Abstract
Obesity is a major health problem and is known to be closely associated with metabolic diseases. Abnormal hepatic accumulation of fat causes fatty liver or hepatic steatosis, and long-term consumption of a high-fat diet is known to be a key obesity-causing factor. Recent studies have demonstrated that probiotics such as Lactobacillus strains, exert an anti-obesity effect by regulating adipogenesis. However, it is still unknown how the consumption of probiotics can reduce abdominal fat volume by regulating the hepatic expression of lipogenic genes. Therefore, we evaluated the effect of long-term ingestion of L. plantarum LMT1-48 on the expression of lipogenic genes in high-fat diet (HFD)-fed mice. We observed that treatment of 3T3-L1 adipocytes with L. plantarum LMT1-48 extract inhibited their differentiation and lipid accumulation by downregulating lipogenic genes, namely, PPARγ, C/EBPα, FAS, and FABP4. Interestingly, administration of L. plantarum LMT1-48 reduced liver weight and liver triglycerides concurrently with the downregulation of the lipogenic genes PPARγ, HSL, SCD-1, and FAT/CD36 in the liver, resulting in the reduction of body weight and fat volume in HFD-fed obese mice. Notably, we also observed that the administration of at least 106 CFU of L. plantarum LMT1-48 significantly lowered body weight and abdominal fat volume in modified diet-fed mouse models. Collectively, these data suggest that L. plantarum LMT1-48 is a potential healthy food for obese people.
Subject terms: Microbiology, Obesity
Introduction
Obesity is a major health problem and causes many diseases, including cardiovascular diseases, type 2 diabetes, and liver diseases1,2. In particular, obesity is closely associated with abnormal accumulation of free fatty acids (FFA) in the liver, resulting in fatty liver or hepatic steatosis3–5. Fatty liver is classified as microvesicular and macrovesicular fat through the histological accumulation of >5% triglycerides in hepatocytes6. The major causes of fatty liver are related to drug use, metabolic syndrome, and alcohol consumption7,8. Insulin resistance is the primary cause of metabolic disorders leading to fat accumulation. Insulin-suppressed hormone-sensitive lipases (HSLs) in adipocytes are activated due to insulin resistance, while triglycerides in adipocytes are released into the blood in the form of fatty acids. Free fatty acids released into the blood increase the fatty acid inflow into the liver, whereby hepatic fat accumulation increases. The mechanism underlying the development of nonalcoholic fatty liver is not fully understood yet, but hepatic fat accumulation is considered a feature of insulin resistance with abdominal obesity and is closely related to metabolic syndrome9,10.
Consequently, long-term consumption of high-fat enriched diets increases the body weight as well as hepatic fat accumulation and hepatic adipocyte proliferation in mammals, thereby leading to obesity11,12. Interestingly, recent studies have demonstrated that human probiotic strains play an important role in modulating immune responses13,14 and exert anti-obesity effects in diet-induced obese mice15. In particular, Lactobacillus species exhibits an anti-obesity effect by regulating the expression of genes related to adipogenesis16,17. The expression of lipogenic genes is known to play a pivotal role in obesity-induced metabolic complications. Peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα) are the major activators of adipogenesis18, and fatty acid synthase (FAS) and fatty acid-binding protein 4 (FABP4) are involved in the formation of mature adipocytes19. Fatty acid translocase (FAT)/CD36 plays an important role in the uptake and oxidation of intracellular long-chain fatty acids20. HSL is a key enzyme involved in the mobilization of fatty acids, overall energy homeostasis, and hydrolysis of fatty acids21, and stearoyl-CoA desaturase-1 (SCD-1) is the main enzyme involved in the biosynthesis of fatty acids22. Thus, lipogenic genes are the key regulators of the synthesis23 and accumulation24 of fatty acids and differentiation of adipocytes25. However, it is still unknown how the ingestion of probiotics can reduce abdominal fat volume by regulating the hepatic expression of lipogenic genes. Therefore, we assessed the functional effects of long-term ingestion of L. plantarum LMT1-48 by evaluating the expression of lipogenic genes in the livers of high-fat diet (HFD)-fed mice.
Results
Inhibitory effect of the cell extract of L. plantarum LMT1-48 on 3T3-L1 adipocytes
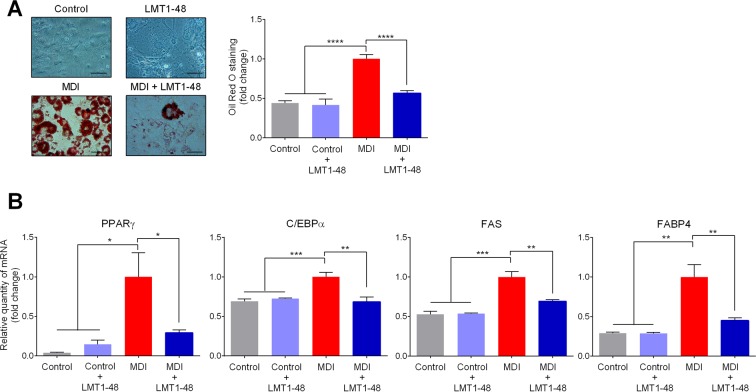
Obesity is associated with an increase in adipocyte number and size25, and activation of adipogenesis factors26. Therefore, we investigated the mechanism by which adipocytes were regulated in response to treatment with L. plantarum LMT1-48. For this, we used 3T3-L1 cells and differentiated them into adipocytes by treating them with MDI reagent. Cellular viability was evaluated to assess for the toxicity of the L. plantarum LMT1-48 extract, and no harmful effect of the extract on the cellular viability was detected (Supple. Fig. S1A,B). The differentiation of 3T3-L1 cells was significantly inhibited in the presence of the L. plantarum LMT1-48 extract (Fig. 1A, Left panel of the staining data). Oil Red O staining was carried out to assess for the inhibitory effect of L. plantarum LMT1-48 and the results were compared with those of the MDI-treated control group (Fig. 1A, Right panel).
Figure 1.
Inhibitory effect of L. plantarum LMT1-48 in 3T3-L1 adipocytes. (A) 3T3-L1 cells were stimulated with MDI reagent for 48 h to induce differentiation. These cells were then treated with the L. plantarum LMT1-48 extract (final concentration of 200 µg/ml). After differentiation, 3T3-L1 cells were stained with Oil red O reagent. To quantitate the inhibitory effect of L. plantarum LMT1-48 on the differentiation, Oil Red O stained fat droplets were dissolved in isopropanol, and the amount of the dissolved stain was spectrophotometrically measured at 520 nm by an ELISA reader. (B) Meanwhile, the mRNA levels of the lipogenic genes PPARγ, C/EBPα, FAS, and FABP4 in differentiated 3T3-L1 cells were quantitated. Two independent experiments were performed with n = 5 for each experiment. Error bars represent standard error of the mean (Mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001.
Further, we used quantitative RT-PCR to assess whether the L. plantarum LMT1-48 extract altered the expression of the genes related to adipocyte differentiation. Among these genes, PPARγ, C/EBPα, FAS, and FABP4, were significantly upregulated in the presence of MDI; however, they were downregulated in response to the treatment with the L. plantarum LMT1-48 extract (Fig. 1B). These results indicate that the L. plantarum LMT1-48 extract inhibited the differentiation of and lipid accumulation in 3T3-L1 adipocytes by downregulating lipogenic genes.
Anti-obesity effect of L. plantarum LMT1-48 in HFD-fed mice
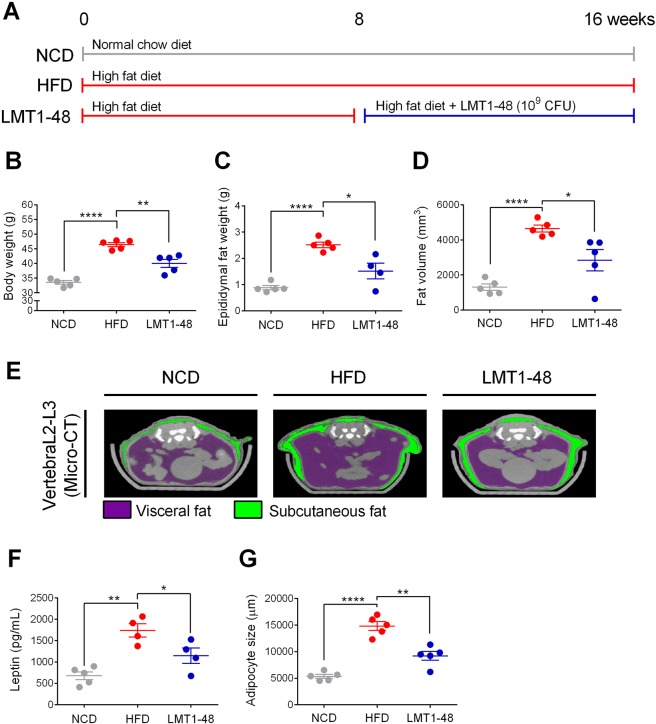
To assess for the anti-obesity effect of L. plantarum LMT1-48 in HFD-fed mice, we designed three different feeding conditions as shown in Fig. 2A. Long-term HFD feeding increased the body weights of mice (46.4 ± 1.6 g) compared with those of the NCD-fed mice (33.5 ± 1.4 g). Administration of L. plantarum LMT1-48 reduced the body weights significantly (40.1 ± 3.0 g) compared with those of the HFD-fed mice (Fig. 2B).
Figure 2.
Anti-obesity effect of L. plantarum LMT1-48 in HFD-fed mice. (A) Experimental scheme. (B) Body weight, (C) epididymal fat weight, and (D) fat volume were measured to assess for the anti-obesity effect of L. plantarum LMT1-48 in mice. (E) Representative micro-computed tomography (CT) images of the abdominal fat at L2–L3 region. The visceral and subcutaneous fats are depicted in purple and green colors, respectively. (F) Serum levels of the leptin hormone were measured via ELISA (G) The adipocyte size in the visceral adipose tissue was measured. Two independent experiments were performed with n = 5 for each experiment. Error bars represent standard error of the mean (Mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We also assessed for changes in the epididymal fat pad weight and abdominal fat volume using a micro-CT scanning system. Administration of L. plantarum LMT1-48 significantly decreased the epididymal fat pad weight (Fig. 2C) and abdominal fat volume in HFD-fed mice (Fig. 2D). Representative positron emission tomography (PET/CT) scanning images are shown in Fig. 2E, where visceral and subcutaneous fat are depicted in purple and green colors, respectively (Fig. 2E).
Recent studies have shown that the leptin hormone is secreted from the adipose tissue and is highly associated with obesity27–29. Therefore, we evaluated the plasma levels of leptin and found that LMT1-48 group had lower levels than the HFD group (Fig. 2F). We also measured the size of visceral adipocytes to assess for the anti-obesity effect of L. plantarum LMT1-48 in HFD-fed mice. Histological analysis showed that adipocyte size in the visceral adipose tissue was smaller in LMT1-48 group than in the HFD group (Fig. 2G). These results indicate that administration of L. plantarum LMT1-48 exerts an anti-obesity effect by downregulating plasma levels of leptin, resulting in inhibition of fat accumulation and adipocyte proliferation in HFD-fed mice.
L. plantarum LMT1-48 downregulates lipogenic genes in the livers of HFD-fed mice
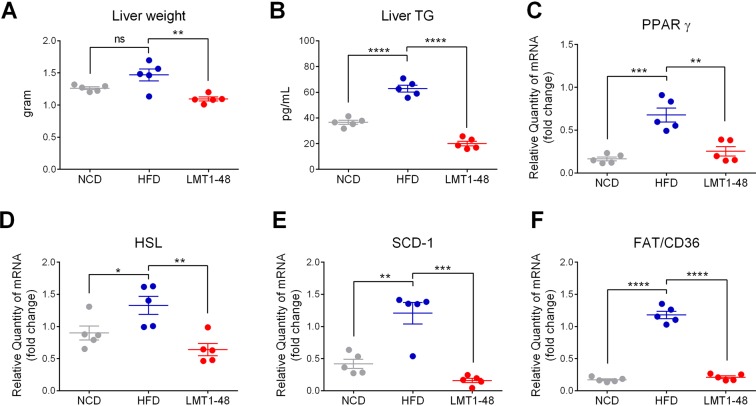
We assessed the expression of lipogenic genes in the livers of HFD-fed mice. Administration of L. plantarum LMT1-48 significantly reduced the liver weight (Fig. 3A), liver triglycerides (Fig. 3B), and expression of the lipogenic genes PPARγ (0.2 ± 0.1 in NCD group, 0.7 ± 0.2 in HFD group, and 0.3 ± 0.1 in LMT1-48 group), HSL (0.9 ± 0.2 in NCD group, 1.3 ± 0.3 in HFD group, and 0.6 ± 0.2 in LMT1-48 group), SCD1 (0.4 ± 0.2 in NCD group, 1.2 ± 0.4 in HFD group, and 0.2 ± 0.1 in LMT1-48 group), and FAT/CD36 (0.2 ± 0.0 in NCD group, 1.2 ± 0.1 in HFD group, and 0.2 ± 0.1 in LMT1-48 group) in the livers of HFD-fed mice (Fig. 3C–F). These results indicate that administration of L. plantarum LMT1-48 has an anti-obesity effect by reducing liver weight and liver triglycerides, and downregulating the expression of lipogenic genes in the livers of HFD-fed obese mice.
Figure 3.
Effect of L. plantarum LMT1-48 on the expression of lipogenic genes in the liver of HFD-fed mice. (A) Liver weight and (B) liver triglycerides were measured to assess the influence of L. plantarum LMT1-48 administration in HFD-fed mice (n = 5 mice per group). (C–F) Hepatic mRNA levels of the lipogenic genes (C) PPARγ, (D) HSL, (E) SCD-1, and (F) FAT/CD36 were measured by quantitative real-time PCR. Two independent experiments were performed with n = 5 for each experiment. Error bars represent standard error of the mean (Mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Anti-obesity effects of L. plantarum LMT1-48 on modified-diet–fed mice
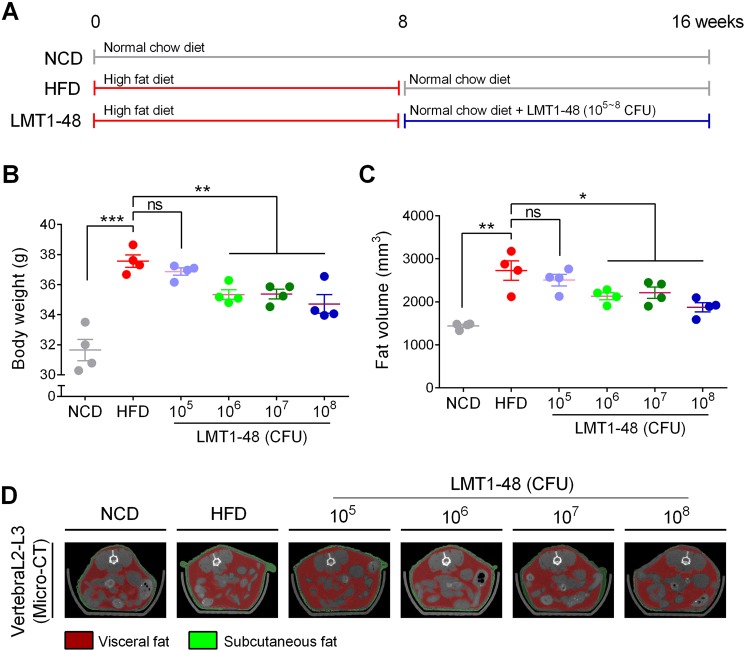
To assess whether the anti-obesity effect of the L. plantarum LMT1-48 extract was dose-dependent, modified-diet–fed mice were administered with different doses of the extract (105, 106, 107, or 108 CFU) per mouse. We designed six different feeding conditions as shown in Fig. 4A. Long-term feeding of HFD followed by normal chow diet led to an increase in body weight in HFD group (37.6 ± 0.8 g) compared with that in the NCD group (31.6 ± 1.4 g, Fig. 4B). Administration of at least 106 CFU of L. plantarum LMT1-48 significantly lowered the body weight (106 CFU: 35.3 ± 0.7 g; 107 CFU: 35.4 ± 0.6 g; 108 CFU: 34.7 ± 1.2 g) in LMT1-48 group, whereas this response was not observed in mice administered with 105 CFU of L. plantarum LMT1-48 (36.9 ± 0.5 g; Fig. 4B). Therefore, we further measured fat volume using a micro-CT scanning system to assess for changes in abdominal fat volume in diet-induced obese mice. Similar to body weight changes, administration of at least 106 CFU of L. plantarum LMT1-48 significantly reduced abdominal fat volume in HFD-fed mice (Fig. 4C). A representative PET/CT image is provided in Fig. 4D, with visceral and subcutaneous fats depicted in red and green colors, respectively. These results indicate that an appropriate amount of the L. plantarum LMT1-48 extract is required to have an anti-obesity effect in HFD-fed mice.
Figure 4.
Anti-obesity effect of L. plantarum LMT1-48 in modified-HFD–fed mice. (A) Experimental scheme. (B) Body weight and (C) fat volume were measured to assess the anti-obesity effect of different concentrations (105, 106, 107, or 108 CFU) of the L. plantarum LMT1-48 extract in modified-HFD–fed mice (n = 4 mice per group). (D) Representative micro-computed tomography (CT) image of the abdominal fat (L2–L3 region). The visceral and subcutaneous fats are depicted in red and green colors, respectively. Two independent experiments were performed with n = 4 for each experiment. Error bars represent standard error of the mean (Mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Obesity is closely associated with liver abnormalities, including nonalcoholic fatty liver disease (NAFLD) and steatohepatitis2,30. Fat accumulation in the liver is generally caused by increased lipotoxicity resulting from high levels of free fatty acids, free cholesterol, and lipid metabolites31, and these, in turn, are regulated by lipogenic genes, such as PPARγ, C/EBPα, FAS, and FABP418,19. Importantly, these lipogenic genes play an important role in obesity-induced metabolic liver diseases19. Additionally, several studies have also demonstrated that liver weight, liver triglycerides, and expression of lipogenic genes are closely associated with obesity2,30, and the administration of lactic acid bacteria (LAB) inhibits the synthesis32 and accumulation33 of fats in HFD-induced obese mice. Therefore, we hypothesized that the administration of L. plantarum LMT1-48 could reduce body fat by regulating the expression of lipogenic genes in the livers of HFD-induced obese mice.
Notably, recent studies have demonstrated that a high amount of lipopolysaccharides (LPS) produced by gram-negative bacteria cause chronic inflammation, and obese people have been reported to have higher levels of gram-negative bacteria in their gastrointestinal tracts34,35. Importantly, LPS is known to leak through damaged intestinal epithelial barrier under inflammatory conditions36 and circulate to the liver through the portal vein. This is accompanied by an increase in the levels of PPARγ37–39, causing obesity and metabolic diseases via an inflammatory immune response40. Importantly, the hepatic expression of PPARγ is highly associated with NAFLD in humans and experimental mouse models41. Moreover, fat accumulation in hepatocytes of nonalcoholic fatty liver is closely related to insulin resistance, and obesity and insulin resistance are also correlated with NAFLD9,10. In general, PPARγ is expressed at very low levels in the liver; however, insulin upregulates the expression of PPAR-γ in the adipose tissue, leading to increased adipogenesis and lipogenesis in the liver during obesity42,43. Although HSL produces fatty acids and glycerol by breaking down triglycerides in the adipose tissue and thereby inhibits fat accumulation44, increased insulin resistance in obese patients reduces HSL levels and inhibits lipolysis activation. Interestingly, in the present study, we observed that the administration of L. plantarum LMT1-48 reduced liver weight and liver triglycerides concurrently with the downregulation of PPARγ and HSL in the liver, resulting in the reduction of body weight and fat volume in HFD-fed mice. Therefore, we speculate that the administration of L. plantarum LMT1-48 may regulate LPS levels along with downregulation of PPARγ and HSL, resulting in reduced body fat and liver weight in HFD-induced obese mice.
Several studies have also demonstrated that short-chain fatty acids (SCFAs) produced by LAB exert an anti-obesity effect through the modulation of the lipid and glucose metabolisms45,46, resulting in reduced adipocyte size47,48. Notably, SCFAs play a potential role in decreasing fat accumulation in the adipose tissue by accelerating the oxidation of fatty acids in HFD-induced obese mice49,50. SCFAs can switch the metabolic state from lipogenesis to fat oxidation by regulating the expression of PPARγ in HFD-induced obese rodents51,52. Interestingly, in the present study, we also observed that treatment with the L. plantarum LMT1-48 extract inhibited adipocyte differentiation and lipid accumulation and downregulated the lipogenic gene PPARγ. However, the mechanism underlying the SCFA-mediated regulation of the expression of the lipogenic genes HSL, SCD-1, and FAT/CD36 has not been clearly studied yet. Based on the present study, we hypothesize that administration of L. plantarum LMT1-48 may help produce certain physiologically active substances, such as SCFAs, which are known to regulate lipid accumulation and adipocyte differentiation through downregulation of lipogenic genes. To address this, we need to further investigate the mechanism through which SCFAs produced by L. plantarum LMT1-48 regulate fat accumulation and differentiation of adipocytes in HFD-induced obese mice.
Recent studies have shown that the amount of LAB is important to maintain the colonization and habitation in the gut53,54, and thus LAB dosage may influence the effectivity of the treatment on obesity55. In particular, most LAB studies have evaluated the efficacy at the dosage of 109 CFU56,57. The reason is that this amount is the maximum viable count of LAB that can be grown58. Therefore, in this study, we evaluated the anti-obesity effect at this dosage and compared the results with those derived from lower doses (105~108 CFU). Interestingly, we observed that the administration of L. plantarum LMT1-48 showed the anti-obesity effect when used at a dosage of >106 CFU but not at 105 CFU. Although further investigation is needed to understand the role of colonization in the LAB-induced anti-obesity effect, we assume that 106 CFU is the minimum dose of colonization in the gut for L. plantarum LMT1-48 to induce the anti-obesity effect.
To summarize, this is the first study that has demonstrated that L. plantarum LMT1-48 exerts an anti-obesity effect in HFD-fed mice through downregulation of the hepatic expression of lipogenic genes along with the consequent reduction in the number of adipocytes. Although further studies are needed to elucidate whether L. plantarum LMT1-48 regulates the body fat mass in humans, our results offer L. plantarum LMT1-48 as a potential probiotic for obese people.
Materials and Methods
Assessment of the inhibitory effect of L. plantarum LMT1-48 on 3T3-L1 adipocytes
3T3-L1 cells (KCBL 10092.1) were obtained from Korean cell line bank (KCBL, Seoul, Korea) and sub-cultured up to 10 passages in Dulbecco’s modified Eagle medium (DMEM, Gibco, NY, USA) containing 10% fetal bovine serum (FBS, Invitrogen, Auckland, New Zealand) and 1% penicillin/streptomycin (P/S, Gibco, NY, USA) at 37 °C in a humidified incubator with 5% CO2. 3T3-L1 cells were seeded at a density of 6 × 104 cells/well in 12-well plates and cultured for 48 h at 37 °C. For adipocyte differentiation, cells were stimulated with MDI (3-Isobutyl-1-methylxanthine, dexamethasone, and insulin) reagent for 48 h at 37 °C. MDI reagent consisted of 0.5 mM 3-isobutyl-1-methylxanthine (IBMX, Sigma, St. Louis, MO, USA), 0.25 nM dexamethasone (Sigma, St. Louis, MO, USA), and 5 μg/mL insulin (Sigma, St. Louis, MO, USA). MDI-stimulated cells were cultured in DMEM containing 10% FBS and 5 μg/mL insulin for 4 days at 37 °C.
To test the inhibitory effect of L. plantarum LMT1-48 on 3T3-L1 adipocytes, the cell extract of L. plantarum LMT1-48 was prepared. Briefly, L. plantarum LMT1-48 strain (KCTC registered identification number: KCTC13024BP; patent registration number: KR101670048B1; GenBank accession number: LC131441.1) was isolated from the traditional Korean fermented food kimchi and cultured in De Man, Rogosa, and Sharpe (MRS; Difco Co., MI, USA) broth for 24 h at 37 °C. Cultured cells were centrifuged at 9358 × g at 4 °C for 10 min and washed thrice with PBS. Cells were mechanically lysed using a beat beater (Bullet Blender, Next Advance, NY, USA) for 3 min, and samples were then placed on ice for another 3 min. The procedure following the lysis and PBS washes were repeated five times. Cell debris was removed by centrifugation at 9358 × g for 5 min at 4 °C, and the supernatant was filtered through a Microcon filter with 0.45-µm pores under sterilized conditions. MDI-treated 3T3-L1 adipocytes were then treated with this cell extract at a final concentration of 200 µg/mL.
Cellular viability assay
The viability test for 3T3-L1 cells was performed using Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan). In brief, 3T3-L1 cells were placed at a density of 5 × 103 cells/well in 96-well plates and cultured for 24 h at 37 °C. Subsequently, the cells were further cultured with L. plantarum LMT1-48 extract alone (25, 50, 100, 200, 400, or 800 μg/mL) or with a combination of L. plantarum LMT1-48 extract (200 µg/mL) and MDI reagent for 48 h at 37 °C. The CCK-8 reagent (10 μL) was administered to the cells, which were then incubated for another 2 h at 37 °C. The absorbance was measured at 450 nm using an ELISA reader (Biotek, VT, USA).
Oil red O staining
After assessing the inhibitory effect of L. plantarum LMT1-48 on 3T3-L1 adipocytes, we assessed its effect on the adipogenic differentiation by Oil red O staining. Briefly, cells were washed twice with PBS and fixed with 10% formaldehyde (Merck, NJ, USA) for 5 min at room temperature. Afterward, cells were treated with fresh 10% formaldehyde for another 1 h at room temperature. Subsequently, formaldehyde was removed and cells were stained with Oil red O (Sigma, St. Louis, MO, USA) for 1 h at room temperature. Next, Oil red O solution was removed and 3T3-L1 adipocytes were washed 4 times with dH2O. Images of stained fat droplets were captured using a microscope from Olympus (Tokyo, Japan). For quantitation, stained fat droplets were dissolved in 1 ml of 100% isopropanol for 10 min at room temperature, and the absorbance was measured at 520 nm using an ELISA reader (Biotek, VT, USA).
Animal models
Male C57BL/6N mice were purchased from OrientBio Inc. (Seongnam-si, Korea). All the mice were maintained at a specialized pathogen-free facility at CHA University (Seongnam-si, Korea) and used at 5 weeks of age for the experiments. All the animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee of CHA University (IACUC170127).
We designed three different treatment groups based on diet-induced obesity and used at least 5 mice per group. Mice in the negative control group (NCD group) were fed with a normal chow diet (Teklad global certified and irradiated 18% protein rodent diet, 2918 C, Envigo, UK) for 16 weeks. Mice in the positive control group (HFD group) were fed with an HFD (DIO rodent purified diet with 45% energy from fat-red, 58V8, Test Diet, UK) for 16 weeks. To evaluate the anti-obesity effect of L. plantarum LMT1-48, mice were fed with an HFD for 8 weeks and then administered L. plantarum LMT1-48 (109 CFU/day) concurrently with HFD for another 8 weeks (LMT1-48 group).
Further, we designed six different modified diet-fed obese animal models. Mice in the negative control group were treated as described above. Mice in the positive control group (HFD group) were fed with HFD for 8 weeks and then supplied with a normal chow diet for another 8 weeks. To evaluate the anti-obesity effect of L. plantarum LMT1-48, mice were fed with HFD for 8 weeks and then administrated L. Plantarum LMT1-48 (105, 106, 107, or 108 CFU/day) concurrently with HFD for another 8 weeks (LMT1-48 group). L. plantarum LMT1-48 strain was freshly prepared and orally administered every day. After 16 weeks, mice were sacrificed, and the visceral fat and liver were collected to assess the adipocyte size and expression of lipogenic genes.
Micro-computed tomography (CT) analysis
The body fat mass of 5 mice from each group was analyzed with a micro-imaging system (NFR Polaris G90, NanoFocusRay co. Ltd., Suwon-si, Korea). Three-dimensional (3D) images were reconstructed using the 3D analysis software Amira 5.4.1 (Visage Imaging GmbH, Germany). Adipose tissue regions were drawn on the abdominal subcutaneous and visceral fat tissues at the level of the vertebra (L2–L3 region).
Measurement of adipocyte size
The visceral fat tissues were collected from the experimental animals and fixed in 10% neutral buffered formalin for 48 h. The samples were dehydrated before embedding in paraffin wax and then sectioned with a thickness of 4 μm. Sections were mounted on slides for hematoxylin and eosin (H&E) staining. Digital images were captured at 100× magnification using an inverted microscope (Olympus, Tokyo, Japan), and images were analyzed using Image J software (http://imagej.nih.gov/ij/).
Measurement of leptin hormone in mouse serum
To analyze hormone levels in sera, blood was collected from the caudal vena cava using a clot activator-coated tube and centrifuged at 2,000 × g for 15 min at 4 °C. Leptin levels in sera were determined using a mouse leptin enzyme-linked immunosorbent assay (ELISA) kit (Abcam Inc., Cambridge, UK).
Measurement of triglyceride in mouse liver
To analyze triglyceride levels in liver, liver tissues was homogenized in lysis buffer containing 5% NP-40 (BioVision, CA, USA) by using a tissue homogenizer. Triglycerides were measured with the Triglyceride Quantification Colorimetric/Fluorometric Kit (BioVision, CA, USA), following the manufacturer’s instructions.
Quantitative real-time PCR
To quantitate the expression of lipogenic genes in 3T3-L1 adipocytes, total RNA was isolated from cultured 3T3-L1 cells using the TRIzol reagent (Invitrogen, CA, USA), and 50 ng of total RNA was used for cDNA synthesis using RocketScript™ Cycle RT PreMix (Bioneer, Daejeon, Korea). Gene expression was detected using SYBR green (Takara, Tokyo, Japan) on a CFX96 real-time system (Bio-rad, CA, USA). The primers used were as follows: PPARγ, forward 5′-GCATGGTGCCTTCGCTGA-3′ and reverse 5′-TGGCATCTCTGTGTCAACCATG-3′; C/EBPα, forward 5′-CAAGAACAGCAACGAGTACCG-3′ and reverse 5′-GTCACTGGTCAACTCCAGCAC-3′, FAS, forward 5′-TGGGTTCTAGCCAGCAGAGT-3′ and reverse 5′-ACCACCAGAGACCGTTATGC-3′; and FABP4, forward 5′-AGTGGGCTTTGCCACAA-3′ and reverse 5′-GGTGATTTCATCGAATTCCA-3′. The expression levels of target genes were normalized to that of GAPDH (forward 5′-AACGACCCCTTCATTGAC-3′ and reverse 5′-TCCACGACATACTCAGCAC-3′).
To assess the expression levels of lipogenic genes in the liver, total RNA was isolated from the liver using the TRIzol reagent. Samples were homogenized using a homogenizer (Biomasher, Tokyo, Japan), and cDNA was synthesized from 1 μg of total RNA using RocketScript™ Cycle RT PreMix. Gene expression was detected using SYBR green on a CFX96 real-time system. The primers used were as follows: PPARγ, forward 5′-GCATGGTGCCTTCGCTGA-3′ and reverse 5′-TGGCATCTCTGTGTCAACCATG-3′; HSL, forward 5′-GGCTCACAGTTACCATCTCACC-3′ and reverse 5′-GAGTACCTTGCTGTCCTGTCC-3′; SCD-1, forward 5′-TGGGTTGGCTGCTTGTG-3′ and reverse 5′-GCGTGGGCAGGATGAAG-3′; and FAT/CD36, forward 5′-GATGTGGAACCCATAACTGGATTCAC-3′ and reverse 5′-GGTCCCAGTCTCATTTAGCCACAGTA-3′. Expression of GAPDH (forward 5′-GAGACCTTCAACACCCC-3′ and reverse 5′-GTGGTGGTGAAGCTGTAGCC-3′) was used as an internal control. The inverse log of ΔΔCT was then calculated.
Statistical analysis
Data from all the experiments were analyzed using Prism software (Prism version 7; GraphPad Software, San Diego, CA). An unpaired Student’s t-test was used to compare the means of the experimental groups. A P-value < 0.05 was considered statistically significant.
Supplementary information
Author contributions
All the authors contributed to the preparation of this manuscript. T.K. and Y.-H.K. conceived, designed, and supervised the experiments. W.C. performed the experiments, analyzed the data, and wrote the paper. H.D., H.J., D.R., S.S. and Y.K. performed the experiments. H.J. advised on the experiments. Y.-H.K. analyzed the data and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Tai Hoon Kim and Yeung-Hyen Kim.
Supplementary information
is available for this paper at 10.1038/s41598-020-57615-5.
References
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2007;13:3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1139–1142. doi: 10.1172/JCI24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J. Hepatol. 2015;7:1450–1459. doi: 10.4254/wjh.v7.i11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel V, Sanyal AJ. Drug-induced steatohepatitis. Clin. Liver Dis. 2013;17:533–546, vii. doi: 10.1016/j.cld.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 9.Cassader M, et al. Postprandial triglyceride-rich lipoprotein metabolism and insulin sensitivity in nonalcoholic steatohepatitis patients. Lipids. 2001;36:1117–1124. doi: 10.1007/s11745-001-0822-5. [DOI] [PubMed] [Google Scholar]
- 10.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 11.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 12.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am. J. Clin. Nutr. 2001;73:444S–450S. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 14.Rocha-Ramirez LM, et al. Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J. Immunol. Res. 2017;2017:4607491. doi: 10.1155/2017/4607491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi WJ, et al. Antiobesity Effects of Lactobacillus plantarum LMT1-48 Accompanied by Inhibition of Enterobacter cloacae in the Intestine of Diet-Induced Obese Mice. J. Med. Food. 2019;22:560–566. doi: 10.1089/jmf.2018.4329. [DOI] [PubMed] [Google Scholar]
- 16.Park DY, Ahn YT, Huh CS, Jeon SM, Choi MS. The inhibitory effect of Lactobacillus plantarum KY1032 cell extract on the adipogenesis of 3T3-L1 Cells. J. Med. Food. 2011;14:670–675. doi: 10.1089/jmf.2010.1355. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, et al. Isolated exopolysaccharides from Lactobacillus rhamnosus GG alleviated adipogenesis mediated by TLR2 in mice. Sci. Rep. 2016;6:36083. doi: 10.1038/srep36083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madsen MS, Siersbaek R, Boergesen M, Nielsen R, Mandrup S. Peroxisome proliferator-activated receptor gamma and C/EBPalpha synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell. Biol. 2014;34:939–954. doi: 10.1128/MCB.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moseti Dorothy, Regassa Alemu, Kim Woo-Kyun. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. International Journal of Molecular Sciences. 2016;17(1):124. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coburn CT, et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 21.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.R200009-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J. Biol. Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 23.Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes & Metab. 2004;30:294–309. doi: 10.1016/s1262-3636(07)70121-0. [DOI] [PubMed] [Google Scholar]
- 24.Girard J, Perdereau D, Foufelle F, Prip-Buus C, Ferre P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB journal: Off. Publ. Federation Am. Societies Exp. Biol. 1994;8:36–42. doi: 10.1096/fasebj.8.1.7905448. [DOI] [PubMed] [Google Scholar]
- 25.Salans LB, Cushman SW, Weismann RE. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J. Clin. Invest. 1973;52:929–941. doi: 10.1172/JCI107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz-Ojeda Francisco, Rupérez Azahara, Gomez-Llorente Carolina, Gil Angel, Aguilera Concepción. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. International Journal of Molecular Sciences. 2016;17(7):1040. doi: 10.3390/ijms17071040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 28.Jequier E. Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 29.Considine RV, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 30.Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity-associated liver disease. J. Clin. Endocrinol. Metab. 2008;93:S74–80. doi: 10.1210/jc.2008-1399. [DOI] [PubMed] [Google Scholar]
- 31.Perla Francesco, Prelati Maurizia, Lavorato Michela, Visicchio Daniele, Anania Caterina. The Role of Lipid and Lipoprotein Metabolism in Non‐Alcoholic Fatty Liver Disease. Children. 2017;4(6):46. doi: 10.3390/children4060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yonejima Y, Ushida K, Mori Y. Effect of lactic acid bacteria on lipid metabolism and fat synthesis in mice fed a high-fat diet. Biosci. Microbiota Food Health. 2013;32:51–58. doi: 10.12938/bmfh.32.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyoshi M, Ogawa A, Higurashi S, Kadooka Y. Anti-obesity effect of Lactobacillus gasseri SBT2055 accompanied by inhibition of pro-inflammatory gene expression in the visceral adipose tissue in diet-induced obese mice. Eur. J. Nutr. 2014;53:599–606. doi: 10.1007/s00394-013-0568-9. [DOI] [PubMed] [Google Scholar]
- 34.Creely SJ, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas Rde C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012;108:801–809. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 37.Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2013;56:461–468. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu Chien-Chao, Ching Yung-Hao, Li Yen-Peng, Liu Ju-Yun, Huang Yen-Te, Huang Yi-Wen, Yang Sien-Sing, Huang Wen-Ching, Chuang Hsiao-Li. Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients. 2017;9(11):1220. doi: 10.3390/nu9111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettinelli P, Videla LA. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metab. 2011;96:1424–1430. doi: 10.1210/jc.2010-2129. [DOI] [PubMed] [Google Scholar]
- 40.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 41.Liss KH, Finck BN. PPARs and nonalcoholic fatty liver disease. Biochimie. 2017;136:65–74. doi: 10.1016/j.biochi.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu JH, et al. Suppression of PPARgamma-mediated monoacylglycerol O-acyltransferase 1 expression ameliorates alcoholic hepatic steatosis. Sci. Rep. 2016;6:29352. doi: 10.1038/srep29352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1195–1205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- 44.Reid BN, et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwiertz A, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obes. (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9:1–15. doi: 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takemura N, Okubo T, Sonoyama K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp. Biol. Med. (Maywood) 2010;235:849–856. doi: 10.1258/ebm.2010.009377. [DOI] [PubMed] [Google Scholar]
- 48.Lin HV, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.den Besten G, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.den Besten G, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 51.Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. doi: 10.1371/journal.pone.0063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.den Besten G, et al. Protection against the metabolic syndrome by guar gum-derived short-chain fatty acids depends on peroxisome proliferator-activated receptor gamma and glucagon-like peptide-1. PLoS One. 2015;10:e0136364. doi: 10.1371/journal.pone.0136364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vieira AT, Teixeira MM, Martins FS. The role of probiotics and prebiotics in inducing gut immunity. Front. Immunol. 2013;4:445. doi: 10.3389/fimmu.2013.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derrien M, van Hylckama Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Suwal S, et al. The Probiotic Effectiveness in Preventing Experimental Colitis Is Correlated With Host Gut Microbiota. Front. Microbiol. 2018;9:2675. doi: 10.3389/fmicb.2018.02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim SM, Jeong JJ, Woo KH, Han MJ, Kim DH. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 2016;36:337–348. doi: 10.1016/j.nutres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Wu CC, et al. Effect of Lactobacillus plantarum Strain K21 on High-Fat Diet-Fed Obese Mice. Evid. Based Complement. Altern. Med. 2015;2015:391767. doi: 10.1155/2015/391767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiraldi C, et al. High cell density cultivation of probiotics and lactic acid production. Biotechnol. Bioeng. 2003;82:213–222. doi: 10.1002/bit.10557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.