Abstract
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) is a powerful and sensitive method used in gene expression analysis. Suitable reference genes, which are stable under all experimental circumstances and tissues significantly improve the accuracy of qRT-PCR data. In this study, the stability of six genes, namely, 18S ribosomal RNA (18s), beta-actin (actb), elongation factor 1-alpha (ef1α), glyceraldehyde-3-phosphate-dehydrogenase (gapdh), cathepsin D (ctsd), and beta-2-microglobulin (b2m) were evaluated as potential references for qRT-PCR analysis. The genes were examined in the hypothalamus-pituitary-ovary-liver (HPOL) axis throughout turbot ovarian development via using the geNorm, NormFinder and BestKeeper algorithms. Results showed that the most stable reference genes were ef1α, actb, and ctsd in the hypothalamus, pituitary, ovary and liver, respectively. The best-suited gene combinations for normalization were 18s, ef1α, and ctsd in the hypothalamus; actb, ctsd, and 18s in the pituitary; actb, and ctsd in the ovary; gapdh and ctsd in the liver. Moreover, the expression profile of estrogen receptor α (erα) manifested no significant difference normalization to the aforementioned best-suited gene during turbot ovarian development. However, no single gene or pair of genes is suitable as an internal control and account for the amplification differences among the four tissues during ovarian development. In summary, these results provide a basic data for the optimal reference gene selection and obtain highly accurate normalization of qRT-PCR data in HPOL axis-related gene expression analysis during turbot ovarian development.
Subject terms: Molecular biology, Reproductive biology
Introduction
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) is widely used in gene expression analysis because of the advantages of its sensitivity and the accurate detection of mRNA at extremely low transcription levels1. The successful application of qRT-PCR depends on accurate transcript normalization via the selection of suitable reference genes. The reference genes minimize errors attributed to the use of biological samples, such as experimental operations, and RNA and cDNA qualities2. The ideal reference gene for qRT-PCR exhibits a stable expression level in all target tissues/cells and should be no affected by various experimental conditions or treatments. In general, the commonly used reference genes mainly include glyceraldehyde-3-phosphate-dehydrogenase (gapdh), 18S ribosomal RNA (18s), beta actin (actb), elongation factor 1-alpha (ef1α), cathepsin D (ctsd), and beta-2-microglobulin-like (b2m) in turbot3,4 and other fish species5–8. However, these classical reference genes vary at the transcription level in different tissues, at different developmental stages and experimental conditions or treatments9–11. Inappropriate reference genes that serve as internal controls can affect the accuracy of qRT-PCR results and cause the significantly different conclusion5,12,13. Actually, no single reference gene is universally applicable in gene expression studies. Numerous literatures have identified that two or more reference genes are generally required for qRT-PCR to minimize experimental errors caused by instability of a single gene under all experimental conditions14–16.
Expression stability is the most important characteristic of reference genes. Several softwares include geNorm, Normfinder and Bestkeeper are commonly used to determine optimal reference genes by calculating the stability values of these genes under different conditions17–19. geNorm identifies the expression stability of a candidate gene by calculate M value and pairwise variation (Vn/n + 1). NormFinder confirms the best-suited reference genes via estimating the inter and intragroup expression variations of candidate genes. BestKeeper evaluates the stability of candidate genes by calculating the standard deviation (SD) and correlation coefficient (r). In general, two or more software programs are used together during data analysis to identify discrepancies between the outputs of different algorithms5,20,21.
Turbot is a widely cultured economic marine fish owing to its high market value in various Asian and European countries. In our previous studies, we cloned and identified the functional properties of hypothalamus-pituitary-ovary-liver (HPOL) axis-related genes, gonadotrophin receptors and estrogen receptors (ers) during the ovarian development of turbot22–24. The 18s, actb and ctsd are the most stable housekeeping gene used for turbot luteinizing hormone receptor, ers, growth hormone and insulin-like growth factor expression analysis22,24,25. Thus, no single reference gene is universally applicable in turbot HPOL axis related genes expression analysis. Meanwhile, the biochemical composition of turbot eggs and ovarian fluid, that is related to egg quality was evaluated throughout the reproductive cycle26–28. To further investigate and fully understand the endocrine, paracrine and autocrine mechanisms during the reproductive cycle of female turbot, we need to select the new or different suitable reference genes for HPOL axis-related genes functions evaluation during ovarian development. Therefore, we aimed to investigate appropriate reference gene used for transcriptional expression analysis in turbot hypothalamus, pituitary, ovary and liver during ovarian development. The six reference genes including, 18s, bact, gapdh, ef1α, ctsd, and b2m were selected and expression stability through ovarian development were analyzed via using geNorm, NormFinder and BestKeeper algorithms, respectively. Previous study has identified that estrogen receptor α (ERα) is a subtype of estrogen receptors that is widely expressed in all tissues and has high mRNA levels in the hypothalamus, pituitary, ovary and liver of turbot22. To further confirm the ideal reference genes and investigate the function of HPOL axis-related genes. Thus, we use erα mRNA expression as an example to evaluate the effect of the selected reference genes on data normalization. The stability analysis results will provide helpful guidelines for optimal reference gene selection and make it possible to obtain more reliable results of target gene expression during turbot ovarian development.
Materials and Methods
Animal management and sampling
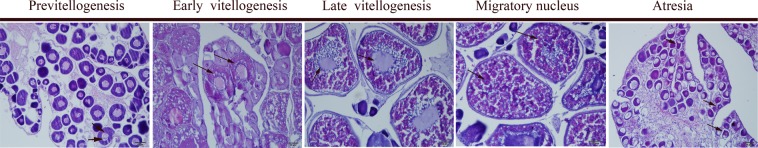
Female turbots were collected from Tianyuan Aquaculture Co., Ltd. of Yantai, China. Fishes were reared in polyethylene tanks and fed with the basal diet for one week to acclimate to the environmental conditions based on our previous study23. The fishes were fasted for 24 h and anesthetized with 100 mg/L tricaine methane sulfonate (MS-222, Sigma, St. Louis, MO) before sampling. Subsequently, hypothalamus, pituitary, liver, and ovary were collected from each fish and stored in liquid nitrogen for RNA extraction. Meanwhile, the ovaries were fixed in Bouin’s solution for histology. After cutting ovarian sections and mounting on glass slides, the sections were stained with hematoxylin and eosin for the identification of the ovary developmental stages based on our previous study23. The ovary developmental stages were classified into pre-vitellogenesis (Prevtg), early vitellogenesis (Evtg), late vitellogenesis (Latvtg), migratory-nucleus (Mig-nucl), atresia (Atre) and described in Fig. 1. All sampling procedures and subsequent experimental protocol were conducted and approved in accordance with the guidelines established by the Institutional Animal Care and Use Committee at Yellow Sea Fisheries Research Institute.
Figure 1.
Development and types of turbot oocyte in ovarian stage. Previtellogenesis, arrow indicates nucleoli at the periphery of the germinal vesicle. Early vitellogenesis, arrow indicates gradually accumulations of yolk granules in the central region of the oocyte. Late vitellogenesis, arrow indicates the yolk granules almost fill the ooplasm, and the nucleus has not yet begun to migrate peripherally. Migratory nucleus, arrow indicates the oocytes, and the yolk granules have attained their maximum size just prior to spawning, and the nucleus is not evident. Atresia, arrow indicates the oocytes have shrinkage or collapse.
RNA extraction and cDNA synthesis
Total RNA was extracted using a MiniBEST Universal RNA extraction kit (Takara Biotech, China) based on the manufacturer’s instructions. The yield and purity of total RNA were determined at 260 nm (A260) absorbance and 260/280 nm (A260/280) ratio by using NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific). The RNA integrity and RNA integrity number (RIN) were assessed by agarose gel and 2100 Bioanalyzer (Agilent Technologies), respectively. The RIN is a number on a scale from 1 to 10, value of 10 indicates intact and non-fragmented RNA, and value of 1 represents completely degraded RNA29. In addition, the total RNA was treated with DNase I (Qiagen) for 30 min at 37 °C to avoid contamination of genomic DNA. Meanwhile, a negative control without cDNA was also included ensure that the reagents were not contaminated. Total RNA (1 μg) from each sample was reverse transcribed via a PrimeScriptTM RT reagent kit with cDNA Eraser (Takara Biotech, China). The resulting cDNA was diluted (1:10) with sterile deionized water and used in qRT-PCR via ABI 7900HT thermocycler (Applied Biosystems).
Primer design and PCR efficiency
Six reference genes namely, gapdh, 18s, actb, ef1a, ctsd, b2m and one target gene erα were used in this study. The full gene names, functions, and accession numbers were listed in Table 1. Primer pairs were designed with Primer 5 software and synthesized by Sangon Biotech (Shanghai) Co., Ltd., China. The cycling conditions for the PCR reaction were 95 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 60 °C for 30 s, and 72 °C for 30 s, and a final extension of 72 °C for 5 min. The PCR efficiencies (E) and coefficient of determination (r2) were established on the basis of the slopes of the standard curves generated from a 10-fold dilution series of purified PCR fragments (1:10,000 dilution) as templates. E was calculated using the formula E (%) = (10−1/slope−1) × 100, and values between 90% and 110% were considered acceptable. All primers information about E, r2 and product lengths are listed in Table 2.
Table 1.
Candidate reference genes used in this study.
| Abbreviation | Reference gene name | Gene function | Accession number |
|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate-dehydrogenase | Glycolysis enzyme | DQ848904 |
| 18S | 18S ribosomal RNA | Ribosomal subunits | EF126038 |
| ACTB | Beta actin | Cytoskeletal structural protein | AY008305 |
| EF1A | Elongation factor 1-alpha | Translational elongation | AF467776 |
| CTSD | Cathepsin D | Endoproteolytic aspartic proteinase | EU077233 |
| B2M | Beta-2-microglobulin-like | Cytoskeletal protein | DQ848854 |
Table 2.
Primers and related information of the candidate reference genes and the target gene.
| Gene | Primer pairs (5′-3′) | Efficiency (%) | Coefficient of determination (r2) | product size (bp) |
|---|---|---|---|---|
| GAPDH |
F: GTATTGGCCGTCTGGTCCT R: GGGAGACCTCACCGTTGTAA |
98.0 | 0.997 | 144 |
| 18S |
F: GTGGAGCGATTTGTCTGGTT R: CTCAATCTCGTGTGGCTGAA |
96.5 | 1.000 | 130 |
| ACTB |
F: CATGTACGTTGCCATCCAAG R: ACCAGAGGCATACAGGGACA |
104.9 | 0.996 | 138 |
| EF1A |
F: CGGCCACCTGATCTACAAGT R: GCCTTCAGTTTGTCCAGCA |
90.9 | 0.997 | 123 |
| CTSD |
F: GAAGAAGGTGGAGCAGAACG R: TGCGGGTGATGTTGATGTAG |
96.8 | 0.999 | 137 |
| B2M |
F: GGCAGTTCCATCTGACCAAG R: ATGTTTGACTCCCAGGCGTA |
92.8 | 0.998 | 112 |
| ERα |
F: GCCACCACTATCTGGAAACC R: CCTGACTCCCCCAAACTGTA |
90.9 | 0.996 | 115 |
Quantitative real time RT-PCR
qRT-PCR was performed in triplicate using ABI 7900HT thermocycler (Applied BioSystems, USA) according to the manufacturer’s instructions. Total reaction volume was 20 μL, including 2 μL of the cDNA sample, 10 μL of SYBR® Primix Ex Taq II (Takara Biotech, China), 0.4 μL of ROX Reference Dye (50×), 0.8 μL of the forward/reverse PCR primers (10 μM), and 6 μL of nuclease-free water. The reaction program was 95 °C for 30 s, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. A negative control without cDNA was included in each assay. Melt curves were obtained by increasing the temperature from 60 °C to 95 °C at increments of 0.5 °C to confirm that only one product was amplified. The amplicons were run on a 3% high resolution buffered agarose gel with a 50 bp ladder (50, 100, 150, 200, 300, 400 and 500) and visualized with ethidium bromide (1 × TAE buffer at 90 V for 1 hour).
Statistical analysis
The stability of Ct values was analyzed via geNorm, NormFinder and BestKeeper software. One-way ANOVA was conducted using SPSS 16.0 software (SPSS Inc., USA). The level of significance was chosen at P < 0.05 and Duncan’s test was conducted when necessary for multiple comparisons. Results were expressed as mean ± SEM (standard error of the mean).
Results
qRT-PCR amplification of candidate reference genes
The yield, purity and integrity of total RNA totally qualify for the experimental requirements (Supplemental data, S1,S2). The six reference genes, namely, gapdh, 18s, actb, ef1a, ctsd, and b2m were amplified from the target samples via qRT-PCR. Melting curve analysis was performed after thermocycling to determine the specifics of the PCR amplifications. The E values of the six candidate reference genes ranged from 92.8% to 104.9% and the r2 of the standard curves ranged from 0.996 to 1 (Table 2). The negative control without cDNA manifest no amplicons on the gel (Supplemental data, S3). The reference genes were reliably amplified and checked via agarose gel electrophoresis (Supplemental data, S4).
Ct values of the reference genes in HPOL tissues during turbot ovarian development
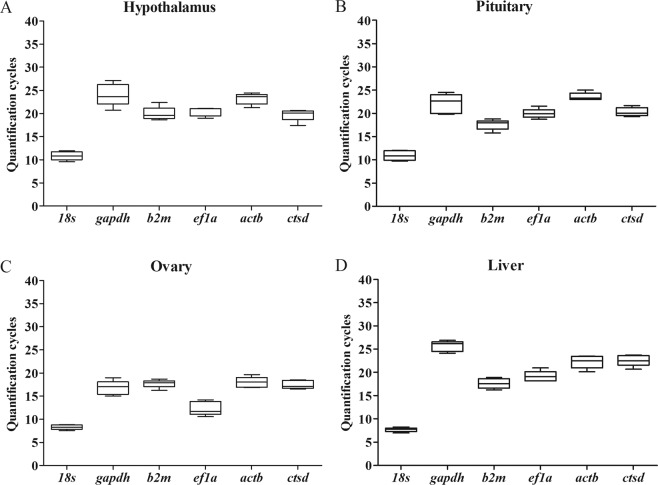
The Ct is defined as the number of cycles required for the fluorescent signal to cross the threshold. Ct levels are inversely proportional to the amount of target nucleic acid in the sample, low values indicate a high target amount, and high values indicate the opposite30. During the turbot ovarian development, the expression of the six reference genes varied in tissue-dependent manner (Fig. 2). 18s and actb showed the lowest Ct variation in the hypothalamus (0.65) and pituitary (0.60), respectively, whereas gapdh exhibited the highest Ct variation in the hypothalamus (1.78) and pituitary (1.74). The 18s showed the lowest level of changes in expression in the ovary (0.40) and liver (0.32), whereas ef1a showed the highest change in expression in the ovary (1.24) and liver (1.20). The average Ct values of the six reference genes in the hypothalamus, pituitary, ovary, and liver throughout ovarian development are included in Table 3.
Figure 2.
The range of expression of the candidate reference genes in hypothalamus, pituitary, ovary and liver of female turbot. The horizontal line in the box plots represents the median. The lower and upper boundaries of the box represent the 25th and 75th percentiles, respectively. The whiskers represent the minimum and maximum data points.
Table 3.
Ct values of candidate reference genes expressed in hypothalamus, pituitary, ovary and liver during ovarian development of turbot.
| Tissues | Stages | GAPDH | 18S | ACTB | EF1A | CTSD | B2M |
|---|---|---|---|---|---|---|---|
| Hypothalamus | Prevtg | 23.30 | 9.72 | 21.30 | 19.03 | 17.45 | 22.37 |
| Evtg | 20.76 | 10.89 | 22.86 | 21.05 | 20.47 | 19.98 | |
| Latvtg | 27.14 | 10.48 | 23.84 | 19.97 | 19.98 | 19.65 | |
| Mig-nucle | 23.68 | 11.95 | 24.45 | 21.08 | 20.10 | 19.15 | |
| Atre | 25.44 | 11.5 | 23.69 | 21.05 | 20.65 | 18.65 | |
| Pituitary | Prevtg | 24.46 | 10.12 | 23.01 | 19.60 | 19.71 | 18.00 |
| Evtg | 22.68 | 12.02 | 23.71 | 21.55 | 20.09 | 17.46 | |
| Latvtg | 20.10 | 10.85 | 23.26 | 20.03 | 20.74 | 19.91 | |
| Mig-nucle | 23.57 | 11.91 | 24.99 | 19.88 | 21.67 | 19.75 | |
| Atre | 19.80 | 9.73 | 23.00 | 18.70 | 19.27 | 15.77 | |
| Ovary | Prevtg | 18.98 | 8.85 | 18.04 | 11.57 | 16.95 | 16.24 |
| Evtg | 15.03 | 8.09 | 16.87 | 11.71 | 16.50 | 17.98 | |
| Latvtg | 15.66 | 8.70 | 17.06 | 10.56 | 17.07 | 17.89 | |
| Mig-nucle | 17.00 | 8.31 | 18.44 | 13.49 | 18.56 | 17.81 | |
| Atre | 17.18 | 9.57 | 19.62 | 14.22 | 18.35 | 18.70 | |
| Liver | Prevtg | 26.91 | 7.86 | 21.74 | 18.18 | 22.55 | 17.57 |
| Evtg | 26.18 | 7.00 | 20.15 | 19.27 | 23.45 | 18.37 | |
| Latvtg | 24.17 | 7.75 | 22.53 | 20.97 | 20.70 | 16.96 | |
| Mig-nucle | 26.31 | 8.26 | 23.44 | 18.92 | 23.76 | 18.92 | |
| Atre | 24.82 | 7.57 | 23.50 | 16.26 | 22.41 | 16.26 |
Prevtg: previtellogenesis; Evtg: early vitellogenesis; Latvtg: late vitellogenesis; Mig-nucle: migratory nucleus; Atre: atresia.
Expression stability of candidate reference genes analyzed by geNorm
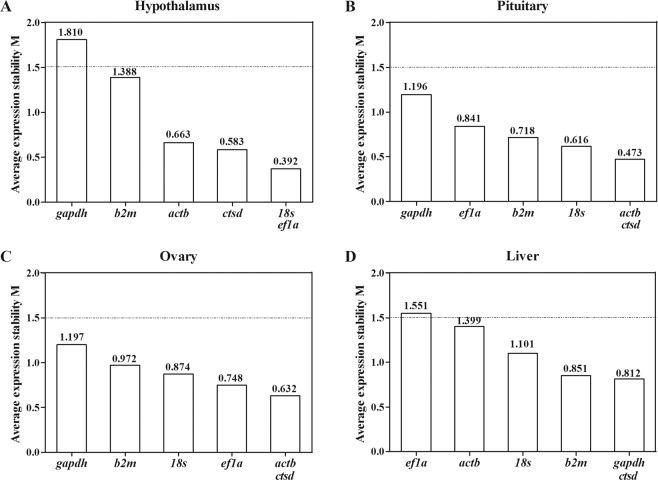
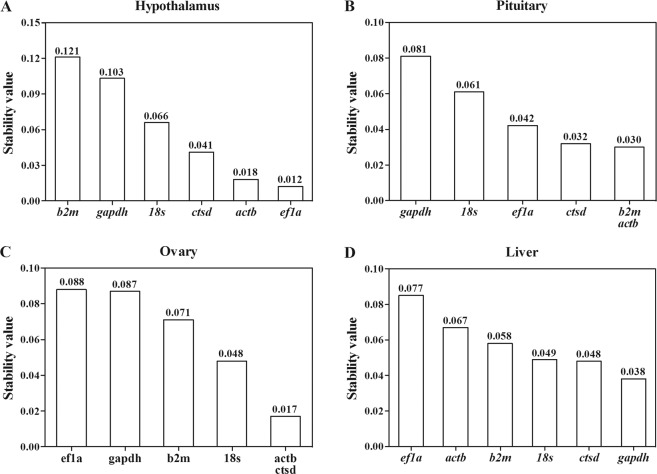
geNorm was used to calculate the expression stability index (M) of the six reference genes through the pairwise comparison of variations in expression ratios. A low value of M indicates a stable gene expression. In general, genes with an M value above 1.5 were not considered to be stably expressed. In the hypothalamus, 18s and ef1a exhibited the lowest M values and were therefore considered the most stable genes, followed by ctsd, actb, and b2m in terms of decreasing stability (Fig. 3A). However, gapdh was the least stable with M value of 1.810 in the hypothalamus (Fig. 3A). In the pituitary, actb and ctsd were ranked as the most stable genes, followed in decreasing stability by 18s, b2m, ef1a, and gapdh (Fig. 3B). In the ovary, actb and ctsd were the most stable genes, and ef1a, 18s, b2m and gapdh showed decreasing stability (Fig. 3C). In the liver, ctsd and gapdh were considered as the most stable genes, whereas ef1a appeared to be the least stable with an M value of 1.551 (Fig. 3D).
Figure 3.
Gene expression stability and ranking orders of the candidate reference genes in hypothalamus (A), pituitary (B), ovary (C), and liver (D) during ovarian development of turbot analyzed by geNorm.
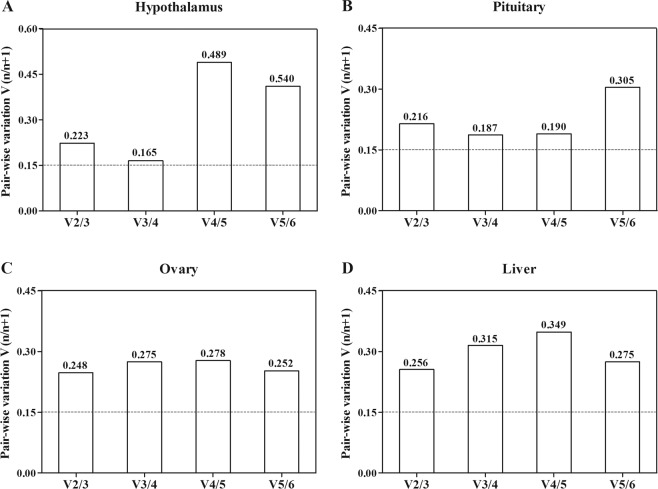
The optimal number of genes required for data normalization was also analyzed using geNorm through the calculation of pairwise variations (Vn/n+1) between sequential normalization factors n and n + 1. A threshold value of 0.15 has been suggested as the variation below which the inclusion of additional genes is not required for normalization. In the current study, all of the Vn/n+1 values obtained were higher than 0.15. The lowest Vn/n+1 values were 0.165 (V3/4) in the hypothalamus (Fig. 4A), 0.187 (V3/4) in the pituitary (Fig. 4B), 0.248 (V2/3) in the ovary (Fig. 4C), and 0.187 (V2/3) in the liver (Fig. 4D).
Figure 4.
The number of the candidate reference genes required for accurate normalization in hypothalamus (A), pituitary (B), ovary (C), and liver (D) during ovarian development of turbot determined by geNorm.
Considering the M and Vn/n+1 values, the optimal reference genes for accurate normalization were three genes (18s, ef1a, ctsd) in the hypothalamus, three genes (actb, ctsd, 18s) in the pituitary, two genes (actb, ctsd) in the ovary, and two genes (gapdh, ctsd) in the liver.
Expression stability of candidate reference genes analyzed by NormFinder
NormFinder was used to estimate and combine the intergroup and intragroup expression variations of reference genes and ultimately obtain a stability value. A low value, equated to a stable gene expression. In the hypothalamus, the stability ranking was ef1a > actb > ctsd > 18s > gapdh > b2m (Fig. 5A). In the pituitary, the stability ranking was b2m = actb > ctsd > ef1a > 18s > gaph (Fig. 5B). In the ovary, the stability ranking was actb = ctsd > 18s > b2m > gapdh > ef1a (Fig. 5C). In the liver, the stability ranking was gapdh > ctsd > 18s > b2m > actb > ef1a (Fig. 5D).
Figure 5.
Gene expression stability and ranking orders of the candidate reference genes in hypothalamus (A), pituitary (B), ovary (C), and liver (D) during ovarian development of turbot determined by Normfinder.
Expression stability of candidate reference genes analyzed by BestKeeper
BestKeeper was used to calculate the standard deviation (SD) and correlation coefficient (r) of the six reference genes (Table 4). Only genes with SD < 0.95 were included into the calculation of r. High values of r (close to 1.0) indicated stably expressed genes. In the hypothalamus, 18s exhibited the highest r value and was ranked as the most stable gene. 18s was followed in decreasing stability by ef1a, ctsd, and actb. In the pituitary, 18s was also considered as the most stable gene, followed by ctsd and actb. In the ovary, BestKeeper ranked actb and ctsd as the first two stable genes similar to the calculation by geNorm and NormFinder. In the liver, b2m appeared to be the most stable gene, followed by gapdh and ctsd as the two most stable genes.
Table 4.
Standard deviations (SD) and correlation coefficient (r) of the reference genes based on their quantification cycle values analyzed by Bestkeeper.
| Tissue | Factor | 18S | GAPDH | B2M | EF1A | ACTB | CTSD |
|---|---|---|---|---|---|---|---|
| Hypothalamus | SD | 0.69 | 1.78 | 0.97 | 0.75 | 0.92 | 0.91 |
| r | 0.96 | — | — | 0.95 | 0.91 | 0.93 | |
| Pituitary | SD | 0.83 | 1.74 | 0.77 | 0.67 | 0.61 | 0.73 |
| r | 0.95 | — | 0.82 | 0.71 | 0.83 | 0.86 | |
| Ovary | SD | 0.38 | 1.14 | 0.59 | 1.23 | 0.84 | 0.78 |
| r | 0.79 | — | 0.58 | — | 0.93 | 0.83 | |
| Liver | SD | 0.28 | 0.94 | 0.82 | 0.78 | 1.06 | 0.82 |
| r | 0.48 | 0.71 | 0.94 | 0.60 | — | 0.71 |
Note: Standard deviations above 0.95 are grayed and discarded from the calculation of correlation coefficient. Lower values of correlation coefficient indicate least stably expressed genes, and higher values of correlation coefficient indicate more stably expressed genes.
Effect of normalization on ERα using different reference genes
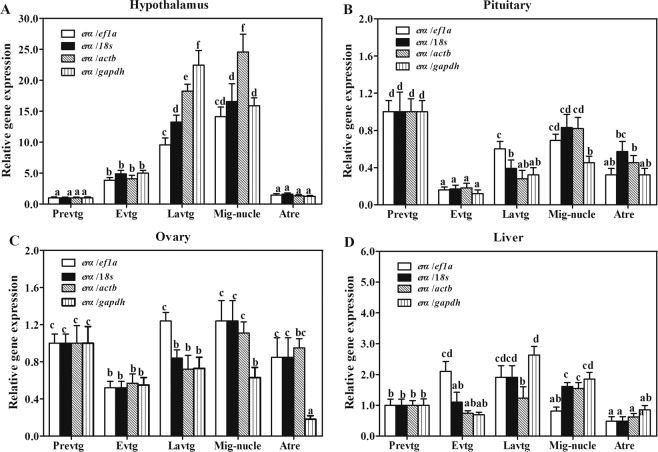
The expression of erα mRNAs levels were normalized to ef1a, 18s, actb, gapdh in the turbot hypothalamus, pituitary, ovary and liver during ovarian development. The expression profiles of erα in the hypothalamus were consistent when ef1a was used as reference controls, but showed different results when 18s, actb, and gapdh were used as the reference genes at the Latvtg and Mig-nucle stages (Fig. 6A). Similar results were observed er expression in the pituitary and ovary when actb or 18s was used as the reference controls (Fig. 6B,C). However, the expression profiles of hepatic erα were consistent when using gapdh or 18s as reference controls, but different with the result using actb or ef1a as reference gene at evtg, lavtg and mig-nucle stages (Fig. 6D).
Figure 6.
Relative quantification of the target gene erα in hypothalamus (A), pituitary (B), ovary (C), and liver (D) during ovarian development of turbot using different reference genes. Prevtg: previtellogenesis; Evtg: early vitellogenesis; Latvtg: late vitellogenesis; Mig-nucle: migratory nucleus; Atre: atresia. Data are presented as means ± SEM. Bars with different superscripts are statistically different (P < 0.05, n = 3).
Discussion
Relative quantification is widely used in analyzing target gene transcription levels on the basis of normalization with suitable reference genes. Selecting one or more appropriate reference genes as internal controls for data normalization is necessary because universal reference genes do not exist that have constant expression. In general, the mRNA levels of target genes are usually analyzed with two or more internal controls12,31. Numerous studies have clearly demonstrated that reference genes are tissue-specific, conditional, and development-dependent10,11,31. Robledo et al.4 (2014) recommended ubiquitin (ub) and ribosomal protein S4 (rps4) for the normalization of gonadal development in turbot samples (30, 45, 60, 75, 90, 105, 120, 135 days post fertilization). Our preliminary experiment showed that the Ct values of ub and rps4 significantly varied during turbot ovarian development and manifested unstable reference genes analyzed by geNorm, BestKeeper and NormFinder (Supplemental data, S5,S6). Thus, we do not include the two genes in the current study. Meanwhile, actb was regarded as an appropriate internal standard for the normalization of the immune-relevant genes of juvenile turbot3. The Ct values of the selected six reference genes indicate abundant target nucleic acid in the specimen, while 18s had the lowest Ct value. In the present study, the stability rankings performed by geNorm and NormFinder were more similar than the rankings calculated by BestKeeper, especially in terms of the determination of the most and least stable genes. Such difference is probably due to the fact that geNorm and NormFinder evaluating the stability of reference genes according to the variation of Ct values and BestKeeper calculating stability values according to the correlation coefficient of Ct values. The similar results of reference genes stability were reported in chicken four tissues31.
The ovary is an HPOL axis target organ that displays morphological and functional differences during different developmental stages32. Numerous studies have attempted to validate the expression stability of reference genes in the ovaries of vertebrates, including fish species. Moreover, actb, ctsd, ef1α, and cathepsin Z were regarded as the most stable gene at the maturation stages of the ovary in tilapia5. In the current study actb and ctsd were the two most suitable gene combinations for normalization in the ovary of turbot during development stages. Furthermore, using the best-suited gene can produce the similar expression profiles of erα during turbot ovarian development. The significantly difference of erα expression occurred at the lavtg and mig-nucle stages via using different reference genes in the current study. The lavtg and mig-nucle stages are the key steps during oocyte maturation in teleosts, and some reference genes may be involved in the regulation of oocyte maturation. It has identified ctsd is a lysosomal enzyme responsible for the limited cleavage of the endocytosed vitellogenin for yolk protein production during ovarian follicle growth and maturation33. Thus, these results further confirmed that the selection of suitable reference genes for data normalization in turbot is necessary.
The hypothalamus, pituitary, and liver are important organs that play key roles in regulating the development and maturation of the ovary in teleost. The selection of suitable reference genes for normalization in these tissues has been rarely explored. In the present study, ef1α/18s/ctsd, actb/ctsd, and gapdh/ctsd were the most suitable in the hypothalamus, pituitary, and liver of female turbot during ovarian development, respectively. No gene or pair of genes is actually suitable as an internal control to account for amplification differences among tissues. To find an internal standard for the cross-tissue type analysis of gene expression Gilsbach et al. (2006), and Nagler et al. (2007, 2012) added enhanced green fluorescent protein (eGFP) in vitro transcribed RNA to the total RNA before cDNA synthesis34–36. The in vitro reference gene (eGFP) could be consistently amplified in all samples. The target gene was then normalized to eGFP by dividing the absolute value of the gene by the absolute value of eGFP.
In conclusion, the expression stability of the six candidate reference genes (18s, actb, ef1α, gapdh, ctsd, b2m) were evaluated in HPOL tissues during turbot ovarian developmental stages using qRT-PCR. The combination of three reference genes in the hypothalamus (18s, ef1α, ctsd) and pituitary (actb, ctsd, 18s), two reference genes in the ovary (actb, ctsd) and liver (gapdh, ctsd) could be used for data normalization during ovarian development. However, no gene or pair of genes is suitable as an internal control to account for amplification differences among the four tissues during turbot reproductive cycle. These findings could provide suitable reference genes for the standardization of qRT-PCR data in studies of the roles of HPOL axis during turbot ovarian development. In addition, adding an exogenous reference gene may be more appropriate than selecting an endogenous reference gene for the cross-tissue type analysis of gene expression in the future studies
Supplementary information
Acknowledgements
This work was supported by National Natural Science Foundation of China (31972811, 31302205), Shandong Major Science and Technology Innovation Project (2018YFJH0703) and China Agriculture Research System (CARS-47). We sincerely thanks to Professor James J Nagler (University of Idaho) for helpful comments on the manuscript.
Author contributions
In this paper, Yunhong Gao mainly finished the stability of six reference genes analysis experiment and wrote the manuscript. Yuntao Gao and Zhen Meng mainly undertaken samples collection and management. Bin Huang provide partly funding support. Yudong Jia provided the funding support and experiment design and revised the manuscript. All authors joined the analysis and interpretation of data and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-57633-3.
References
- 1.Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation strategies and considerations. Gen. Immun. 2005;6:279–84. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 2.Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;4:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 3.Dang W, Sun L. Determination of internal controls for quantitative real time RT-PCR analysis of the effect of Edwardsiella tarda infection on gene expression in turbot (Scophthalmus maximus) Fish. Shellfish. Immun. 2011;30:720–728. doi: 10.1016/j.fsi.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Robledo D, et al. Analysis of qPCR reference gene stability determination methods and a practical approach for efficiency calculation on a turbot (Scophthalmus maximus) gonad dataset. BMC Genomics. 2014;15:648. doi: 10.1186/1471-2164-15-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deloffre LAM, Andrade A, Filipe AI, Canario AVM. Reference genes to quantify gene expression during oogenesis in a teleost fish. Gene. 2012;506:69–75. doi: 10.1016/j.gene.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 6.Wang E, et al. Evaluation and selection of appropriate reference genes for real-time quantitative PCR analysis of gene expression in Nile Tilapia (Oreochromis niloticus) during vaccination and infection. Inter. J. Mol. Sci. 2015;16(5):9998–10015. doi: 10.3390/ijms16059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Lu J, Wang Y, Wang S. Reference gene validation for quantification of gene expression during final oocyte maturation induced by diethylstilbestrol and di-(2-ethylhexyl)-phthalate in common carp. J. Enviro Sci. 2016;46:47–54. doi: 10.1016/j.jes.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Dong Z, et al. Evaluation of reference genes for quantitative real-time PCR analysis of gene expression in Hainan medaka (Oryzias curvinotus) Gen. Rep. 2019;14:94–99. doi: 10.1016/j.genrep.2018.11.008. [DOI] [Google Scholar]
- 9.Dhorne-Pollet S, Thélie A, Pollet N. Validation of novel reference genes for RT-qPCR studies of gene expression in Xenopus tropicalis during embryonic and post-embryonic development. Dev. Dynam. 2013;242:709–17. doi: 10.1002/dvdy.23972. [DOI] [PubMed] [Google Scholar]
- 10.McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008;9:102. doi: 10.1186/1471-2199-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Øvergård A, Nerland AH, Patel S. Evaluation of potential reference genes for real time RT-PCR studies in Atlantic halibut (Hippoglossus Hippoglossus L.); during development, in tissues of healthy and NNV-injected fish, and in anterior kidney leucocytes. BMC Mol. Biol. 2010;11:36. doi: 10.1186/1471-2199-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao S, et al. Evaluation of putative internal reference genes for gene expression normalization in Nannochloropsis sp. by quantitative real-time RT-PCR. Biochem. Bioph Res. 2012;424:118–23. doi: 10.1016/j.bbrc.2012.06.086. [DOI] [PubMed] [Google Scholar]
- 13.Fuentes EN, Safian D, Valdés JA, Molina A. Isolation and selection of suitable reference genes for real-time PCR analyses in the skeletal muscle of the fine flounder in response to nutritional status: assessment and normalization of gene expression of growth-related genes. Fish. Physiol. Biochem. 2013;39:765–77. doi: 10.1007/s10695-012-9739-5. [DOI] [PubMed] [Google Scholar]
- 14.Ma Q, Zhuang Z, Feng W, Liu S, Tang Q. Evaluation of reference genes for quantitative real-time PCR analysis of gene expression during early development processes of the tongue sole (Cynoglossus semilaevis) Acta Oceanologica Sin. 2015;34:90–97. doi: 10.1007/s13131-015-0725-5. [DOI] [Google Scholar]
- 15.Mitter K, et al. Evaluation of candidate reference genes for QPCR during ontogenesis and of immune-relevant tissues of European seabass (Dicentrarchus labrax) Comp. Biochem. Physiol. B. 2009;153:340–347. doi: 10.1016/j.cbpb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Yang CG, et al. Evaluation of reference genes for quantitative real-time RT-PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus) Gene. 2013;527:183–92. doi: 10.1016/j.gene.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-Time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Can. Res. 2004;64:5245–50. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffl MW, Tichopad A, Prgonmet C, Neuvians T. Determination of stable housekeeping genes, differentially regulated target genes and samples integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Let. 2004;26:509–15. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 19.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Gen. Biol. 2002;3:31–34. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibbelet S, Scharsack JP, Becker S. Housekeeping genes for quantitative expression studies in the three-spined stickleback Gasterosteus aculeatus. BMC Mol. Biol. 2008;9:18. doi: 10.1186/1471-2199-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Sun L, Xiao Z, Hu Y. Quantitative real time RT-PCR study of pathogen-induced gene expression in rock bream (Oplegnathus fasciatus): Internal controls for data normalization. Mar. Genomics. 2014;15:75–84. doi: 10.1016/j.margen.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Hu P, Meng Z, Jia Y. Molecular characterization and quantification of estrogen receptors in turbot (Scophthalmus maximus) Gen. Comp. Endocrinol. 2018;257:38–49. doi: 10.1016/j.ygcen.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Jia Y, Meng Z, Niu H, Hu P, Lei J. Molecular cloning, characterization, and expression analysis of luteinizing hormone receptor gene in turbot (Scophthalmus maximus) Fish. Physiol. Biochem. 2014;40:1639–50. doi: 10.1007/s10695-014-9954-3. [DOI] [PubMed] [Google Scholar]
- 24.Jia Y, Sun A, Meng Z, Liu B, Lei J. Molecular characterization and quantification of the follicle-stimulating hormone receptor in turbot (Scophthalmus maximus) Fish. Physiol. Biochem. 2016;42:179–191. doi: 10.1007/s10695-015-0128-8. [DOI] [PubMed] [Google Scholar]
- 25.Jia YD, Jing QQ, Gao Y, Huang B. Involvement and expression of growth hormone/insulin-like growth factor member mRNAs in the ovarian development of turbot (Scophthalmus maximus) Fish. Physiol. Biochem. 2019;45((3)):955–964. doi: 10.1007/s10695-018-0604-z. [DOI] [PubMed] [Google Scholar]
- 26.Jia Y, Meng Z, Liu X, Lei J. Biochemical composition and quality of turbot (Scophthalmus maximus) eggs throughout the reproductive season. Fish. Physiol. Biochem. 2014;40:1093–1104. doi: 10.1007/s10695-014-9908-9. [DOI] [PubMed] [Google Scholar]
- 27.Jia Y, Meng Z, Liu X, Lei J. Molecular components related to egg quality during the reproductive season of turbot (Scophthalmus maximus) Aqua Res. 2014;46:2565–2572. doi: 10.1111/are.12406. [DOI] [Google Scholar]
- 28.Jia YD, Niu HX, Meng Z, Liu X, Lei J. Biochemical composition of the ovarian fluid and its effects on the fertilization capacity of turbot Scophthalmus maximus during the spawning season. J. Fish. Biol. 2015;86:1612–1620. doi: 10.1111/jfb.12676. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder A, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bubner B, Baldwin IT. Use of real-time PCR for determining copy number and zygosity in transgenic plants. Plant. Cell Rep. 2004;23:263–271. doi: 10.1007/s00299-004-0859-y. [DOI] [PubMed] [Google Scholar]
- 31.Bagés S, Estany J, Tor M, Pena RN. Investigating reference genes for quantitative real-time PCR analysis four chicken tissues. Gene. 2015;561:82–7. doi: 10.1016/j.gene.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Gillies K, Krone SM, Nagler JJ, Schultz IR. A computational model of the rainbow trout hypothalamus-pituitary-ovary-liver axis. PLoS Comput. Biol. 2016;12:1–27. doi: 10.1371/journal.pcbi.1004874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carnevali O, Cionna C, Tosti L, Lubzens E, Maradonna F. Role of cathepsins in ovarian follicle growth and maturation. Gen. Comp. Endocrinol. 2006;146:195–203. doi: 10.1016/j.ygcen.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Gilsbach R, Kouta M, Bonisch H, Bruss M. Comparison of in vitro and in vivo reference genes for internal standardization of real-time PCR data. BioTec. 2006;40:173–77. doi: 10.2144/000112052. [DOI] [PubMed] [Google Scholar]
- 35.Nagler JJ, Cavileer T, Sullivan J, Cyr DG. The complete nuclear estrogen receptor family in the rainbow trout: Discovery of the novel ERα2 and both ERβ isoforms. Gene. 2007;392:164–173. doi: 10.1016/j.gene.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagler. JJ, et al. Estrogen receptor mRNA expression patterns in the liver and ovary of female rainbow trout over a complete reproductive cycle. Gen. Comp. Endocrinol. 2012;178:556–561. doi: 10.1016/j.ygcen.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.