Abstract
The Schizothoracinae fishes, endemic species in the Tibetan Plateau, are considered as ideal models for highland adaptation and speciation investigation. Despite several transcriptome studies for highland fishes have been reported before, the transcriptome information of Schizothoracinae is still lacking. To obtain comprehensive transcriptome data for Schizothoracinae, the transcriptome of a total of 183 samples from 14 representative Schizothoracinae species, were sequenced and de novo assembled. As a result, about 1,363 Gb transcriptome clean data was obtained. After the assembly, we obtain 76,602–154,860 unigenes for each species with sequence N50 length of 1,564–2,143 bp. More than half of the unigenes were functionally annotated by public databases. The Schizothoracinae fishes in this work exhibited diversified ecological distributions, phenotype characters and feeding habits; therefore, the comprehensive transcriptome data of those species provided valuable information for the environmental adaptation and speciation of Schizothoracinae in the Tibetan Plateau.
Subject terms: RNA sequencing, Ichthyology, Transcriptomics
| Measurement(s) | RNA • transcriptome • sequence_assembly • sequence feature annotation |
| Technology Type(s) | RNA sequencing • sequence assembly process • sequence annotation |
| Sample Characteristic - Organism | Schizothorax oconnori • Schizothorax lissolabiata • Schizopyge nukiangensis • Schizothorax plagiostomus • Schizothorax labiatus • Schizothorax davidi • Ptychobarbus kaznakovi • Gymnocypris namensis • Gymnocypris przewalskii • Gymnocypris eckloni • Gymnocypris selincuoensis • Schizopygopsis younghusbandi • Schizopygopsis pylzovi • Platypharodon extremus • Oxygymnocypris stewartii |
| Sample Characteristic - Environment | lake • drainage basin |
| Sample Characteristic - Location | Tibetan Plateau |
Machine-accessible metadata file describing the reported data: 10.6084/m9.figshare.11475294
Background & Summary
The Tibetan Plateau, the world’s largest and highest plateau, has unique geographical and climatic characteristics, such as the high altitude, dramatic difference in day and night temperature, strong solar radiation1. Due to the special geographical environment, many highland species that are distributed in and around the Tibetan Plateau have gradually formed unique characteristics to tolerate harsh living conditions during the long-term evolution2. The Schizothoracinae fishes, members of family Cyprinidae, are endemic to Asian highlands including 15 genera and ca. 100 species3. In China, more than 70 species, account for over 80% of the world’s Schizothoracine fishes, are mainly distributed in lakes and rivers of the Tibetan Plateau and adjacent areas4. According to the morphological characteristics, the Schizothoracine fishes can be divided into three groups: the primitive group, the specialized group and the highly specialized group5. Several researches on the morphology, archaeology and molecular biology of Schizothoracine fishes on the Tibetan Plateau have shown that there is close correlation between the species diversity and the uplift of the Tibetan Plateau6,7 and the morphological traits of Schizothoracine fishes is related with specific periods of geological evolution of the Tibetan Plateau such as pharyngeal teeth, scales and whiskers5. Therefore, the Schizothoracine fishes are considered as good model species for the investigations on highland adaptation and speciation. More genomic and transcriptome data are required to decipher the relationship of the speciation and the uplift of the Tibetan Plateau for the Schizothoracine fishes.
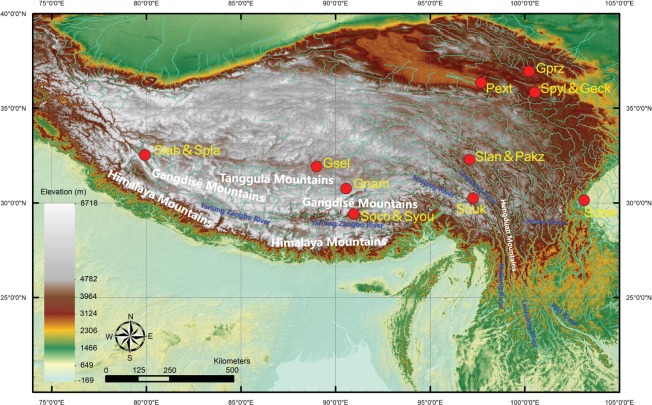
Recent advances in sequencing technologies have offered the opportunity to obtain the genomes of numerous highland animals, enabling us to better understand the adaptive evolution mechanism of highland fish species. So far, the vast majority of the genome researches on the environmental adaptation were performed on highland terrestrial animal (e.g., yak8 and Tibetan antelope9). Few study was reported on highland fish, especially for Schizothoracinae fishes. One of the major reasons was the complexity of the genome, such as high content of repeats and polyploidy10. Transcriptome sequencing is a good choice to construct the sequence dataset for transcribed genes in many polyploidy cases11. Despite several transcriptome analyses on highland adaptation have reported in Schizothoracine fishes before12–16, the species and tissues used for transcriptome sequencing were still limited. There is a great demand for more transcriptome sequencing data for the adaptation and evolution of Schizothoracine fishes in the Tibetan Plateau. In this work, we obtained and released a total of ∼1.36 Tb of high-quality transcriptome data for 183 samples of 14 representative Schizothoracine fish covering 5 genera from 6 drainage systems and 3 lakes in the Tibetan Plateau (Tables 1, 2 and Fig. 1). The distribution, ecological position and phenotype difference making the transcriptome of those Schizothoracine species invaluable genetic resources for the adaptation and speciation of endemic fish in the Tibetan Plateau.
Table 1.
Sample information for the species in the study.
| Genus | Species | Abbreviations | Geographic region | Drainage | Partial morphological feature | |
|---|---|---|---|---|---|---|
| Pairs of whiskers | Body scales | |||||
| Schizothorax | S. oconnori | Soco | Gongga, Tibet, China | YarlungZangbo River | 2 | small scale |
| S. lissolabiatus | Slis | Changdu, Tibet, China | Lancang River | 2 | small scale | |
| S. nukiangensis | Snuk | Bomi, Tibet, China | Nujiang River | 2 | small scale | |
| S. plagiostomus | Spla | Ali, Tibet, China | Shiquan River | 2 | small scale | |
| S. labiatus | Slab | Ali, Tibet, China | Shiquan River | 2 | small scale | |
| S. davidi | Sdav | Ganzi, Sichuan, China | Jinsha River | 2 | small scale | |
| Ptychobarbus | P. kaznakovi | Pkaz | Changdu, Tibet, China | Lancang River | 1 | moderate degeneration |
| Gymnocypris | G. namensis | Gnam | Bange, Tibet, China | Lake Namtso | 0 | absence |
| G. przewalskii | Gprz | Haibei, Qinghai, China | Lake Qinghai | 0 | absence | |
| G. eckloni | Geck | Xunhua, Qinghai, China | Yellow River | 0 | absence | |
| G. selincuoensis | Gsel | Bange, Tibet, China | Lake Siling Co | 0 | absence | |
| Schizopygopsis | S. younghusbandi | Syou | Lazi, Tibet, China | YarlungZangbo River | 0 | absence |
| S. pylzovi | Spyl | Xunhua, Qinghai, China | Yellow River | 0 | absence | |
| Platypharodon | P. extremus | Pext | Gonghe, Qinghai, China | Yellow River | 0 | absence |
Table 2.
Sample collected for the transcriptome sequencing.
| Species | The number of samples | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Liver | Spleen | Skin | Swim bladder | Gut | Eye | Gill | Kidney | Heart | Brain | Gonads | Vibrissa | Fat | Blood | Total | |
| S. oconnori | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| S. lissolabiatus | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | 14 |
| S. nukiangensis | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | 1 | 1 | — | 1 | 13 |
| S. plagiostomus | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | — | — | 1 | 12 |
| S. labiatus | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | 1 | — | 1 | 13 |
| S. davidi | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | 14 |
| P. kaznakovi | 1 | 1 | 1 | 1 | — | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | 13 |
| G. namensis | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | — | 1 | 13 |
| G. przewalskii | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | — | 13 |
| G. eckloni | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | 14 |
| G.selincuoensis | 1 | 1 | 1 | — | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | 1 | 13 |
| S. younghusbandi | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | — | — | 12 |
| S. pylzovi | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | — | — | 12 |
| P. extremus | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | — | — | 12 |
| Total | 14 | 14 | 14 | 13 | 13 | 14 | 14 | 14 | 14 | 13 | 13 | 13 | 7 | 3 | 10 | 183 |
The abbreviations of species were identical with those in Table 1. The short line represented the absence of the sample in the transcriptome sequencing.
Fig. 1.
Sample sites of 14 Schizothoracine species in our study. The abbreviations of species were identical with those in Table 1. The altitude was represented by the color bar from white (high alititude) to green (low altitude).
Methods
Sample collection
To select representative Schizothoracine species in our study, we chose 14 species of 5 genera in Schizothoracine fishes representing the three specialized group based on the previous morphology study5. The primitive group in our study contains 6 species in Schizothorax genus, such as Schizothorax oconnori (S. oconnori), Schizothorax lissolabiatus (S. lissolabiatus), Schizothorax nukiangensis (S. nukiangensis), Schizothorax plagiostomus (S. plagiostomus), Schizothorax labiatus (S. labiatus) and Schizothorax davidi (S. davidi). The specialized group contains Ptychobarbus kaznakovi in Ptychobarbus genus. The highly specialized group contains 7 species in 3 genera, such as Gymnocypris namensis (G. namensis), Gymnocypris przewalskii (G. przewalskii), Gymnocypris eckloni (G. eckloni) and Gymnocypris selincuoensis (G. selincuoensis) of the Gymnocypris genus, Schizopygopsis younghusbandi (S. younghusbandi), and Schizopygopsis pylzovi (S. pylzovi) of the Schizopygopsis genus, Platypharodon extremus (P. extremus) in the Platypharodon genus. The samples were collected from the six major rivers and three lakes of the Tibetan Plateau including Yarlung Zangbo River, Shiquan River, Lancang River, Nujiang River, Jinsha River, Yellow River, Lake Namtso, Lake Qinghai, Lake Siling Co (Fig. 1 and Table 1). We noted that the Schizothoracine species in this work exhibited obvious morphology diversification, especially on the whiskers and scales. For example, Gymnocypris, Schizopygopsis and Platypharodon species were naked, while small scales were observed in the Schizothorax and Ptychobarbus genus (Table 1).
All individuals were narcotized with MS-222 (Solarbio, Beijing, China) for a few minutes before the sample collection. A total of 183 tissues were collected from 14 representative Schizothoracine fish in our study, including muscle, liver, spleen, gonads, skin, swim bladder, gut, eye, gill, kidney, heart, brain, blood, fat, vibrissa (Table 2). All tissues were immediately frozen in liquid nitrogen after the dissection and then stored at −80 °C until total RNA isolation.
RNA extraction and sequencing
Total RNA was isolated from each sample using RNAiso Plus (TaKaRa, Dalian, China) according to the manufacturer’s instructions and was determined with a photometer for RNA sample integrity (Thermo Scientific, USA). RNA samples passing the quality criteria (see technical validation for detail) were used for the library preparation and RNA sequencing. All samples were sequenced on an Illumina HiSeq X Ten platform with 150 bp paired-end mode. In preset research, a total of more than 10 billion raw PE reads were obtained from all libraries. After filtering by removal of adaptor sequences, contaminated reads and poor–quality reads, we obtained approximately 1.4 Tb of clean data with Q20 bases larger than 96.94%. The average of 7.6 Gb sequencing data were obtained for samples (Supplementary Table S1). The transcriptome data for Oxygymnocypris stewarti in the Oxygymnocypris genus that reported in our previous studies17 were also used for comparision in the work.
De novo assembly of fish transcriptome
We firstly utilized publicly available program Trinity software version 2.5.118 with default parameters for de novo assembly of fish transcripts. The length of <200 bp contigs from each assembly libraries were discarded for subsequent analysis. Next, the redundancies of the transcripts for each species in the dataset were eliminated using the CD-HIT-EST program included in the cd-hit-v4.6.6 package19, with parameters -c 0.98 -n 11 -d 0 -M 0 -T 8 in the final assembly and the longest transcript in each cluster was considered as unigenes. After assembly, the unigene numbers for 15 Schizothoracine species ranged from 76,602 to 154,860 (Table 3). Of these, the highest number of unigenes was observed in P. kaznakovi, and the lowest in S. labiatus. The GC contents of transcripts for all species were rather stable around 40–42%. The N50 length of unigenes ranged from 1,564 to 2,143 bp, with an average of 1,250 bp for all fish transcriptome. As shown in Fig. 2, the unigene length distribution is comparable for all Schizothoracine species, and the average length ranged from 1,120 to 1,392 bp.
Table 3.
The statistics of the de novo transcriptome assembly.
| Species | Total size (Mb) | GC (%) | Unigene | Transcript | ||||
|---|---|---|---|---|---|---|---|---|
| Sequence number | N50 length (bp) | Longest (bp) | Sequence number | N50 length (bp) | Longest (bp) | |||
| S. oconnori | 117.00 | 0.415 | 88,676 | 1,948 | 36,581 | 831,353 | 1,527 | 36,694 |
| S. lissolabiatus | 104.06 | 0.422 | 79,073 | 1,946 | 33,187 | 667,802 | 1,573 | 33,187 |
| S. nukiangensis | 107.46 | 0.419 | 84,638 | 1,835 | 30,806 | 743,518 | 1,420 | 30,806 |
| S. plagiostomus | 98.95 | 0.419 | 83,169 | 1,725 | 17,902 | 736,405 | 1,255 | 17,910 |
| S. labiatus | 99.98 | 0.416 | 76,602 | 1,905 | 43,720 | 670,792 | 1,432 | 43,720 |
| S. davidi | 109.44 | 0.42 | 83,757 | 2,043 | 24,328 | 689,222 | 1,589 | 24,340 |
| P. kaznakovi | 173.48 | 0.409 | 154,860 | 1,564 | 77,434 | 1,363,461 | 1,198 | 77,434 |
| G. namensis | 107.09 | 0.415 | 84,464 | 1,825 | 23,933 | 813,474 | 1,294 | 23,933 |
| G. przewalskii | 105.49 | 0.413 | 78,762 | 1,974 | 28,230 | 751,137 | 1,409 | 28,231 |
| G. eckloni | 113.00 | 0.412 | 87,248 | 1,891 | 23,925 | 849,836 | 1,411 | 23,925 |
| G. selincuoensis | 122.36 | 0.406 | 106,851 | 1,588 | 25,730 | 1,187,251 | 914 | 25,730 |
| S. younghusbandi | 101.23 | 0.414 | 81,029 | 1,820 | 23,570 | 723,624 | 1,329 | 23,570 |
| S. pylzovi | 97.96 | 0.418 | 80,542 | 1,724 | 26,467 | 751,215 | 1,202 | 26,467 |
| P. extremus | 101.78 | 0.417 | 85,919 | 1,674 | 24,119 | 843,423 | 1,122 | 24,119 |
| O. stewartii# | 106.52 | 0.422 | 77,069 | 2,143 | 25,942 | 639,444 | 1,920 | 25,942 |
Note that the total size means the total base amount of all transcripts for species.
#The transcriptome data for Oxygymnocypris stewarti was reported in our previous studies17.
Fig. 2.
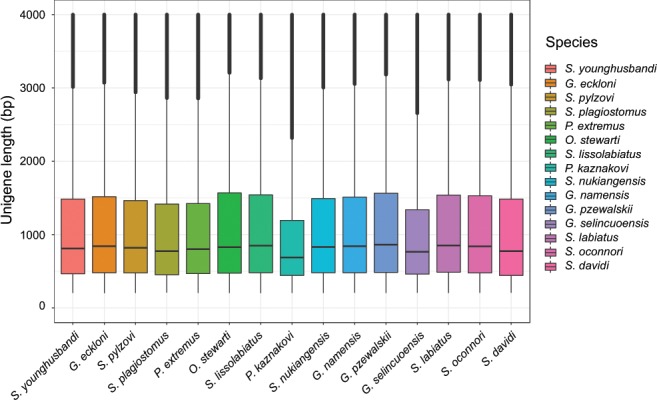
Length distribution of unigenes for all species.
The assembled transcriptome sequences were analyzed by the BUSCO pipeline. BUSCO were generally used in the evaluation of the completeness of a genome assembly, we applied BUSCO version3.0.2 to assess the quality of transcriptome assembly in our work. As a result, we found that more than 98% of the 2,586 BUSCO genes of vertebrates were detected in our transcriptome and 85–92% were completely identified depends on species (Fig. 3), suggesting the transcriptome represented a rather high level of completeness of the conserved genes. Meanwhile, we found that a high fraction of duplicated BUSCO for all species (Fig. 3), which was consistent with the fact that the majority of the Schizothoracine fish were polyploidy.
Fig. 3.
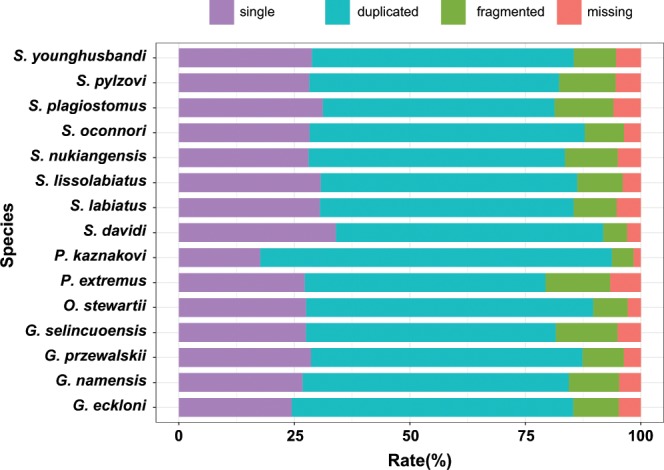
BUSCO statistics of assembled transcripts for species. The rate of single, duplicated, fragmented and missing BUSCO genes were colored by purple, blue, green and pink.
Functional annotation of transcriptome
To annotate the assembled unigenes, we searched the homologous sequences for all unigenes against four public available function databases (Blast-X search: E-value cutoff of 1 × 10−10), including NCBI nonredundant protein database (NR), Swiss-Prot, KEGG pathway database and KOG database. Only the best hits with the highest sequence homology was used for annotation. Then, the gene ontology (GO) terms analysis of the predicted protein based on the NR in NCBI was performed with the Blast2GO software version3.1 with default parameters. We found that at least 40.2% of unigenes per species were annotated based on proteins in four public databases (Table 4 and Supplementary Fig. S1). Meanwhile, we found that high match efficiency was observed the longer assembled unigenes (≥2,000 bp) compared to shorter unigenes (≤500 bp) during the annotation process, the same result was reported in other animal20.
Table 4.
Functional annotation summary for species.
| Species | NR | Swiss-port | KOG | GO | KEGG | Total | Ratio |
|---|---|---|---|---|---|---|---|
| S. oconnori | 45,296 | 29,701 | 40,793 | 28,842 | 28,816 | 46,972 | 52.97% |
| S. lissolabiatus | 45,091 | 30,793 | 41,064 | 30,203 | 29,922 | 46,516 | 58.83% |
| S. nukiangensis | 46,557 | 31,077 | 42,380 | 30,450 | 30,185 | 48,122 | 56.86% |
| S. plagiostomus | 49,111 | 33,194 | 44,034 | 34,896 | 32,267 | 51,264 | 61.64% |
| S. labiatus | 43,749 | 29,702 | 39,846 | 28,668 | 28,837 | 44,956 | 58.69% |
| S. davidi | 47,898 | 32,467 | 42,544 | 35,628 | 31,610 | 50,962 | 60.85% |
| P. kaznakovi | 58,392 | 34,174 | 49,960 | 33,669 | 33,253 | 62,216 | 40.18% |
| G. namensis | 44,310 | 29,970 | 40,147 | 28,721 | 29,102 | 45,732 | 54.14% |
| G. przewalskii | 43,104 | 29,502 | 39,141 | 28,524 | 28,628 | 44,387 | 56.36% |
| G. eckloni | 45,847 | 31,699 | 41,648 | 30,754 | 30,813 | 47,353 | 54.27% |
| G. selincuoensis | 49,768 | 32,165 | 44,381 | 31,049 | 31,239 | 51,828 | 48.50% |
| S. younghusbandi | 46,369 | 33,008 | 42,487 | 31,612 | 32,070 | 47,533 | 58.66% |
| S. pylzovi | 44,777 | 31,296 | 41,101 | 30,088 | 30,408 | 46,094 | 57.23% |
| P. extremus | 46,694 | 32,136 | 42,756 | 30,766 | 31,231 | 48,074 | 55.95% |
| O. stewartii# | 43,212 | 29,426 | 38,495 | 32,099 | 28,597 | 46,009 | 59.70% |
The hit number for NR, Swiss-port, KOG, GO, KEGG were summarized. The ratio means the percentage of annotated unigenes to the total assembly sequences.
#The transcriptome data for Oxygymnocypris stewarti was reported in our previous studies17.
Data Records
The sequencing and assembly data of transcriptome for all samples were deposited into public repositories: The transcriptome sequencing data generated in this work were deposited as SRP186751 in NCBI Sequence Read Archive21; The assembly of sequencing data were deposited in TSA as GHYM0000000022, GHYL0000000023, GHYK0000000024, GHYJ0000000025, GHYI0000000026, GHYH0000000027, GHYG0000000028, GHYF0000000029, GHYE0000000030, GHYD0000000031, GHYC0000000032, GHYB0000000033, GHYA0000000034, GIBO0000000035, and GHXZ0000000036; The transcriptome annotation information and predicted coding and protein sequences for unigenes were uploaded to figshare37.
Technical Validation
RNA integrity
The transcriptome for twelve tissues from three fish individuals were sequenced. In before constructing RNA-Seq libraries, the concentration and quality of total RNA were evaluated using NanoVue Plus spectrophotometer (GE Healthcare, NJ, USA). The total amount of RNA, RNA integrity and rRNA ratio were used to estimate the quality, content and degradation level of RNA samples. In the present study, RNAs samples with a total RNA amount ≥ 10 μg, RNA integrity number ≥ 8, and rRNA ratio ≥ 1.5 were finally subjected to construct the sequencing library.
Quality filtering of Illumina sequencing raw reads
The raw sequencing reads generated from the Illumina platform were rigorously cleaned by the following procedures as in the previous study38. Firstly, adaptors in the reads were filtered out; secondly, reads with more than 10% of N bases were filtered out; thirdly, reads with more than 50% of the low-quality bases (phred quality score < =5) were filtered out. If any end of the pair was classified as low quality, both pairs were discarded. The initially generated raw sequencing reads were also evaluated regarding quality distribution, GC content distribution, base composition, average quality score at each position and other metrics.
Supplementary information
Acknowledgements
We thank Mr. Qiyong Liu for his help in sample collection. This work was supported by the special finance of Tibet autonomous region (No. 2017CZZX003, 2017CZZX004 and XZNKY-2018-C-040), the National Natural Science Foundation of China (No. 31560144 and 31602207), and the National Key Research and Development Program of China (No. 2016YFC1200500).
Author contributions
Haiping Liu and Zenbo Mu conceived and managed the study. Chaowei Zhou, Haiping Liu and Yanchao Liu collected the samples and extracted the RNA; Chaowei Zhou, Shijun Xiao, Ming Zou and Yu Zou assembled and annotated the transcriptome; Chaowei Zhou, Shijun Xiao, Haiping Liu and Zenbo Mu wrote the manuscript. Also, all authors read, edited and approved the final manuscript.
Code availability
No specific code or script was used in this work. All commands used in the data processing were executed as the manual and usage instrument of the corresponding bioinformatics software.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chaowei Zhou, Shijun Xiao and Yanchao Liu.
Supplementary information
is available for this paper at 10.1038/s41597-020-0361-6.
References
- 1.Zheng D. The system of physico-geographical regions of the Qinghai-Xizang (Tibet) Plateau. Science in China Series D-Earth Sciences. 1996;39:8. [Google Scholar]
- 2.Wen L. Uplift of the Tibetan Plateau influenced the morphological evolution of animals. J. Agric. Sci. 2014;6:244. [Google Scholar]
- 3.Mirza, M. R. A contribution to the systematics of the schizothoracine fishes (Pisces: Cyprinidae) with the description of three new tribes. Pak. J. Zool. 23, 339–341 (1991).
- 4.Yue, P., Shan, X. & Lin, R. Fauna sinica. Osteichthyes cypriniformes III. (Science Press, Beijing) (in Chinese) (2000).
- 5.Cao, W., Chen, Y., Wu, Y. & Zhu, S. Origin and evolution of schizothoracine fishes in relation to the upheaval of the Xizang Plateau. (1980).
- 6.He D, Chen Y. Molecular phylogeny and biogeography of the highly specialized grade schizothoracine fishes (Teleostei: Cyprinidae) inferred from cytochrome b sequences. Sci. Bull. 2007;52:777–788. doi: 10.1007/s11434-007-0123-2. [DOI] [Google Scholar]
- 7.Yang T, et al. New schizothoracine from Oligocene of Qaidam Basin, northern Tibetan Plateau, China, and its significance. J. Vertebr. Paleontol. 2018;38:e1442840. doi: 10.1080/02724634.2018.1442840. [DOI] [Google Scholar]
- 8.Qiu Q, et al. The yak genome and adaptation to life at high altitude. Nat. Genet. 2012;44:946. doi: 10.1038/ng.2343. [DOI] [PubMed] [Google Scholar]
- 9.Ge R-L, et al. Draft genome sequence of the Tibetan antelope. Nat. Commun. 2013;4:1858. doi: 10.1038/ncomms2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu, Y. Fishes of the Qinghai-Xizang plateau. (Sichuan Publishing House of Science & Technology, 1992).
- 11.Ye H, et al. De novo assembly of Schizothorax waltoni transcriptome to identify immune-related genes and microsatellite markers. RSC Adv. 2018;8:13945–13953. doi: 10.1039/C8RA00619A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong C, Tian F, Zhao K. Genomic signature of highland adaptation in fish: a case study in Tibetan Schizothoracinae species. BMC Genom. 2017;18:948. doi: 10.1186/s12864-017-4352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong C, Fei T, Zhang C, Zhao K. Comprehensive transcriptomic analysis of Tibetan Schizothoracinae fish Gymnocypris przewalskii reveals how it adapts to a high altitude aquatic life. BMC Evol. Biol. 2017;17:74. doi: 10.1186/s12862-017-0925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong C, Tian F, Zhang C, Zhao K. The microRNA repertoire of Tibetan naked carp Gymnocypris przewalskii: A case study in Schizothoracinae fish on the Tibetan Plateau. PLoS One. 2017;12:e0174534. doi: 10.1371/journal.pone.0174534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu M, et al. Divergent adaptation to Qinghai-Tibetan Plateau implicated from transciptome study of Gymnocypris dobula and Schizothorax nukiangensis. Biochem. Syst. Ecol. 2017;71:97–105. doi: 10.1016/j.bse.2017.02.003. [DOI] [Google Scholar]
- 16.Barat A, Sahoo PK, Kumar R, Goel C, Singh AK. Transcriptional response to heat shock in liver of snow trout (Schizothorax richardsonii)–a vulnerable Himalayan Cyprinid fish. Funct. Integr. Genomics. 2016;16:203–213. doi: 10.1007/s10142-016-0477-0. [DOI] [PubMed] [Google Scholar]
- 17.Liu HP, et al. The sequence and de novo assembly of Oxygymnocypris stewartii genome. Sci. Data. 2019;6:190009. doi: 10.1038/sdata.2019.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, et al. Transcriptome analysis reveals diversified adaptation of Stipa purpurea along a drought gradient on the Tibetan Plateau. Funct. Integr. Genomics. 2015;15:295–307. doi: 10.1007/s10142-014-0419-7. [DOI] [PubMed] [Google Scholar]
- 21.2019. NCBI Sequence Read Archive. SRP186751
- 22.Zhou C, Xiao S, Liu Y. 2019. TSA: Ptychobarbus kaznakovi strain adult, transcriptome shotgun assembly. Genbank. GHYM00000000
- 23.Zhou C, Xiao S, Liu Y. 2019. TSA: Schizopygopsis younghusbandi strain adult, transcriptome shotgun assembly. Genbank. GHYL00000000
- 24.Zhou C, Xiao S, Liu Y. 2019. TSA: Schizopygopsis pylzovi strain adult, transcriptome shotgun assembly. Genbank. GHYK00000000
- 25.Zhou C, Xiao S, Liu Y. 2019. TSA: Gymnocypris przewalskii strain adult, transcriptome shotgun assembly. Genbank. GHYJ00000000
- 26.Zhou C, Xiao S, Liu Y. 2019. TSA: Gymnocypris selincuoensis strain adult, transcriptome shotgun assembly. Genbank. GHYI00000000
- 27.Zhou C, Xiao S, Liu Y. 2019. TSA: Gymnocypris namensis strain adult, transcriptome shotgun assembly. Genbank. GHYH00000000
- 28.Zhou C, Xiao S, Liu Y. 2019. TSA: Gymnocypris eckloni strain adult, transcriptome shotgun assembly. Genbank. GHYG00000000
- 29.Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax oconnori strain adult, transcriptome shotgun assembly. Genbank. GHYF00000000
- 30.Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax labiatus strain adult, transcriptome shotgun assembly. Genbank. GHYE00000000
- 31.Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax davidi strain adult, transcriptome shotgun assembly. Genbank. GHYD00000000
- 32.Zhou C, Xiao S, Liu Y. 2019. TSA: Platypharodon extremus strain adult, transcriptome shotgun assembly. Genbank. GHYC00000000
- 33.Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax lissolabiata strain adult, transcriptome shotgun assembly. Genbank. GHYB00000000
- 34.Zhou C, Xiao S, Liu Y. 2019. TSA: Schizopyge nukiangensis strain adult, transcriptome shotgun assembly. Genbank. GHYA00000000
- 35.Zhou C, Xiao S, Liu Y. 2019. TSA: Oxygymnocypris stewartii strain adult, transcriptome shotgun assembly. Genbank. GIBO00000000
- 36.Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax plagiostomus strain adult, transcriptome shotgun assembly. Genbank. GHXZ00000000
- 37.ChaoWei Z, ShiJun X, YanChao L, HaiPing L. 2019. The unigene of comprehensive transcriptome for endemic Schizothoracinae fish in the Tibetan Plateau. figshare. [DOI]
- 38.Liu H, et al. Draft genome of Glyptosternon maculatum, an endemic fish from Tibet Plateau. Gigascience. 2018;7:giy104–giy104. doi: 10.1093/gigascience/giy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- 2019. NCBI Sequence Read Archive. SRP186751
- Zhou C, Xiao S, Liu Y. 2019. TSA: Ptychobarbus kaznakovi strain adult, transcriptome shotgun assembly. Genbank. GHYM00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Schizopygopsis younghusbandi strain adult, transcriptome shotgun assembly. Genbank. GHYL00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Schizopygopsis pylzovi strain adult, transcriptome shotgun assembly. Genbank. GHYK00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Gymnocypris przewalskii strain adult, transcriptome shotgun assembly. Genbank. GHYJ00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Gymnocypris selincuoensis strain adult, transcriptome shotgun assembly. Genbank. GHYI00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Gymnocypris namensis strain adult, transcriptome shotgun assembly. Genbank. GHYH00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Gymnocypris eckloni strain adult, transcriptome shotgun assembly. Genbank. GHYG00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax oconnori strain adult, transcriptome shotgun assembly. Genbank. GHYF00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax labiatus strain adult, transcriptome shotgun assembly. Genbank. GHYE00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax davidi strain adult, transcriptome shotgun assembly. Genbank. GHYD00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Platypharodon extremus strain adult, transcriptome shotgun assembly. Genbank. GHYC00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax lissolabiata strain adult, transcriptome shotgun assembly. Genbank. GHYB00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Schizopyge nukiangensis strain adult, transcriptome shotgun assembly. Genbank. GHYA00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Oxygymnocypris stewartii strain adult, transcriptome shotgun assembly. Genbank. GIBO00000000
- Zhou C, Xiao S, Liu Y. 2019. TSA: Schizothorax plagiostomus strain adult, transcriptome shotgun assembly. Genbank. GHXZ00000000
- ChaoWei Z, ShiJun X, YanChao L, HaiPing L. 2019. The unigene of comprehensive transcriptome for endemic Schizothoracinae fish in the Tibetan Plateau. figshare. [DOI]
Supplementary Materials
Data Availability Statement
No specific code or script was used in this work. All commands used in the data processing were executed as the manual and usage instrument of the corresponding bioinformatics software.