Abstract
CorA proteins belong to 2-TM-GxN family of membrane proteins, and play a major role in Mg2+ transport in prokaryotes and eukaryotic mitochondria. The selection of substrate is believed to occur via the signature motif GxN, however there is no consensus how strict this selection within the family. To answer this question, we employed fluorescence-based transport assays on three different family members, namely CorA from bacterium Thermotoga maritima, CorA from the archeon Methanocaldococcus jannaschii and ZntB from bacterium Escherichia coli, reconstituted into proteoliposomes. Our results show that all three proteins readily transport Mg2+, Co2+, Ni2+ and Zn2+, but not Al3+. Despite the similarity in cation specificity, ZntB differs from the CorA proteins, as in the former transport is stimulated by a proton gradient, but in the latter by the membrane potential, confirming the hypothesis that CorA and ZntB proteins diverged to different transport mechanisms within the same protein scaffold.
Subject terms: Ion channels, Membrane proteins
Introduction
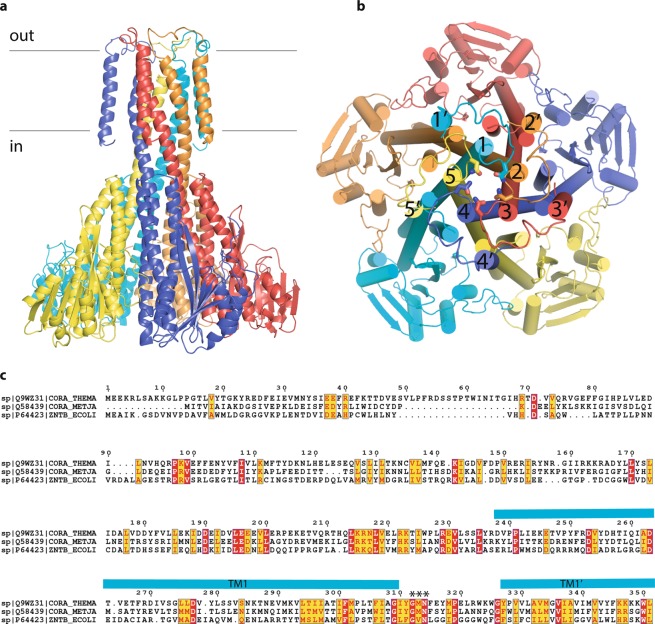
Magnesium is one of the essential metal ions, which is invariantly required for every cell, as it is involved in numerous metabolic reactions and also plays additional roles, for example as a stabilizer of highly charged adenosine triphosphate and lipidic bilayer, where it compensates the negative charge of phosphate groups1. Since magnesium is normally present in biological systems in its ionic form as Mg2+, it cannot readily cross the biological membrane, thus it is channeled via membrane-embedded proteins2. In prokaryotes, this is often done via MgtE and CorA families of proteins2,3. The latter are homo- or hetero- pentamers2,4–7, possess large cytoplasmic domains, which are believed to play a regulatory function8,9 and the transmembrane part, which consists of two α-helices per protomer, arranged as an inner and an outer pentamer, while the loops connecting them bear the signature motif GxN, which is at the same time is the selectivity filter6,10,11 (Fig. 1).
Figure 1.
The general organization of CorA family of proteins as exemplified by TmCorA (pdb code 4I0U). (a) side view, each protomer is color-coded; the position of membrane is indicated with black lines; (b) view from the extracellular part, transmembrane helices are numbered with the numerals, those with ′ indicate the outer transmembrane helix of each protomer. The asparagine side chains of GxN motif are shown as sticks; (c) the sequence alignment of TmCorA, MjCorA and EcZntB. Essentially conserved amino acids are in red. The turquoise bars show the position of the long helices (numbered in panel b) forming the channel and of the periphery helices (indicated with ′ in panel b). The signature motif/selectivity filter is indicated with *. The sequence alignment was produced with T-coffee42 (http://tcoffee.crg.cat/apps/tcoffee/index.html) and annotated with Espript 3.0 (ref. 43) (http://espript.ibcp.fr).
Most of the efforts in the structural characterization on CorA family of proteins have been focused on CorAs from Thermotoga maritima (TmCorA) and Methanocoldococcus jannaschii (MjCorA)4,6,8,9,12–15. The initial functional characterization of the family was done with orthologous CorA from Salmonella typhimurium16,17 which was later extended by the vast amount of data on TmCorA generated by different groups in an attempt to understand its transport mechanism. This led to a situation that only TmCorA is characterized in a great detail by various techniques (including but not limited to transport assays, electrophysiology, Molecular Dynamics simulations, mutagenesis) but for other members such characterization is rather scarce. With the goal to extend such a functional characterization on other members of CorA family we performed the extensive in vitro functional characterization of CorA from M. jannaschii and of homologous zinc transporter ZntB18 in comparison with CorA from T. maritima. Our results show that similarly to TmCorA, both MjCorA and EcZntB are not highly selective, and support the hypothesis that CorA and ZntB proteins might utilize different transport mechanisms.
Results
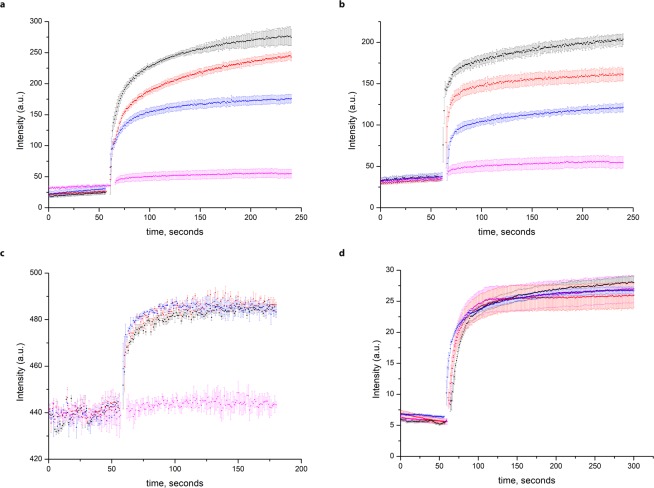
Purified CorA proteins from T. maritima and M. jannaschii and ZntB from E.coli (Supplementary Fig. 2) were reconstituted into proteoliposomes and their transport activity was assayed as described previously18. Both TmCorA and MjCorA readily transported Zn2+, Cd2+, Co2+ and Ni2+ similarly to EcZntB as seen in the experiments with the Fluozin-1 dye (Fig. 2a,b); all three proteins readily transport Mg2+ as registered with Fluozin-3 dye (Fig. 2c), but are not capable to transport Al3+ as seen in the experiments with morin dye (Fig. 2d).
Figure 2.
Transport of different cations assayed by the fluorophores trapped inside the proteoliposomes. Dequenching of Fluozin-1 dye fluorescence with (a) TmCorA and (b) MjCorA (added at 1 min and color-coded: black—25 μM Zn2+, red—25 μM Cd2+, blue—100 μM Ni2+, magenta—empty liposomes with 25 μM Zn2+). c Dequenching of FluoZin-3 fluorescence during transport of Mg2+ via red—ZntB, blue—TmCorA, black—MjCorA, magenta—empty liposomes. 100 μM Mg2+ was added at 1 min. (d) Incapability of these proteins to transport Al3+ as registered by morin dye (the same coloring as in (c), 200 μM Al3+ was added at 1 min). Error bars represent s.e.m. from three or more technical replicates of independent batches of proteoliposomes.
Surprisingly the transport activity of TmCorA and MjCorA was not efficiently inhibited by Hexamminecobalt(III) chloride, the known CorA inhibitor19,20 (Fig. 3).
Figure 3.
The incapability of hexamminecobalt(III) chloride (CoHex) to inhibit the transport of Zn2+ by proteoliposomes with (a) TmCorA, (b) MjCorA and (c) ZntB. Black—25 μM Zn2+ with no added CoHex, red— 25 μM Zn2+ + 1 μM CoHex, blue—25 μM Zn2+ + 10 μM CoHex, green—25 μM Zn2+ + 50 μM CoHex, magenta—empty liposomes with 25 μM Zn2+ + 50 μM CoHex. All substrates were simultaneously added at 1 min. Higher concentrations of CoHex led to the collapse of proteoliposomes (see Supplementary Fig. 1). Error bars represent s.e.m. from three or more technical replicates of independent batches of proteoliposomes.
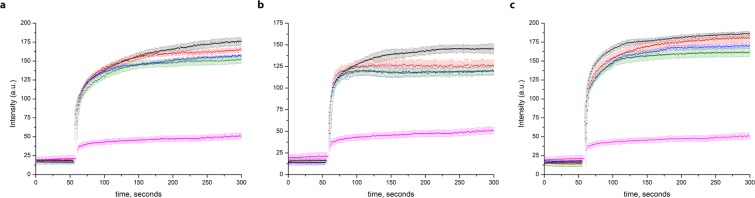
To test whether either of CorA has a preference for Co2+ over Mg2+ we performed competition assay experiments. We compared the transport of Co2+ by TmCorA and MjCorA in the absence of Mg2+ and in the presence of 100–1000 µM of Mg2+ (Fig. 4). Whereas transport of Co2+ via MjCorA was significantly affected already by addition of 100 µM of Mg2+ (Fig. 4a) transport of Co2+ via TmCorA was not much affected even in the presence of 1 mM of Mg2+ (Fig. 4b). This result supports the previous hypothesis that TmCorA might be a Co2+-selective channel21.
Figure 4.
transport competition assays with Co2+ and Mg2+ in the proteoliposomes, loaded with FluoZin-1, with (a) MjCorA and (b) TmCorA, (black—100 μM CoSO4 with no added MgSO4, red—100 μM CoSO4 + 100 μM MgSO4, blue —100 μM CoSO4 + 200 μM MgSO4, green—100 μM CoSO4 + 500 μM MgSO4, light blue—100 μM CoSO4 + 1000 μM MgSO4, magenta—empty liposomes with 100 μM CoSO4 + 1000 μM MgSO4). All substrates were simultaneously added at 1 min. Error bars represent s.e.m. from three or more technical replicates of independent batches of proteoliposomes.
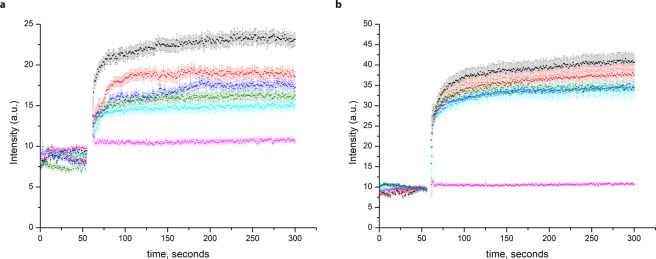
In stark contrast with ZntB, both TmCorA and MjCorA do not seem to transport protons as seen in the experiments with ACMA dye (Fig. 5), supporting our previous hypothesis that ZntB and CorA proteins evolved to use different transport mechanisms despite the same general architecture.
Figure 5.
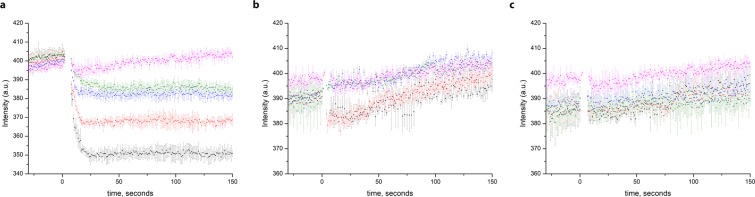
Quenching of the pH-dependent fluorophore ACMA at different Zn2+ concentrations in proteoliposomes with (a) EcZntB (b) TmCorA and (c) MjCorA (addition of Zn2+ after baseline stabilization, black—50 μM Zn2+, red—25 μM Zn2+, blue—5 μM Zn2+, green—1 μM Zn2+, magenta—empty liposomes with 50 μM Zn2+). Error bars represent s.e.m. from three or more technical replicates of independent batches of proteoliposomes.
This is further corroborated by the fact that ZntB and CorA respond differently to the presence of membrane potential: a creation of membrane potential of −116 mV by addition of valinomycin to proteoliposomes leads to the enhanced transport via CorA but not ZntB proteins (Fig. 6).
Figure 6.
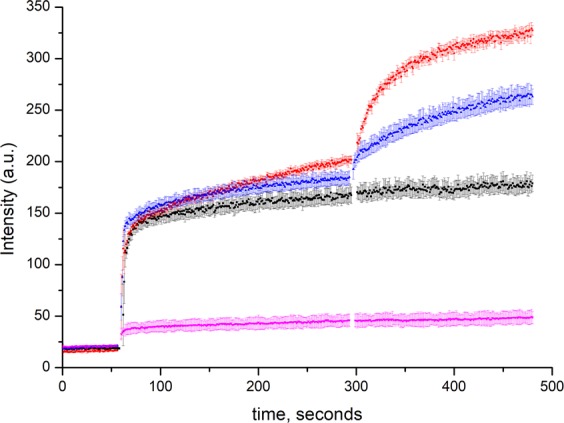
Effect of membrane potential on the transport of Zn2+ (added after 1 min) assayed by the fluorophore FluoZin-1 trapped inside the proteoliposomes with EcZntB (black), TmCorA (red) and MjcorA (blue) and empty liposomes (magenta). 1 μM of valinomycin were added after 5 min. Error bars represent s.e.m. from three or more technical replicates of independent batches of proteoliposomes.
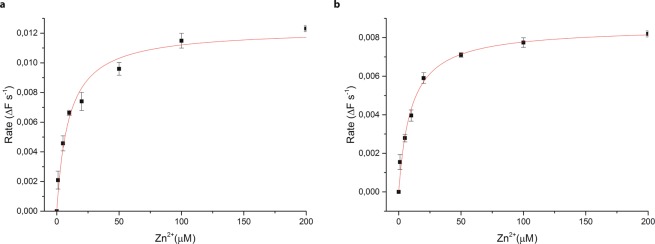
The rates of transport, as well as Km values of 9.5 μM and 9.9 μM for TmCorA and MjCorA respectively (Fig. 7), are similar to the previously reported Km value of 7.5 μM for EcZntB18.
Figure 7.
Rate of transport dependence on Zn2+ concentration in (a) TmCorA (b) MjCorA. The solid lines represent the fit to the Michaelis–Menten equation (based on FluoZin-1 experiments). Error bars represent s.e.m. from three or more technical replicates of independent measurements.
Discussion
CorA proteins are the most characterized representatives of 2-TM-GxN family of transporters/channels, which additionally includes ZntB, Alr, Mrs2 and other proteins22–26. However there is an obvious knowledge imbalance within CorA subfamily itself, where basically only TmCorA is extensively characterized both functionally17,27 and structurally4,8,9,12,14. In an attempt to mend it we performed the extensive in vitro functional characterization using fluorescence-based transport assays on two other representatives of 2-TM-GxN family, for which the full-length structures are available, namely on archaeal MjCorA6 and bacterial EcZntB18 in parallel with TmCorA. Using these fluorescence-based transport assays on proteins reconstituted into proteoliposomes we tried to answer whether there is a high substrate specificity within subfamilies – a controversial issue originating from an array of different experiments, including whole cell uptakes, isothermal titration calorimetry and patch clamp experiments and structural models of CorA and ZntB proteins which do not always agree4,7,18–21,28–30. Our results show that despite there is a strict selection of divalent over trivalent cations (Fig. 2) for all studied members, the uptake of divalent ions is rather promiscuous. For example, it was assumed that ZntB proteins are strictly selective for Zn2+ over Mg2+ and vice versa for CorA. To monitor the intraliposomal accumulation of Mg2+ we used Fluozin-3 dye and not Mag-fura 2 dye as we found the performance of the latter not satisfactory in our experimental setup. The transport of Mg2+ via ZntB is comparable with one via TmCorA and MjCorA (Fig. 2c). Similarly, both TmCorA and MjCorA readily transport Zn2+ (Fig. 2a,b). Furthermore, for TmCorA it has been shown that in absence of any Mg2+ (which is rather a non-physiological) it becomes rather a non-selective divalent channel30. Taking into account similarity among studied cations it is feasible to assume that the single main recognition pattern is indeed the hydration radius (close to 2.1 Å) and octahedral arrangement of water molecules in the first hydration shell of a cation as it was proposed earlier18,31,32, however that fails to explain the fact why TmCorA prefers Co2+ as its substrate even in the presence of 1 mM Mg2+ (Fig. 4b). To this moment the most plausible explanation is the presence of additional recognition patterns – such as threonine residues inside the pore8, however more systematic study involving more members of both subgroup A (supposedly Co2+ -selective channels) and B (supposedly true Mg2+ -channels)33 is necessary. This result also disfavors the explanation that since TmCorA reside in water habitat it should just prefer Mg2+ (ref. 15), as the exact elemental pattern in its preferential dwelling (hot springs and hydrothermal vents) might be very different from the normal water composition34. If this is correct then basically the preferred substrate will be dictated by the environmental milieu – organisms exposed to high Co2+ and low Mg2+ evolved to scavenge Co2+ more efficiently; the observation that some enzymes of T. maritima are cobalt-dependent35,36 supports this hypothesis. Furthermore, the results of complementation assay of TmCorA in the salmonella strain devoid of all Mg2+ transporters19, thermostability assays and competition studies21 all indicate that Mg2+ is not the most preferred substrate for TmCorA and electrophysiological measurements on TmCorA expressed in oocytes revealed that its affinity for Co2+ is ~ 10 times higher than for Mg2+ (ref. 37).
Another evidence for possible extra selectivity features is the metallotransportosome of Cupriavidus metallidurans, which encodes three different CorA proteins (CorA1-CorA3, with sequence identity below 7%) as well as ZntB protein7,38. Interestingly, CmCorA1 and CmCorA2 are involved in Ni2+ import, whereas all three forms can transport Zn2+ and CmCorA123 heterotrimer is responsible for the import of Co2+ (ref. 7). Clearly, the structural and functional in vitro characterization of C. metallidurans transporters will be essential to pinpoint residues responsible for such substrate specificity.
A puzzling observation we made is that hexamminecobalt(III) chloride (CoHex) is not a potent inhibitor (Fig. 3) of CorA and ZntB proteins at least under our experimental conditions. The previously reported IC50 values of 0.5–1 µM for StCorA obtained with the radioactive 63Ni2+ whole cell uptakes20 were taken as a reference, however CoHex concentrations up to 50 µM showed little impact on the transport uptake by TmCorA and MjCorA (Fig. 3). We noticed that for the homologous yeast Mrs2 channel39 and for TmCorA reconstituted in liposomes19 the inhibition of transport was observed with the cobalt hexamine concentration of 1 mM. Furthermore, two-electrode voltage clamp experiments revealed the similar value of Km of 0.9 ± 0.4 mM30. We tried performing our uptake experiments in the presence of 0.2–1 mM range of CoHex, however at such high concentrations it led to the collapse of proteoliposomes (Supplementary Fig. 1). We can only speculate why it did not work in our case (different lipid composition, reconstitution ratio, etc.) but it might be as well as in the published work with TmCorA in the presence of 1 mM CoHex the authors observed the collapse of proteoliposomes and not the inhibition effect. Furthermore, in the aforementioned work on Mrs2 complete inhibition was not observed39. Interestingly, thermal shift assays studies on MjCorA revealed that CoHex exerts the stabilization effect on the protein (stabilization from 76.7 to ~ 85 °C), albeit it is considerably lower than Co2+ ion itself (stabilization up to 95 °C)40. Altogether this might indicate that CoHex indeed binds to CorA proteins but either not with the high affinity or not exactly at the selectivity filter.
Our results also provide further evidence that CorA and ZntB proteins diverged to use different transport mechanisms. First of all, CorA proteins seem not to utilize any proton gradient in contrast with ZntB (Fig. 5). In EcZntB proteoliposomes under conditions of equal pH inside and outside, 9-amino-6-chloro-2-methoxyacridine (ACMA) dye senses the buildup of pH gradient upon Zn2+ transport and the fluorescence is quenched (Fig. 5a). In case of TmCorA and MjCorA apparently there is no co-transport of H+ thus the fluorescence is more or less at the same level (Fig. 5b,c).
In line with the previous reports19,41 the influx of Mg2+ via CorA proteins is driven by the membrane potential (Fig. 6). Addition of valinomycin to CorA proteoliposomes loaded with 25 mM potassium chloride, leads to the fast escape of potassium ions and build-up of membrane potential to the −116 mV enhancing the transport of divalent cations. The similar behavior of TmCorA was shown before in the experiments with Mag-fura 2 dye19, however the enhancement of transport was less pronounced. This discrepancy could be caused by difference in the experimental setup and liposome preparation and / or fluorescent properties of different dyes. In the yeast mitochondria expressing Mrs2 (in KCl buffer), the influx of Mg2+ was significantly reduced upon addition of valinomycin, which dissipated mitochondrial membrane potential39.
The emerging picture is that in the 2-TM-GxN family the homo- pentameric fold evolved for recognition of similar divalent cations - such as Mg2+, Co2+, Ni2+, Zn2+. However, in the particular milieus, where a certain cation is prevailing, specificity might have evolved. Furthermore, for not yet discovered reasons, some members, such as CorA and Mrs2 evolved to be highly-conductive magnesium channels24,30, whereas others such as ZntB and Alr proteins became proton-coupled sympoters18,22. Clearly there are still open questions, such as what is the actual mode of CoHex binding to CorA proteins, and how some members can be involved in the transport of divergent Al3+ cation (1.9 Å first hydration shell radius vs ~ 2.1 Å for aforementioned cations). The elucidation of structures as well as thorough functional characterization of other members of 2-TM-GxN is necessary to answer such questions and to fully understand the transport of ions in this family of proteins.
Methods
Cloning
TmCorA and MjCorA were cloned into pNIC28-Bsa4 vector encoding an N-terminal 6xHis-tag and a tobacco etch virus protease cleavage site. The full-length CorA genes were amplified from genomic DNAs of Thermotoga maritima and Methanocaldococcus jannaschii (DSMZ, Germany). The expression vector was constructed using ligation independent cloning with primers for TmCorA (forward 5′-TACTTCCAATCCATGGAGGAAAAGAGGCTGTCTGC-3′ and reverse 5′-TATCCACCTTTACTGTCACAGCCACTTCTTTTTCTTG-3′) and MjCorA (forward 5′-TACTTCCAATCCATGATTACGGTAATTGCTATAGC-3′ and reverse 5′-TATCCACCTTTACTGCTAAATCCATCCTGACCTTC-3′).
Protein expression and membrane vesicle preparation
TmCorA, MjCorA and EcZntB proteins were expressed in the same way according to the previously established protocol18: expression of target protein was performed in a 5-l flask containing 2 l of LB medium (10 g l−1 Bacto trypton, 5 g l−1 Bacto yeast extract, 10 g l−1 NaCl), supplemented with 50 ug ml−1 kanamycin and 34 ug ml−1 chloramphenicol. The E. coli BL-21(DE3) cells with the needed plasmid were grown at 37 °C, 200 rpm to an OD600 of 0.8, with an induction by addition of 0.1 mM IPTG. After 3 h of expression the cells were collected by centrifugation (15 min, 7,446 g, 4 °C), washed in buffer A (50 mM Tris/HCl, pH 8.0) and resuspended in the buffer B (50 mM Tris/HCl, pH 8.0, 250 mM NaCl, 10% glycerol). Membrane vesicles were prepared as described previously18 and were either prepared immediately, or the resuspended cells were stored at −80 °C after flash freezing in liquid nitrogen. Before membrane vesicle preparation, 1 mM MgSO4 and 50–100 ug ml−1 DNase were added to the cells. The cells were lysed by high-pressure disruption (Constant Cell Disruption System Ltd, UK, two passages at 25 kPsi for E. coli cells, 5 °C) and cell debris was removed by low-speed centrifugation (30 min, 12,074 g, 4 °C). Membrane vesicles were collected by ultracentrifugation (120 min, 193,727 g, 4 °C), and resuspended in buffer C (50 mM Tris/HCl, pH 8.0, 150 mM NaCl, 15% glycerol) to a final volume of 5 ml per 1 l of cell culture. Subsequently, the membrane vesicles were aliquoted, flash frozen in liquid nitrogen and stored at −80 °C.
Protein purification
Protein purification was done as described previously18. Membrane vesicles were thawed rapidly and solubilized in buffer D (50 mM Tris/HCl, pH 8.0, 150 mM NaCl, 10 mM imidazole, 10% glycerol, 1% (w/v) n-dodecyl-β-D-maltopyranoside (DDM, Anatrace)) for 1 h at 4 °C, while gently rocking. Unsolubilized material was removed by centrifugation (30 min, 442,907 g, 4 °C). The supernatant was incubated for 1 h at 4 °C under gently rocking with Ni2+-sepharose resin (column volume of 0.5 ml), which had been equilibrated with 10 CV of buffer E (50 mM Tris/HCl, pH 8.0, 250 mM NaCl, 50 mM imidazole, 0.03% DDM). Subsequently, the suspension was poured into a 10-ml disposable column (Bio-Rad) and the flow through was collected. The column material was washed with 10 ml of buffer E. The target protein was eluted in three fractions of buffer F (50 mM Tris/HCl, pH 8.0, 250 mM NaCl, 500 mM imidazole, 0.03% (w/v) DDM) of 200, 750 and 500 µl, respectively. 2 mM of EDTA was added to the second elution fraction to remove co-eluted Ni2+ ions and any residual divalent cations. Subsequently, the second elution fraction was purified by size-exclusion chromatography using a Superdex 200 10/300 gel filtration column (GE-Healthcare), equilibrated with buffer G (50 mM Tris/HCl, pH 8.0, 250 mM NaCl, 0.03% (w/v) DDM). After size-exclusion chromatography, the fractions containing the target protein were combined and used directly for proteoliposome reconstitution.
Reconstitution into proteoliposomes
Reconstitution in proteoliposomes was performed as described previously18: polar lipids of E. coli and egg phosphatidylcholine (in 3:1 (w/w) ratio) were dissolved in chloroform, then dried in a rotary evaporator and subsequently resuspended in buffer containing 50 mM KPi, pH 7.5 to the concentration of 20 mg ml−1. After three freeze-thaw cycles, large unilamellar vesicles (LUVs) were obtained and stored in liquid nitrogen. To prepare proteoliposomes, LUVs were extruded through a 400-nm-diameter polycarbonate filter (Avestin, 11 passages). Obtained liposomes were diluted to 4 mg ml−1 in buffer H (50 mM HEPES, pH 7.5) or buffer I (50 mM HEPES, pH 6.5) and subsequently destabilized beyond Rsat with Triton X-100. The target purified protein was added to the liposomes at a weight ratio of 1:250 (protein/lipid), followed by detergent removal using Bio-beads (50 mg ml−1, four times after 0.5 h, 1 h, 2 h and overnight incubation). Afterwards, proteoliposomes were collected by centrifugation (25 min, 285,775 g, 4 °C) and resuspended in buffer H or buffer I to a lipid concentration of 10 mg ml−1. Finally, after three freeze-thaw cycles, obtained proteoliposomes were stored in liquid nitrogen until subsequent experiments.
Fluorescent transport assays
Transport of metals was measured according to the previously established protocol18. Zinc transport was measured with the Zn2+-sensitive fluorophore FluoZin-1 (ThermoFisher, USA). To avoid bleaching of the fluorophore, the sample was shielded from the direct light as much as possible. FluoZin-1 (stock concentration 3 mM in H2O) was added to a final concentration of 5 μM to the proteoliposomes. FluoZin-1 encapsulation was performed by three freeze-thaw cycles and subsequent extrusion through 0.4 µm polycarbonate filters. Extravesicular dye was removed from approximately 500 μl of liposome suspension by size exclusion chromatography on a 2 ml Sephadex G-75 column equilibrated with buffer H or I. Proteoliposomes were collected by ultracentrifugation (25 min, 285,775 g, 4 °C), and the supernatant was removed. Proteoliposomes were resuspended with 10 μl buffer H or I per 2.5 mg of proteoliposomes (protein to lipid ratio 1:250). Transport assays were initiated by the addition of 10 mM stock solution of zinc acetate to the desired final concentration. For each measurement, 0.3 mg of proteoliposomes was diluted in 1 ml of desired buffer. A fluorescence time course was measured in a 1-ml cuvette with a stirrer (350 rpm) using an excitation wavelength of 490 nm and an emission wavelength of 525 nm. Experiments with empty liposomes were performed in parallel as controls. Initial transport rates (ΔF s−1) were calculated by performing a linear regression on the transport data between 1 and 10 s after addition of zinc acetate. The resulting data was fitted to a Michaelis-Menten equation. All measurements were at least triplicated.
To investigate the inhibition effect of hexamminecobalt (III) chloride (CoHex), the proteoliposomes loaded with FluoZin-1 were preincubated with various concentrations of CoHex from 1 μM to 1 mM for 3 minutes, after that 25 μM zinc acetate was added. All other steps were performed in the similar way as described above. Experiments with empty liposomes were performed in parallel as controls. All measurements were triplicated. To check the ability of target proteins to transport Al3+, the proteoliposomes were prepared the same way as for FluoZin-1, but instead loaded with 3 μM morin (Sigma-Aldrich). A fluorescence time course was measured in a 1-ml cuvette with a stirrer using an excitation wavelength of 420 nm and an emission wavelength of 500 nm; 50 μM AlCl3 was added after 1 minute of equilibration time. Experiments with empty liposomes were performed in parallel as controls. All measurements were triplicated. Magnesium transport was measured by FluoZin-3 (ThermoFisher, USA). All preparations of the proteoliposomes were the same as with FluoZin-1 except FluoZin-3 (stock concentration 1 mM in H2O) was added to a final concentration of 5 μM to the proteoliposomes. A fluorescence time course was measured in a 1-ml cuvette with a stirrer using an excitation wavelength of 494 nm and an emission wavelength of 516 nm. After 3 minute of the baseline’s stabilisation 100 μM MgSO4 was added. Experiments with empty liposomes were performed in parallel as controls. All measurements were triplicated. H+ transport assays were performed as described previously18: the lumenal buffer of the proteoliposomes was exchanged for buffer J (5 mM HEPES pH 6.7) by resuspension of the liposomes in this buffer followed by three freeze-thaw cycles and extrusion through 0.4 μm polycarbonate filters. Proteoliposomes were collected by ultracentrifugation (25 min, 285,775 g, 4 °C), and the supernatant was removed. Proteoliposomes were resuspended with 10 μl buffer J per 2.5 mg of proteoliposomes (protein to lipid ratio 1:250). For each measurement, 0.3 mg of proteoliposomes was diluted in 1 ml of buffer K (5 mM HEPES, pH 6.7, 150 nM ACMA). A fluorescence time course was measured in a 1-ml cuvette with a stirrer using an excitation wavelength of 419 nm and an emission wavelength of 483 nm; zinc acetate was added after 3 minutes of equilibration time. Experiments with empty liposomes were performed in parallel as controls. All measurements were triplicated.
Data analysis
The structural figures were produced with an open source version of Pymol (https://github.com/schrodinger/pymol-open-source). The sequence alignment was produced with T-coffee42 (http://tcoffee.crg.cat/apps/tcoffee/index.html) and annotated with Espript 3.0 (ref. 43) (http://espript.ibcp.fr). The statistical analysis was performed in Excel (Microsoft Corp.) and the final graphs were produced in Origin Pro 7 (OriginLab Corp.)
Supplementary information
Acknowledgements
This research was supported by Dutch Scientific Organization, grant # 723.014.002 to A.G. The authors cordially thank Prof Dr Dirk J Slotboom for scientific discussions.
Author contributions
A.S. performed all experiments. A.S. and A.G. analyzed the data and wrote the manuscript.
Data availability
All data reported in this research are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-57869-z.
References
- 1.Wolf FI, Cittadini A. Chemistry and biochemistry of magnesium. Mol. Aspects Med. 2003;24:3–9. doi: 10.1016/S0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 2.Payandeh J, Pfoh R, Pai EF. The structure and regulation of magnesium selective ion channels. Biochim. Biophys. Acta. 2013;1828:2778–2792. doi: 10.1016/j.bbamem.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Pohland A-C, Schneider D. Mg2+ homeostasis and transport in cyanobacteria - at the crossroads of bacterial and chloroplast Mg2+ import. Biol. Chem. 2019;0:22469. doi: 10.1515/hsz-2018-0476. [DOI] [PubMed] [Google Scholar]
- 4.Eshaghi S, et al. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science. 2006;313:354–357. doi: 10.1126/science.1127121. [DOI] [PubMed] [Google Scholar]
- 5.Wachek M, Aichinger MC, Stadler JA, Schweyen RJ, Graschopf A. Oligomerization of the Mg2+-transport proteins Alr1p and Alr2p in yeast plasma membrane. FEBS J. 2006;273:4236–4249. doi: 10.1111/j.1742-4658.2006.05424.x. [DOI] [PubMed] [Google Scholar]
- 6.Guskov A, et al. Structural insights into the mechanisms of Mg2+ uptake, transport, and gating by CorA. Proc. Natl. Acad. Sci. USA. 2012;109:18459–18464. doi: 10.1073/pnas.1210076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzberg M, Bauer L, Kirsten A, Nies DH. Interplay between seven secondary metal uptake systems is required for full metal resistance of Cupriavidus metallidurans. Metallomics. 2016;8:313–326. doi: 10.1039/C5MT00295H. [DOI] [PubMed] [Google Scholar]
- 8.Nordin N, et al. Exploring the structure and function of Thermotoga maritima CorA reveals the mechanism of gating and ion selectivity in Co2+/Mg2+ transport. Biochemical Journal. 2013;451:365–374. doi: 10.1042/BJ20121745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payandeh J, Pai EF. A structural basis for Mg2+ homeostasis and the CorA translocation cycle. The EMBO Journal. 2006;25:3762–3773. doi: 10.1038/sj.emboj.7601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Sharma M, Qin H, Gao FP, Cross TA. Ligand binding in the conserved interhelical loop of CorA, a magnesium transporter from Mycobacterium tuberculosis. J. Biol. Chem. 2009;284:15619–15628. doi: 10.1074/jbc.M901581200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palombo I, Daley DO, Rapp M. Why is the GMN motif conserved in the CorA/Mrs2/Alr1 superfamily of magnesium transport proteins? Biochemistry. 2013;52:4842–4847. doi: 10.1021/bi4007397. [DOI] [PubMed] [Google Scholar]
- 12.Lunin VV, et al. Crystal structure of the CorA Mg2+ transporter. Nature. 2006;440:833–837. doi: 10.1038/nature04642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleverley RM, et al. The Cryo-EM structure of the CorA channel from Methanocaldococcus jannaschii in low magnesium conditions. Biochim. Biophys. Acta. 2015;1848:2206–2215. doi: 10.1016/j.bbamem.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthies D, et al. Cryo-EM Structures of the Magnesium Channel CorA Reveal Symmetry Break upon Gating. Cell. 2016;164:747–756. doi: 10.1016/j.cell.2015.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowatz T, Maguire ME. Loss of cytosolic Mg2+ binding sites in the Thermotoga maritima CorA Mg2+ channel is not sufficient for channel opening. Biochim Biophys Acta Gen Subj. 2019;1863:25–30. doi: 10.1016/j.bbagen.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hmiel SP, Snavely MD, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 1986;168:1444–1450. doi: 10.1128/JB.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snavely MD, Florer JB, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. 1989;171:4761–4766. doi: 10.1128/JB.171.9.4761-4766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gati, C., Stetsenko, A., Slotboom, D. J., Scheres, S. H. W. & Guskov, A. The structural basis of proton driven zinc transport by ZntB. Nature Communications 2017 8:1 8, 1313 (2017). [DOI] [PMC free article] [PubMed]
- 19.Payandeh J, et al. Probing structure-function relationships and gating mechanisms in the CorA Mg2+ transport system. J. Biol. Chem. 2008;283:11721–11733. doi: 10.1074/jbc.M707889200. [DOI] [PubMed] [Google Scholar]
- 20.Kucharski LM, Lubbe WJ, Maguire ME. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J. Biol. Chem. 2000;275:16767–16773. doi: 10.1074/jbc.M001507200. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y, et al. Co2+ selectivity of Thermotoga maritima CorA and its inability to regulate Mg2+ homeostasis present a new class of CorA proteins. J. Biol. Chem. 2011;286:16525–16532. doi: 10.1074/jbc.M111.222166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDiarmid CW, Gardner RC. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J. Biol. Chem. 1998;273:1727–1732. doi: 10.1074/jbc.273.3.1727. [DOI] [PubMed] [Google Scholar]
- 23.Worlock AJ, Smith RL. ZntB is a novel Zn2+ transporter in Salmonella enterica serovar Typhimurium. J. Bacteriol. 2002;184:4369–4373. doi: 10.1128/JB.184.16.4369-4373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindl R, Weghuber J, Romanin C, Schweyen RJ. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys. J. 2007;93:3872–3883. doi: 10.1529/biophysj.107.112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papp-Wallace KM, Maguire ME. Bacterial homologs of eukaryotic membrane proteins: the 2-TM-GxN family of Mg(2+) transporters. Mol. Membr. Biol. 2007;24:351–356. doi: 10.1080/09687680701441883. [DOI] [PubMed] [Google Scholar]
- 26.Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol., Cell Physiol. 2010;298:C407–29. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 27.Smith RL, Gottlieb E, Kucharski LM, Maguire ME. Functional similarity between archaeal and bacterial CorA magnesium transporters. J. Bacteriol. 1998;180:2788–2791. doi: 10.1128/JB.180.10.2788-2791.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan K, et al. Structure and electrostatic property of cytoplasmic domain of ZntB transporter. Protein Sci. 2009;18:2043–2052. doi: 10.1002/pro.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Q, et al. X-ray crystallography and isothermal titration calorimetry studies of the Salmonella zinc transporter ZntB. Structure. 2011;19:700–710. doi: 10.1016/j.str.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalmas O, et al. A repulsion mechanism explains magnesium permeation and selectivity in CorA. Proc. Natl. Acad. Sci. USA. 2014;111:3002–3007. doi: 10.1073/pnas.1319054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guskov A, Eshaghi S. The mechanisms of Mg2+ and Co2+ transport by the CorA family of divalent cation transporters. Curr Top Membr. 2012;69:393–414. doi: 10.1016/B978-0-12-394390-3.00014-8. [DOI] [PubMed] [Google Scholar]
- 32.Kitjaruwankul S, Wapeesittipan P, Boonamnaj P, Sompornpisut P. Inner and Outer Coordination Shells of Mg2+ in CorA Selectivity Filter from Molecular Dynamics Simulations. J. Phys. Chem. B. 2016;120:406–417. doi: 10.1021/acs.jpcb.5b10925. [DOI] [PubMed] [Google Scholar]
- 33.Niegowski D, Eshaghi S. The CorA family: structure and function revisited. Cell. Mol. Life Sci. 2007;64:2564–2574. doi: 10.1007/s00018-007-7174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douville E, et al. The rainbow vent fluids (36°14′N, MAR): the influence of ultramafic rocks and phase separation on trace metal content in Mid-Atlantic Ridge hydrothermal fluids. Chemical Geology. 2002;184:37–48. doi: 10.1016/S0009-2541(01)00351-5. [DOI] [Google Scholar]
- 35.Wojciechowski CL, Cardia JP, Kantrowitz ER. Alkaline phosphatase from the hyperthermophilic bacterium T. maritima requires cobalt for activity. Protein Sci. 2002;11:903–911. doi: 10.1110/ps.4260102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima M, Imamura H, Shoun H, Wakagi T. Unique metal dependency of cytosolic alpha-mannosidase from Thermotoga maritima, a hyperthermophilic bacterium. Arch. Biochem. Biophys. 2003;415:87–93. doi: 10.1016/S0003-9861(03)00222-4. [DOI] [PubMed] [Google Scholar]
- 37.Dalmas, O., Sompornpisut, P., Bezanilla, F. & Perozo, E. Molecular mechanism of Mg2+-dependent gating in CorA. Nature Communications 2017 8:1 5, 3590–11 (2014). [DOI] [PMC free article] [PubMed]
- 38.Große C, et al. Characterization of the Δ7 Mutant of Cupriavidus metallidurans with Deletions of Seven Secondary Metal Uptake Systems. mSystems. 2016;1:313. doi: 10.1128/mSystems.00004-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolisek M, et al. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. The EMBO Journal. 2003;22:1235–1244. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kean J, et al. Characterization of a CorA Mg2+ transport channel from Methanococcus jannaschii using a Thermofluor-based stability assay. Mol. Membr. Biol. 2008;25:653–663. doi: 10.1080/09687680802541169. [DOI] [PubMed] [Google Scholar]
- 41.Froschauer EM, Kolisek M, Dieterich F, Schweigel M, Schweyen RJ. Fluorescence measurements of free [Mg2+] by use of mag-fura 2 in Salmonella enterica. FEMS Microbiol. Lett. 2004;237:49–55. doi: 10.1016/j.femsle.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 43.Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–4. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this research are available from the corresponding author on reasonable request.