Abstract
Background
The increase in prevalence of colorectal cancer among young patients coupled with an older retirement age in developed countries means that more patients are being diagnosed with colorectal cancer while still at work. The aim of this study was to develop prediction models for return to work by 1 and 2 years after the start of sick leave.
Methods
This was a retrospective registry‐based cohort study of data from a nationwide occupational health service in the Netherlands. Only employed patients with colonic or rectal cancer treated with curative intent were included. Two predictor variable models were developed using multivariable logistic regression with backward selection. Calibration, discrimination and explained variance were used to assess model performance, and internal validation by bootstrapping was performed.
Results
Median time to return to work for 317 included patients was 423 (95 per cent c.i. 379 to 467) days. Two‐thirds of patients had returned to work by 2 years after the start of the sick leave. Presence of metastases, adjuvant treatment, stoma, emotional distress and postoperative complications were predictors of not returning to work in the 1‐year model. In the 2‐year model, presence of metastases, emotional distress, postoperative complications, company size and the trajectory of the return‐to‐work process were predictors.
Conclusion
Almost 70 per cent of patients with colorectal cancer in this population returned to work within 2 years after the start of sick leave. The models can be used to guide patients early in colorectal cancer treatment about the likelihood of returning to work, and to identify and modify barriers that could facilitate this.
Almost 70 per cent of patients with colorectal cancer in this population returned to work within 2 years after the start of the sick leave period. The models in this study can be used to guide patients with colorectal cancer early in their treatment about their likelihood of returning to work, and to identify and modify barriers that could facilitate this.
Most return at two years
Antecedentes
El aumento de la prevalencia del cáncer colorrectal (colorectal cancer, CCR) en pacientes jóvenes, junto con una edad de jubilación más avanzada en los países desarrollados, significa un mayor número de pacientes en edad laboral diagnosticados de CCR. El objetivo de este estudio fue desarrollar modelos de predicción del regreso al trabajo uno y dos años después del inicio de la baja por enfermedad para el tratamiento de CCR.
Métodos
Se trata de un estudio de cohortes retrospectivo basado en el registro de datos nacionales de salud laboral en los Países Bajos. Solo se incluyeron pacientes con cáncer de colon o recto tratados con intención curativa. Se desarrollaron dos modelos de variables de predicción utilizando regresión logística multivariante con selección de variables hacia atrás. Para evaluar el rendimiento del modelo se utilizaron la calibración, la discriminación y la varianza explicada y se realizó la validación interna mediante bootstrap.
Resultados
La mediana de tiempo de regreso al trabajo de 317 pacientes incluidos fue de 423 días (i.c. del 95%: 378,6‐467,4). Dos tercios de los pacientes habían regresado al trabajo dos años después del inicio del período de baja por enfermedad. La presencia de metástasis, el tratamiento adyuvante, el estoma, la angustia emocional y las complicaciones postoperatorias fueron factores predictivos de no volver a trabajar en el modelo de un año. En el modelo de dos años, la presencia de metástasis, la angustia emocional, las complicaciones postoperatorias, el tamaño de la empresa y la trayectoria del proceso de retorno al trabajo fueron factores predictores del regreso laboral.
Conclusión
Casi el 70% de los pacientes con CCR en esta población volvieron a trabajar dentro de los dos años posteriores al inicio del periodo de baja por enfermedad. Los modelos se pueden usar para guiar a los pacientes con CCR al inicio de su tratamiento sobre su probabilidad de regresar al trabajo, así como para identificar y modificar las barreras que podrían facilitarlo.
Introduction
Improvements in colorectal cancer management have led to more patients being diagnosed at an early stage, with improved survival1, 2, 3, 4, 5, 6, 7. As a result, more patients with colorectal cancer will resume normal life after treatment, including a return to work8. The increased incidence of colorectal cancer in young patients, coupled with the increasing retirement age in many countries, will lead to more patients being diagnosed with colorectal cancer while still in work9, 10.
Ability to return to work and to stay at work is important both to the patient and society in general11, 12, 13, 14, 15. The impact of colorectal cancer on a patient's ability to return to work has not been studied extensively16, 17. Psychosocial and occupational factors, such as self‐efficacy or workplace environment, may have an impact on return to work after a cancer diagnosis in general18, 19. Previous research has mainly concentrated on other cancer types, where other disease‐specific factors may play a role20, 21. Factors influencing rates of return to work among patients with colorectal cancer may be modifiable, such as workplace environment, or non‐modifiable, such as age, (neo)adjuvant therapy and co‐morbidity21, 22, 23, 24, 25, 26, 27.
Reported return‐to‐work rates for patients with colorectal cancer range from 60 to 89 per cent26, 27, 28, 29, 30. The average duration of sick leave for patients with colorectal cancer in the Netherlands is unknown31, 32. More evidence is required about the modifiable and non‐modifiable barriers for patients to return to work19, 20. The aim of this study was to develop and internally validate prediction models for return to work by 1 and 2 years after the start of the sick leave among patients with colorectal cancer.
Methods
This was a retrospective registry‐based cohort study. Data were extracted from the nationwide Dutch Occupational Health Service (ArboNed) registry33. Data were collected from the medical records of occupational health physicians providing sickness guidance to employees of 70 000 contracted companies from a variety of economic sectors. The database included more than 1 million employees in the Netherlands. This study was approved by the Medical Ethical Committee of the Amsterdam University Medical Centres, VU University Amsterdam (registration number 2016.092). The TRIPOD checklist was used to ensure transparent reporting of these prediction models34.
Study population
Included patients were sourced from the ArboNed registry. Inclusion criteria were being at least 18 years old with colonic or rectal cancer treated with curative intent, between January 2012 and December 2014. This included patients with potentially curable metastatic disease. Exclusion criteria were: patients with a diagnosis of recurrent colonic or rectal cancer, and those with another cancer diagnosis during the sick leave.
Sick leave regulation in the Netherlands
Regulation and guidance regarding sick leave for employees is mostly coordinated by Occupational Health Services in the Netherlands. An occupational health physician should be consulted within 6 weeks of starting sick leave, but the responsibility for return to work resides with the employer and employee. During the first 2 years of sick leave, the occupational health physician reviews the patient every 6 weeks to monitor recovery and potential for return to work, and to give advice. Employers are obliged to pay at least 70 per cent of the salary of the sick employee during these 2 years. After 2 years, if a return to work has not been possible, the employee is eligible for a disability pension from the social security agency of the Netherlands. The employer is then no longer responsible for payments35, 36.
Outcome measures
Return to work was calculated as a dichotomous outcome (did or did not return to work) by 1 and 2 years after the start of the sick leave. Return to work was defined as full and sustainable, which means at least 28 days of full work resumption after the sick leave ended with no loss of earning capacity.
Predictors of return to work
Potential predictors of return to work were selected based on clinical knowledge and previous studies, and also whether the data were available from the occupational health physician registry. All consultations were reviewed by the researcher and all candidate predictors were scored based on the information from these consultations. A second reviewer scored 10 per cent of all patients independently. All disagreements were resolved by discussion. Candidate predictors of return to work were categorized as person‐, disease‐ or treatment‐, or occupation‐related factors. Person‐related factors included: age (continuous)16, 21, 22, 23, sex (male, female)22, 23, marital status (married, single, widower, co‐habiting)22, 23 and medical history (no, yes)16, 21, 22, 23. Disease‐ or treatment‐related factors included: type of diagnosis (colonic, rectal)22, 23, 27, presence of metastases (curative treatment without metastases, curative treatment with metastases)27, neoadjuvant treatment (no, yes)16, 21, 22, 23, adjuvant treatment (no, yes)16, 21, 22, 23, stoma (no, yes)22, 23, emotional distress (no, yes)22, 23, fatigue (no, yes)22, 23, pain (no, yes) and postoperative complications (no, yes)16, 22, 23, 27. Occupation‐related factors included: type of work (physical, not physical, combination)37, type of contract (full‐time (36 h or more per week), part‐time (less than 36 h but more than 12 h per week), flexible)38, type of employment (permanent, temporary contract), company size (micro (fewer than 10 employees), small (11–50 employees), medium (51–250 employees), large (251 or more employees))39, relationship with employer (bad, good) and the trajectory of the return to work (direct, phased).
Missing data
Before data extraction it was decided that, if no information regarding neoadjuvant therapy, adjuvant therapy, stoma, emotional distress or postoperative complications was available in the medical records, these factors would be scored as ‘not reported’ and included in the analysis as not present. This is in line with routine practice, as confirmed by the involved occupational health physicians, not to specifically record the absence of each of these variables. For all other predictors not reported in the medical record, the assumption was made that data were missing. To ensure the quality of the applied models, given the approach taken for the five predictors specified above, all other predictors with missing values were excluded from the analysis.
Statistical analysis
Analyses were performed using SPSS® version 22.0 (IBM, Armonk, New York, USA) unless indicated otherwise.
Model development
Two prediction models were developed: return to work by 1 year and by 2 years after the start of sick leave. Collinearity between co‐variables was tested based on the correlation between candidate predictors. The candidate predictor type of diagnosis was excluded from the analysis owing to the assumed correlation with neoadjuvant therapy and adjuvant therapy. There was no assumption of correlation for all other candidate predictors. The variable age was tested for linearity by means of spline curves and was subsequently considered as a continuous variable in the analysis40. All candidate predictors were analysed in a separate univariable analysis. Then, multivariable logistic regression modelling with backward selection of variables with P ≤ 0·100 was undertaken. Odds ratios and 95 per cent confidence intervals were calculated for the predictors in the models before and after internal validation.
Model performance
Calibration was assessed visually using a smooth calibration curve, and statistically using the Hosmer and Lemeshow goodness‐of‐fit test. Predicted probabilities were calculated for return to work by 1 and 2 years for each patient by using the linear predictor41. Discrimination was estimated using the area under the receiver operating characteristic (ROC) curve (AUC) (C‐index of the model), which indicated the model's ability to discriminate between patients with a high versus low probability of returning to work. AUC values of 0·70–0·79 were considered to indicate acceptable discrimination, 0·80–0·89 as excellent and 0·90 or more as outstanding discrimination41. Finally, the explained variance was calculated in terms of Nagelkerke's R 2 value.
Validation
Internal validation by bootstrapping was done using Stata® version 14 (StataCorp, College Station, Texas, USA). The modelling process was repeated in 250 bootstrap samples. Testing the 250 bootstrap models for the original data and calculating the linear predictor slope established the overoptimism of the models developed in this study. The average difference between the linear predictor slope in the bootstrap samples and the original data was used as a shrinkage factor to correct the regression coefficients of the original model, and report the optimism‐corrected AUC and Nagelkerke's R 2 value42.
Results
Some 317 patients with colorectal cancer were identified in the registry, 175 patients with colonic and 142 with rectal cancer. Patient characteristics are summarized in Table 1. These characteristics were also selected as candidate predictors for development of the prediction models. The other candidate predictors with missing values were not selected for analysis; the percentage of missing values for each excluded variable is shown in Table S1 (supporting information). The median time until return to full‐time and sustainable work was 423 (95 per cent c.i. 379 to 467) days (Fig. 1) and that until the first day of return to work was 273 (239 to 307) days. In total, 223 patients with colorectal cancer (70·3 per cent) returned to work fully. Return to work rates by 1 and 2 years, and reasons for not returning to work after 2 years are shown in Table 2.
Table 1.
Characteristics of the study population
| No. of patients* (n = 317) | |
|---|---|
| Age (years) † | 54·4(7·7) |
| Sex ratio (F : M) | 105 : 212 |
| Diagnosis | |
| Colonic cancer | 175 (55·2) |
| Rectal cancer | 142 (44·8) |
| Aim of treatment | |
| Curative (no metastases) | 260 (82·0) |
| Curative (metastases present) | 57 (18·0) |
| Neoadjuvant therapy | |
| No | 4 (1·3) |
| Yes | 124 (39·1) |
| Not reported | 189 (59·6) |
| Adjuvant therapy | |
| No | 48 (15·1) |
| Yes | 117 (36·9) |
| Not reported | 152 (47·9) |
| Stoma | |
| No | 37 (11·7) |
| Yes | 164 (51·7) |
| Not reported | 116 (36·6) |
| Emotional distress | |
| No psychological distress | 75 (23·7) |
| Psychological distress | 90 (28·4) |
| Not reported | 152 (47·9) |
| Postoperative complications | |
| No | 28 (8·8) |
| Yes | 128 (40·4) |
| Not reported | 161 (50·8) |
| Type of work | |
| Physical | 90 (28·4) |
| Non‐physical | 126 (39·7) |
| Combination | 101 (31·9) |
| Type of employment contract | |
| Part time | 90 (28·4) |
| Full time | 187 (59·0) |
| Flexible/0 h | 40 (12·6) |
| Company size (no. of employees) | |
| < 10 | 97 (30·6) |
| 10–50 | 154 (48·6) |
| 51–250 | 56 (17·7) |
| ≥ 251 | 10 (3·2) |
| Trajectory of return to work | |
| Direct | 130 (41·0) |
| Phased | 187 (59·0) |
With percentages in parentheses unless indicated otherwise;
values are mean(s.d.).
Figure 1.
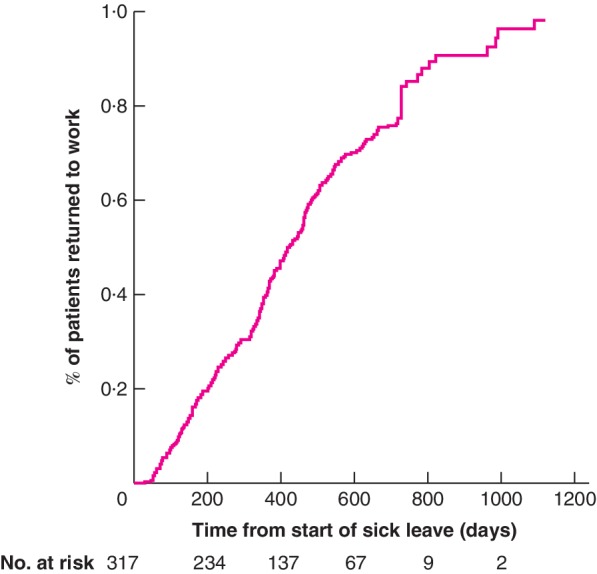
Return to work for all 317 patients with colorectal cancer
Table 2.
Median time until return to work and reasons for not returning
| No. of patients (n = 317)* | |
|---|---|
| Time until full return to work (days) † | 423 (379, 467) |
| Time until first day of return to work (days) † | 273 (239, 307) |
| Returned or did not return to work after total sick leave period | |
| Returned to work | 233 (73·5) |
| Full and sustainable | 223 (70·3) |
| Partial | 10 (3·2) |
| Did not return to work | 84 (26·5) |
| Registered work disabled | 18 (5·7) |
| Contract terminated | 32 (10·1) |
| Retired | 12 (3·8) |
| Termination of contract of occupational health service with employer | 11 (3·5) |
| Died | 2 (0·6) |
| Reason unknown | 9 (2·8) |
| 1‐year follow‐up | |
| Returned to work | 118 (37·2) |
| Not returned to work | 199 (62·8) |
| 2‐year follow‐up | |
| Returned to work | 214 (67·5) |
| Not returned to work | 103 (32·5) |
With percentages in parentheses unless indicated otherwise;
values are median (95 per cent c.i.).
Univariable analysis
Table 3 shows the results of univariable analysis for return to work by 1 and 2 years. The candidate predictors presence of metastases, adjuvant therapy, stoma, emotional distress and postoperative complications predicted not returning to work by 1 year. Presence of metastases, emotional distress and the trajectory of the return predicted not returning to work by 2 years.
Table 3.
Results of univariable logistic regression analysis for return to work by 1 and 2 years
| Return to work by 1 year | Return to work by 2 years | |||
|---|---|---|---|---|
| Odds ratio | P | Odds ratio | P | |
| Patient‐related factors | ||||
| Sex (M versus F) | 1·43 (0·88, 2·31) | 0·145 | 0·87 (0·54, 1·46) | 0·631 |
| Age (per year) | 0·99 (1·00, 1·03) | 0·742 | 0·98 (0·95, 1·01) | 0·280 |
| Disease‐ and treatment‐related factors | ||||
| Presence of metastases (yes versus no) | 0·34 (0·17, 0·69) | 0·003 | 0·38 (0·21, 0·69) | 0·001 |
| Neoadjuvant therapy (yes versus no) | 0·62 (0·39, 1·01) | 0·053 | 1·02 (0·63, 1·65) | 0·943 |
| Adjuvant therapy (yes versus no) | 0·34 (0·20, 0·56) | < 0·001 | 0·78 (0·48, 1·27) | 0·323 |
| Stoma (yes versus no) | 0·37 (0·23, 0·59) | < 0·001 | 0·76 (0·48, 1·22) | 0·259 |
| Emotional distress (yes versus no) | 0·44 (0·26, 0·76) | 0·003 | 0·42 (0·25, 0·70) | 0·001 |
| Postoperative complications (yes versus no) | 0·51 (0·32, 0·83) | 0·006 | 0·68 (0·43, 1·10) | 0·118 |
| Occupation‐related factors | ||||
| Type of work | ||||
| Physical versus not physical | 0·78 (0·45, 1·35) | 0·372 | 1·02 (0·56, 1·85) | 0·959 |
| Physical versus combination | 0·61 (0·34, 1·10) | 0·097 | 0·60 (0·33, 1·09) | 0·092 |
| Type of contract | ||||
| Part‐time versus full‐time | 0·86 (0·51, 1·44) | 0·558 | 0·84 (0·49, 1·45) | 0·531 |
| Part‐time versus flexible | 0·81 (0·37, 1·75) | 0·589 | 0·61 (0·28, 1·33) | 0·213 |
| Company size (no.of employees) | ||||
| 10–50 versus < 10 | 0·87 (0·52, 1·47) | 0·612 | 0·84 (0·49, 1·47) | 0·548 |
| 51–250 versus < 10 | 0·83 (0·42, 1·63) | 0·583 | 0·73 (0·36, 1·47) | 0·380 |
| ≥ 251 versus < 10 | 0·37 (0·08, 1·85) | 0·226 | 0·41 (0·11, 1·51) | 0·179 |
| Trajectory of return to work (direct versus phased) | 0·69 (0·44, 1·10) | 0·119 | 2·87 (1·77, 4·66) | < 0·001 |
Values in parentheses are 95 per cent confidence intervals.
Prediction model for return to work by 1 year
Multivariable analysis identified the following variables as independent predictors of not returning to work by 1 year: presence of metastases, adjuvant therapy, stoma, emotional distress and postoperative complications (Table 4). The P value for the Hosmer and Lemeshow test was 0·736. Before internal validation, the AUC of the model was 0·76 (95 per cent c.i. 0·70 to 0·81) and Nagelkerke's R 2 was 0·26. After internal validation with bootstrapping (250 samples), the AUC of the model was 0·73 (0·67 to 0·78) and Nagelkerke's R 2 was 0·16. The bootstrapped ROC curve is shown in Fig. 2 a and the calibration plot of this model in Fig. 3 a. A worked example using the 1‐year prediction model for return to work is available in Appendix S1 (supporting information).
Table 4.
Results of multivariable logistic regression analysis for return to work by 1 year
| Before internal validation | After internal validation | |||||
|---|---|---|---|---|---|---|
| Regression coefficient | Odds ratio | P | Regression coefficient | Odds ratio | P | |
| Presence of metastases (yes versus no) | –0·84 | 0·43 (0·20, 0·94) | 0·034 | –0·84 | 0·43 (0·18, 1·04) | 0·062 |
| Adjuvant therapy (yes versus no) | –1·56 | 0·21 (0·11, 0·39) | < 0·001 | –1·56 | 0·21 (0·11, 0·40) | < 0·001 |
| Stoma (yes versus no) | –1·30 | 0·27 (0·15, 0·49) | < 0·001 | –1·30 | 0·27 (0·15, 0·49) | < 0·001 |
| Emotional distress (yes versus no) | –0·75 | 0·47 (0·26, 0·86) | 0·010 | –0·75 | 0·47 (0·25, 0·89) | 0·020 |
| Postoperative complications (yes versus no) | –0·52 | 0·60 (0·34, 1·06) | 0·068 | –0·52 | 0·60 (0·35, 1·01) | 0·055 |
| Constant | 1·16 | 1·16 | ||||
| P (Hosmer and Lemeshow test) | 0·736 | |||||
| Nagelkerke's R 2 | 0·26 | 0·16 | ||||
| AUC | 0·76 (0·70, 0·81) | 0·73 (0·67. 0·78) | ||||
Values in parentheses are 95 per cent confidence intervals. AUC, area under the curve.
Figure 2.
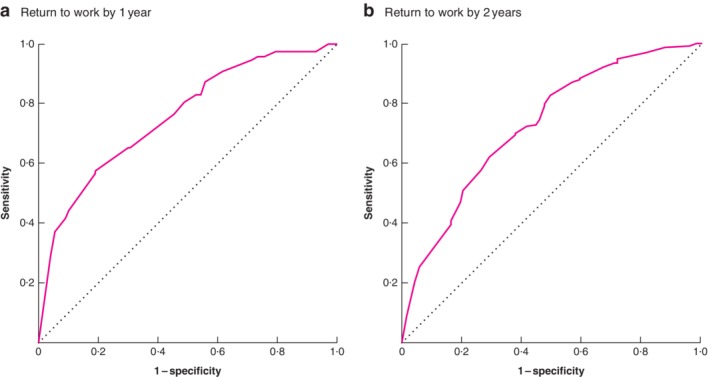
Bootstrapped receiver‐operating characteristic (ROC) curves for the models predicting return to work by 1 and 2 years Return to work by a 1 year and b 2 years.
Figure 3.
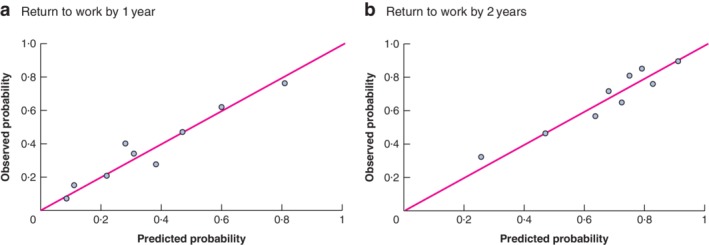
Calibration plots for the models predicting return to work by 1 and 2 years Return to work by a 1 year and b 2 years.
Prediction model for return to work by 2 years
Multivariable analysis revealed that the following variables predicted return to work by 2 years: presence of metastases, emotional distress, postoperative complications, company size and the trajectory of the return to work (Table 5). The P value for the Hosmer and Lemeshow test was 0·513. Before internal validation, the AUC of the model was 0·73 (95 per cent c.i. 0·67 to 0·79) and Nagelkerke's R 2 was 0·20. After internal validation with bootstrapping (250 samples), the AUC of the model was 0·71 (0·65 to 0·77) and Nagelkerke's R 2 was 0·12. The bootstrapped ROC curve and calibration plot for this model are shown in Figs 2 b and 3 b respectively. Calculation of the probability of return to work by 2 years for a fictitious patient in shown in Appendix S1 (supporting information).
Table 5.
Results of multivariable logistic regression analysis for return to work by 2 years
| Before internal validation | After internal validation | |||||
|---|---|---|---|---|---|---|
| Regression coefficient | Odds ratio | P | Regression coefficient | Odds ratio | P | |
| Presence of metastases (yes versus no) | –0·95 | 0·39 (0·21, 0·72) | 0·003 | –0·95 | 0·39 (0·22, 0·67) | 0·001 |
| Emotional distress (yes versus no) | –0·95 | 0·39 (0·22, 0·67) | 0·001 | –0·95 | 0·39 (0·22, 0·67) | 0·001 |
| Postoperative complications (yes versus no) | –0·60 | 0·55 (0·32, 0·93) | 0·026 | –0·60 | 0·55 (0·31, 0·97) | 0·039 |
| Company size (no. of employees) | ||||||
| 10–50 versus < 10 | –0·28 | 0·76 (0·41, 1·38) | 0·362 | –0·28 | 0·76 (0·39, 1·46) | 0·403 |
| 51–250 versus < 10 | –0·60 | 0·55 (0·25, 1·18) | 0·125 | –0·60 | 0·55 (0·24, 1·27) | 0·162 |
| ≥ 251 versus < 10 | –1·31 | 0·27 (0·07, 1·12) | 0·070 | –1·31 | 0·27 (0·07, 1·11) | 0·069 |
| Trajectory of return to work (direct versus phased) | 1·26 | 3·52 (2·07, 6·00) | < 0·001 | 1·26 | 3·52 (1·52, 5·62) | 0·001 |
| Constant | 1·08 | 1·08 | ||||
| P (Hosmer and Lemeshow test) | 0·513 | |||||
| Nagelkerke's R 2 | 0·20 | 0·12 | ||||
| AUC | 0·73 (0·67, 0·79) | 0·71 (0·65, 0·77) | ||||
Values in parentheses are 95 per cent confidence intervals. AUC, area under the curve.
Discussion
Return to work after cancer treatment can be considered a surrogate marker of recovery in general. The ability to work is rated as the third most important aspect of quality of life43, 44. The present study has developed two models that can be used in daily practice to advise patients very early during treatment for colorectal cancer about their likelihood of returning to work by 1 and 2 years after diagnosis. Early identification of barriers that may prevent a return to work could allow employment modification of the barrier to allow a return to work that may have otherwise been impossible.
Disease‐ and treatment‐related factors were found to play a role in return to work within the first year, which is not surprising as this interval coincides with the treatment period. Thereafter, occupation‐related factors influenced return to work. It has been found previously that psychosocial and other occupation‐related factors such as beliefs and expectations about long‐term illness influence return to work for cancer survivors18, 45, 46, 47, 48, 49. There were insufficient data available to analyse all these factors fully in the present study, which may explain the low variance of these models. However, acceptable discrimination before and after internal validation was seen for both prediction models41, 50.
In the present study, only 37·2 per cent of included patients had returned to work by 1 year, which is lower than reported previously. This may be a result of different definitions of return to work26, 27, 28, 29, 30. Alternatively, the lower rate of return to work by 1 year may be explained by differences in sick‐leave regulations between countries. Patients may be less motivated to return to work when state‐funded welfare is available, and so the importance of patient motivation should not be underestimated51. In the UK, for example, employers must provide Statutory Sick Pay for up to 28 weeks; thereafter, the state is responsible for at least 70 per cent of the compensation51. In the Netherlands, the employer is obliged to pay the salary of the sick employee for 2 years after the start of sick leave. The rate of return to work of 67·5 per cent at 2 years after the start of the sick leave in the present study is comparable to results in other countries (60–83 per cent)52, 53.
This study was limited by a relatively small data set, which can cause overfitting of prediction models, resulting in underestimation of the probability of an event for low‐risk patients and overestimation for high‐risk patients42, 54. However, the use of internal validation by bootstrap resampling addresses the stability and quality of selected predictors, such that the risk of overfitting of these models is decreased. Another potential limitation was that the data were not originally collected for research purposes. This resulted in poorer data quality, meaning that some candidate predictors could not be assessed adequately and were therefore not included in the analysis. In addition, for some candidate predictors it was assumed that no reference in the occupational health medical report meant that this factor was not present. The occupational health physicians involved in this study indicated that it is routine practice not to specifically record the absence of each of these variables. This assumption may have had an impact on the results, and collection of longitudinal data for research purposes is recommended in future prospective studies. Furthermore, most categorical predictors were scored as dichotomous variables, resulting in less distinct information within the variable. However, dichotomization of variables makes the models more user‐friendly, allowing quicker assessment of the likelihood of return to work.
An evidence‐based guideline for recovery after colorectal cancer treatment in the Netherlands has been developed. Potential benefits and harms of the use of the screening tools in this study population need to be evaluated by others in future studies and external validation of these models performed. These studies should also include relevant occupational factors and patients' own expectations regarding ability to work. It would also be useful to develop separate models for colonic and rectal cancer to reflect the different characteristics and treatment options for tumours at each site.
Supporting information
Appendix S1 Two cases of fictitious patients using the 1‐ and 2‐year return‐to‐work prediction models
Table S1 Percentages of missing values per excluded candidate predictor
Acknowledgements
The authors thank ArboNed for providing the data. J.A.F.H. received grants from the Dutch Research Council (NWO), ZonMw and Samsung during the conduct of the study, and a fee from Olympus, outside the submitted work. H.J.B. received personal fees from Olympus, Stryker, Medtronic and Applied Medical, outside the submitted work. J.R.A. holds a Chair in Insurance Medicine paid by the Dutch Social Security Agency, he is a stockholder of Evalua, and received grants from ZonMw/NWO, Instituut Gak, Employee Insurance Agency (UWV), Ministry of Social Affairs and Employment (SZW), Ministry of Health, Welfare and Sport (VWS), Pfizer, Achmea and Healthcare Insurance Board (CVZ)/Zorg Instituut, outside the submitted work.
Disclosure: The authors declare no other conflict of interest.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 2. de Boer AG, Taskila TK, Tamminga SJ, Feuerstein M, Frings‐Dresen MH, Verbeek JH. Interventions to enhance return‐to‐work for cancer patients. Cochrane Database Syst Rev 2015; (9)CD007569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GLOBOCAN. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012; 2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx [accessed 12 June 2018].
- 4. Holleczek B, Rossi S, Domenic A, Innos K, Minicozzi P, Francisci S et al; EUROCARE‐5 Working Group . On‐going improvement and persistent differences in the survival for patients with colon and rectum cancer across Europe 1999–2007 – results from the EUROCARE‐5 study. Eur J Cancer 2015; 51: 2158–2168. [DOI] [PubMed] [Google Scholar]
- 5. Douaiher J, Ravipati A, Grams B, Chowdhury S, Alatise O, Are C. Colorectal cancer – global burden, trends, and geographical variations. J Surg Oncol 2017; 115: 619–630. [DOI] [PubMed] [Google Scholar]
- 6. Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP et al Colorectal cancer screening: a global overview of existing programmes. Gut 2015; 64: 1637–1649. [DOI] [PubMed] [Google Scholar]
- 7. Elferink MAG, Toes‐Zoutendijk E, Vink GR, Lansdorp‐Vogelaar I, Meijer GA, Dekker E et al [National population screening for colorectal carcinoma in the Netherlands: results of the first years since the implementation in 2014]. Ned Tijdschr Geneeskd 2018; 162: D2283. [PubMed] [Google Scholar]
- 8. Choi KS, Kim EJ, Lim JH, Kim SG, Lim MK, Park JG et al Job loss and reemployment after a cancer diagnosis in Koreans – a prospective cohort study. Psychooncology 2007; 16: 205–213. [DOI] [PubMed] [Google Scholar]
- 9.Netherlands Cancer Registry. https://www.cijfersoverkanker.nl/selecties/Dataset_1/img5b1f85619dbd1 [accessed 12 June 2018].
- 10. Hurd MD, Smith JP, Zissimopoulos JM. The effects of subjective survival on retirement and social security claiming. J Appl Economet 2004; 19: 761–775. [Google Scholar]
- 11. Amir Z, Wynn P, Whitaker S, Luker K. Cancer survivorship and return to work: UK occupational physician experience. Occup Med (Lond) 2009; 59: 390–396. [DOI] [PubMed] [Google Scholar]
- 12. Kennedy F, Haslam C, Munir F, Pryce J. Returning to work following cancer: a qualitative exploratory study into the experience of returning to work following cancer. Eur J Cancer Care (Engl) 2007; 16: 17–25. [DOI] [PubMed] [Google Scholar]
- 13. Barofsky I. The status of psychosocial research in the rehabilitation of the cancer patient. Semin Oncol Nurs 1992; 8: 190–201. [DOI] [PubMed] [Google Scholar]
- 14. Rønning B, Wyller TB, Jordhøy MS, Nesbakken A, Bakka A, Seljeflot I et al Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol 2014; 5: 26–32. [DOI] [PubMed] [Google Scholar]
- 15. Luciani A, Jacobsen PB, Extermann M, Foa P, Marussi D, Overcash JA et al Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008; 31: 424–430. [DOI] [PubMed] [Google Scholar]
- 16. Van den Brink M, van den Hout WB, Kievit J, Marijnen CA, Putter H, van de Velde CJ et al The impact of diagnosis and treatment of rectal cancer on paid and unpaid labor. Dis Colon Rectum 2005; 48: 1875–1882. [DOI] [PubMed] [Google Scholar]
- 17. Steiner JF, Nowels CT, Main DS. Returning to work after cancer: quantitative studies and prototypical narratives. Psychooncology 2010; 19: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feuerstein M, Todd BL, Moskowitz MC, Bruns GL, Stoler MR, Nassif T et al Work in cancer survivors: a model for practice and research. J Cancer Surviv 2010; 4: 415–437. [DOI] [PubMed] [Google Scholar]
- 19. Spelten ER, Sprangers MA, Verbeek JH. Factors reported to influence the return to work of cancer survivors: a literature review. Psychooncology 2002; 11: 124–131. [DOI] [PubMed] [Google Scholar]
- 20. van Muijen P, Weevers NL, Snels IA, Duijts SF, Bruinvels DJ, Schellart AJ et al Predictors of return to work and employment in cancer survivors: a systematic review. Eur J Cancer Care (Engl) 2013; 22: 144–160. [DOI] [PubMed] [Google Scholar]
- 21. Bains M, Munir F, Yarker J, Bowley D, Thomas A, Armitage N et al The impact of colorectal cancer and self‐efficacy beliefs on work ability and employment status: a longitudinal study. Eur J Cancer Care (Engl) 2012; 21: 634–641. [DOI] [PubMed] [Google Scholar]
- 22. Gordon LG, Beesley VL, Lynch BM, Mihala G, McGrath C, Graves N et al The return to work experiences of middle‐aged Australian workers diagnosed with colorectal cancer: a matched cohort study. BMC Public Health 2014; 14: 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lynch BM, Mihala G, Beesley VL, Wiseman AJ, Gordon LG. Associations of health behaviours with return to work outcomes after colorectal cancer. Support Care Cancer 2016; 24: 865–870. [DOI] [PubMed] [Google Scholar]
- 24. Chen L, Glimelius I, Neovius M, Ekberg S, Martling A, Eloranta S et al Work loss duration and predictors following rectal cancer treatment among patients with and without prediagnostic work loss. Cancer Epidemiol Biomarkers Prev 2016; 25: 987–994. [DOI] [PubMed] [Google Scholar]
- 25. Hauglann BK, Saltytė Benth J, Fosså SD, Tveit KM, Dahl AA. A controlled cohort study of sickness absence and disability pension in colorectal cancer survivors. Acta Oncol 2014; 53: 735–743. [DOI] [PubMed] [Google Scholar]
- 26. Gordon L, Lynch BM, Newman B. Transitions in work participation after a diagnosis of colorectal cancer. Aust N Z J Public Health 2008; 32: 569–574. [DOI] [PubMed] [Google Scholar]
- 27. Carlsen K, Harling H, Pedersen J, Christensen KB, Osler M. The transition between work, sickness absence and pension in a cohort of Danish colorectal cancer survivors. BMJ Open 2013; 3: e002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen L, Glimelius I, Neovius M, Eloranta S, Ekberg S, Martling A et al Risk of disability pension in patients following rectal cancer treatment and surgery. Br J Surg 2015; 102: 1426–1432. [DOI] [PubMed] [Google Scholar]
- 29. Bhalla A, Williams JP, Hurst NG, Speake WJ, Tierney GM, Tou S et al One‐third of patients fail to return to work 1 year after surgery for colorectal cancer. Tech Coloproctol 2014; 18: 1153–1159. [DOI] [PubMed] [Google Scholar]
- 30. Sanchez KM, Richardson JL, Mason HR. The return to work experiences of colorectal cancer survivors. AAOHN J 2004; 52: 500–510. [PubMed] [Google Scholar]
- 31. Nederlandse Vereniging voor Verzekeringsgeneeskunde . Verzekeringsgeneeskunde protocol Darmkanker en Diabetes Mellitus; 2009. https://www.nvvg.nl/files/43/02_Boekje_Darmkanker_en_Diabetes_Mellitus.pdf [accessed 16 May 2018].
- 32. Landelijke werkgroep Gastro Intestinale Tumoren . Landelijke Richtlijn Coloncarcinoom, versie 3.0 2014 https://www.oncoline.nl/colorectaalcarcinoom [accessed 16 May 2018].
- 33. ArboNed . Een gezond en vitaal werkend Nederland https://www.arboned.nl/over-arboned [accessed 16 May 2018].
- 34. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015; 162: 735–736. [DOI] [PubMed] [Google Scholar]
- 35. De Staatssecretaris van Sociale Zaken en Werkgelegenheid , de Krom P. Regeling verzekeringsgeneeskundige protocollen arbeidsongeschiktheidswetten http://wetten.overheid.nl/BWBR0019502/2013-01-01 [accessed 16 May 2018].
- 36. De Minister van Landbouw, Nijverheid en Handel , Talma AS. Regeling verzekeringsgeneeskundige protocollen arbeidsongeschiktheidswetten Gegeven op het Loo, den 5den Juni 1913. http://wetten.overheid.nl/BWBR0001888/2016-07-01#AfdelingTweede [accessed 16 May 2018].
- 37. Centraal Bureau voor de Statistiek . Standaard Bedrijfs Indeling 2008 Versie 2018 Structuur: tweede digit en vijfde digit Centraal Bureau voor de Statistiek januari 2018 https://www.cbs.nl/nl-nl/onze-diensten/methoden/classificaties/activiteiten/sbi-2008-standaard-bedrijfsindeling-2008 [accessed 16 May 2018].
- 38. Centraal bureau voor de Statistiek . https://www.cbs.nl/nl-nl/onze-diensten/methoden/begrippen?tab=v#id=voltijdbaan [accessed 16 May 2018].
- 39. European Commission . Commission Staff Working Document on the Implementation of Commission Recommendation of 6 May 2003 Concerning the Definition of Micro, Small and Medium‐sized Enterprises. European Commission: Brussels, 2009. [Google Scholar]
- 40. Steenland K, Deddens JA. A practical guide to dose–response analyses and risk assessment in occupational epidemiology. Epidemiology 2004; 15: 63–70. [DOI] [PubMed] [Google Scholar]
- 41. Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. John Wiley & Sons: Hoboken, 2013. [Google Scholar]
- 42. Steyerberg E. Clinical Prediction Models: a Practical Approach to Development, Validation, and Updating. Springer Science & Business Media: New York, 2008. [Google Scholar]
- 43. Kremer AM, Chorus AM, Wevers CW. TNO Arbeid Kanker en werk. PlantijnCasparie: Hoofddorp, 2002. [Google Scholar]
- 44. Bowling A. What things are important in people's lives? A survey of the public's judgements to inform scales of health related quality of life. Soc Sci Med 1995; 41: 1447–1462. [DOI] [PubMed] [Google Scholar]
- 45. Loisel P, Anema JR. Handbook of Work Disability Prevention and Management. Springer: New York, 2013. [Google Scholar]
- 46. Reiso H, Nygård JF, Jørgensen GS, Holanger R, Soldal D, Bruusgaard D. Back to work: predictors of return to work among patients with back disorders certified as sick: a two‐year follow‐up study. Spine (Phila Pa 1976) 2003; 28: 1468–1473. [DOI] [PubMed] [Google Scholar]
- 47. Ekbladh E, Haglund L, Thorell LH. The worker role interview – preliminary data on the predictive validity of return to work of clients after an insurance medicine investigation. J Occup Rehabil 2004; 14: 131–141. [DOI] [PubMed] [Google Scholar]
- 48. Nieuwenhuijsen K, Bos‐Ransdorp B, Uitterhoeve LL, Sprangers MA, Verbeek JH. Enhanced provider communication and patient education regarding return to work in cancer survivors following curative treatment: a pilot study. J Occup Rehabil 2006; 16: 647–657. [DOI] [PubMed] [Google Scholar]
- 49. de Boer AG, Verbeek JH, Spelten ER, Uitterhoeve AL, Ansink AC, de Reijke TM et al Work ability and return‐to‐work in cancer patients. Br J Cancer 2008; 98: 1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. CJEM 2006; 8: 19–20. [DOI] [PubMed] [Google Scholar]
- 51. Candon D. The effects of cancer on older workers in the English labour market. Econ Hum Biol 2015; 18: 74–84. [DOI] [PubMed] [Google Scholar]
- 52. Werner EL, Merkus SL, Mæland S, Jourdain M, Schaafsma F, Canevet JP et al Physicians' assessments of work capacity in patients with severe subjective health complaints: a cross‐sectional study on differences between five European countries. BMJ Open 2016; 6: e011316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anema JR, Schellart AJ, Cassidy JD, Loisel P, Veerman TJ, van der Beek AJ. Can cross country differences in return‐to‐work after chronic occupational back pain be explained? An exploratory analysis on disability policies in a six country cohort study. J Occup Rehabil 2009; 19: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N et al Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010; 21: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Two cases of fictitious patients using the 1‐ and 2‐year return‐to‐work prediction models
Table S1 Percentages of missing values per excluded candidate predictor


