Abstract
Host‐plant selection is a key factor driving the ecology and evolution of insects. While the majority of phytophagous insects is highly host specific, generalist behavior is quite widespread among bees and presumably involves physiological adaptations that remain largely unexplored. However, floral visitation patterns suggest that generalist bees do not forage randomly on all available resources. While resource availability and accessibility as well as nectar composition have been widely explored, pollen chemistry could also have an impact on the range of suitable host‐plants. This study focuses on particular pollen nutrients that cannot be synthesized de novo by insects but are key compounds of cell membranes and the precursor for molting process: the sterols. We compared the sterol composition of pollen from the main host‐plants of three generalist bees: Anthophora plumipes, Colletes cunicularius, and Osmia cornuta, as well as one specialist bee Andrena vaga. We also analyzed the sterols of their brood cell provisions, the tissues of larvae and nonemerged females to determine which sterols are used by the different species. Our results show that sterols are not used accordingly to foraging strategy: Both the specialist species A. vaga and the generalist species C. cunicularius might metabolize a rare C27 sterol, while the two generalist species A. plumipes and O. cornuta might rather use a very common C28 sterol. Our results suggest that shared sterolic compounds among plant species could facilitate the exploitation of multiple host‐plants by A. plumipes and O. cornuta whereas the generalist C. cunicularius might be more constrained due to its physiological requirements of a more uncommon dietary sterol. Our findings suggest that a bee displaying a generalist foraging behavior may sometimes hide a sterol‐specialized species. This evidence challenges the hypothesis that all generalist free‐living bee species are all able to develop on a wide range of different pollen types.
Keywords: bee–flower interactions, generalization, insects, physiological constraints, sterols
We compared the sterol composition of pollen from the main host‐plants of three generalist bees: Anthophora plumipes, Colletes cunicularius, and Osmia cornuta, as well as one specialist bee Andrena vaga. We also analyzed the sterols of their brood cell provisions, the tissues of larvae, and nonemerged females to determine which sterols are used by the different species. Our findings suggest that a bee displaying a generalist foraging behavior might sometimes hide a sterol specialized species.
1. INTRODUCTION
Plant–insect interactions range from antagonism to mutualism and from specialization to generalization (Mayhew, 1997; Wcislo & Cane, 1996, and references therein; Lengyel, Gove, Latimer, Majer, & Dunn, 2009). While generalist species exploit plants from more than one family, the majority of phytophagous insects is highly host specific relying on a single genus, subfamily or family of plants for their development (Bernays & Chapman, 1994). Specialization among plant‐feeding insects could be partly explained by the limited neural capacity to forage on diverse plant species with different morphologies and by the physiological challenge of digesting tissues from unrelated plants (Janz & Nylin, 2008). Whereas specialization offers obvious evolutionary advantages such as physiological efficiency, optimal foraging, and efficient host discrimination (Janz & Nylin, 2008, and references therein), a minority of herbivorous insects has taken a different evolutionary route and exploits numerous host plants. Such generalist behavior involves processing multiple sensory and chemical signals (Bernays, 2001; Riffell, 2011) and also potentially requires adaptations related to host recognition, foraging, and digestion (Finlay‐Doney & Walter, 2012). However, ecological generalization does not imply that generalists forage randomly on all available plants (Praz, Müller, & Dorn, 2008; Sedivy, Müller, & Dorn, 2011; Thorsteinson, 1960). They can exploit plants to which they might be preadapted behaviorally and/or ecologically (Haider, Dorn, & Müler, 2013; Janz & Nylin, 2008) or forage on multiple hosts that individually fill only a part of their physiological requirements. In the present work, we explore how generalist bees may be partly constrained in their floral choices by their physiological requirements and by the chemical composition of their pollen host.
While <10% of all herbivorous insects feed on plants belonging to more than three different plant families (Bernays & Graham, 1988), half of all free‐living bee species (i.e., excluding cuckoo bees) forage on a wide range of host plants (e.g., Colletes nigricans visiting the flowers of up to 15 different plant families; Müller & Kuhlmann, 2008), making bees a pertinent model to understand ecological generalization. Bees rely on floral resources for their development, principally on pollen and nectar (Michener, 2007). Several studies have provided evidence that generalist bees face high interspecific variation in pollen composition (e.g., alkaloids; Gosselin et al., 2013; essential amino acids; Weiner, Hilpert, Werner, Linsenmair, & Bluthgen, 2010; sterols; Vanderplanck et al., 2018) and do not show equivalent development on all pollen diets, with for instance an increase of larval mortality and a decrease in the mass of individual offspring on Asteraceae pollen (e.g., Levin & Haydak, 1957; Sedivy et al., 2011; Vanderplanck et al., 2018). Pollen nutritional content may consequently represent an important constraint in host‐plant selection for generalist bees. While attention has mainly been paid to proteins and amino acids, sterols remain poorly studied despite their importance for numerous physiological processes in bees (e.g., pupation, ovary development) (Behmer & Nes, 2003; Cohen, 2004). Sterols are requisite nutrients since insects cannot synthesize these essential components de nova for hormone production, gene expression, and cell membrane stability (Behmer & Nes, 2003; Cohen, 2004). While cholesterol (C27H46O) is typically used as the primary sterol, plant phytosterols (C28 or C29) are not directly used because of their additional carbon(s) (Behmer & Nes, 2003). Physiological pathways of conversion from phytosterols to cholesterol (i.e., dealkylation) occur in basal clades of the Hymenoptera (i.e., Symphyta), but more derived members like bees seem to have lost this ability (Behmer & Nes, 2003). Alternative pathways could be used to cope with this lack of cholesterol such as mutualistic interactions with endosymbiots or synthesis of particular molting hormones (e.g., makisterone A with an additional carbon) from particular phytosterols (Behmer & Nes, 2003; Cohen, 2004).
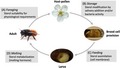
To evaluate the importance of sterols in bee host‐plant interaction and the sterolic requirement of bees (Figure 1a,b), we compared the sterol profiles of host pollen and brood cell provisions of three generalist bees (Anthophora plumipes, Apidae; Colletes cunicularius, Colletidae and Osmia cornuta, Megachilidae) and one specialist bee (Andrena vaga, Andrenidae). Additionally, we aimed to identify (a) the sterols that are assimilated by larvae during pollen feeding by investigating sterol profiles of larval tissues, and (b) the sterols that are metabolized for molting by comparing sterol profiles of tissues of larvae and nonemerged females (Figure 1c,d). We expect generalist bees to forage on host‐pollen that shows a common sterol profile and/or that they are able to metabolize different sterols. On the contrary, the specialist species could forage on host‐pollen that has a peculiar sterol profile and could be constrained to metabolize just one specific sterol.
Figure 1.
The importance of pollen sterols for host‐plant selection and the life cycle of a bee, here illustrated for the generalist bee Osmia cornuta. The main steps of sterol modification, assimilation, and metabolization are indicated in the gray boxes
2. MATERIALS AND METHODS
2.1. Bee species and plant species
We selected four spring univoltine bee species that are common in Belgium: (a) A. vaga (Andrenidae), a specialist on willow; (b) three generalist species: A. plumipes (Apidae), C. cunicularius (Colletidae), and O. cornuta (Megachilidae) (Figure 2). These solitary species can live in the same habitat and therefore potentially have access to the same plant community. They belong to phylogenetically distant bee lineages (Danforth, Cardinal, Praz, Almeida, & Michez, 2013) and display different life‐history traits.
Figure 2.

Bees on one of their preferred host‐plants. (a) Anthophora plumipes on Muscari botryoides (photograph by Kurt Geeraerts), (b) Colletes cunicularius on Salix caprea (photograph by Henk Wallays), (c) Andrena vaga on Salix fragilis (photograph by Maxime Drossart), and (d) Osmia cornuta on Erica sp. (photograph by Kurt Geeraerts)
Andrena vaga Panzer (Andrenidae) is a pollen specialist bee that becomes active between mid‐March and the beginning of May (Rezkova, Zakova, Zakova, & Straka, 2012; Westrich, 1989). Females collect large pollen loads predominantly on Salix trees (Bischoff, Feltgen, & Breckner, 2003; Vanderplanck, Bruneau, & Michez, 2009; Westrich, 1989). Andrena vaga builds deep nests in sandy soils in sunny locations (Westrich, 1989).
Anthophora plumipes (Pallas) (Apidae) is a pollen generalist that is active between the end of April and the beginning of June (Westrich, 1989). Females make short and frequent foraging flights and collect small pollen loads from a wide range of characteristically deep‐throated flowers species from Berberidaceae, Boraginaceae (e.g., Pulmonaria officinalis, Symphytum officinale), Fabaceae (e.g., Trifolium repens), Iridaceae, Lamiaceae (e.g., Lamium album, Lamium purpureum, Glechoma hederacea), Liliaceae, Papaveraceae (Corydalis sp.), Primulaceae (Primula sp.), Rosaceae, and Scophulariaceae (Westrich, 1989). However, it has a strong preference for Lamiaceae. Anthophora plumipes builds shallow nests in dry soil with an open entrance protected from the rain.
Colletes cunicularius (L.) (Colletidae) is a pollen generalist that is active in early spring from March to May (Bischoff et al., 2003). Females mainly exploit Salix spp. (Salicaceae) but also collect pollen from alternative host‐plants, including Asteraceae (Cichorioideae), Brassicaceae, Cistaceae, Ericaceae, Fabaceae (Cytisus sp. and Ilex sp.), Grossulariaceae, Resedaceae, Rhamnaceae, Rosaceae (Prunus sp., Sorbus sp. and Pyrus sp.), and Sapindaceae (Acer sp.) (Müller & Kuhlmann, 2008). This exploitation of alternative host‐plants occurs particularly toward the end of the relatively short flowering season of willows (Bischoff et al., 2003; Müller & Kuhlmann, 2008). Colletes cunicularius builds deep nests in sandy soil in sunny locations and is commonly observed in syntopy with A. vaga (Müller, Krebs, & Amiet, 1997; Vereecken, Toffin, Gosselin, & Michez, 2006; Westrich, 1989).
Osmia cornuta (Latreille) (Megachilidae) is a pollen generalist that has an early seasonal flight period that usually lasts from the beginning of March until the beginning of May (Westrich, 1989). Females collect pollen from plants belonging to many different families including Brassicaceae, Ericaceae, Papaveraceae (Corydalis sp.), Ranunculaceae (Anemone sp.), Rosaceae (e.g., Pyrus sp.), Salicaceae (Salix sp.), Sapindaceae (Acer sp.), and some monocots (Haider, Dorn, Sedivy, & Müller, 2014; Marquez, Bosch, & Vicens, 1994; Westrich, 1989). Osmia cornuta nests in a great variety of preexisting cavities (e.g., in the wall or hollow bamboo stalks) where it brought mud as construction material (Westrich, 1989).
We selected four main host plants for each pollen generalist as well as two widespread and common willow species for the pollen specialist (Table 1). Pollen was collected from the stamens of different flowers by using a turning fork (around 100 mg of fresh pollen) and cleaned under a binocular microscope (i.e., removal of trichomes, anthers, dust, or filaments). All the plants were collected during the bee flying period and from the same area (i.e., one population) to avoid intraspecific variation. During the flying period, we collected brood cell provisions for each species by digging up the nest (for the three ground nesting species: A. vaga, A. plumipes, and C. cunicularius) or by opening bamboo stalks (for the stem nesting species: O. cornuta). The brood cell provisions were collected from closed nest cells with an egg on top. We avoided analyzing brood cell provisions with developed larvae. We collected new cells to sample larvae (nondefecating larval stage) 1 month after the flying period, whereas nonemerged females were collected at least 2 months latter (i.e., overwintering diapause as adults). At least three nests per species were used for each sampling session (i.e., brood cell provisions, larvae, nonemerged females) to yield enough material. Moreover, we ensured that several host‐plants were available near the nesting site of generalist species to allow for mixing behavior and avoid biased data (Table 1).
Table 1.
Four selected bee species, sampled localities, degree of floral specialization, and the main host‐plant from the sampled population. Classification APGIII (2009)
| Bee species | Locality | Specialization | Plant species analyzed |
|---|---|---|---|
| Andrena vaga | Belgium, Blaton | Specialist (Westrich, 1989) |
Salix caprea (Salicaceae) Salix fragilis (Salicaceae) |
| Anthophora plumipes | Belgium, Malines | Generalist (10 plant families; Westrich, 1989) |
Lamium album (Lamiaceae) Pulmonaria officinalis (Boraginaceae) Symphytum officinale (Boraginaceae) Salix caprea (Salicaceae) |
| Colletes cunicularius | Belgium, Blaton | Generalist (11 plant families; Müller & Kuhlmann, 2008) |
Cytisus scoparius (Fabaceae) Prunus avium (Rosaceae) Salix caprea (Salicaceae) Sorbus aucuparia (Rosaceae) |
| Osmia cornuta | Belgium, Mons | Generalist (8 plant families; Westrich, 1989) |
Erica carnea (Ericaceae) Muscari botryoïdes (Asparagaceae) Pyrus communis (Rosaceae) Salix caprea (Salicaceae) |
2.2. Sterol analyses
Before each analysis, lyophilized floral pollen and fresh brood cell provisions were carefully homogenized and divided into a minimum of three samples (i.e., 20 mg per analytical replicate). We also removed blind guts from the larvae to avoid bias due to pollen remains as well as wings and legs (mainly chitin) from the female bodies prior to analyses (i.e., single individual per analytical replicate).
Sterols were quantified by GC‐FID after extraction and purification according to the method described by Vanderplanck, Michez, Vancraenenbroeck, and Lognay (2011). The multi‐step procedure can be summarized as follows: (a) saponification with 2 M methanolic potassium hydroxide, (b) extraction of the unsaponifiable portion with diethylether and several water washings, (c) solvent evaporation, (d) fractionation of the unsaponifiable portion by TLC, (e) trimethylsilylation of the sterols (scrapped from the silicagel), and (f) separation by GC. The total sterol content was determined considering all peaks above the limit of quantification; (LOQ = 9.6 ng/1.2 µl injected) whose retention time was between cholesterol and betulin (internal standard). Individual sterols were quantified on the basis of peak areas from analyses. Under the present analytical conditions applied, campesterol and 24‐methylenecholesterol co‐eluted. Therefore, the results are pooled for these two compounds. Compounds were identified according to their retention times in comparison with those of sunflower oil as reference. The identifications were corroborated by GC/MS (Vanderplanck et al., 2011).
2.3. Data analyses
To determine whether the host‐plants of each bee species have significantly different sterol composition, data were first square root transformed and standardized using the Wisconsin double standardization (“wisconsin” function, R‐package vegan, Oksanen et al., 2018) prior to the multivariate analysis (i.e., bee species as categorical variable with four levels, all host‐plants were considered equally for a given bee species). The Wisconsin double standardization is a method which first standardizes the data by sterol maximum standardization and afterward by sample total standardization (i.e., normalization to percent abundance). We then performed a perMANOVA using the Bray–Curtis dissimilarities as a measure of ecological distance and 999 permutations (“adonis” function, R‐package vegan). An advantage of this method is that the procedure is less dependent on data distribution than constrained methods. When perMANOVA returned significant p‐value (p < .05), multiple pairwise comparisons were conducted on the data to detect precisely the differences and p‐values were adjusted using Bonferroni's correction to avoid increases of type error I due to multiple testing. Indicator Species Analyses (Indval; Dufrêne & Legendre, 1997) were finally performed using the “indval” function from the labdsv package (Roberts, 2012) to identify the pollen sterols that were indicator of host‐plants exploited by a given bee species. This analysis calculates an indicator value based on relative abundance (specificity) and relative frequency (fidelity) for each sterol to identify the compound(s) with the highest indicator value for each sample. A p‐value was calculated for each sterol‐bee combination to assess whether pollen sterols are significantly found in association with a given bee species. All p‐values were adjusted using Holm's correction, to avoid increases of type error I due to multiple testing. Both similarities and dissimilarities were visually assessed on a nonmetric multidimensional scaling (nMDS) ordination using the “metaMDS” function from the package vegan. This function transforms the data using the Wisconsin double standardization, applies Bray–Curtis dissimilarities, runs NMDS multiple times with random starts to avoid local optima, and rotates the axes of the final configuration so that the variance of points is maximized on the first dimension. We determined the appropriate number of axes to use by obtaining stress values for ten replicates NMDS runs for each number of dimensions between one and four. We set the maximum number of random starts for each run at 500. For the final number of dimensions, we selected the lowest number of axes that had a stress value ≤0.2 (conventional cutoff; McCune & Grace, 2002). Similar statistical procedure was used to compare the host‐pollen (i.e., plant species as categorical variable with 11 levels), brood cell provisions, tissues of larvae, and nonemerged females among the four bee species (i.e., bee species as categorical variable with four levels).
To detect sterol(s) that might be involved in growth and developmental processes of the selected bee species, we compared the tissues from larvae and nonemerged females for each bee species using a perMANOVA on transformed data (i.e., square root and Wisconsin double standardization). Similarity percentage analyses were then performed in R using the “simper” function from the vegan package to identify the compounds that were responsible for detected differences between larvae and nonemerged females. Statistical results were summarized and displayed on back‐to‐back horizontal bar plots. All data analyses and visualization were performed in R version 3.4.0 (R Core Team, 2017).
3. RESULTS
3.1. Sterols in pollen and brood cell provisions
Pollen of the 11 targeted floral species displayed concentrations of total sterols ranging from 1.17 (Muscari botryoides) to 26.85 (Pyrus communis) mg per g of lyophilized matter (F 10,39 = 23.13, p < .001, Table 2). Pollen from Pyrus communis, Prunus avium, and Pulmonaria officinalis showed a significantly higher sterol concentration than pollen from Muscari botryoides, Erica carnea, and Lamium album whereas the other species displayed intermediate concentrations (Table 2). Pollen of Ericaceae (E. carnea), Fabaceae (Cytisus scoparius), Lamiaceae (L. album), and Salicaceae (Salix caprea and Salix fragilis) displayed high concentrations of C29 sterols (β‐sitosterol and δ5‐avenasterol) whereas 24‐methylenecholesterol (C28 sterol) and campesterol (same fraction) are the most abundant sterolic consituents in Boraginaceae (P. officinalis and Symphytum officinale), Asparagaceae (M. botryoides), and Rosaceae (P. avium, P. communis and Sorbus aucuparia), followed by δ5‐avenasterol and β‐sitosterol (Table 2). The analyses show that all plants significantly differed from each other (F 10,39 = 21.15, p < .001; multiple pairwise comparisons, p < .05), except P. avium, S. aucuparia, and S. officinale whose pollen displayed similar phytosterolic composition (multiple pairwise comparisons, p > .05). The occurrence of δ7‐avenasterol in pollen is indicative of E. carnea (p = .009, indicator value = 0.254) while δ5‐avenasterol in pollen is indicative of L. album (p = .009, indicator value = 0.186), 24‐methylenecholesterol and stigmasterol of P. officinalis (24‐methylenecholesterol: p = .009, indicator value = 0.269; stigmasterol: p = .015, indicator value = 0.269), and cholestenone of S. fragilis (p = .009, indicator value = 0.315).
Table 2.
Sterolic compositions of floral pollen from the 11 plant models
| Species | n | Cholesterol | Desmosterol | 24‐methylenechol. and campesterol | Stigmasterol | β‐sitosterol | δ5‐avenasterol | Cholestenone | δ7‐stigmasterol | δ7‐avenasterol | Total content (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytisus scoparius | 5 | 2.48 ± 0.32 | 0.09 ± 0.09 | 17.33 ± 3.27 | 0.80 ± 0.35 | 46.76 ± 4.03 | 30.13 ± 1.36 | 1.10 ± 1.27 | 0.75 ± 1.03 | 0.56 ± 0.11 | 5.24 ± 0.72 |
| Erica carnea | 5 | 3.16 ± 0.55 | 1.16 ± 0.92 | 7.63 ± 0.66 | 0.97 ± 0.59 | 54.09 ± 5.33 | 19.52 ± 1.40 | 0.21 ± 0.29 | 7.03 ± 2.11 | 6.23 ± 1.40 | 1.79 ± 0.59 |
| Lamium album | 5 | 6.10 ± 0.90 | 1.73 ± 1.66 | 4.77 ± 2.31 | 0.05 ± 0.11 | 44.66 ± 2.95 | 41.87 ± 2.66 | 0.65 ± 0.61 | 0.00 ± 0.00 | 0.18 ± 0.24 | 3.06 ± 1.41 |
| Muscari botryoides | 5 | 10.37 ± 7.05 | 2.32 ± 0.90 | 40.74 ± 8.08 | 4.27 ± 1.27 | 28.09 ± 7.19 | 5.40 ± 1.72 | 0.00 ± 0.00 | 7.19 ± 2.61 | 1.64 ± 0.51 | 1.17 ± 0.39 |
| Prunus avium | 3 | 0.37 ± 0.07 | 0.03 ± 0.03 | 89.92 ± 1.00 | 1.13 ± 0.11 | 5.74 ± 0.66 | 2.47 ± 0.13 | 0.23 ± 0.10 | 0.00 ± 0.00 | 0.11 ± 0.05 | 11.94 ± 0.47 |
| Pulmonaria officinalis | 5 | 2.95 ± 0.15 | 0.36 ± 0.03 | 88.97 ± 1.14 | 2.14 ± 1.07 | 2.94 ± 0.20 | 2.60 ± 0.16 | 0.04 ± 0.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.43 ± 1.17 |
| Pyrus communis | 6 | 2.79 ± 2.26 | 2.58 ± 2.80 | 85.57 ± 3.33 | 0.28 ± 0.38 | 5.17 ± 1.61 | 1.74 ± 1.08 | 0.45 ± 0.24 | 1.43 ± 3.06 | 0.01 ± 0.01 | 26.85 ± 19.85 |
| Salix caprea | 5 | 1.07 ± 0.34 | 0.22 ± 0.36 | 4.89 ± 0.18 | 0.33 ± 0.09 | 44.41 ± 1.97 | 42.10 ± 1.96 | 1.84 ± 0.74 | 3.22 ± 2.10 | 1.90 ± 0.42 | 6.52 ± 0.44 |
| Salix fragilis | 5 | 1.07 ± 0.44 | 0.03 ± 0.02 | 4.05 ± 0.28 | 0.09 ± 0.14 | 50.08 ± 3.24 | 33.64 ± 2.86 | 5.92 ± 3.71 | 2.23 ± 1.51 | 2.89 ± 1.30 | 5.59 ± 0.75 |
| Sorbus aucuparia | 3 | 2.10 ± 0.42 | 0.08 ± 0.07 | 81.68 ± 4.77 | 4.81 ± 8.00 | 5.16 ± 0.76 | 4.98 ± 3.94 | 0.00 ± 0.00 | 0.71 ± 0.32 | 0.48 ± 0.42 | 8.61 ± 1.83 |
| Symphytum officinale | 3 | 1.39 ± 1.11 | 0.00 ± 0.00 | 47.07 ± 15.03 | 3.62 ± 1.39 | 24.83 ± 9.81 | 16.29 ± 9.01 | 0.00 ± 0.00 | 4.42 ± 3.38 | 2.39 ± 0.91 | 9.62 ± 9.81 |
The concentrations of individual sterols are expressed as percentage of total sterolic content (mean ± SD). Major sterols (>5%) are indicated in bold.
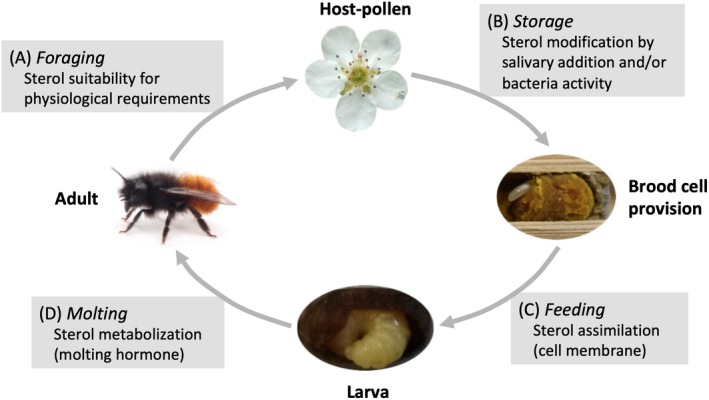
Statistical analysis using the foraging bee species as explicative variable detected a significant difference in pollen phytosterolic composition among the four species (F 3,52 = 7.22, p < .001). Pairwise comparisons reveal that the specialist species A. vaga significantly differed from the three generalist ones (i.e., A. plumipes, C. cunicularius, O. cornuta). When structuring the data according to the foraging behavior (i.e., generalist species vs. specialist species), a significant association was detected between 24‐methylenecholesterol (C28 sterol) and pollen used by generalist bee species (Indicator Compound Analysis, p = .009, indicator value = 0.744) whereas occurrence of cholestenone (C27 sterol) in pollen is indicative of host plants foraged on by A. vaga, the specialist bee species (Indicator Compound Analysis, p = .009, indicator value = 0.719; Figure 3a).
Figure 3.
nMDS ordination plot based on Bray–Curtis distances calculated on abundances (mg/g) of sterolic compounds in (a) pollen from the host‐plants, with centroids per plant species and per bee species (n are mentioned in Table 2) (b) brood cell provisions, with centroids per bee species (n are mentioned in Table 3). Sterols in red are indicative (indicative value in %)
The results were slightly different for brood cell provisions (i.e., larval food) with the statistical analyses supporting two groups (F 3,25 = 13.02, p = .001; Figure 3b). The first group consists of brood cell provisions of A. vaga and C. cunicularius that are significantly associated with cholestenone (C27 sterol; p = .009, indicator value = 0.839) whereas the second group is composed of brood cell provisions of A. plumipes and O. cornuta that are significantly associated with cholesterol (C27 sterol; p = .009, indicator value = 0.767; Figure 3b, Table 3).
Table 3.
Sterolic compositions of brood cell provisions, larval tissues, and nonemerged females
| Species | n | Cholesterol | Desmosterol | 24‐methylenechol. and campesterol | Stigmasterol | β‐sitosterol | δ5‐avenasterol | Cholestenone | δ7‐stigmasterol | δ7‐avenasterol | Total content (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Andrena vaga | |||||||||||
| Brood cell provisions | 10 | 0.59 ± 0.35 | 0.00 ± 0.00 | 3.94 ± 0.41 | 0.19 ± 0.13 | 61.06 ± 4.14 | 28.17 ± 4.02 | 2.40 ± 1.44 | 2.18 ± 2.99 | 1.47 ± 1.35 | 6.046 ± 2.89 |
| Larvae | 6 | 1.48 ± 0.56 | 0.09 ± 0.07 | 12.47 ± 1.96 | 0.83 ± 0.32 | 42.34 ± 4.63 | 22.26 ± 11.71 | 19.58 ± 9.25 | 0.26 ± 0.39 | 0.68 ± 0.81 | 0.51 ± 020 |
| Nonemerged females | 15 | 6.07 ± 8.65 | 0.35 ± 0.22 | 15.86 ± 3.78 | 0.24 ± 0.83 | 32.00 ± 6.37 | 40.06 ± 10.36 | 3.48 ± 2.79 | 0.30 ± 0.97 | 1.64 ± 3.62 | 0.84 ± 0.38 |
| Anthophora plumipes | |||||||||||
| Brood cell provisions | 4 | 3.36 ± 1.52 | 31.90 ± 10.43 | 25.43 ± 9.05 | 0.79 ± 0.24 | 25.20 ± 9.82 | 12.76 ± 5.04 | 0.07 ± 0.10 | 0.32 ± 0.17 | 0.17 ± 0.12 | 2.20 ± 0.79 |
| Larvae | 5 | 1.60 ± 0.76 | 10.20 ± 12.75 | 55.74 ± 15.44 | 0.14 ± 0.20 | 18.62 ± 5.65 | 13.45 ± 2.99 | 0.14 ± 0.15 | 0.07 ± 0.10 | 0.05 ± 0.07 | 6.30 ± 1.86 |
| Nonemerged females | 5 | 0.77 ± 0.66 | 0.00 ± 0.00 | 73.96 ± 4.77 | 9.25 ± 20.67 | 7.39 ± 2.62 | 8.46 ± 2.84 | 0.17 ± 0.38 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.94 ± 0.77 |
| Colletes cunicularius | |||||||||||
| Brood cell provisions | 10 | 0.13 ± 0.17 | 0.01 ± 0.01 | 27.07 ± 27.41 | 0.14 ± 0.15 | 32.88 ± 15.85 | 34.41 ± 11.03 | 2.36 ± 1.07 | 1.01 ± 1.34 | 2.00 ± 1.85 | 3.08 ± 0.70 |
| Larvae | 6 | 2.59 ± 1.67 | 0.07 ± 0.11 | 6.51 ± 2.07 | 1.05 ± 0.68 | 51.99 ± 3.90 | 22.66 ± 10.32 | 9.90 ± 8.82 | 4.13 ± 2.75 | 1.08 ± 1.33 | 0.81 ± 0.51 |
| Nonemerged females | 10 | 0.91 ± 0.40 | 0.10 ± 0.11 | 8.54 ± 0.46 | 0.24 ± 0.12 | 63.02 ± 4.16 | 26.07 ± 3.62 | 0.74 ± 0.28 | 0.20 ± 0.26 | 0.18 ± 0.10 | 1.22 ± 0.33 |
| Osmia cornuta | |||||||||||
| Brood cell provisions | 5 | 7.82 ± 13.02 | 0.08 ± 0.19 | 40.46 ± 34.86 | 0.40 ± 0.33 | 25.61 ± 14.37 | 22.60 ± 13.07 | 0.41 ± 0.41 | 0.46 ± 0.30 | 2.15 ± 1.69 | 8.77 ± 6.35 |
| Larvae | 5 | 0.91 ± 0.29 | 0.12 ± 0.15 | 51.37 ± 17.36 | 0.73 ± 1.31 | 32.15 ± 10.11 | 14.00 ± 7.33 | 0.29 ± 0.31 | 0.14 ± 0.09 | 0.29 ± 0.15 | 2.98 ± 0.87 |
| Nonemerged females | 5 | 1.64 ± 1.64 | 0.24 ± 0.11 | 31.34 ± 15.68 | 0.39 ± 0.18 | 31.23 ± 17.92 | 34.56 ± 11.69 | 0.10 ± 0.08 | 0.22 ± 0.14 | 0.27 ± 0.05 | 1.02 ± 0.32 |
The concentrations of individual sterols are expressed as percentage of total sterolic content (mean ± SD). Major sterols (>5%) are indicated in bold.
3.2. Sterol in bees
3.2.1. Sterol assimilation
Larvae of the four bee species displayed high concentrations of C29 sterolic compounds like β‐sitosterol and δ5‐avenasterol in their tissues, as well as high concentrations of 24‐methylenecholesterol (C28 sterol) and campesterol (same fraction) compared to the other detected sterols (Table 3). These compounds were also abundant in the brood cell provisions (Table 3) and quite common in pollen (Table 2). Such occurrence in larval and adult tissues from the four bee species suggests their assimilation regardless of the bee specialization (Table 3). Besides these common sterols, tissues of A. plumipes larvae also contained high concentration of desmosterol whereas larval tissues of A. vaga and C. cunicularius contained high concentrations of cholestenone (Table 3).
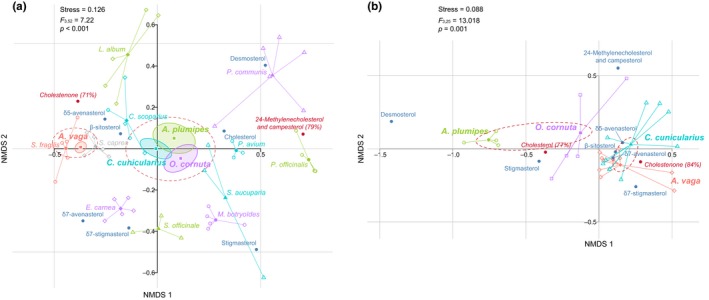
Statistical analysis detected a significant difference in sterolic composition of larval tissues among the four species (F 3,18 = 10.28, p < .001) and the pairwise comparisons structure the data in two distinct groups. The first group consists of the larvae of A. vaga and C. cunicularius that are significantly associated with cholestenone (C27 sterol) (p = .009, indicator value = 0.833) whereas the second group is composed of the larvae of A. plumipes and O. cornuta that are significantly associated with 24‐methylenecholesterol (C28 sterol; p = .009, indicator value = 0.818; Figure 4, Table 3).
Figure 4.
nMDS ordination plot based on Bray–Curtis distances calculated on abundances (mg/g) of sterolic compounds in larval tissues, with centroids per bee species (n are mentioned in Table 3). Sterols in red are indicative (indicative value in %)
3.2.2. Sterol metabolization
The 24‐methylenecholesterol was the most abundant sterol in both larval tissues and nonemerged females of A. plumipes, followed by either stigmasterol (nonemerged females) or β‐sitosterol (larvae), and δ5‐avenasterol. Regarding O. cornuta, tissues from both larvae and nonemerged females contained the same major phytosterols, namely 24‐methylenecholesterol, β‐sitosterol, and δ5‐avenasterol, but in slightly different ratios (Table 3). For both A. vaga and C. cunicularius, the most abundant sterols identified in larval tissues and nonemerged females were β‐sitosterol and δ5‐avenasterol followed by 24‐methylenecholesterol for nonemerged females and cholestenone for larvae (Table 3).
Except for O. cornuta, a significant difference was detected between nonemerged females and larvae (p < .05). For A. plumipes, larval tissues were more concentrated in 24‐methylenecholesterol (SIMPER, contribution to overall dissimilarity: 20.13%). This suggests a metabolization of this C28 sterol during the molting (Figure 5). The desmosterol was also more abundant in larvae compared to the nonemerged females but was too variable to be involved in key metabolic pathway such as molting. Despite no significant difference between nonemerged females and larvae was detected for O. cornuta, likely because of the high variation in sterol concentration within sample type, the 24‐methylenecholesterol remains an excellent candidate for precursor of molting hormone (SIMPER, contribution to overall dissimilarity: 14.22%) (Figure 5). For both A. vaga and C. cunicularius, larval tissues were more concentrated in cholestenone (SIMPER, contribution to overall dissimilarity: 24.25% for A. vaga; 15.52% for C. cunicularius). This suggests a metabolization of this C27 during the molting (Figure 5, Table 3). Other sterols were more abundant in larvae compared to the nonemerged females (i.e., stigmasterol for A. vaga and δ7‐stigmasterol for C. cunicularius) but were too variable to be involved in key metabolic pathway such as molting.
Figure 5.
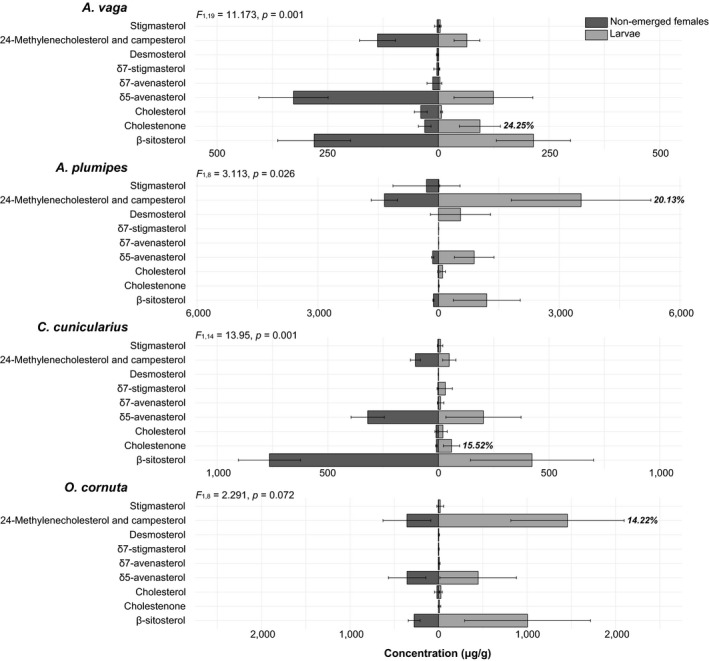
Sterolic compositions (μg/g; mean ± SD) of tissues for nonemerged female (dark gray) and larvae (light gray) of Andrena vaga, Anthophora plumipes, Colletes cunicularius, and Osmia cornuta. Molecules with the highest percentage contribution (SIMPER) to the defined difference between development stages are given for each bee species (%)
4. DISCUSSION
The results show that the sterolic profile of pollen was quite variable among the host‐plant species. However, some sterols were common (β‐sitosterol, δ5‐avenasterol, and 24‐methylenecholesterol) and were also abundant in brood cell provisions as well as in the tissues of larvae and nonemerged females. This highlighted their importance in bee nutrition as well as their assimilation in bee tissues. As bee tissues displayed a sterolic composition similar to the food (i.e., pollen and brood cell provisions), bees are likely to lack dealkylation pathways.
Regarding sterol metabolization, A. plumipes and O. cornuta might rely on the 24‐methylenecholesterol for the synthesis of their molting hormone. As this pollen sterol is quite spread among plant species, such metabolic pathway would allow them to exploit a large range of host‐plant, so that they could be considered as true generalist species. This is not the case of C. cunicularius that seemed to display a similar metabolic pathway than A. vaga, the specialist bee species. Both species might likely use the cholestenone, a quite uncommon pollen sterol, as precursor of their molting hormone. Although this sterol was only found in high relative abundance in pollen of S. fragilis, these two bee species could be capable of concentrating into their tissues this minor dietary sterol in preference to others that are available in much larger amounts, as it has been already shown for other insect species (reviewed in Clayton, 1964). Despite its apparent generalist foraging behavior, C. cunicularius might then be constrained by the dietary sterol it is specialized on. This bee species, while clearly a generalist under the traditional definition, could be considered to have a much more constrained diet than the other two generalists investigated here. All these highlights are hereafter discussed in regards of the extant literature.
4.1. The significance of sterol variability in pollen of flowering plants
Like in many plants, the pollen of M. botryoides, P. avium, P. officinalis, P. communis, S. aucuparia, and S. officinale shows a high level of 24‐methylenecholesterol (Lusby, Buchmann, & Feldlaufer, 1993; Nes & Schmidt, 1988; Roger et al., 2016). Occasionally, however, β‐sitosterol is the major sterol (Standifer, Devys, & Barbier, 1968) as we found in C. scoparius, E. carnea, L. album, S. caprea, and S. fragilis. Despite these differences in major phytosterols, the 11 plant species investigated herein contain the same sterols but in different ratios. Such similarity in pollen composition could allow bees to display a generalist foraging behavior (i.e., foraging on plants from unrelated clades) and might be selected at wide geographic scale since it directly benefits the generalist bees and promotes generalization in pollination systems. However, some plants display particular pollen sterolic profile with large absolute amounts of less conventional sterols such as cholestenone in the pollen of S. fragilis. This has been already shown for the pollen of heather (Calluna vulgaris, Ericaceae) that contains large amount of stigmasterol, as well as for the pollen of cottonwood (Populus fremontii, Salicaceae) and cat's ear (Hypochoeris radicata, Asteraceae) (Standifer et al., 1968) that both display high level of cholesterol.
Although S. caprea and S. fragilis display similar sterolic composition as also observed for species belonging to Rosaceae and, to a lesser extent, to Boraginaceae; Standifer et al. (1968) found little evidence that taxonomy could be used to predict pollen sterol profile since three species belonging to Salicaceae (e.g., Populus genus, Salix genus) varied widely in the content of C27, C28, and C29 sterols. This sterolic diversity is not limited to pollen but concerns all plant parts. Little is known about the functional significance of this variation. One hypothesis is that sterol profiles may reflect adaptations to local abiotic conditions but this explanation is not always sufficent (Behmer & Nes, 2003). Another hypothesis is that phytosterol profiles may function as a unique defence against insect herbivores, for example, in grasshoppers (Behmer & Nes, 2003). Sterolic composition of pollen, and more globally pollen nutrients, may therefore affect both generalist and specialist bee species (Gosselin et al., 2013; Praz et al., 2008; Sedivy et al., 2011; Weiner et al., 2010).
One strong hypothesis is that particular sterolic compounds could filter through the available spectrum of floral visitors (i.e., nutritional compound for effective pollinators and toxic repellent for robbers or noneffective visitors) and thereby promote tight association with obligate specialists. Such specialization in pollination systems presents advantages for both bees and plants since it reduces pollinator competition and improves plant pollination efficiency by restricting the range of visitors to a specialist guild (Suzuki, Dohzono, & Hiei, 2007). Other floral traits are known to support pollination specificity such as nectar and floral scent (Johnson, Hargreaves, & Brown, 2006; Shuttleworth & Johnson, 2009). Growing evidence suggests that pollination syndromes are not limited to morphological traits but convergent suites of floral chemical traits could also act as filters in host‐plant selection and therefore pollination systems (Johnson et al., 2006; Shuttleworth & Johnson, 2009; Vanderplanck et al., 2017; Weiner et al., 2010). This hypothesis is strongly supported by the bee abilities to detect pollen nutritional quality and discriminate among hosts (Vaudo, Patch, Mortensen, Tooker, & Grozinger, 2016). Several studies have shown that bumblebees preferentially forage on plant species providing protein‐rich pollen (Hanley, Franco, Pichon, Darvill, & Goulson, 2008; Kitaoka & Nieh, 2009; Leonhardt & Blüthgen, 2012; Rasheed & Harder, 1997; Robertson, Mountjoy, Faulkner, Roberts, & Macnair, 1999). The composition and concentration in amino acids also seem to impact foraging decision and behavior of bees (Alm, Ohnmeiss, Lanza, & Vriesenga, 1990; Hanley et al., 2008; Leonhardt & Blüthgen, 2012; Weiner et al., 2010). Moreover, addition of lipid extracts from pollen to substitutes such as cellulose powder has been shown to stimulate pollen foraging in honeybee (Pernal & Currie, 2002). Such active extracts are known to contain phytosterols or steroids (Hügel, 1962; Louveaux, 1959) as well as free fatty acids (Hopkins, Jevans, & Boch, 1969; Lepage & Boch, 1968), strengthening the hypothesis of a potential role of pollen sterol in bee foraging decision.
4.2. The pollen sterols as a constrain for floral choices
High concentrations of β‐sitosterol, δ5‐avenasterol, and 24‐methylenecholesterol were found in larval and adult tissues of the four bee species, suggesting their assimilation and involvement in structural roles such as membrane inserts (Behmer & Nes, 2003). Because these phytosterols are common in pollen of a wide taxonomic range of angiosperms (Barbier, Hügel, & Lederer, 1960; Lusby et al., 1993; Nes & Schmidt, 1988; Standifer et al., 1968), they might not represent a constraint for host‐plant selection. As the sterolic composition of larval and adult tissues reflects this one of the food resources (i.e., floral pollen and brood cell provisions), the four bee species do probably not dealkylate their dietary sterols.
The comparison between larvae and nonemerged females suggested that cholestenone (C27 sterol) would be a good candidate for precursor of molting hormone in A. vaga and C. cunicularius whereas 24‐methylenecholesterol would be used as precursor of molting hormone in A. plumipes and O. cornuta. Regarding these results, we may suggest that A. vaga and C. cunicularius could synthesize a C27 ecdysteroid such as 20‐hydroxyecdysone using cholestenone as a precursor while A. plumipes and O. cornuta could rather synthesize a C28 ecdysteroid such as makisterone A. Such implication of the cholestenone (C27 sterol) in the 20‐hydroxyecdysone synthesis has been already described in Manduca sexta (Grieneisen, Warren, & Gilbert, 1993, and references therein) whereas the use of alternative phytosterols (i.e., C28 sterols) to synthesize makisterone A for molting has been described for Drosophila melanogaster (Redfern, 1986) and Bombus terrestris (Regali, 1996). Although our data are very suggestive, experiments involving radioactive labeling techniques and dietary supply remain nevertheless needed to fully validate our interpretation and demonstrate the dietary requirement for a given sterol in the four bee species.
In light of previous similar studies, bees seem to have a sterolic physiology that is relatively conserved since all bee models studied to date lack dealkylation ability and use C27 or C28 as precursor of 20‐hydroxyecdysone or makisterone A, respectively (Apis mellifera, Megachile rotundata, Diadasia rinconis and B. terrestris; in Feldlaufer, Herbert, Svoboda, & Thompson, 1986; Svoboda & Lusby, 1986; Feldlaufer, Lusby, Weirich, Svoboda, & Buchmann, 1993 and Regali, 1996, respectively). This could be partly explained by their ecological specialization on pollen. Other insect groups such as flies display higher diversity in their ecologies and sterol physiologies. For example, the fruit fly D. melanogaster is an ecological generalist but other Drosophila species are specialized on fruit, mushrooms, cacti, flowers, or even the excretory pores of land crabs (Markow & O'Grady, 2005). Among these specialist flies, Drosophila pachea represents an unprecedented model since it is specialized on senita cacti (Lophocereus schottii, Cactaceae) and requires an uncommon dietary sterol from its host plant, the lathosterol (Kircher, Heed, Russell, & Groove, 1967). Lang et al. (2012) showed that D. pachea evolved an obligate specialization on senita cacti through changes in a single enzyme. Even for the generalist grasshopper Schistocerca americana, metabolic constraints presumably restrict the spectrum of phytosterols capable of supporting normal growth and development (Behmer & Elias, 1999). These sterol metabolic constraints are a shared trait among grasshopper species that suffer high levels of mortality when they accumulate unsuitable sterols (Behmer & Elias, 1999, 2000), which may also occur in bees (Vanderplanck et al., 2018).
Overall our results show that shared sterolic profiles among floral species could facilitate exploitation of a wide range of host‐plants by two generalist bees (A. plumipes and O. cornuta) but that the generalist C. cunicularius might be more constrained in its floral choices by a quite rare dietary sterol. In this regard, C. cunicularius might share a similar sterol requirement with the specialist A. vaga and not with the other generalist bees, which could be verify using dietary supply experiments as well as isotopic tracer techniques (reviewed in Clayton, 1964). Our findings suggest that bees with a generalist foraging pattern such as C. cunicularius could hide sterol specialists that might be highly specific in terms of sterol preferences, irrespective of the plant taxonomy, assuming the hypothesis of the improbability of ecological generalization in nature (Loxdale, Lushai, & Harvey, 2011).
CONFLICT OF INTEREST
None declared.
AUTHORS' CONTRIBUTIONS
DM conceived and designed the study. MV coordinated the study and carried out laboratory work with the help of P‐LZ. MV, P‐LZ, and GL participated in data analysis. MV carried out the statistical analyses and drafted the manuscript. DM and GL critically revised the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
ACKNOWLEDGMENTS
We would like to thank L. Crépin, D. Evrard, and A. Pinczewski for their kind help during sampling as well as S. Vancraenenbroeck and G. Carreaux for their contribution to the chemical analyses. This research was funded by the “Fonds de la Recherche Fondamentale et Collective” (FRFC project 2.4613.10, FRS‐FNRS, Belgium). P.‐L. Zerck was Ph.D. student funded by the Belgian Fund for Scientific Research (FRS‐FNRS). MV received financial support from the Belgian FRS‐FNRS through a postdoctoral grant.
Vanderplanck M, Zerck P‐L, Lognay G, Michez D. Generalized host‐plant feeding can hide sterol‐specialized foraging behaviors in bee–plant interactions. Ecol Evol. 2020;10:150–162. 10.1002/ece3.5868
DATA AVAILABILITY STATEMENT
Data supporting the results: Dryad: https://doi.org/10.5061/dryad.cz8w9gj03.
REFERENCES
- Alm, J. , Ohnmeiss, T. E. , Lanza, J. , & Vriesenga, L. (1990). Preference of cabbage white butteries and honey bees for nectar that contains amino acids. Oecologia, 84, 53–57. [DOI] [PubMed] [Google Scholar]
- APGIII (2009). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society, 161, 105–121. [Google Scholar]
- Barbier, M. , Hügel, M. F. , & Lederer, E. (1960). Isolation of 24‐methylene cholesterol from the pollen of different plants. Bulletin de la Société de Chimie Biologique, 42, 91–97. [PubMed] [Google Scholar]
- Behmer, S. T. , & Elias, D. O. (1999). The nutritional significance of sterol metabolic constraints in the generalist grasshopper Schistocerca americana . Journal of Insect Physiology, 45, 339–348. [DOI] [PubMed] [Google Scholar]
- Behmer, S. T. , & Elias, D. O. (2000). Sterol metabolic constraints as a factor contributing to the maintenance of diet mixing in grasshoppers (Orthoptera: Acrididae). Physiological and Biochemical Zoology, 73, 219–230. 10.1086/316728 [DOI] [PubMed] [Google Scholar]
- Behmer, S. T. , & Nes, W. D. (2003). Insect sterol nutrition and physiology: A global overview. Advances in Insect Physiology, 31, 1–72. [Google Scholar]
- Bernays, E. A. (2001). Neural limitations of phytophagous Insects: Implications for diet breadth and host affiliation. Annual Review of Entomology, 46, 703–727. [DOI] [PubMed] [Google Scholar]
- Bernays, E. A. , & Chapman, R. F. (1994). Host plant selection by phytophagous insects. New York, NY: Chapman & Hall. [Google Scholar]
- Bernays, E. A. , & Graham, M. (1988). On the evolution of host specificity in phytophagous arthropods. Ecology, 69, 886–892. 10.2307/1941237 [DOI] [Google Scholar]
- Bischoff, I. , Feltgen, K. , & Breckner, D. (2003). Foraging strategy and pollen preferences in Andrena vaga (Panzer) (Hym. Andrenidae) and Colletes cunicularius (L.) (Hym. Colletidae). Journal of Hymenoptera Research, 12(2), 220–237. [Google Scholar]
- Clayton, R. B. (1964). The utilization of sterols by insects. Journal of Lipid Research, 5, 3–19. [PubMed] [Google Scholar]
- Cohen, A. C. (2004). Insect diet: Science and technology. Boca Raton, FL: CRC. [Google Scholar]
- R Core Team (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R-project.org/ [Google Scholar]
- Danforth, B. N. , Cardinal, S. , Praz, C. , Almeida, E. A. B. , & Michez, D. (2013). The impact of molecular data on our understanding of bee phylogeny and evolution. Annual Review of Entomology, 58, 57–78. 10.1146/annurev-ento-120811-153633 [DOI] [PubMed] [Google Scholar]
- Dufrêne, M. , & Legendre, P. (1997). Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs, 67, 345–366. 10.2307/2963459 [DOI] [Google Scholar]
- Feldlaufer, M. F. , Herbert, E. W. , Svoboda, J. A. , & Thompson, M. J. (1986). Biosynthesis of makisterone A and 20‐hydroxyecdysone from labeled sterols by the honey bee, Apis mellifera . Archives of Insect Biochemistry and Physiology, 3, 415–421. 10.1002/arch.940030502 [DOI] [Google Scholar]
- Feldlaufer, M. F. , Lusby, W. R. , Weirich, G. F. , Svoboda, J. A. , & Buchmann, S. L. (1993). Neutral sterols and ecdysteroids of the solitary cactus bee Diadasia rinconis cockerell (Hymenoptera: Anthophoridae). Archives of Insect Biochemistry and Physiology, 23, 91–98. 10.1002/arch.940230205 [DOI] [Google Scholar]
- Finlay‐Doney, M. , & Walter, G. H. (2012). The conceptual and practical implications of interpreting diet breadth mechanistically in generalist predatory insects. Biological Journal of the Linnean Society, 107, 737–763. 10.1111/j.1095-8312.2012.01991.x [DOI] [Google Scholar]
- Gosselin, M. , Michez, D. , Vanderplanck, M. , Roelants, D. , Glauser, G. , & Rasmont, P. (2013). Does Aconitum septentrionale chemically protect floral rewards to the advantage of specialist bumblebees? Ecological Entomology, 38, 400–407. [Google Scholar]
- Grieneisen, M. L. , Warren, J. T. , & Gilbert, L. I. (1993). Early steps in ecdysteroid biosynthesis: Evidence for teh involvement of cytochrome P450 enzymes. Insect Biochemistry and Molecular Biology, 23, 13–23. [DOI] [PubMed] [Google Scholar]
- Haider, M. , Dorn, S. , & Müler, A. (2013). Intra‐ and interpopulational variation in the ability of a solitary bee species to develop on non‐host‐pollen: Implications for host range expansion. Functional Ecology, 27, 255–263. 10.1111/1365-2435.12021 [DOI] [Google Scholar]
- Haider, M. , Dorn, S. , Sedivy, C. , & Müller, A. (2014). Phylogeny a, d floral hosts of a predominantly polen generalist group of mason bees (Megachilidae: Osmiini). Biological Journal of the Linnean Society, 111(1), 78–91. [Google Scholar]
- Hanley, M. E. , Franco, M. , Pichon, S. , Darvill, B. , & Goulson, D. (2008). Breeding system, pollinator choice and variation in pollen quality in British herbaceous plants. Functional Ecology, 22, 592–598. 10.1111/j.1365-2435.2008.01415.x [DOI] [Google Scholar]
- Hopkins, G. , Jevans, A. W. , & Boch, R. (1969). Occurence of octadeca‐trans‐2, cis‐9, cis‐12‐trienoic acid in pollen attractive to the honey bee. Canadian Journal of Biochemistry, 47, 433–436. [DOI] [PubMed] [Google Scholar]
- Hügel, M. F. (1962). Etude de quelques constituants du pollen. Annales de l'Abeille, 5, 97–133. [Google Scholar]
- Janz, N. , & Nylin, S. (2008). The oscillation hypothesis of host‐plant range and speciation In Tilmon K. J. (Ed.), Specialization, speciation and radiation: The evolutionary biology of herbivorous insects (pp. 203–215). Berkeley, CA: University of California Press. [Google Scholar]
- Johnson, S. D. , Hargreaves, A. L. , & Brown, M. (2006). Dark, bitter‐tasting nectar functions as filter of flower visitors in a bird‐pollinated plant. Ecology, 87(11), 2709–2716. [DOI] [PubMed] [Google Scholar]
- Kircher, H. W. , Heed, W. B. , Russell, J. S. , & Groove, J. (1967). Senita cactus alkaloids: Their significance to Sonoran desert Drosophila ecology. Journal of Insect Physiology, 13, 1869–1874. 10.1016/0022-1910(67)90023-6 [DOI] [Google Scholar]
- Kitaoka, T. K. , & Nieh, J. C. (2009). Bumble bee pollen foraging regulation: Role of pollen quality, storage levels, and odor. Behavioral Ecology and Sociobiology, 63, 501–510. [Google Scholar]
- Lang, M. , Murat, S. , Clark, A. G. , Gouppil, G. , Blais, C. , Matzkin, L. M. , … Orgogozo, V. (2012). Mutations in the neverland gene turned Drosophila pachea into an obligate specialist species. Science, 337(6102), 1658–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel, S. , Gove, A. D. , Latimer, A. M. , Majer, J. D. , & Dunn, R. R. (2009). Ants sow the seeds of global diversification in flowering plants. PLoS ONE, 4(5), e5480 10.1371/journal.pone.0005480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt, S. D. , & Blüthgen, N. (2012). The same, but different: Pollen foraging in honeybee and bumblebee colonies. Apidologie, 43, 449–464. 10.1007/s13592-011-0112-y [DOI] [Google Scholar]
- Lepage, M. , & Boch, R. (1968). Pollen lipids attractive to honeybees. Lipids, 3, 530–534. 10.1007/BF02530897 [DOI] [PubMed] [Google Scholar]
- Levin, M. D. , & Haydak, M. H. (1957). Comparative value of different pollens in the nutrition of Osmia lignaria Say (Hymenoptera: Apoidea). Bee World, 38, 221–226. 10.1080/0005772X.1957.11095007 [DOI] [Google Scholar]
- Louveaux, J. (1959). Recherches sur la récolte du pollen par les abeilles. Annales des Abeilles, 2, 7–111. [Google Scholar]
- Loxdale, H. D. , Lushai, G. , & Harvey, J. A. (2011). The evolutionary improbability of ‘generalism’ in nature, with special reference to insects. Biological Journal of the Linnean Society, 103, 1–18. 10.1111/j.1095-8312.2011.01627.x [DOI] [Google Scholar]
- Lusby, W. R. , Buchmann, S. L. , & Feldlaufer, M. F. (1993). Pollen sterols from three species of Sonoran cacti. Lipids, 28, 469–470. 10.1007/BF02535947 [DOI] [Google Scholar]
- Markow, T. A. , & O'Grady, P. M. (2005). Evolutionary genetics of reproductive behavior in Drosophila: Connecting the dots. Annual Reviews of Genetics, 39, 263–291. [DOI] [PubMed] [Google Scholar]
- Marquez, J. , Bosch, J. , & Vicens, N. (1994). Pollens collected by wild and managed populations of the potential orchard pollinator Osmia cornuta (Latr.) (Hym., Megachilidae). Journal of Applied Entomology, 117, 353–359. [Google Scholar]
- Mayhew, P. J. (1997). Adaptive patterns of host‐plant selection by phytophagous insects. Oikos, 79(3), 417–428. 10.2307/3546884 [DOI] [Google Scholar]
- McCune, B. , & Grace, J. B. (2002). Analysis of ecological communities. Glenden Beach, OR: MjM Software Design. [Google Scholar]
- Michener, C. D. (2007). The bees of the world (2nd ed.). Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- Müller, A. , Krebs, A. , & Amiet, F. (1997). Bienen: Mitteleuropäische Gattung, Lebensweise, Beobachtung. Augsburg, Germany: Naturbuch Verlag. [Google Scholar]
- Müller, A. , & Kuhlmann, M. (2008). Pollen hosts of western palaearctic bees of the genus Colletes (Hymenoptera: Colletidae): The Asteraceae paradox. Biological Journal of the Linnean Society, 95, 719–733. 10.1111/j.1095-8312.2008.01113.x [DOI] [Google Scholar]
- Nes, W. D. , & Schmidt, J. O. (1988). Isolation of 25(27)‐dehydrolanostenol from Cerens gigantens and its biosynthetic implications. Phytochemistry, 27, 1705–1708. [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Friendly, M. , Kindt, R. , Legendre, P. , McGlin, D. , … Wagner, H. (2018). vegan: Community ecology package. R package version 2.5‐3. https://CRAN.R-project.org/package=vegan [Google Scholar]
- Pernal, S. F. , & Currie, R. W. (2002). Discrimination and preferences for pollen‐based cues by foraging honeybees, Apis mellifera L. Animal Behaviour, 63(2), 369–390. 10.1006/anbe.2001.1904 [DOI] [Google Scholar]
- Praz, C. J. , Müller, A. , & Dorn, S. (2008). Specialized bees fail to develop on non‐host pollen: Do plants chemically protect their pollen? Ecology, 89, 795–804. 10.1890/07-0751.1 [DOI] [PubMed] [Google Scholar]
- Rasheed, S. A. , & Harder, L. D. (1997). Economic motivation for plant species preferences of pollen‐collecting bumble bees. Economic Entomology, 22, 209–219. 10.1046/j.1365-2311.1997.t01-1-00059.x [DOI] [Google Scholar]
- Redfern, C. P. F. (1986). Changes in patterns of ecdysteroid secretion by the ring gland of Drosophila in relation to the sterol composition of the diet. Experientia, 42, 307–309. 10.1007/BF01942516 [DOI] [Google Scholar]
- Regali, A. (1996). Contribution à l'étude des besoins alimentaires en stéroïdes de Bombus terrestris (L.). Thesis, University of Mons‐Hainaut, Belgium, 143 pp. [Google Scholar]
- Rezkova, K. , Zakova, M. , Zakova, Z. , & Straka, J. (2012). Analysis of nesting behavior based on daily observation of Andrena vaga (Hymenoptera: Andrenidae). Journal of Insect Behaviour, 25, 24–47. 10.1007/s10905-011-9274-8 [DOI] [Google Scholar]
- Riffell, J. A. (2011). The neuroecology of a pollinator's buffet: Olfactory preferences and learning in insect pollinators. Integrative and Comparative Biology, 51(5), 781–793. 10.1093/icb/icr094 [DOI] [PubMed] [Google Scholar]
- Roberts, D. W. (2012). labdsv: Ordination and multivariate analysis for ecology. R package version 1.5‐0. https://CRAN.R-project.org/package=labdsv [Google Scholar]
- Robertson, A. W. , Mountjoy, C. , Faulkner, B. E. , Roberts, M. V. , & Macnair, M. R. (1999). Bumblebee selection of Mimulus guttatus flowers: The effects of pollen quality and reward depletion. Ecology, 80, 2594–2606. [Google Scholar]
- Roger, N. , Moerman, R. , Carvalheiro, L. G. , Aguirre‐Guitiérrez, J. , Jacquemart, A.‐L. , Kleijn, D. , … Michez, D. (2016). Impact of pollen resources drift on common bumblebees in NW Europe. Global Change Biology, 23(1), 68–76. [DOI] [PubMed] [Google Scholar]
- Sedivy, C. , Müller, A. , & Dorn, S. (2011). Closely related pollen generalist bees differ in their ability to develop on the same pollen diet: Evidence for physiological adaptations to digest pollen. Functional Ecology, 25(3), 718–725. 10.1111/j.1365-2435.2010.01828.x [DOI] [Google Scholar]
- Shuttleworth, A. , & Johnson, S. D. (2009). The importance of scent and nectar filters in a specialized wasp‐pollination system. Functional Ecology, 23, 931–940. 10.1111/j.1365-2435.2009.01573.x [DOI] [Google Scholar]
- Standifer, L. N. , Devys, M. , & Barbier, M. (1968). Pollen sterols – A mass spectrographic survey. Phytochemistry, 7, 1361–1365. 10.1016/S0031-9422(00)85638-1 [DOI] [Google Scholar]
- Suzuki, K. , Dohzono, I. , & Hiei, K. (2007). Evolution of pollinator generalization in bumblebee‐pollinated plants. Plant Species Biology, 22, 141–159. 10.1111/j.1442-1984.2007.00187.x [DOI] [Google Scholar]
- Svoboda, J. A. , & Lusby, W. R. (1986). Sterols of phytophagous and omnivorous species of hymenoptera. Archives of Insect Biochemistry and Physiology, 3, 13–18. 10.1002/arch.940030103 [DOI] [Google Scholar]
- Thorsteinson, A. J. (1960). Host selection in phytophagous insects. Annual Review of Entomology, 5, 193–218. 10.1146/annurev.en.05.010160.001205 [DOI] [Google Scholar]
- Vanderplanck, M. , Bruneau, E. , & Michez, D. (2009). Oligolectisme et décalage phénologique entre plante hôte et pollinisateur: Étude de deux espèces printanières psammophiles, Colletes cunicularius (L.) (Hymenoptera, Colletidae) et Andrena vaga (PANZER) (Hymenoptera, Andrenidae). Osmia, 3, 23–27. [Google Scholar]
- Vanderplanck, M. , Decleves, S. , Roger, N. , Decroo, C. , Caulier, G. , Glauser, G. , … Michez, D. (2018). Is non‐host pollen suitable for generalist bumblebees? Insect Science, 25(2), 259–272. 10.1111/1744-7917.12410 [DOI] [PubMed] [Google Scholar]
- Vanderplanck, M. , Michez, D. , Vancraenenbroeck, S. , & Lognay, G. (2011). Micro‐quantitative method for analysis of sterol levels in honeybees and their pollen loads. Analytical Letters, 44(10), 1807–1820. 10.1080/00032719.2010.526271 [DOI] [Google Scholar]
- Vanderplanck, M. , Vereecken, N. J. , Grumiau, L. , Esposito, F. , Lognay, G. , Wattiez, R. , & Michez, D. (2017). The importance of pollen chemistry in evolutionary host shifts of bees. Scientific Reports, 7, 43058 10.1038/srep43058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudo, A. , Patch, H. M. , Mortensen, D. A. , Tooker, J. F. , & Grozinger, M. (2016). Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 4035–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecken, N. , Toffin, E. , Gosselin, M. , & Michez, D. (2006). Observations relatives à la biologie et à la nidification de quelques abeilles sauvages psammophiles d'intérêt en Wallonie; 1. Observations printanières. Parcs et Réserves, 61(1), 8–13. [Google Scholar]
- Wcislo, W. T. , & Cane, J. H. (1996). Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural ennemies. Annual Review of Entomology, 41, 257–286. [DOI] [PubMed] [Google Scholar]
- Weiner, C. N. , Hilpert, A. , Werner, M. , Linsenmair, K. E. , & Bluthgen, N. (2010). Pollen amino acids and flower specialisation in solitary bees. Apidologie, 41(4), 476–487. 10.1051/apido/2009083 [DOI] [Google Scholar]
- Westrich, P. (1989). Die Wildbienen Baden‐Württembergs. Suttgart, Germany: Eugen Ulmer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the results: Dryad: https://doi.org/10.5061/dryad.cz8w9gj03.


