Abstract
Cross-coupling reactions have developed into powerful approaches for carbon–carbon bond formation. In this work, a Ni-catalyzed migratory Suzuki–Miyaura cross-coupling featuring high benzylic or allylic selectivity has been developed. With this method, unactivated alkyl electrophiles and aryl or vinyl boronic acids can be efficiently transferred to diarylalkane or allylbenzene derivatives under mild conditions. Importantly, unactivated alkyl chlorides can also be successfully used as the coupling partners. To demonstrate the applicability of this method, we showcase that this strategy can serve as a platform for the synthesis of terminal, partially deuterium-labeled molecules from readily accessible starting materials. Experimental studies suggest that migratory cross-coupling products are generated from Ni(0/II) catalytic cycle. Theoretical calculations indicate that the chain-walking occurs at a neutral nickel complex rather than a cationic one. In addition, the original-site cross-coupling products can be obtained by alternating the ligand, wherein the formation of the products has been rationalized by a radical chain process.
Subject terms: Catalyst synthesis, Catalytic mechanisms, Homogeneous catalysis, Density functional theory, Reaction mechanisms
Migratory cross-coupling reactions are powerful tools to form bonds at predictable positions. Here the authors report a nickel-catalyzed migratory Suzuki–Miyaura cross-coupling of unactivated alkyl electrophiles with aryl and vinyl boron reagents and provide experimental and computational mechanistic evidence.
Introduction
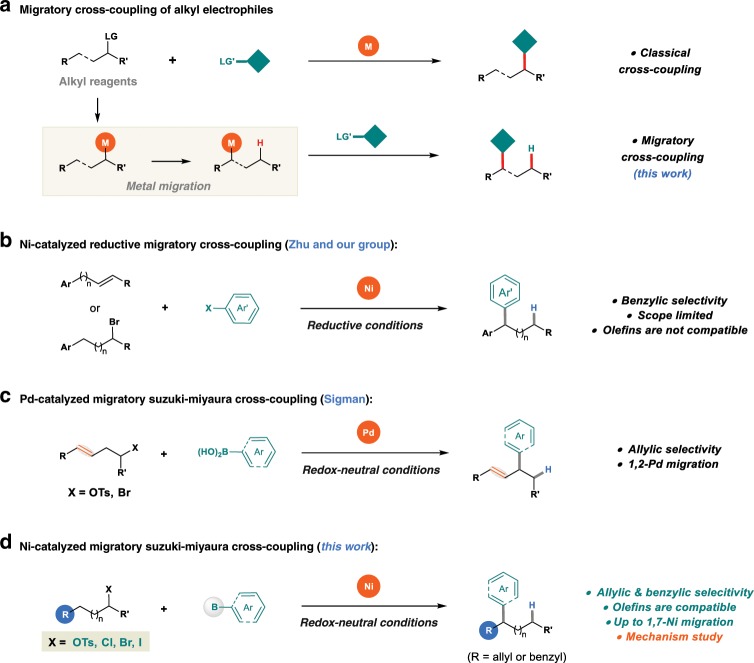
Transition metal-catalyzed cross-coupling reactions have developed into powerful approaches for carbon–carbon bond formation, and have revolutionized synthetic strategies in medicinal chemistry and material science1–5. Historically, the successful cross-coupling of alkyl electrophiles represents a landmark of this research field6–9. The β-hydrogen-containing, electronically unactivated alkyl partners is generally recognized to be more challenging than their aryl and vinyl analogs10–12. Over the past two decades, significant effort has been devoted to avoiding β-hydride elimination and constructing chemical bonds at the original position in an efficient and selective manner13–16. Chain-walking highlights great opportunities in bond formation at new positions, the exploration of transformations involving chain-walking have stimulated considerable interest in the synthetic community recently17,18. In this context, the concept of migratory cross-coupling has been initially described by the Baudoin group in palladium-catalyzed C–H bond functionalization reactions (Fig. 1a)19–21.
Fig. 1. Transition metal-catalyzed migratory cross-coupling.
a Migratory cross-coupling of alkyl electrophiles. b Ni-catalyzed reductive migratory cross-coupling. c Pd-catalyzed migratory Suzuki–Miyaura cross-coupling. d The approach developed in this study.
Recently, it has been reported that nickel catalysis22 exhibits unique performance in migratory transformations23. Particularly, a set of reductive migratory cross-coupling with aryl halides have been disclosed by Zhu24,25 and our group26,27 independently, wherein a benzylic selectivity is achieved and a series of pharmaceutically relevant 1,1-diarylalkanes can be efficiently constructed (Fig. 1b). In the past decade, extensive efforts have been devoted to the mechanistic studies of nickel-catalyzed cross-coupling of alkyl electrophiles13,14,28–30. While the well-known Ni(0/II)10,31,32 or Ni(II/IV)8,33 catalytic cycles are proposed in some cases, a radical chain process and bonding formation from a Ni(III) intermediate is particularly prevailed in those reactions with a nitrogen-based ligand29,30,34. In contrast, there are still quite limited reports on the mechanism of the nickel-catalyzed migratory cross-coupling reactions25,35. Compared with classical cross-coupling reactions, the major difference of migratory cross-coupling is a metal chain-walking process involved in the catalytic cycle. More elementary steps in catalytic cycle mean more complicated in mechanism, which increases difficulty into the mechanistic investigations as well. As a result, mechanistic information on the details for catalytic cycle, nickel chain-walking process and the origin of regioselectivity and so on, is still scarce, which seriously restricts further reaction design.
Furthermore, the current methods have several limitations in their substrate scope. First, in our previous investigations, the aryl coupling partners were restricted to electron-neutral and -rich aryl bromides26,27. Second, functionalization at benzylic position after chain-walking has been well-studied, but the synthetically useful allylic positions are unachievable24,26. Besides, the use of inexpensive, abundant nucleophiles for a redox-neutral migratory coupling reaction remains underdeveloped. For example, the migratory Suzuki–Miyaura cross-coupling of alkyl electrophiles has only been demonstrated by Sigman with palladium catalysis, wherein only allylic products and 1,2-palladium migration could be efficiently achieved (Fig. 1c)36,37. Moreover, the especially useful, unactivated alkyl chlorides38–40 have not been successfully employed in a migratory coupling event to date.
Here, we report our work on developing the general, Ni-catalyzed migratory Suzuki–Miyaura cross-coupling of unactivated alkyl electrophiles with both aryl and vinyl boron reagents (Fig. 1d). Experimental and computational studies are performed to reveal the detailed mechanism.
Results
Reaction optimization
We launched the investigation with the cross-coupling of 3-phenylpropyl tosylate (1a) with aryl boronic acid 2a. The preliminary study demonstrated that the generation of active nickel catalyst was not efficient via reduction of Ni(II) salts by 2a. A series of Ni(II) precatalyst activation methods were tested in the following studies41. The addition of 25 mol% Et3SiH, proved to be an efficient method to generate the active nickel catalyst, which offering the coupled products in 82% isolated yield (Table 1, entries 1–5, and Supplementary Fig. 3). Base is also crucial to the success of this transformation. The importance of LiOH was highlighted by the failure of other bases which only afford trace amount product (Table 1, entry 5, and Supplementary Table 1).
Table 1.
Reaction optimizationa.
| Entry | Deviation from standard conditions | Yield, % | 3a:4a |
|---|---|---|---|
| 1 | No | 89 (82)b | 27:1 |
| 2 | No Et3SiH | 6 | 4:1 |
| 3 | Zn instead of Et3SiH | 35 | 12:1 |
| 4 | ZnMe2, MeMgBr instead of Et3SiH | Trace | – |
| 5 | KF, Na2CO3, Cs2CO3, NaOH instead of LiOH | Trace | – |
| 6 | No TBAB | 8 | 2:1 |
| 7 | NiCl2 instead of NiI2 | Trace | – |
| 8 | NiBr2 instead of NiI2 | 46 | 21:1 |
| 9 | Ni(COD)2, no Et3SiH | 12 | 2:1 |
| 10 | L1 | 28 | 2:1 |
| 11 | L2 | 81 | 51:1 |
| 12 | L3 | 78 | 35:1 |
| 13 | L4 | 76 | 1:77 |
| 14 | NiI2/BC (5 mol%) | 72 | 19:1 |
| 15 | 60 °C, 24 h | 71 | 8:1 |
| 16 | 60 °C, no Et3SiH | 10 | 6:1 |
aStandard conditions: NiI2 (15.6 mg, 0.05 mmol, 10 mol%), BC (18.0 mg, 0.05 mmol, 10 mol%), Et3SiH (20 μL, 0.13 mmol, 25 mol%), 1a (145.0 mg, 0.5 mmol, 1.0 equiv), 2a (127.5 mg, 0.75 mmol, 1.5 equiv), LiOH (29.9 mg, 1.25 mmol, 2.5 equiv), TBAB (161.2 mg, 0.5 mmol, 1.0 equiv), DMA (4 mL). Yields were determined by GC with 1,3,5-trimethoxybenzene as the internal standard
bIsolated yield
The addition of TBAB greatly improves the efficiency, which due to the in situ conversion of the alkyl tosylate into alkyl bromide (Table 1, entry 6). Nickel iodide is superior to other nickel precatalysts (Table 1, entries 7–9). Dimethyl substitution on 1,10-phenanthroline or 2,2′-bipyridine backbones are crucial for the migratory selectivity (Table 1, entries 10–13). Notably, the original-site Suzuki–Miyaura cross-coupling42,43 product 4a could also be obtained in a good yield and excellent selectivity when the 5,5′-dimethylbipyridine (L4) was used (Table 1, entry 13), which is consistent with our previous reductive cross-couplings26. Solvent evaluation indicated that only amide solvents could deliver the migratory product 3a (Supplementary Table 1). Decreasing the catalyst loading to 5 mol% resulted in moderately lower yield and selectivity (Table 1, entry 14). Increasing the temperature to 60 °C led to worse selectivity (Table 1, entries 15 and 16).
Substrate scope
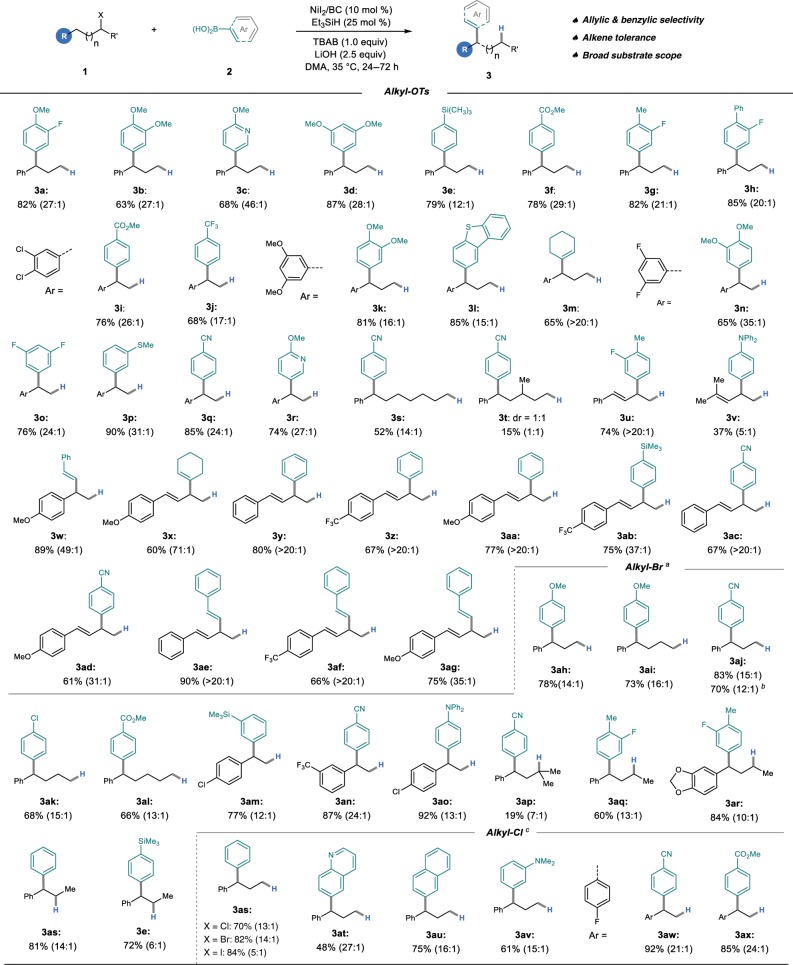
With the optimal conditions in hand, we next shifted our attention to investigating the generality of the Ni-catalyzed migratory Suzuki–Miyaura cross-coupling reaction. First, the scope of alkyl tosylates, which are readily accessible from the corresponding alcohols, was examined. As shown in Fig. 2 (top part), all examples furnished the migratory coupling products in good to excellent yields and regioselectivity under the standard conditions. A class of 1,1-diarylalkanes was prepared accordingly, which are widely encountered in biologically relevant molecules44,45. To our delight, both electronic-rich and deficient aryl coupling partners performed smoothly under this redox-neutral conditions. Moreover, homoallyl (3v) and homostyrenyl tosylates were also suitable substrates for this nickel-catalyzed system. Vinyl (3m and 3×) and styrenyl (3w, 3ae-3ag) boronic acids coupled with the homostyrenyl tosylates, to provide the skipped diene products with high regioselectivity (3×, 3ae-3ag). It should be noted that in Knochel’s study, an olefin coordination with nickel could facilitate reductive elimination at the original position32.
Fig. 2. Scope of the methodology.
Isolated yields were shown. Ratios of 3:4 are determined by GCMS. aWithout TBAB Additive. b10 mmol scale. cKI (0.38 mmol, 0.75 equiv), LiOH (0.75 mmol, 1.5 equiv), the mixture was stirred at 80 °C.
The scope of the nickel-catalyzed migratory reaction could also be extended to alkyl halides. As illustrated in Fig. 2 (3ah–3e), both primary and secondary alkyl bromides were able to yield the benzylic coupling products efficiently under the conditions without TBAB. The tertiary bromide was also able to provide the migratory product, albeit in low yield under the current conditions (3ap). The coupling reaction could also proceed when the iodide analog was employed, but with poor regioselectivity under the same conditions (3as). Gratifyingly, the more challenging chloride electrophiles were also converted to the benzylic arylation products efficiently in the presence of KI under an elevated temperature (80 °C) (Supplementary Table 2), which dramatically broadens the application of this migratory strategy in cascade or iterative synthesis with polyhalogenated materials46. In addition, a gram-scale experiment showed the scalability of this method (3aj). All 1,2- to 1,7-Ni migration (3s) coupling products could be successfully accessed under the redox-neutral conditions. Finally, a range of functional groups such as trimethylsilyl (SiMe3), ester (CO2Me), amines (NMe2, NPh2), thioether (SMe), cyano (CN), double bonds (C=C), pyridinyl, and quinolyl were well tolerated in this transformation. Notably, the double bonds can survive in this protocol makes it different from the strategy of using olefin and stoichiometric silanes as the susbstrates25.
Aryl boronic esters could also be employed as the coupling partner to deliver the migratory coupling products (Fig. 3a). It is noteworthy that 3,5-disubstituted aryl boronic esters can be prepared directly from the corresponding arenes by a highly efficient iridium-catalyzed borylation47.
Fig. 3. Expansion of substrate scope.
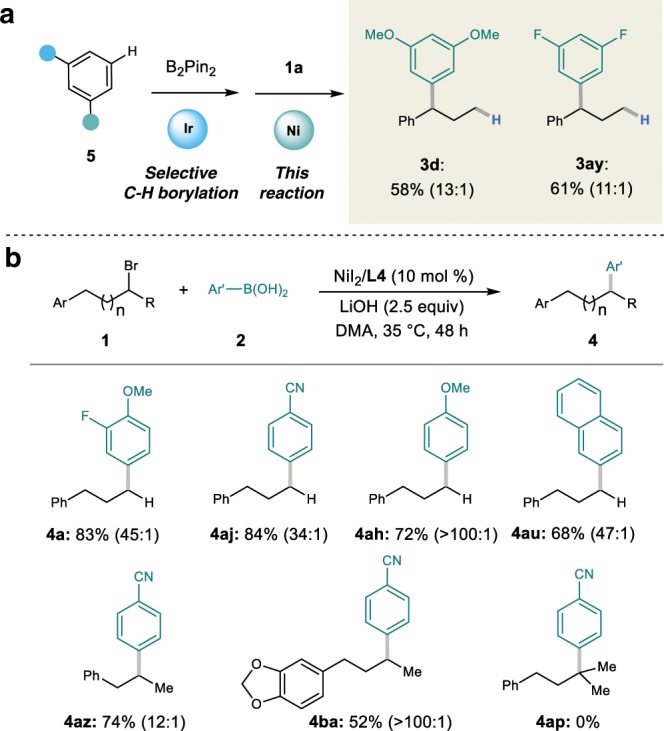
a Migratory Suzuki–Miyaura cross-coupling of aryl borate. b Original-site Suzuki–Miyaura cross-coupling.
In addition, the scope of the original-site Suzuki–Miyaura cross-coupling of alkyl bromides with aryl boronic acids was also studied. As shown in Fig. 3b, the electronic property of the aryl boronic acids did not affect the reactivity. Both primary and secondary alkyl bromides could successfully yield the corresponding cross-coupling products. However, tertiary alkyl bromide shows no reactivity. It should be noted that the migratory benzylic cross-coupling products were also detected in most cases, but no terminal coupling products were observed in the reactions with secondary alkyl bromides due to the limitation of our instruments.
Mechanism discussion
The application of nickel catalyst constitutes a great advance in the cross-coupling of alkyl electrophiles8,13. The nitrogen-based ligands enable nickel-catalyzed reactions prefer to go through single-electron transfer (SET) processes, which increases the difficulty in mechanism investigation. Although preliminary mechanistic studies towards nickel-catalyzed migratory cross-coupling have been done by Zhu’s24 and our group26, several important concerns are still unclear, e.g., the origin of regioselectivity, the electronic character of nickel catalyst in chain-walking process and why olefins can dissociate from the catalyst during chain-walking, etc. This homogenous, redox-neural cross-coupling reaction provides a good platform to obtain insights into the details of chain-walking, which would supply a rational basis for the further development of transformations involving nickel migration.
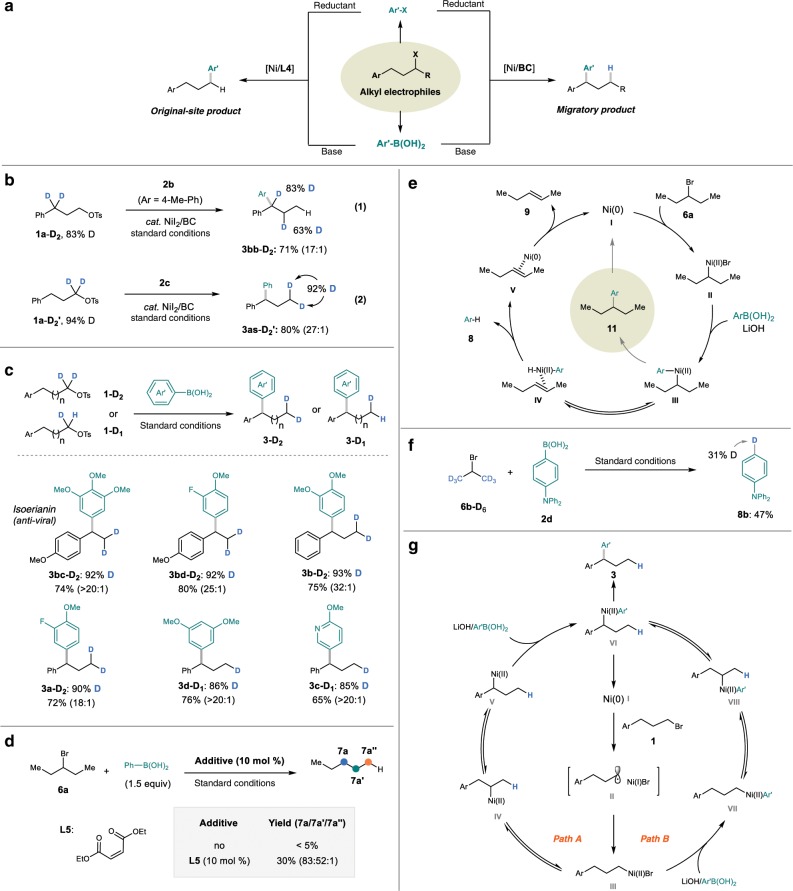
Moreover, this Suzuki–Miyaura reaction and our prior reductive condition reactions share many similarities, such as the same ligands, solvent, temperature, and the same selectivity (Fig. 4a). These similarities imply that these two type reactions share some commences in mechanism. The mechanistic study from the redox-neutral reaction can help us to understand the more complicated reductive ones.
Fig. 4. Mechanism discussion.
a Ni-catalyzed migratory cross-coupling of alkyl electrophiles with aryl coupling partners. b Experiments of D-labeled alkyl bromide with aryl group. c Synthesis of terminal D-labeled molecules. d Investigation the role of aryl group. e Proposed mechanism of D-labeled alkyl bromide without aryl group. f Experiments of D-labeled alkyl bromide without aryl group. g Proposed mechanism.
Even though significant achievements have been made in mechanistic studies on nickel-catalyzed cross-coupling of alkyl electrophiles with an array of metal reagents48,49 or another electrophiles14. However, reports on systematical mechanistic studies on the nickel-catalyzed Suzuki–Miyaura cross-coupling of alkyl electrophiles are still limited to date50,51.
As a result, after achieving this nickel-catalyzed migratory Suzuki–Miyaura cross-coupling reaction, we started to investigate its mechanism.
First of all, to gain additional insight into the nickel migration, deuterium-labeled tosylates were synthesized and examined in this reaction. First, the reaction of 1a-D2 with 2b furnished the D-migrated product 3bb-D2 with 100% benzylic deuterium retention and 63% D-incorporation at the homobenzylic position (Fig. 4b-1). These results suggest that the formation of migratory coupling products involve a key step of nickel chain-walking. Besides, the reaction of 1a-D2’ with 2c delivered the migratory product 3as-D2’ with 98% deuterium retention under the standard conditions (Fig. 4b-2). These observations reflect that this method can serve as a platform to synthesize terminal partially D-labeled compounds. Deuterium-labeled molecules have been drawing increasing attention in medicinal chemistry recently52,53, and partially D-labeled methyl groups (CDH2 and CD2H) were demonstrated to be useful in spectroscopic investigations and metabolic studies54. As a consequence, a series of 2D and 1D-labeled 1,1-diarylalkane derivatives were prepared from the readily accessible α-D substituted tosylates (Fig. 4c).
Next, only trace amount of cross-coupling products were detected under the standard conditions when an alkyl bromide without an aryl group substituent (6a) was employed (Fig. 4d). This finding inspires us to investigate the role of the aryl group on the alkyl electrophiles. Further study found that using an electron-deficient olefin L5 as ligand, the yield of the cross-coupling products could be improved to 30% (Fig. 4d). These results are agreed with the fact that reductive elimination from Ni(II) species is difficult, which can be promoted by the electron-deficient olefin ligands32,55. Therefore, the aryl group plays a significant role of promoting the reductive elimination.
Consequently, a catalytic cycle involving an alkyl-Ni(II)-Ar intermediate is proposed to rationalize this Suzuki–Miyaura reaction. As depicted in Fig. 4e, inefficient reductive elimination of the Ni(II) complex III leads to the formation of β-H elimination complex IV. Subsequent reductive elimination generates an arene (8) and an olefin (9).
The corresponding deborylation and olefin side products in the reaction of 6a with 2c were detected under our optimal conditions. A deuterium-labeled isopropyl bromide (6b-D6) was examined under the standard conditions, the deborylation product (8b) was isolated in 47% yield with 31% D-incorporation (Fig. 4f)56,57. These results suggest that the hydrogen atom of the deborylation product is partly from the alkyl electrophile, which agrees with the proposed catalytic cycle (Fig. 4e) and reveals that the aryl group of the alkyl electrophile plays the role of promoting the carbon–carbon bond formation at benzylic position.
In addition, several reports demonstrated the possibility of reductive elimination from the Bn-Ni(II)-Ar intermediates to construct diarylalkanes58, and a very recent report from the Fu group experimentally established the feasibility of Ni(II) chain-walking59. Based on these results, we proposed a catalytic cycle for the Ni-catalyzed migratory Suzuki–Miyaura reaction. As illustrated in Fig. 4g, the reaction is initiated by a Ni(0) species (I), which generates an alkyl-nickel(II) intermediate (III) by stepwise oxidative addition. There are two possibilities in the following steps: (A) a rapid chain-walking occurs to form a thermodynamically stable benzylic nickel(II) complex (V), and following transmetalation with a boronic acid to generate a benzyl-Ni(II)-Ar intermediate (VI); (B) transmetalation occurs before chain-walking, leading to the formation of the intermediate VI as well. Finally, the desired products 3 are produced from VI via reductive elimination.
These two pathways differ in the order of transmetalation and chain-walking. In order to differentiate these two pathways and to obtain details on the catalytic cycle, kinetic studies were conducted next.
Kinetic studies
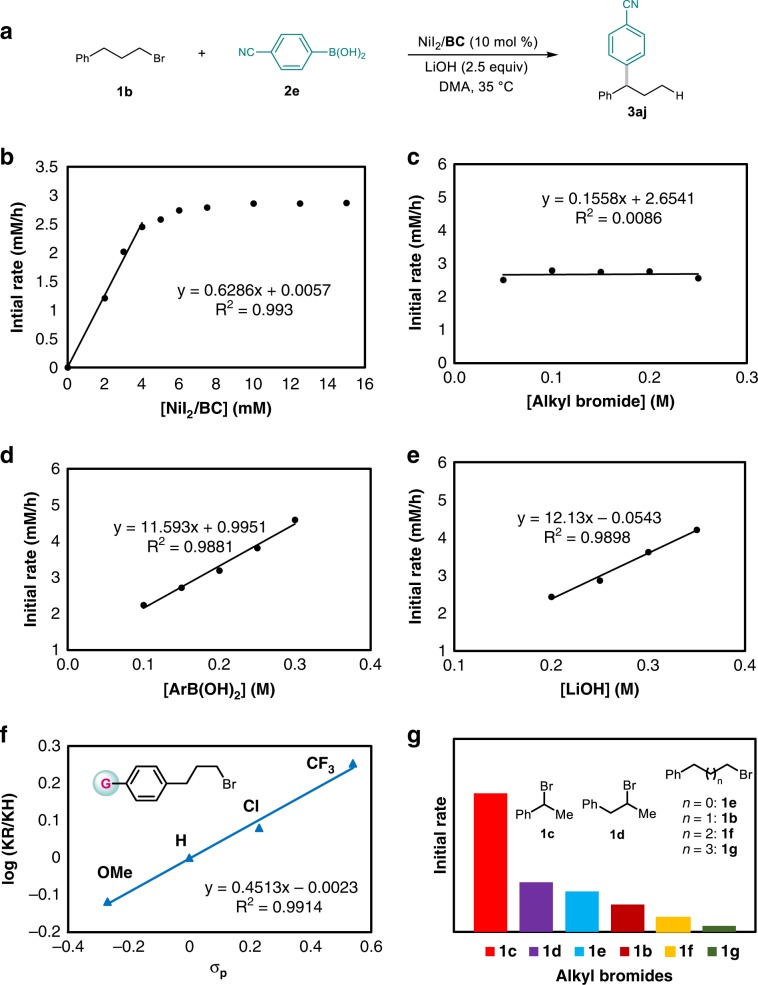
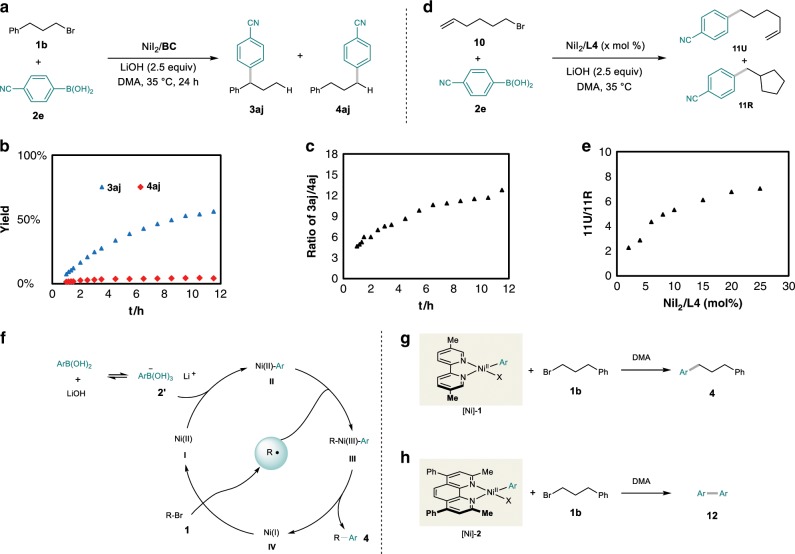
To simplify the studies, we chose the reaction of alkyl bromide 1b with 4-cyanophenylboronic acid (2e) as a model reaction (Fig. 5a), which does not require the addition of silane. The dependence of the initial rate on nickel catalyst, alkyl bromide, boronic acid, and base concentration was evaluated in each case by monitoring the formation of product by gas chromatography. As illustrated in Fig. 5, a complex kinetic picture with first order in Ni at low catalyst loading and saturation kinetics at higher loadings was observed (Fig. 5b), possibly indicative of off-cycle pathways, which is also observed in a previous report. 60 The reaction exhibits a zeroth-order dependence on the concentration of alkyl bromide (Fig. 5c), and first-order dependence on the concentration of both aryl boronic acid (Fig. 5d) and base (Fig. 5e).
Fig. 5. Kinetic profiles.
a Model reaction. b Reaction order in catalyst concentration. c Reaction order in alkyl bromide concentration. d Reaction order in aryl boronic acid concentration. e Reaction order in base concentration. f Hammett plots of alkyl bromides. g Initiate reaction rate of different alkyl bromides.
The above results reveal that: (1) the oxidative addition of alkyl bromide is a rapid and non-rate-determining step. A similar phenomenon was disclosed by Lei in the Ni-catalyzed oxidative coupling of arylzinc reagents61. (2) the transmetalation is the rate-determining step (RDS).
To further validate reaction pathway, relative rate constants were determined for the reaction of 2e with different (p-RC6H4)(CH2)3Br (R=MeO, H, Cl, and CF3). The reactions with different alkyl bromides were conducted independently. To our delight, plotting the log(kR/kH) values against the Hammett σp constants resulted in a satisfactory linear correlation (Fig. 5f), and the electronic properties of the aryl groups on alkyl electrophiles indeed affect the reactivity. A positive ρ value (ρ = +0.45) was detected, indicating that more electron-deficient benzyl-Ni(II) species show faster reactivity. This finding suggest the transmetalation occurs with a benzyl-Ni intermediate, which agrees with that the nickel chain-walking occurs prior to transmetalation, which is consistent with the path A, and rules out the path B (Fig. 4g).
Finally, the initiate rates of different alkyl bromides were also investigated (Fig. 5g). Among of these, secondary benzyl bromide (1c) exhibits the fastest rate, secondary unactivated alkyl bromide (1d) shows faster rate than the corresponding primary one (1e), which indicates the C–Br bond cleavage involving a SET process. Moreover, the alkyl bromide with longer carbon chain exhibits slower rate (compare 1e with 1b, 1f and 1g). These results indicate that chain-walking also affects the RDS, which is also agreed with path A and inconsistent with path B.
Computational studies
Extensive computational studies have been done on chain-walking process in the palladium-catalyzed reactions62, in contrast, very limited reports on the nickel-catalyzed ones63. In order to get more details on the nickel chain-walking process of this reaction, density functional theory (DFT) calculations were carried out.
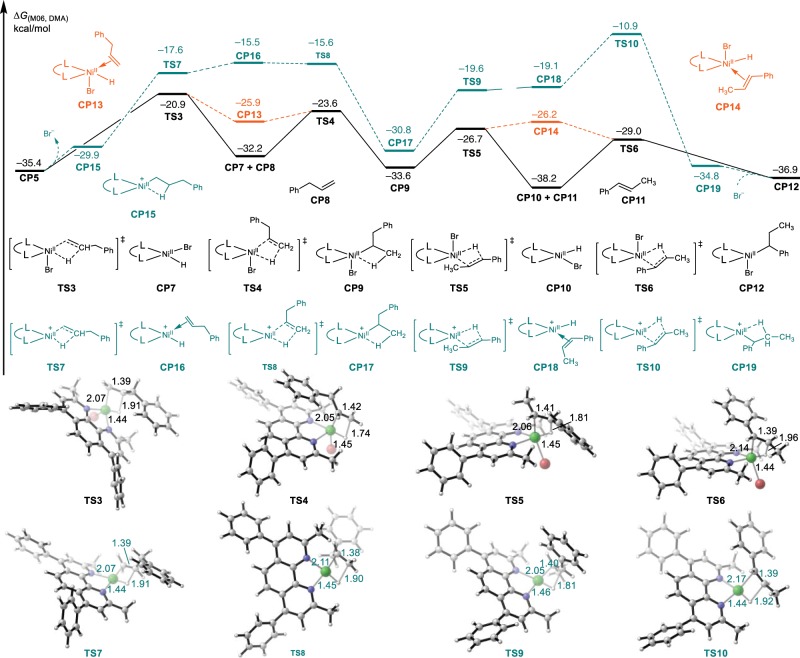
With the presence of BC ligand and reductant additive of Et3SiH species, the catalyst precursor NiI2 would generate Ni0 species by ligand exchange and reduction. We first calculated the oxidative addition of alkyl halides with Ni(0) species64,65. As shown in Fig. 6, active catalyst Ni(0) species CP1 was chosen as the relative zero point for the free energy profiles, which is coordinated by a BC ligand and one solvent molecule (N, N-dimethylacetamide). Subsequent ligand exchange between the alkyl bromide and solvent molecule results in the formation of an intermediate CP2, with a large energy releasing (13.6 kcal/mol). The homolytic C–Br bond dissociation via TS1 generates a 3-phenylpropyl radical CP4 and an open-shell bromonickel(I) intermediate CP3. The radical rebounds to the nickel intermediate to produce a 3-phenylpropylnickel(II) intermediate CP5. The oxidative addition via a three-membered ring transition state TS2, needs to overcome a barrier of 22.0 kcal/mol, 7.5 kcal/mol higher than that of open-shell Ni(0)/Ni(I) pathway. The above results indicate that the alkyl-Ni(II) complex formation via a SET pathway is favorable.
Fig. 6. The calculated reaction energy profile of alkyl-Ni(II) complex formation steps.
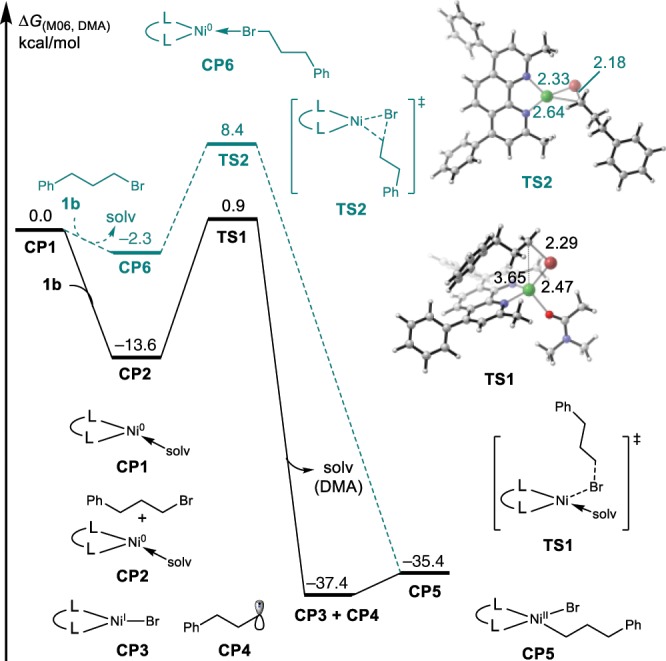
Energy values are given in kcal/mol and represent the relative free energies calculated by the M06 method in N,N-dimethylacetamide. The values of bond length are given in ångstrom.
After the formation of 3-phenylpropylnickel(II) complex, the following chain-walking process by iterative β-hydride elimination and migratory insertion were taken into account. As illustrated in Fig. 7, the first β-hydride elimination process via TS3 generates a planar Ni(II)-H species CP7 and allylbenzene (CP8), which can insert to Ni–H bond to form a secondary C–Ni(II) species via TS4. Notably, the relative free energy of allylbenzene coordinated Ni(II) species CP13 is 6.3 kcal/mol higher than CP7, which suggests that the olefin could dissociate from the nickel complex during the chain-walking process. Subsequent iterative β-hydride elimination and migratory insertion through TS5 and TS6 finally generates a 1-phenylpropylnickel(II) intermediate CP12. The calculated energy profile shows that the conversion of CP5 to CP12 is a reversible process: the highest energy barrier is first β-hydride elimination (14.5 kcal/mol) in the forward step (from CP5 to CP12), and last alkene insertion (16.0 kcal/mol) in the reverse step (from CP12 to CP5), with the same transition state TS3. Both the above two barriers are lower than that of transmetalation step (Fig. 8a, from CP21 to CP25 via the transition state TS13, with a barrier of 22.0 kcal/mol).
Fig. 7. The calculated reaction energy profile of nickel chain-walking process.
Energy values are given in kcal/mol and represent the relative free energies calculated by the M06 method in N, N-dimethylacetamide.
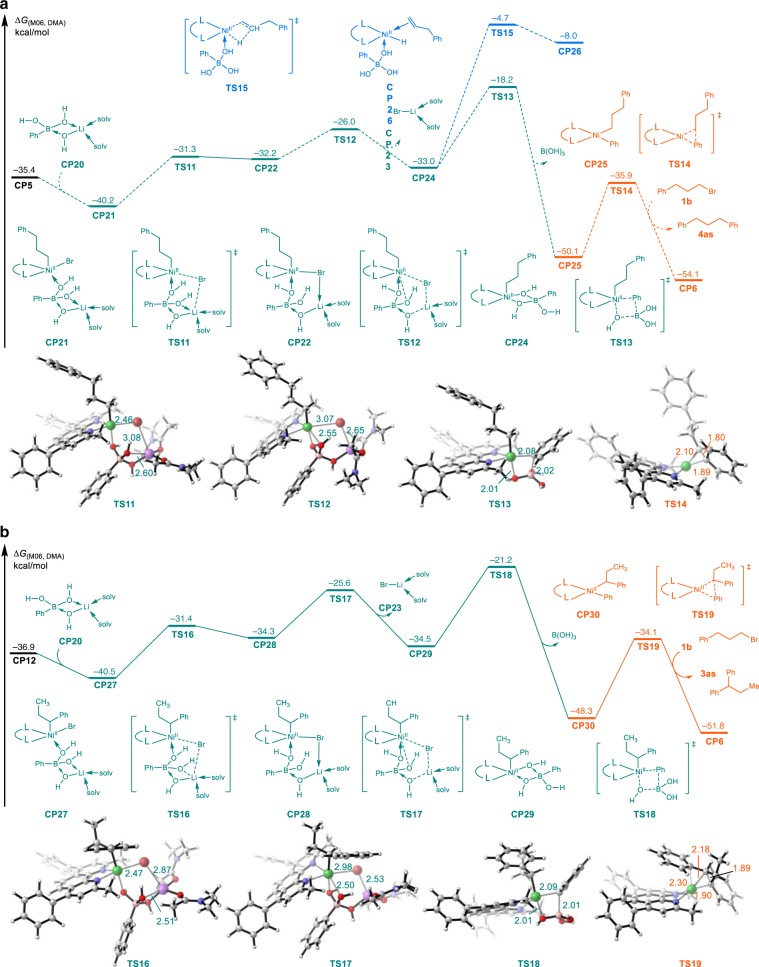
Fig. 8. Calculated reaction energy profile.
a Chain-walking and original-site product formation process. Energy values are given in kcal/mol and represent the relative free energies calculated by the M06 method in N, N-dimethylacetamide. The values of bond length are given in ångstrom. b migratory product formation process. Energy values are given in kcal/mol and represent the relative free energies calculated by the M06 method in N, N-dimethylacetamide. The values of bond length are given in ångstrom.
In addition, the cationic tetracoordinated nickel chain-walking process was also considered. In the cationic process, the initial state and final state (from CP5 to CP12) are same as the ones in charge neutral process. The highest energy barrier changed to be the last alkene insertion (24.5 kcal/mol) in the forward process (from CP5 to CP12) and the first β-hydride elimination (26.0 kcal/mol) in the reverse process (from CP12 to CP5), with the same transition state TS10, which are higher than that in the neutral chain-walking process. Therefore, the cationic nickel chain-walking process is determined to be unfavorable. Energy profile of subsequent transmetalation and C–C bond formation is shown in Fig. 8b. Considering charge neutralization and coordination number, we choose the lithium phenylboronate CP20, which is generated via the coordination of hydroxyl to PhB(OH)2, as active boron reagents (See Supplementary Fig. 55 for details)65,66. Coordination between 1-phenylpropylnickel(II) intermediate CP12 and lithium phenylboronate compounds CP20 leads to the formation of CP27, which is able to generate a neutral Ni(II) complex CP29 via a stepwise process involving transition states TS16 (O–Li cleavage and coordinate Br–Li bond formation) and TS17 (O–Ni formation and Ni–Br cleavage). Subsequent intramolecular transmetalation through a four-membered ring transition state TS18 generates a phenyl-Ni(II)-benzyl intermediate CP30, which is exothermic by 27.0 kcal/mol. The energy barrier of transmetalation after chain-walking is 19.3 kcal/mol, which is 3.0 kcal/mol lower than that of direct transmetalation via transition state TS13. Finally, reductive elimination releases migratory product 3as from CP30, via transition state TS19 and with an energy barrier of 14.2 kcal/mol. The RDS is intramolecular transmetalation and the overall activation energy is 19.3 kcal/mol.
First of all, we found that the formation of the original-site coupling product was not concurrent with the migratory product (Fig. 9a, b), in other words, the regioselectivity varied during the reaction conduction (Fig. 9c). This finding reveals that the formation of the two products is probably from different catalytic cycles. Next, a radical probe experiment was carried out. The reaction of 6-bromo-1-hexene (10) with 2e was studied in our reaction conditions. We found that the ratio of uncyclized cross-coupling product (11U) with the 5-exo-cyclized coupling product (11R) gradually increased along with the catalyst loading (Fig. 9d,e). These results indicate that a radical chain process is operating in this transformation, which is in accordance with the reductive cross-electrophiles coupling reactions34.
Fig. 9. The study of original-site products.
a Model reaction. b Time course of product 3aj and 4aj. c Regioselectivity of migratory product with original-site product. d radical clock experiment. f Effect of catalyst loading on the ratio of 11U/11R. f Proposed mechanism of original-site product. g Proposed process on reductive cross-coupling by Ni/L4. h Proposed process on reductive cross-coupling by Ni/BC.
Collectively, a catalytic cycle involving a radical chain process is proposed for the original-site Suzuki–Miyaura cross-coupling reaction. As depicted in Fig. 9f, the reaction is initiated by a Ni(II) species (I), and an aryl-Ni(II) species (II) is generated after transmetalation. The complex II reacts with an alkyl radical to generate a Ni(III) intermediate (III), which delivers the product (4) and a Ni(I) species (IV) via reductive elimination. The Ni(I) species IV reacts with an alkyl electrophile (1) to yield the alkyl radical and regenerate the Ni(II) catalyst.
Different ligands enable different regioselectivity, a phenomenon which also observed in our previous reductive conditions26,27. The mechanism of reductive cross-electrophile coupling of alkyl halides with aryl halides has been thoroughly studied by Weix and coworkers34. They have demonstrated that Ar–Ni(II) complex was able to react with the alkyl halide, initiating a radical chain process. Thereby, we think the reaction of Ni(II) complex [Ni]-1 with the alkyl bromide (1b) is also the initiation of the radical chain process in our reaction (Fig. 9g). Moreover, in our prior reductive conditions, the electron-deficient aryl bromides could not efficiently yield the cross-coupling products, but homocoupling products (12) of the aryl bromides were observed instead. We conjecture that Ar–Ni(II) complexes [Ni]-2 could also generate in the reactions with BC ligand, but [Ni]-2 with 1b was not able to trigger the radical chain, thus leading to the formation of 12 (Fig. 9h). In other words, the selectivity of the products derives from the reactivity of the nickel complexes towards the alkyl electrophiles. The less steric 5,5′-dmbpy (L4) is favorable for subsequent SET. Therefore, L4 coordinated [Ni]-1 is easier to generate the active Ni(III) compared with BC coordinated [Ni]-2. Instead, the Ni(0)/Ni(II) catalytic cycle operates in the reaction with BC as the ligand. If a stronger oxidant is used, it is supposed to overcome such steric effects and trigger the SET process. This is probably the reason that when more reactive alkyl iodides are used, poor regioselectivity was detected (Fig. 2, 3as). Thus, the selectivity of the final products is determined by the ligand and the reactivity of the substrates.
Discussion
In summary, we have developed a nickel-catalyzed migratory Suzuki–Miyaura cross-coupling reaction, featuring high benzylic or allylic selectivity. This method offers a protocol to rapidly access the diarylalkanes and allylbenzenes from a series of aryl/vinyl-bearing alkyl electrophiles and sp2-C boron-based nucleophiles. This approach is characterized by a broad substrate scope and excellent regioselectivity. In addition, the more challenging, but especially useful unactivated alkyl chlorides have been successfully used in the migratory cross-coupling. More potential applications in synthesis are addressed by the combination with selective borylation of C–H bonds. Furthermore, this protocol can serve as a platform to access terminal partially deuterium-labeled diarylmethine pharmacophores from the readily accessible precursors.
Mechanistically, the results of Hammett plots suggest that the transmetalation of boronic acid is with a benzyl-Ni(II) species. The DFT calculation suggest that oxidative addition of alkyl halides with Ni(0) species is prone to involve an SET pathway, the neutral nickel complex is more readily chain-walking than the cationic one, and the olefin is readily dissociated from the catalyst during chain-walking. In addition, the studies towards the original-site cross-coupling reveal that the formation of the product is likely to involve a radical chain process. Both Ni(0)/Ni(II) catalytic cycle and radical process are present in these reactions, but the selectivity of the products is controlled by the catalyst and the substrate. We believe this study will greatly advance the future studies toward transformations involving nickel migration.
Methods
General procedure
Under N2 atmosphere, into an oven-dried 10 mL reaction tube equipped with a magnetic stir bar and sealed with a rubber stopper sequentially added NiI2 (15.6 mg, 0.05 mmol, 10 mol%), BC (18.0 mg, 0.05 mmol, 10 mol%), anhydrous DMA (4 mL), and Et3SiH (20 μL, 0.13 mmol, 25 mol%). The mixture was stirred at 35 °C for 30 min, then TBAB (161.2 mg, 0.5 mmol, 1.0 equiv), LiOH (29.9 mg, 1.25 mmol, 2.5 equiv), alkyl tosylate (0.5 mmol, 1 equiv), and aryl boronic acid (0.75 mmol, 1.5 equiv) were added in this order. The resulting mixture was stirred at 35 °C (if aryl boronic pinacol ester was used, stirred at 60 °C; if Alkyl bromide was used, TBAB was not need) and monitored by GC until the alkyl tosylate disappeared. After the reaction was complete, the mixture was quenched by saturated brine and extracted with ethyl acetate (20 mL × 3). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The resulting crude product was separated on a silica gel column affording the cross-coupling product.
Supplementary information
Acknowledgements
We thank Profs Qianghui Zhou, Wen-Bo Liu, and Aiwen Lei at Wuhan University for lending lab space and sharing the basic instruments. We are grateful for the financial support from National Natural Science Foundation of China (21702151, 21871211, 21822303, and 21703159) and the Fundamental Research Funds for Central Universities (2042019kf0208, 2018CDXZ0002 and 2018CDPTCG0001/4). We thank Dr Joshua Buss at University of Wisconsin-Madison for his very helpful suggestions on the manuscript preparation.
Source data
Author contributions
Yuqiang Li designed and carried out most of the chemical reactions and analyzed the data. Long Peng, Yangyang Li, Binzhi Zhao, Wang Wang, Hailiang Pang, and Yi Deng supported the design and performance of synthetic experiments. Guoyin Yin designed and supervised the project. Yixin Luo, Ruopeng Bai, and Yu Lan conducted the calculations. Yuqiang Li, Yixin Luo, Ruopeng Luo, Yu Lan, and Guoyin Yin wrote the manuscript. All authors discussed the results and commented on the manuscript, and approved the final version of the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information file. The raw data underlying Supplementary Figs. 3 with 55 are provided as a Supplementary Figures file. The raw data underlying Supplementary Tables 1 with 2 are provided as a Supplementary Tables file. The raw data for Absolute Calculation Energies, Enthalpies, and Free Energies are provided as a Source Data file. Additional data are available from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Donghui Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Lan, Email: lanyu@cqu.edu.cn.
Guoyin Yin, Email: yinguoyin@whu.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-14016-1.
References
- 1.Liu C, Zhang H, Shi W, Lei A. Bond formations between two nucleophiles: transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 2011;111:1780. doi: 10.1021/cr100379j. [DOI] [PubMed] [Google Scholar]
- 2.Johansson Seechurn CC, Kitching MO, Colacot TJ, Snieckus V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 nobel prize. Angew. Chem. Int. Ed. 2012;51:5062. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- 3.Colacot Thomas., editor. New Trends in Cross-Coupling. Cambridge: Royal Society of Chemistry; 2014. [Google Scholar]
- 4.Knappke CE, et al. Reductive cross-coupling reactions between two electrophiles. Chem. Eur. J. 2014;20:6828. doi: 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Dai Y, Gong H. Nickel-catalyzed reductive couplings. Top. Curr. Chem. 2016;374:43. doi: 10.1007/s41061-016-0042-2. [DOI] [PubMed] [Google Scholar]
- 6.Terao J, Kambe N. Cross-coupling reaction of alkyl halides with grignard reagents catalyzed by Ni, Pd, or Cu complexes with π-carbon ligand(s) Acc. Chem. Res. 2008;41:1545. doi: 10.1021/ar800138a. [DOI] [PubMed] [Google Scholar]
- 7.Tollefson EJ, Hanna LE, Jarvo ER. Stereospecific nickel-catalyzed cross-coupling reactions of benzylic ethers and esters. Acc. Chem. Res. 2015;48:2344. doi: 10.1021/acs.accounts.5b00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki T, Kambe N. Ni-catalyzed C-C couplings using alkyl electrophiles. Top. Curr. Chem. 2016;374:66. doi: 10.1007/s41061-016-0067-6. [DOI] [PubMed] [Google Scholar]
- 9.Kaga A, Chiba S. Engaging radicals in transition metal-catalyzed cross-coupling with alkyl electrophiles: Recent advances. ACS Catal. 2017;7:4697. doi: 10.1021/acscatal.7b01405. [DOI] [Google Scholar]
- 10.Netherton MR, Fu GC. Nickel-catalyzed cross-couplings of unactivated alkyl halides and pseudohalides with organometallic compounds. Adv. Synth. Catal. 2004;346:1525. doi: 10.1002/adsc.200404223. [DOI] [Google Scholar]
- 11.Frisch AC, Beller M. Catalysts for cross-coupling reactions with non-activated alkyl halides. Angew. Chem. Int. Ed. 2005;44:674. doi: 10.1002/anie.200461432. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph A, Lautens M. Secondary alkyl halides in transition-metal-catalyzed cross-coupling reactions. Angew. Chem. Int. Ed. 2009;48:2656. doi: 10.1002/anie.200803611. [DOI] [PubMed] [Google Scholar]
- 13.Hu X. Nickel-catalyzed cross coupling of non-activated alkyl halides: a mechanistic perspective. Chem. Sci. 2011;2:1867. doi: 10.1039/c1sc00368b. [DOI] [Google Scholar]
- 14.Weix DJ. Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 2015;48:1767. doi: 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Wang S, Xue W, Gong H. Nickel-catalyzed reductive coupling of aryl bromides with tertiary alkyl halides. J. Am. Chem. Soc. 2015;137:11562. doi: 10.1021/jacs.5b06255. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, et al. Ni-catalyzed reductive coupling of electron-rich aryl iodides with tertiary alkyl halides. J. Am. Chem. Soc. 2018;140:14490. doi: 10.1021/jacs.8b09473. [DOI] [PubMed] [Google Scholar]
- 17.Vasseur A, Bruffaerts J, Marek I. Remote functionalization through alkene isomerization. Nat. Chem. 2016;8:209. doi: 10.1038/nchem.2445. [DOI] [PubMed] [Google Scholar]
- 18.Sommer H, Juliá-Hernández F, Martin R, Marek I. Walking metals for remote functionalization. ACS Cent. Sci. 2018;4:153. doi: 10.1021/acscentsci.8b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renaudat A, et al. Palladium-catalyzed β arylation of carboxylic esters. Angew. Chem. Int. Ed. 2010;49:7261. doi: 10.1002/anie.201003544. [DOI] [PubMed] [Google Scholar]
- 20.Baudoin O. Selectivity control in the palladium-catalyzed cross-coupling of alkyl nucleophiles. Chimia. 2016;70:768. doi: 10.2533/chimia.2016.768. [DOI] [PubMed] [Google Scholar]
- 21.Dupuy S, Zhang K-F, Goutierre A-S, Baudoin O. Terminal-selective functionalization of alkyl chains by regioconvergent cross-coupling. Angew. Chem. Int. Ed. 2016;55:14793. doi: 10.1002/anie.201608535. [DOI] [PubMed] [Google Scholar]
- 22.Tasker SZ, Standley EA, Jamison TF. Recent advances in homogeneous nickel catalysis. Nature. 2014;509:299. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen-Müller, D., Sahoo, B., Sun, S. Z. & Martin, R. Tackling remote sp3 C-H functionalization via Ni-catalyzed “chain-walking” reactions. Isr. J. Chem. 59, 1 (2019).
- 24.Chen F, et al. Remote migratory cross-electrophile coupling and olefin hydroarylation reactions enabled by in situ generation of NiH. J. Am. Chem. Soc. 2017;139:13929. doi: 10.1021/jacs.7b08064. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Cai Y, Zhu S. Mild and regioselective benzylic C-H functionalization: Ni-catalyzed reductive arylation of remote and proximal olefins. J. Am. Chem. Soc. 2017;139:1061. doi: 10.1021/jacs.6b11962. [DOI] [PubMed] [Google Scholar]
- 26.Peng L, et al. Ligand-controlled nickel-catalyzed reductive relay cross-coupling of alkyl bromides and aryl bromides. ACS Catal. 2017;8:310. doi: 10.1021/acscatal.7b03388. [DOI] [Google Scholar]
- 27.Peng L, Li Z, Yin G. Photochemical nickel-catalyzed reductive migratory cross-coupling of alkyl bromides with aryl bromides. Org. Lett. 2018;20:1880. doi: 10.1021/acs.orglett.8b00413. [DOI] [PubMed] [Google Scholar]
- 28.Lu X, et al. Practical carbon–carbon bond formation from olefns through nickel-catalyzed reductive olefn hydrocarbonation. Nat. Commun. 2016;7:11129. doi: 10.1038/ncomms11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schley ND, Fu GC. Nickel-catalyzed Negishi arylations of propargylic bromides: a mechanistic investigation. J. Am. Chem. Soc. 2014;136:16588. doi: 10.1021/ja508718m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao C, Jia X, Wang X, Gong H. Ni-catalyzed reductive coupling of alkyl acids with unactivated tertiary alkyl and glycosyl halides. J. Am. Chem. Soc. 2014;136:17645. doi: 10.1021/ja510653n. [DOI] [PubMed] [Google Scholar]
- 31.Knochel P. Ni(II)-catalyzed cross-coupling between polyfunctional arylzinc derivatives and primary alkyl iodides. J. Am. Chem. Soc. 1998;120:11186. doi: 10.1021/ja982520o. [DOI] [Google Scholar]
- 32.Giovannini R, Studemann T, Devasagayaraj A, Dussin G, Knochel P. New efficient nickel-catalyzed cross-coupling reaction between two Csp3 centers. J. Org. Chem. 1999;64:3544. doi: 10.1021/jo982317b. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Zou L, Bai R, Lan Y. Ni(I)–Ni(III) vs. Ni(II)–Ni(IV): Mechanistic study of Ni-catalyzed alkylation of benzamides with alkyl halides. Org. Chem. Front. 2018;5:615. doi: 10.1039/C7QO00850C. [DOI] [Google Scholar]
- 34.Biswas S, Weix DJ. Mechanism and selectivity in nickel-catalyzed cross-electrophile coupling of aryl halides with alkyl halides. J. Am. Chem. Soc. 2013;135:16192. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaydou M, Moragas T, Juliá-Hernández F, Martin R. Site-selective catalytic carboxylation of unsaturated hydrocarbons with CO2 and water. J. Am. Chem. Soc. 2017;139:12161. doi: 10.1021/jacs.7b07637. [DOI] [PubMed] [Google Scholar]
- 36.Stokes BJ, Opra SM, Sigman MS. Palladium-catalyzed allylic cross-coupling reactions of primary and secondary homoallylic electrophiles. J. Am. Chem. Soc. 2012;134:11408. doi: 10.1021/ja305403s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stokes BJ, Bischoff AJ, Sigman MS. Pd(quinox)-catalyzed allylic relay suzuki reactions of secondary homostyrenyl tosylates via alkene-assisted oxidative addition. Chem. Sci. 2014;5:2336. doi: 10.1039/C4SC00602J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Z, Fu GC. Alkyl-alkyl Suzuki cross-coupling of unactivated secondary alkyl chlorides. Angew. Chem. Int. Ed. 2010;49:6676. doi: 10.1002/anie.201003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atack TC, Cook SP. Manganese-catalyzed borylation of unactivated alkyl chlorides. J. Am. Chem. Soc. 2016;138:6139. doi: 10.1021/jacs.6b03157. [DOI] [PubMed] [Google Scholar]
- 40.Borjesson M, Moragas T, Martin R. Ni-catalyzed carboxylation of unactivated alkyl chlorides with CO2. J. Am. Chem. Soc. 2016;138:7504. doi: 10.1021/jacs.6b04088. [DOI] [PubMed] [Google Scholar]
- 41.Rezazadeh S, Devannah V, Watson DA. Nickel-catalyzed C-alkylation of nitroalkanes with unactivated alkyl iodides. J. Am. Chem. Soc. 2017;139:8110. doi: 10.1021/jacs.7b04312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirchhoff JH, Netherton MR, Hills ID, Fu GC. Boronic acids: new coupling partners in room-temperature suzuki reactions of alkyl bromides. Crystallographic characterization of an oxidative-addition adduct generated under remarkably mild conditions. J. Am. Chem. Soc. 2002;124:13662. doi: 10.1021/ja0283899. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Fu GC. Suzuki cross-couplings of unactivated secondary alkyl bromides and iodides. J. Am. Chem. Soc. 2004;126:1340. doi: 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]
- 44.Malhotra B, Gandelman K, Sachse R, Wood N, Michel M. The design and development of fesoterodine as a prodrug of 5- hydroxymethyl tolterodine (5-Hmt), the active metabolite of tolterodine. Curr. Med. Chem. 2009;16:4481. doi: 10.2174/092986709789712835. [DOI] [PubMed] [Google Scholar]
- 45.Vanjari R, Singh KN. Utilization of methylarenes as versatile building blocks in organic synthesis. Chem. Soc. Rev. 2015;44:8062. doi: 10.1039/C5CS00003C. [DOI] [PubMed] [Google Scholar]
- 46.Rossi R, Bellina F, Lessi M. Selective palladium-catalyzed Suzuki-Miyaura reactions of polyhalogenated heteroarenes. Adv. Synth. Catal. 2012;354:1181. doi: 10.1002/adsc.201100942. [DOI] [Google Scholar]
- 47.Murphy JM, Liao X, Hartwig JF. Meta halogenation of 1,3-disubstituted arenes via iridium-catalyzed arene borylation. J. Am. Chem. Soc. 2007;129:15434. doi: 10.1021/ja076498n. [DOI] [PubMed] [Google Scholar]
- 48.Breitenfeld J, Ruiz J, Wodrich MD, Hu X. Bimetallic oxidative addition involving radical intermediates in nickel-catalyzed alkyl-alkyl kumada coupling reactions. J. Am. Chem. Soc. 2013;135:12004. doi: 10.1021/ja4051923. [DOI] [PubMed] [Google Scholar]
- 49.Diccianni JB, Katigbak J, Hu C, Diao T. Mechanistic characterization of (xantphos)Ni(I)-mediated alkyl bromide activation: oxidative addition, electron transfer, or halogen-atom abstraction. J. Am. Chem. Soc. 2019;141:1788. doi: 10.1021/jacs.8b13499. [DOI] [PubMed] [Google Scholar]
- 50.Dudnik AS, Fu GC. Nickel-catalyzed coupling reactions of alkyl electrophiles, including unactivated tertiary halides, to generate carbon-boron bonds. J. Am. Chem. Soc. 2012;134:10693. doi: 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Z, Wilsily A, Fu GC. Stereoconvergent amine-directed alkyl-alkyl suzuki reactions of unactivated secondary alkyl chlorides. J. Am. Chem. Soc. 2011;133:8154. doi: 10.1021/ja203560q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atzrodt J, Derdau V, Kerr WJ, Reid M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. 2018;57:1758. doi: 10.1002/anie.201704146. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, et al. General and practical potassium methoxide/disilane-mediated dehalogenative deuteration of (hetero)arylhalides. J. Am. Chem. Soc. 2018;140:10970. doi: 10.1021/jacs.8b07597. [DOI] [PubMed] [Google Scholar]
- 54.Xia H-M, Zhang F-L, Ye T, Wang Y-F. Selective α-monomethylation by an amine-borane/N,N-dimethylformamide system as the methyl source. Angew. Chem. Int. Ed. 2018;57:11770. doi: 10.1002/anie.201804794. [DOI] [PubMed] [Google Scholar]
- 55.Huang C-Y, Doyle AG. Nickel-catalyzed Negishi alkylations of styrenyl aziridines. J. Am. Chem. Soc. 2012;134:9541. doi: 10.1021/ja3013825. [DOI] [PubMed] [Google Scholar]
- 56.Verheyen T, et al. Ketone synthesis by a nickel-catalyzed dehydrogenative cross-coupling of primary alcohols. J. Am. Chem. Soc. 2019;141:6869. doi: 10.1021/jacs.9b03280. [DOI] [PubMed] [Google Scholar]
- 57.Yi Y-Q-Q, et al. Nickel-catalyzed C-N bond reduction of aromatic and benzylic quaternary ammonium triflates. Chem. Commun. 2016;52:10894. doi: 10.1039/C6CC04531F. [DOI] [PubMed] [Google Scholar]
- 58.KC S, et al. Ni-catalyzed regioselective alkylarylation of vinylarenes via C(sp3)-C(sp3)/C(sp3)-C(sp2) bond formation and mechanistic studies. J. Am. Chem. Soc. 2018;140:9801. doi: 10.1021/jacs.8b05374. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Yin H, Fu GC. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature. 2018;563:379. doi: 10.1038/s41586-018-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ni S, et al. A radical approach to anionic chemistry: synthesis of ketones, alcohols, and amines. J. Am. Chem. Soc. 2019;141:6726. doi: 10.1021/jacs.9b02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin L, Xin J, Huang Z, He J, Lei A. Transmetalation is the rate-limiting step: quantitative kinetic investigation of nickel-catalyzed oxidative coupling of arylzinc reagents. J. Am. Chem. Soc. 2010;132:9607. doi: 10.1021/ja1045296. [DOI] [PubMed] [Google Scholar]
- 62.Xu L, et al. Mechanism, reactivity, and selectivity in palladium-catalyzed redox-relay Heck arylations of alkenyl alcohols. J. Am. Chem. Soc. 2014;136:1960. doi: 10.1021/ja4109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapat A, Sperger T, Guven S, Schoenebeck F. E-olefins through intramolecular radical relocation. Science. 2019;363:391. doi: 10.1126/science.aav1610. [DOI] [PubMed] [Google Scholar]
- 64.de Aguirre A, Funes-Ardoiz I, Maseras F. Four oxidation states in a single photoredox Ni-based catalytic cycle: a computational study. Angew. Chem. Int. Ed. 2019;58:3898. doi: 10.1002/anie.201814233. [DOI] [PubMed] [Google Scholar]
- 65.Omer HM, Liu P. Computational study of Ni-catalyzed C-H functionalization: factors that control the competition of oxidative addition and radical pathways. J. Am. Chem. Soc. 2017;139:9909. doi: 10.1021/jacs.7b03548. [DOI] [PubMed] [Google Scholar]
- 66.Singh SKS, Sunoj RB. Aliphatic C(sp3)−H bond activation using nickel catalysis: mechanistic insights on regioselective arylation. J. Org. Chem. 2017;82:9619. doi: 10.1021/acs.joc.7b01672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information file. The raw data underlying Supplementary Figs. 3 with 55 are provided as a Supplementary Figures file. The raw data underlying Supplementary Tables 1 with 2 are provided as a Supplementary Tables file. The raw data for Absolute Calculation Energies, Enthalpies, and Free Energies are provided as a Source Data file. Additional data are available from the corresponding authors upon request.