovation in surgery, and informing evidence‐based practice12, 14. Key IDEAL recommendations relate to incremental changes in study design, clinical indications, technique standardization, governance arrangements and outcomes, as the innovation progresses from first‐in‐human to long‐term follow‐up. To date, reviews of the evidence for MSA have not studied its introduction into clinical practice, so compliance with these IDEAL guidelines for evaluating innovative surgical procedures and devices is unknown14. This study therefore aimed to summarize and appraise the reporting of studies of MSA, to understand how this innovative procedure has been introduced and evaluated in relation to the IDEAL recommendations.
Methods
A systematic review was undertaken to identify all published studies reporting MSA insertion. The review was conducted in line with the PRISMA statement15. Methods were based on those described previously14.
Search strategy and study selection
Searches were undertaken in MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), the Cochrane Library, Web of Science and BIOSIS databases, from inception to January 2019. Searches consisted of subject headings and text words, combining terms for ‘magnetic sphincter augmentation’ with ‘gastro‐oesophageal reflux disease’ using the Boolean operator ‘AND’ (Table S1 , supporting information).
Study eligibility
Searches were limited to studies in humans, written in English. All primary research study designs (such as case reports, case series and comparative studies) were eligible for inclusion. Where systematic reviews were identified, reference lists were cross‐checked to ensure that all eligible studies were included. Presentations and conference abstracts were excluded because of the high probability of incomplete data. Studies reporting solely on device removal were excluded. Search results were deduplicated.
Identification and selection of papers
Titles and abstracts were screened independently by two authors. The full‐text versions of papers retained after title and abstract screening were assessed further for eligibility. Disagreements were first discussed between the reviewers, and any unresolved conflicts referred to the wider study team. Reference lists of included papers were searched manually for additional relevant articles. Data from full‐text papers were extracted independently by at least two assessors.
Data collection
Data collection was based on IDEAL recommendations14, and included information about general study characteristics, patient selection, regulatory and governance arrangements, operator and centre expertise, technique description and outcome reporting14. Outcome data were extracted from papers reporting follow‐up of initial studies included in the review to acquire information about long‐term outcomes; however, other data were not included to avoid double‐counting of results.
General study characteristics
The study design, year and journal of publication, country of origin, and number of participating centres and patients, were extracted. The timing of publication of studies in relation to FDA approval was also recorded. If studies involved a comparator group, eligibility criteria were compared with those for MSA, including any matching at baseline or statistical analyses to account for differences. Risk‐of‐bias assessments were undertaken for RCTs16.
Patient selection
Study‐specific inclusion and exclusion criteria for undergoing MSA insertion were documented, and compared with the criteria approved by the FDA in 2012. If different criteria were used, these were recorded along with any rationale. Information regarding those who were eligible to undergo MSA insertion, but did not have the procedure, was also collected.
Regulatory and governance arrangements
Reporting of information about governance approvals (for example, ethics committees, Institutional Review Boards (IRBs) and clinical effectiveness committees) was documented. Articles were assessed for whether patients were informed specifically about the innovative nature of MSA. Details of any funding from the manufacturer or other potential conflicts of interest were also noted.
Operator and centre expertise
Details of the types of centre and number of surgeons undertaking MSA insertion were recorded. Reports of surgeon and team experience with the new procedure, including any details of the learning curve and how it was accounted for, were extracted.
Technique description
All descriptions of the techniques used to insert MSA were extracted verbatim and assessed using a typology, which allows systematic deconstruction of an intervention into its individual components and steps17, 18. Descriptions of each component (such as incisions, dissection, device insertion and reconstruction) were tabulated chronologically, to identify whether: there was clear reporting of what had been performed; modifications occurred over time; and the technique had stabilized.
Outcome selection, measurement and reporting
All outcomes were extracted and categorized into groups: clinical (a clinician's or researcher's assessment of symptoms or signs)19; patient‐reported (a report of the status of a patient's health condition that comes directly from the patient, without interpretation of the patient's response by a clinician or anyone else)20; process (the specific steps that lead to a particular outcome)21; cost and other economic; and adverse events (an untoward medical occurrence as a result of the use of the device)22. The rationale for device removal and techniques used when removal was required were also documented.
In addition to recording details of reporting, adverse event and process data were summarized by calculating the range of rates for each outcome.
Data synthesis
Results were summarized in a narrative synthesis, with descriptive statistics where appropriate. Comparisons were made between studies undertaken before and after marketing approval to establish compliance with FDA‐approved criteria. Because the study did not aim to draw conclusions about the effectiveness of MSA over other treatments, meta‐analyses were not performed.
Results
Characteristics of included studies
Systematic searches identified 974 papers and, after removing duplicates, 587 abstracts were screened (Fig. 1). A total of 39 full‐text papers6, 10, 11, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, published between 2008 and 2019, were finally included. The 39 articles consisted of one RCT, five comparative cohort studies (2 prospective and 3 retrospective), three comparative case–control studies, 25 case series (14 prospective, 11 retrospective) and five case reports (Fig. 2). Four papers23, 24, 25, 26 reported longer‐term outcomes from earlier studies. This review therefore presents the results of the remaining 35 papers in detail unless specified otherwise.
Figure 1.
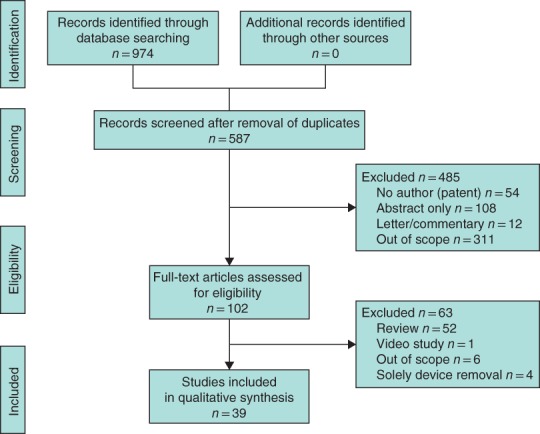
PRISMA diagram showing selection of articles for review Four papers reported longer‐term outcomes from earlier studies; this review therefore presents the results of the remaining 35 papers in detail.
Figure 2.
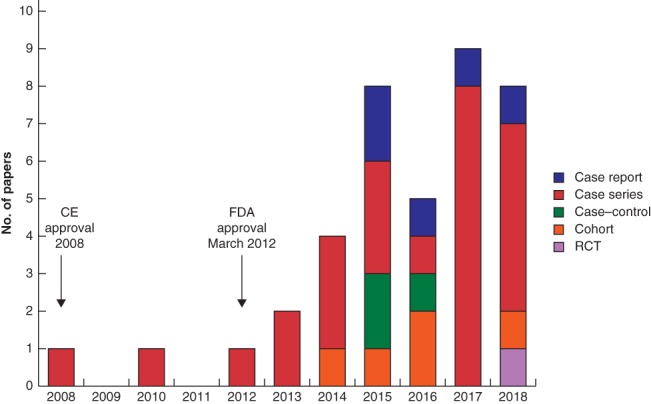
Timeline of publication year and study type relative to marketing approval CE, Conformité Européenne; FDA, Food and Drug Administration.
Two papers were published before FDA approval. The first10 was reported to be a feasibility study aimed at demonstrating the safety and efficacy of MSA, and the second24 reported 1‐ and 2‐year follow‐up of the initial study.
Seventeen studies were reported from a single centre. Thirty‐three studies included at least one author who had published two or more articles on MSA. Nine studies (1 RCT, 5 cohort and 3 case–control studies) included a comparator group (Tables 1 and 2), although only one was randomized. Of these, seven compared MSA with antireflux surgery, one27 compared different types of dissection technique for MSA, and the RCT28 compared MSA with PPIs. Five, including the RCT, reported differences in patient baseline demographics, including hernia size, obesity, age, DeMeester score and disease severity, which were not accounted for in analyses. Although four reported ‘matched’ controls, there were differences in demographics in three29, 30, 31, 33.
Table 1.
Details of nine studies comparing magnetic sphincter augmentation with an alternative technique
| Eligibility criteria | |||||
|---|---|---|---|---|---|
| Reference | Study design | No. of patients | Comparator | MSA | Comparator |
| Louie et al.44 | Cohort* | 66 (34 MSA) | LNF | FDA criteria | FDA criteria |
| Sheu et al.29 | Case–control* | 24 (12 MSA) | LNF | Previous ARS, hiatus hernia > 2 cm, dysmotility, allergy to device, need for future MRI | n.r. |
| Riegler et al.45 | Cohort | 249 (202 MSA) | LNF | FDA criteria plus hernia > 3 cm, Barrett's and stage C or D oesophagitis | FDA criteria plus hernia > 3 cm, Barrett's and stage C or D oesophagitis |
| Reynolds et al.32 | Case–control* | 100 (50 MSA) | LNF | FDA criteria | FDA criteria |
| Asti et al.33 | Cohort | 238 (135 MSA) | LTF | FDA criteria | FDA criteria |
| Warren et al.30 | Cohort* | 415 (201 MSA) | LNF | FDA criteria | FDA criteria |
| Reynolds et al.31 | Case–control* | 119 (52 MSA) | LNF | n.r. | n.r. |
| Tatum et al.27 | Cohort* | 182 (all MSA) | Surgical technique | Patients undergoing MSA without previous surgery | Patients undergoing MSA without previous surgery |
| Bell et al.28 | RCT | 152 (50 MSA) | PPI | FDA criteria | FDA criteria |
Retrospective. MSA, magnetic sphincter augmentation; LNF, laparoscopic Nissen fundoplication; FDA, Food and Drug Administration; ARS, antireflux surgery; n.r., not reported; LTF, laparoscopic Toupet fundoplication; PPI, proton pump inhibitor.
Table 2.
Differences in baseline characteristics in studies comparing magnetic sphincter augmentation with an alternative technique
| Reference | BMI (kg/m2) | Age (years) | GERD‐HRQL score | DeMeester score | Hernia size (cm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MSA | Comparator | MSA | Comparator | MSA | Comparator | MSA | Comparator | MSA | Comparator | |
| Louie et al.44 | 27 | 30 | 54 | 47 | n.r. | n.r. | n.r. | n.r. | 1·4 | 1·5 |
| Sheu et al.29 | 26·6 | 26·6 | 39·3 | 43·8 | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. |
| Riegler et al.45 | 25·7 | 26·1 | 46·6 | 52·8 | 6·4% severe GORD | 61·7% severe GORD | n.r. | n.r. | 1·6% > 3 cm | 45·7% > 3 cm |
| Reynolds et al.32 | 26·4 | 26·7 | 53 | 54 | 19·7 | 18·8 | n.r. | n.r. | 1·5 | 1·6 |
| Asti et al.33 | 23·9 | 25·1 | 44 | 50 | 21·0 | 19·7 | 31·4 | 37·6 | 2 | 2 |
| Warren et al.30 | 32 | 40 | 54 | 52 | 21 | 19 | 34 | 39 | n.r. | n.r. |
| Reynolds et al.31 | 26 | 27 | 53 | 53 | 17 | 19 | n.r. | n.r. | n.r. | n.r. |
| Tatum et al.27 | 26·8 | 27·8 | 55·3 | 63·1 | n.r. | n.r. | 39·9 | 79·3 | 0·64 | 2 |
| Bell et al.28 | 28 | 28 | 46 | 46 | 23·5 | 25·0 | 40·3 | 30·9 | 58% < 3 cm | 49% < 3 cm |
GERD‐HRQL, gastro‐oesophageal reflux disease – health‐related quality of life; MSA, magnetic sphincter augmentation; n.r., not reported; GORD, gastro‐oesophageal reflux disease.
The only RCT included 152 patients, randomized to receive PPI (102) or MSA (50) in a 2 : 1 ratio across 21 US centres. It concluded that patients should be considered for MSA rather than increased PPI doses; however, the overall risk of bias was unclear because no information was provided about the randomization process, allocation concealment or attrition.
Patient selection
All studies involved adult patients aged over 18 years. Twenty‐one followed the FDA eligibility criteria for MSA insertion10. Seven, comprising a total of 427 patients and published after FDA approval, extended these criteria to include patients with a hiatus hernia greater than 3 cm34, 35 or previous gastrointestinal surgery36, 37, 38, 39, 40, to assess whether the device could be used in this population.
Three articles provided information about patients who were recruited but did not receive MSA. Reasons included: insurance requirements, patient preference, allergy to device metals, the potential future need for MRI (initially, this was a contraindication to MSA insertion26), or conversion to conventional antireflux surgery owing to more severe disease at operation than expected.
Regulatory and governance arrangements
Twenty‐six studies reported that ethical approval had been obtained (IRB in 23, ethics committee in 3). Twelve studies were funded by the manufacturer, and 24 stated a conflict of interest in that some or all authors worked at the device company. Only 14 explicitly documented obtaining patient consent for study participation; a further six stated that consent was not needed given the study's retrospective nature.
One premarket study10 documented specific discussions with patients regarding the innovative use of MSA: ‘each patient was informed about the investigational nature of the trial and received detailed information about the study protocol’. One further paper38 reported that the authors ‘advised patients about the novelty of the approach’. None of the seven articles that extended the inclusion criteria off licence to patients outside the FDA‐approved guidelines reported discussing this with patients, although ethical approval was obtained for all seven.
Operator and centre expertise
None of the studies provided any information about the surgical learning curve. Fifteen stated the number of surgeons performing MSA procedures (range 1–4). Seven reported entry criteria for participating surgeons (Table 3). Two studies reported that participating surgeons received training in the use of MSA. A further study reported that specific experience with MSA was required, but did not quantify what this comprised. The remaining five reported only that surgeons were required to have experience with antireflux surgery in general. None reported information about the expertise or training of the wider surgical team (nurses and anaesthetists). Although three studies stated that their hospitals were ‘high volume’ and one a ‘specialist reflux centre’41, no other information about caseload was provided.
Table 3.
Statements of expertise of participating surgeons in included studies
| Reference | Type of expertise described | Statement |
|---|---|---|
| Smith et al.42 | Surgeons' previous experience | ‘Over 20 years of experience treating GORD’ |
|
Ganz et al.46 Lipham et al.25 |
‘Experience with fundoplication’ | |
| Lipham et al.47 | ‘Proficiency in performing laparoscopic Nissen fundoplication and comfortable working at the oesophago‐gastric junction’ | |
| Bell et al.28 | ‘Trained and experienced in MSA’ | |
| Kuckelman et al.39 | Training requirements | ‘All surgeons had completed the training and certification process for MSA placement, including didactics, live case observations, and then proctoring on initial cases’ |
| Louie et al.48 | ‘All participating study centres were required to have undergone training to implant the device and completed a minimum of five LINX™ implants’ |
GORD, gastro‐oesophageal reflux disease; MSA, magnetic sphincter augmentation.
Technique description
Eighteen studies reported at least some information about the technique of MSA insertion, and a further nine cited one or more of these articles (Table S2 , supporting information). Although the first published study10 documented the procedure in detail, no study described every component. Technical details were reported about the incisions (8 studies), hiatal dissection (18), device insertion (18) and crural repair (9). However, these descriptions were heterogeneous, using different wording and describing different anatomical structures. It was therefore difficult to determine whether the procedure or any of its components had evolved with time or whether they had stabilized.
With regard to the dissection technique, the need to identify the vagal trunk, access the retro‐oesophageal window and perform ‘minimal hiatal dissection’ was reported in seven, five and seven studies respectively, although the latter was not defined. One study27 aimed to undertake a comparison of ‘minimal hiatal dissection’ with ‘planned obligatory dissection’. During the study, however, this comparison was abandoned and all subsequent patients underwent obligatory hiatal dissection, with no explanation of why this occurred.
Of the studies providing details about device insertion, six reported how the sizing device should be inserted and three documented the required tightness around the oesophagus. Further information about the insertion technique varied between studies; for example, five reported the device location as between the posterior vagus and oesophagus, and two the retro‐oesophageal window. One study (100 patients)6 reported using a modified version of MSA, whereby the first 30 patients received a first‐generation and the rest a second‐generation device. Differences between the devices were described as the ‘use of a clasp instead of a suture to close the ring’ and a ‘laparoscopic sizing tool’ instead of a ‘colour‐coded sizing device’, although there was no further information and no rationale for the modification was provided. There was no documentation to suggest that patients were made aware of this change of device or that ethical approval was sought. No other studies documented which generation of device was used.
Eleven studies reported that crural repair was undertaken at the discretion of the surgeon, and technical information was included in nine. Of these, two described a posterior cruroplasty using ‘a permanent suture’ and ‘with one or two sutures’, whereas seven stated only that ‘posterior cruroplasty’ was undertaken. Four studies documented a rationale for crural repair: ‘…when the hiatus appeared patulous or a sliding hernia was present’ 6, 42, and ‘if hiatal hernia visible after posterior dissection of hiatus that kept the phreno‐oesophageal membrane intact’31, 32.
Outcome selection, measurement and reporting
Outcome reporting from all 39 papers is summarized in Table 4. Thirty‐eight different outcomes were reported across all studies. No single outcome was measured in all studies. No study provided a rationale for the outcomes selected. Duration of follow‐up ranged from 4 weeks to 5 years; this was not reported in one study. Five‐year data were available from two studies and the duration of follow up was less than 1 year in 14.
Table 4.
Outcome reporting in included studies
| No. of studies reporting outcomes (n = 39) | Range of rates reported (%)* | |
|---|---|---|
| Adverse events | ||
| Device removal | 27 | 0·5–8·3 |
| Device erosion | 8 | 0·1–1·2 |
| Need for dilatation | 22 | 2–67 |
| Readmission to hospital within 30 days | 13 | 1·3–5·4 |
| Mortality | 3 | 0 |
| Clinical | ||
| DeMeester score | 7 | – |
| Manometry | 4 | – |
| Oesophageal pH testing | 10 | – |
| Oesophagogastroduodenoscopy | 13 | – |
| Barium swallow | 7 | – |
| Proton pump inhibitor use | 25 | – |
| Process | ||
| Duration of operation (min) | 16 | 23–184† |
| Duration of hospital stay (days) | 13 | 0–3† |
| Patient‐reported outcomes | ||
| GERD‐HRQL score | 31 | – |
| Other validated patient‐reported outcomes | 7 | – |
| Non‐validated patient‐reported outcome | 5 | – |
| Economic | ||
| Costs associated with device | 1 | – |
Unless indicated otherwise;
values are median (range). GERD‐HRQL, gastro‐oesophageal reflux disease – health‐related quality of life.
Clinical outcomes
Objective clinical outcomes were reported in 25 studies. These included data from DeMeester scores (7 studies), manometry (4), oesophageal pH measurements (10), oesophagogastroduodenoscopy (13) and barium swallows (7). Fifteen of the 25 studies reported obtaining more than one of these objective outcomes. These assessments were undertaken at variable times after surgery, ranging from 1 day to 3 years. Three studies performed these tests beyond 1 year after operation. The objective clinical data were infrequently correlated with the patient‐reported outcomes reported below.
Patient‐reported outcomes
The validated gastro‐oesophageal reflux disease health‐related quality of life (GERD‐HRQL) questionnaire was used in 31 studies. Baseline questionnaires were administered before operation in all 31 studies and at various other time points, ranging from 10 days to 5 years after surgery. Only five studies reported use of the questionnaire after a year. Other assessment measures included a Foregut Symptom Questionnaire (4 studies), Respiratory Index Score (1), Quality of Life in Reflux and Dyspepsia score (1) and Modified Dakkak dysphagia severity score (1). Five remaining studies reported patient‐reported outcomes, including incidence of dysphagia, change in reflux, gas‐related symptoms and symptoms of odynophagia, but did not define these or use a named questionnaire.
Process outcomes
Operating time and duration of hospital stay were reported in 16 and 13 studies, respectively.
Cost and economic outcomes
One study reported an economic outcome: overall hospital charges associated with device insertion.
Adverse events
Twenty‐two studies reported the need for postoperative oesophageal balloon dilatation for dysphagia or odynophagia, eight described cases of device erosion into the oesophagus, and 13 readmission to hospital (for reasons including dehydration, dysphagia, pain, nausea and pulmonary embolus). No intraoperative complications were reported in any paper.
Device removal
Of the 27 studies reporting the need for device removal (total 84 patients), the timing ranged from 21 days to 47 months after insertion. No prespecified criteria or rationale were provided. Reasons for device removal were reported in all studies, and included erosion, migration, development of oesophageal cancer, ongoing refractory symptoms, the need for MRI and patient preference. The surgical approach to device removal was poorly documented in these studies, and included laparoscopic, endoscopic and laparoendoscopic techniques.
Discussion
This comprehensive review of the reporting of an innovative surgical device – MSA of the lower oesophagus – summarizes information from 39 studies published between 2008 and January 2019. Only one small RCT was identified; most studies were case studies or case series with serious shortcomings. Of the nine comparative studies, including one RCT, eight were limited by different selection criteria and unmatched patients at baseline. Information about ethical approval, patient consent and conflicts of interest was often missing. Many studies reported using MSA for indications outside the current FDA regulations. Reporting of the technical aspects of device insertion was either lacking or varied between studies, making it difficult for surgeons to replicate the technique and learn from others' experiences. Currently, therefore, MSA is being used in clinical practice despite a lack of robust evidence to support its effectiveness, potentially placing patients, surgeons and healthcare providers at risk. Although some guidance for evaluating innovative surgical procedures and devices is available (IDEAL recommendations), it was not followed. Pilot work is now required to address the aforementioned issues and enable an RCT comparing MSA with current treatments to be designed optimally, to inform decision‐making and patient care. Moreover, a registry to monitor device insertions, document long‐term outcomes including adverse events, record surgeon experience and allow safe widespread adoption of the device into clinical practice, is required.
The Royal College of Surgeons of England has urged the government to ‘act urgently to reform the lax regulation system governing medical devices, including a compulsory registry of all new implants’12, 59. Such a system would need to strike a balance between avoiding burden and encouraging the development of well founded evidence on safety and efficacy12, 59. There has been a recent call for new surgical procedures and implants to be tested in RCTs before being made routinely available, in line with IDEAL recommendations and existing pathways for introducing pharmaceutical products12. In the UK, the Medicines and Healthcare products Regulatory Agency60 regulates medical devices with the aim of ensuring that they are safe and efficacious. The type of study required to achieve approval is not specified, but the majority do not include a control group, despite the Royal College calling for more RCTs to be undertaken and encouraging surgeons to use IDEAL guidance59. In the USA, invasive devices are now subject to a pivotal clinical study as part of the FDA's premarket approval pathway12. Although MSA did undergo a pivotal study, subsequent publications failed to build on its findings and long‐term outcomes have not been established, particularly when compared with current treatments (PPIs and antireflux surgery).
This is concerning in the context of recent high‐profile devices that have later caused serious health problems despite complying with regulatory processes. One such example is transvaginal mesh, which is no longer offered routinely as the result of an independent review61. Another example is robotic surgery. Although this has been introduced rigorously in some surgical disciplines (robotic prostatectomy has been endorsed by NICE for centres performing more than 150 procedures per year), such evaluation has not been applied uniformly. Conversely, a recent review62 conceded that, despite a lack of evidence to support any benefit, cardiac surgery ‘represents one of the largest markets in the field of robotic surgery’, and the first robotic mitral valve replacement in the UK resulted in the death of the patient63. Following this, guidance regarding the clinical governance, oversight and infrastructure required when introducing new innovations has been published. The guidance reinforces the responsibilities of surgeons and surgical societies in arranging appropriate training and mentorship, and submission of data to national registries, thereby placing patient safety at the core64. Another example of a device that was introduced before full evaluation – and subsequently removed from practice – is the Angelchik prosthesis. Similar to MSA, it gained popularity as a solution to GORD in the 1980s because of its technical simplicity65. Although data from initial non‐randomized studies were promising, an RCT was stopped prematurely owing to adverse events, and long‐term follow‐up detected erosions and migrations necessitating reoperation in 25 per cent of patients66. As a result, use of the device was almost entirely abandoned by the early 2000s, by which time over 25 000 had already been inserted.
Despite this, MSA continues to be used across the world and over 3000 device insertions were undertaken in the USA between 2012 and 201643. A registry that was funded and sponsored by the device manufacturer existed until 2016. However, this did not include all patients and follow‐up was limited to 3 years because it closed prematurely for unknown reasons. Without registries to monitor outcomes of new devices, there can be delays in understanding the short‐ and long‐term risks of new devices and procedures67.
This study has tracked the introduction and evolution of MSA from the first published description to the present day. Despite this, however, there are limitations. Outcomes were not analysed in depth and meta‐analyses were not undertaken. Exclusion of non‐English language papers may mean that important additional findings were missed. Data were extracted verbatim, and the authors assumed that if something was not documented, it did not happen; authors were not contacted individually for further information. It is also possible that the device is used much more widely and other cases have not been published. A final limitation is that one paper was published before IDEAL was introduced in 2009 (and therefore could not have followed these guidelines rigorously), and a further 16 were published before publication of the updated IDEAL‐D framework specific for surgical devices in 2016. More work is needed to establish why the IDEAL guidance has not been followed; despite this, the fundamental principles of IDEAL are not new concepts and form the basis of good research.
This in‐depth analysis of reporting of an innovative invasive procedure corroborates existing evidence12 that surgical procedures are often poorly evaluated before implementation in clinical practice. The long‐term safety of MSA has not been sufficiently demonstrated in the existing 39 published studies owing to inconsistent reporting of outcomes, particularly those detailing long‐term follow‐up. There is a lack of standardized, transparent reporting of how the MSA device should optimally be inserted, making adoption into practice difficult and hampering comparisons between studies. There is a need for robust assessment and reporting to improve the rigour with which innovative surgical procedures are evaluated, to optimize transparency, maximize patient benefit and reduce harms.
Editor's comments
What have we learned from the past? Not much, according to this study by Kirkham et al. The surgical community falls short in the proper evaluation of new surgical devices before implementation in clinical practice. We have to do better in terms of informing patients on experimental treatments and being critical on new technologies. On the other hand, we want to make surgery safer, more accessible and improve the outcomes, and technological improvements are needed to achieve these goals. However, I share the authors' conclusion that ‘magnetic sphincter augmentation is being used in clinical practice despite a lack of robust evidence to support its effectiveness, placing surgeons and patients at risk’. There is a clear need for international registries that evaluate indications and outcomes of new devices and techniques. National surgical bodies and governments should support these registries so they are independent of the industry/companies and surgeons involved in the manufacturing and promotion of new devices, because there will always be a conflict of interest.
B. P. L. Wijnhoven
Editor, BJS
Supporting information
Table S1. Ovid MEDLINE search strategy
Table S2. Descriptions of the key components of MSA insertion
Acknowledgements
The authors thank C. Borwick for help with the search strategy. This research was not registered in an independent, institutional registry. The authors are willing to make the data, analytical methods and study materials available to other researchers. This study was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at the University Hospitals Bristol National Health Service (NHS) Foundation Trust and the University of Bristol. The views expressed are those of the authors and not necessarily those of the UK NHS, NIHR or Department of Health. J.M.B. is an NIHR Senior Investigator. B.G.M. is an NIHR Academic Clinical Lecturer. N.S.B. is a Medical Research Council Clinician Scientist.
Disclosure: The authors declare no conflict of interest.
References
- 1. El‐Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut 2014; 63: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grant AM, Cotton SC, Boachie C, Ramsay CR, Krukowski ZH, Heading RC et al; REFLUX Trial Group . Minimal access surgery compared with medical management for gastro‐oesophageal reflux disease: five year follow‐up of a randomised controlled trial (REFLUX). BMJ 2013; 346: f1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yates RB, Oelschlager BK. Surgical treatment of gastroesophageal reflux disease. Surg Clin North Am 2015; 95: 527–553. [DOI] [PubMed] [Google Scholar]
- 4. Kahrilas PJ, Boeckxstaens G, Smout AJ. Management of the patient with incomplete response to PPI therapy. Best Pract Res Clin Gastroenterol 2013; 27: 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zak Y, Rattner DW. The use of LINX for gastroesophageal reflux. Adv Surg 2016; 50: 41–48. [DOI] [PubMed] [Google Scholar]
- 6. Bonavina L, Saino G, Lipham JC, DeMeester TR. LINX® Reflux Management System in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux. Therap Adv Gastroenterol 2013; 6: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen MY, Huang DY, Wu A, Zhu YB, Zhu HP, Lin LM et al Efficacy of magnetic sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease in short term: a meta‐analysis. Can J Gastroenterol Hepatol 2017; 2017: 9596342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skubleny D, Switzer NJ, Dang J, Gill RS, Shi X, de Gara C et al LINX® magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta‐analysis. Surg Endosc 2017; 31: 3078–3084. [DOI] [PubMed] [Google Scholar]
- 9. Loh Y, McGlone ER, Reddy M, Khan OA. Is the LINX reflux management system an effective treatment for gastro‐oesophageal reflux disease? Int J Surg 2014; 12: 994–997. [DOI] [PubMed] [Google Scholar]
- 10. Bonavina L, Saino GI, Bona D, Lipham J, Ganz RA, Dunn D et al Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg 2008; 12: 2133–2140. [DOI] [PubMed] [Google Scholar]
- 11. Prakash D, Campbell B, Wajed S. Introduction into the NHS of magnetic sphincter augmentation: an innovative surgical therapy for reflux – results and challenges. Ann R Coll Surg Engl 2018; 100: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedrakyan A, Campbell B, Merino JG, Kuntz R, Hirst A, McCulloch P. IDEAL‐D: a rational framework for evaluating and regulating the use of medical devices. BMJ 2016; 353: i2372. [DOI] [PubMed] [Google Scholar]
- 13. Main BG, Adair SR. The changing face of informed consent. Br Dent J 2015; 219: 325–327. [DOI] [PubMed] [Google Scholar]
- 14. Main BG, Blencowe NS, Howes N, Cousins S, Avery KNL, Gormley A et al Protocol for the systematic review of the reporting of transoral robotic surgery. BMJ Open 2018; 8: e019198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCulloch P, Cook JA, Altman DG, Heneghan C, Diener MK; IDEAL Group . IDEAL framework for surgical innovation 1: the idea and development stages. BMJ 2013; 346: f3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I et al A revised tool for assessing risk of bias in randomized trials. Cochrane Database of Systematic Reviews 2016; 10(Suppl 1): 29–31. [Google Scholar]
- 17. Blencowe NS, Mills N, Cook JA, Donovan JL, Rogers CA, Whiting P et al Standardizing and monitoring the delivery of surgical interventions in randomized clinical trials. Br J Surg 2016; 103: 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blencowe NS, Brown JM, Cook JA, Metcalfe C, Morton DG, Nicholl J et al; Members of the MRC Hub for Trials Methodology Research Network Workshop . Interventions in randomised controlled trials in surgery: issues to consider during trial design. Trials 2015; 16: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Food and Drug Administration . Clinical outcome assessment (COA) Qualification Programme https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm284077.htm [accessed 1 February 2019].
- 20. Weldring T, Smith SM. Patient‐reported outcomes (PROs) and patient‐reported outcome measures (PROMs). Health Serv Insights 2013; 6: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mant J. Process versus outcome indicators in the assessment of quality of healthcare. Int J Qual Healthcare 2001; 13: 475–480. [DOI] [PubMed] [Google Scholar]
- 22. The Medical Dictionary . https://medical-dictionary.thefreedictionary.com/adverse+event [accessed 1 February 2019].
- 23. Ganz RA, Edmundowicz SA, Taiganides PA, Lipham JC, Smith CD, DeVault KR et al Long‐term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol 2016; 14: 671–677. [DOI] [PubMed] [Google Scholar]
- 24. Bonavina L, DeMeester T, Fockens P, Dunn D, Saino G, Bona D et al Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one‐ and 2‐year results of a feasibility trial. Ann Surg 2010; 252: 857–862. [DOI] [PubMed] [Google Scholar]
- 25. Lipham JC, DeMeester TR, Ganz RA, Bonavina L, Saino G, Dunn DH et al The LINX® reflux management system: confirmed safety and efficacy now at 4 years. Surg Endosc 2012; 26: 2944–2949. [DOI] [PubMed] [Google Scholar]
- 26. Saino G, Bonavina L, Lipham JC, Dunn D, Ganz RA. Magnetic sphincter augmentation for gastroesophageal reflux at 5 years: final results of a pilot study show long‐term acid reduction and symptom improvement. J Laparoendosc Adv Surg Tech A 2015; 25: 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tatum JM, Alicuben E, Bildzukewicz N, Samakar K, Houghton CC, Lipham JC. Minimal versus obligatory dissection of the diaphragmatic hiatus during magnetic sphincter augmentation surgery. Surg Endosc 2019; 33: 782–788. [DOI] [PubMed] [Google Scholar]
- 28. Bell R, Lipham J, Louie B, Williams V, Luketich J, Hill M et al Laparoscopic magnetic sphincter augmentation versus double‐dose proton pump inhibitors for management of moderate‐to‐severe regurgitation in GERD: a randomized controlled trial. Gastrointest Endosc 2019; 89: 14.e1–22.e1. [DOI] [PubMed] [Google Scholar]
- 29. Sheu EG, Nau P, Nath B, Kuo B, Rattner DW. A comparative trial of laparoscopic magnetic sphincter augmentation and Nissen fundoplication. Surg Endosc 2015; 29: 505–509. [DOI] [PubMed] [Google Scholar]
- 30. Warren HF, Reynolds JL, Lipham JC, Zehetner J, Bildzukewicz NA, Taiganides PA et al Multi‐institutional outcomes using magnetic sphincter augmentation versus Nissen fundoplication for chronic gastroesophageal reflux disease. Surg Endosc 2016; 30: 3289–3296. [DOI] [PubMed] [Google Scholar]
- 31. Reynolds JL, Zehetner J, Nieh A, Bildzukewicz N, Sandhu K, Katkhouda N et al Charges, outcomes, and complications: a comparison of magnetic sphincter augmentation versus laparoscopic Nissen fundoplication for the treatment of GERD. Surg Endosc 2016; 30: 3225–3230. [DOI] [PubMed] [Google Scholar]
- 32. Reynolds JL, Zehetner J, Wu P, Shah S, Bildzukewicz N, Lipham JC. Laparoscopic magnetic sphincter augmentation vs laparoscopic Nissen fundoplication: a matched‐pair analysis of 100 patients. J Am Coll Surg 2015; 221: 123–128. [DOI] [PubMed] [Google Scholar]
- 33. Asti E, Bonitta G, Lovece A, Lazzari V, Bonavina L. Longitudinal comparison of quality of life in patients undergoing laparoscopic Toupet fundoplication versus magnetic sphincter augmentation: observational cohort study with propensity score analysis. Medicine (Baltimore) 2016; 95: e4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rona KA, Reynolds J, Schwameis K, Zehetner J, Samakar K, Oh P et al Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc 2017; 31: 2096–2102. [DOI] [PubMed] [Google Scholar]
- 35. Buckley FP III, Bell RCW, Freeman K, Doggett S, Heidrick R. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc 2018; 32: 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Desart K, Rossidis G, Michel M, Lux T, Ben‐David K. Gastroesophageal reflux management with the LINX® system for gastroesophageal reflux disease following laparoscopic sleeve gastrectomy. J Gastrointest Surg 2015; 19: 1782–1786. [DOI] [PubMed] [Google Scholar]
- 37. Muñoz‐Largacha JA, Hess DT, Litle VR, Fernando HC. Lower esophageal magnetic sphincter augmentation for persistent reflux after Roux‐en‐Y gastric bypass. Obes Surg 2016; 26: 464–466. [DOI] [PubMed] [Google Scholar]
- 38. Hawasli A, Tarakji M, Tarboush M. Laparoscopic management of severe reflux after sleeve gastrectomy using the LINX® system: technique and one year follow up case report. Int J Surg Case Rep 2017; 30: 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuckelman JP, Phillips CJ, Derickson MJ, Faler BJ, Martin MJ. Esophageal magnetic sphincter augmentation as a novel approach to post‐bariatric surgery gastroesophageal reflux disease. Obes Surg 2018; 28: 3080–3086. [DOI] [PubMed] [Google Scholar]
- 40. Melloni M, Lazzari V, Asti E, Bonavina L. Magnetic sphincter augmentation is an effective option for refractory duodeno‐gastro‐oesophageal reflux following Billroth II gastrectomy. BMJ Case Rep 2018; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwameis K, Nikolic M, Morales Castellano DG, Steindl A, Macheck S, Kristo I et al Results of magnetic sphincter augmentation for gastroesophageal reflux disease. World J Surg 2018; 42: 3263–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith CD, DeVault KR, Buchanan M. Introduction of mechanical sphincter augmentation for gastroesophageal reflux disease into practice: early clinical outcomes and keys to successful adoption. J Am Coll Surg 2014; 218: 776–781. [DOI] [PubMed] [Google Scholar]
- 43. Smith CD, Ganz RA, Lipham JC, Bell RC, Rattner DW. Lower esophageal sphincter augmentation for gastroesophageal reflux disease: the safety of a modern implant. J Laparoendosc Adv Surg Tech A 2017; 27: 586–591. [DOI] [PubMed] [Google Scholar]
- 44. Louie BE, Farivar AS, Shultz D, Brennan C, Vallières E, Aye RW. Short‐term outcomes using magnetic sphincter augmentation versus Nissen fundoplication for medically resistant gastroesophageal reflux disease. Ann Thorac Surg 2014; 98: 498–504. [DOI] [PubMed] [Google Scholar]
- 45. Riegler M, Schoppman SF, Bonavina L, Ashton D, Horbach T, Kemen M. Magnetic sphincter augmentation and fundoplication for GERD in clinical practice: one‐year results of a multicenter, prospective observational study. Surg Endosc 2015; 29: 1123–1129. [DOI] [PubMed] [Google Scholar]
- 46. Ganz RA, Peters JH, Horgan S. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013; 368: 2039–2040. [DOI] [PubMed] [Google Scholar]
- 47. Lipham JC, Taiganides PA, Louie BE, Ganz RA, DeMeester TR. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis Esophagus 2015; 28: 305–311. [DOI] [PubMed] [Google Scholar]
- 48. Louie BE, Smith CD, Smith CC, Bell RCW, Gillian GK, Mandel JS et al Objective evidence of reflux control after magnetic sphincter augmentation: one year results from a post approval study. Ann Surg 2019; 270: 302–308. [DOI] [PubMed] [Google Scholar]
- 49. Reynolds JL, Zehetner J, Bildzukewicz N, Katkhouda N, Dandekar G, Lipham JC. Magnetic sphincter augmentation with the LINX device for gastroesophageal reflux disease after U.S. Food and Drug Administration approval. Am Surg 2014; 80: 1034–1038. [PubMed] [Google Scholar]
- 50. Rona KA, Tatum JM, Zehetner J, Schwameis K, Chow C, Samakar K et al Hiatal hernia recurrence following magnetic sphincter augmentation and posterior cruroplasty: intermediate‐term outcomes. Surg Endosc 2018; 32: 3374–3379. [DOI] [PubMed] [Google Scholar]
- 51. Brockmeyer JR, Connolly EE, Wittchow RJ, Kothari SN. Resolution of fundic gland polyposis following laparoscopic magnetic sphincter augmentation and subsequent cessation of proton pump inhibitors. Case Rep Gastrointest Med 2015; 2015: 576263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stadlhuber RJ, Dubecz A, Meining A, Stein HJ. Adenocarcinoma of the distal esophagus in a patient with a magnetic sphincter augmentation device: first of many to come? Ann Thorac Surg 2015; 99: e147–e148. [DOI] [PubMed] [Google Scholar]
- 53. Czosnyka NM, Buckley FP, Doggett SL, Vassaur H, Connolly EE, Borgert AJ et al Outcomes of magnetic sphincter augmentation – a community hospital perspective. Am J Surg 2017; 213: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 54. Asti E, Siboni S, Lazzari V, Bonitta G, Sironi A, Bonavina L. Removal of the magnetic sphincter augmentation device: surgical technique and results of a single‐center cohort study. Ann Surg 2017; 265: 941–945. [DOI] [PubMed] [Google Scholar]
- 55. Warren HF, Brown LM, Mihura M, Farivar AS, Aye RW, Louie BE. Factors influencing the outcome of magnetic sphincter augmentation for chronic gastroesophageal reflux disease. Surg Endosc 2018; 32: 405–412. [DOI] [PubMed] [Google Scholar]
- 56. Warren HF, Louie BE, Farivar AS, Wilshire C, Aye RW. Manometric changes to the lower esophageal sphincter after magnetic sphincter augmentation in patients with chronic gastroesophageal reflux disease. Ann Surg 2017; 266: 99–104. [DOI] [PubMed] [Google Scholar]
- 57. Alicuben ET, Tatum JM, Bildzukewicz N, Samakar K, Samaan JS, Silverstein EN et al Regression of intestinal metaplasia following magnetic sphincter augmentation device placement. Surg Endosc 2019; 33: 576–579. [DOI] [PubMed] [Google Scholar]
- 58. Schwameis K, Schwameis M, Zörner B, Lenglinger J, Asari R, Riegler FM et al Modern GERD treatment: feasibility of minimally invasive esophageal sphincter augmentation. Anticancer Res 2014: 2341–2348. [PubMed] [Google Scholar]
- 59. Royal College of Surgeons . RCS Statement on Medical Device Regulation; 2018. https://www.rcseng.ac.uk/news-and-events/media-centre/press-releases/medical-device-regulation/ [accessed 1 February 2019].
- 60. Gov.uk . Medicines and Healthcare Products Regulatory Agency https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency/about [accessed 1 February 2019].
- 61. The Independent . The Vaginal Mesh Scandal is the New Thalidomide – So Where is the Outcry?; 2017. https://www.independent.co.uk/news/long_reads/transvaginal-mesh-vaginal-procedure-surgery-tvt-gynaecology-thalidomide-womens-health-psychology-a7862126.html [accessed 1 March 2019].
- 62. Sepehripour AH, Garas G, Athanasiou T, Casula R. Robotics in cardiac surgery. Ann R Coll Surg Engl 2018; 100(Suppl 7): 22–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dyer C. Robot Assisted Surgery is Blamed for Heart Patient's Death; 2018. https://www.bmj.com/content/363/bmj.k4791 [accessed 1 August 2019]. [DOI] [PubMed]
- 64. Royal College of Surgeons . Surgical Innovation, New Techniques and Technologies; a Guide to Good Practice; 2019. http://www.rcseng.ac.uk/standardsandguidance [accessed 1 July 2019].
- 65. Mercer SJ, Toh SK, Somers SS. Esophageal adenocarcinoma developing above an Angelchik prosthesis. Dis Esophagus 2007; 20: 546–548. [DOI] [PubMed] [Google Scholar]
- 66. Stewart KC, Urschel JD, Hallgren RA. Reoperation for complications of the Angelchik antireflux prosthesis. Ann Thorac Surg 1994; 57: 1557–1558. [DOI] [PubMed] [Google Scholar]
- 67. Sedrakyan A, Campbell B, Graves S, Cronenwett JL. Surgical registries for advancing quality and device surveillance. Lancet 2016; 388: 1358–1360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Ovid MEDLINE search strategy
Table S2. Descriptions of the key components of MSA insertion


