Abstract
Background and purpose
Muscle‐strengthening, stretching or proprioceptive treatments may slow symptom progression in Charcot—Marie–Tooth (CMT) neuropathy. The aim of the study was to evaluate safety and efficacy of treadmill training in CMT1A.
Methods
We planned a multicenter, prospective, randomized, single‐blind, controlled study. We recruited 53 outpatients affected by CMT1A and randomized them into two treatment groups: one underwent stretching and proprioceptive exercise, whereas the other was additionally treated with treadmill training (TreSPE). Primary outcome measures (OMs) were the walking evaluations and secondary OM was the balance assessment. All participants were assessed at baseline and after 3 and 6 months of treatment.
Results
Most patients showed an improvement in at least one OM after 3 months [42/47 (89.4%)] and 6 months [38/40 (95%)] of treatment. No adverse events were reported in either group.
Conclusions
The most important finding was that both stretching and proprioceptive exercise and treadmill training had an objective benefit on patients affected by CMT disease, without causing overwork weakness. We had a low rate of drop out and did not find deterioration in motor performance. Our results also confirm that applying evidence‐based medicine methods to rehabilitative research is the correct way to test the efficacy of a treatment.
Keywords: aerobic exercise, Charcot–Marie–Tooth disease, evidence‐based medicine, muscle fatigue, rehabilitation
Introduction
Charcot–Marie–Tooth (CMT) neuropathy is the most common inherited neurological disorder. Patients often complain of gait disorders with frequent falls and difficulties in running. To date there is no effective therapy and the efficacy of rehabilitation is unclear. However, physiotherapy may slow the progression of symptoms. In particular, aerobic exercises, muscle‐strengthening, stretching or proprioceptive treatments are possible intervention methods in adult patients affected by CMT disease 1. There is evidence in the literature of the positive effect of aerobic training in patients affected by neuropathy 2, but treadmill training has never been tested in CMT disease. The extent and nature of interventions make research in physical medicine and rehabilitation more complex than in other branches of medicine 3. This is not a trivial point, as the most recent investigations on the effect of rehabilitation in CMT disease managed to find just a few randomized, controlled studies 4. However, several uncontrolled studies showed the positive effect of exercise in CMT disease, testifying that it may include various aspects of rehabilitation 2, 5, 6.
We planned a multicenter, prospective, randomized, single‐blind, controlled study to evaluate the safety and efficacy of aerobic exercise in CMT1A.
We also aimed to understand the maintenance of effects, if any, after the treatment, and to investigate whether a challenging rehabilitation protocol can produce overwork weakness (OW) in patients with CMT disease.
Methods
Patients were recruited in four Italian centers that specialized in hereditary neuropathies. The clinical trial (ClinicalTrials.gov identifier: NCT01289704) was approved by the ethical committees of each center. During the different phases of the project, training and tutorial activities were implemented to enhance synergy and complementarity among the different healthcare professionals. Specific workshops were performed and continuous exchange of information, results and competencies were stimulated, in order to exploit complementarity and synergy of all of the research teams.
Inclusion criteria were clinically and genetically confirmed diagnosis of CMT1A; age between 18 and 75 years; ability to walk without support with or without ankle/foot orthoses; Short Physical Performance Battery (SPPB) scoring between 2 and 10; and ability to sign informed consent.
Exclusion criteria were forms of hereditary neuropathy other than CMT1A; vestibular, psychiatric, cardiovascular and lung disorders or severe arthropathic changes in the lower limbs; and other associated causes of neuropathy.
Between January 2010 and January 2012 we recruited 53 patients affected by CMT1A, who met all of the inclusion criteria and were evaluated at baseline (T0). In each center, a single examiner was responsible for the evaluation.
Protocol description
Participants were blindly randomized into two treatment groups. Randomization was centralized in the coordinating center of Genoa, stratified by center and in blocks of six patients. The first group underwent aerobic exercise on the treadmill along with stretching and proprioceptive exercises (TreSPE). The second group performed only stretching and proprioceptive exercise (SPE). Patients assigned to the SPE group underwent 3 months of treatment consisting of two 60‐min sessions per week of respiratory, proprioceptive and stretching exercise. Patients assigned to the TreSPE group underwent 3 months of treatment consisting of two 60‐min sessions per week of SPE and 30 min of treadmill training. All participants were evaluated at T0 and after 3 months (T1) and 6 months (T2) of treatment. The physiotherapy session lasted for 90 min (treadmill, 30 min; rest, 10 min; respiratory rehabilitation, 25 min; proprioceptive exercises, 25 min), repeated twice a week for 12 weeks. The work on the treadmill consisted of a walk initially performed with a constant load equivalent to 40% of the maximum load reached at the first cardiopulmonary effort test, with 10% increases in the subsequent sessions up to attainment of 70% of the maximum load. The respiratory rehabilitation adopted positive expiratory pressure bottle and expiration with the glottis open in the lateral position. Proprioceptive and postural kinesitherapy to improve balance and coordination were carried out according to the Perfetti method. The balance training consisted of exercises carried out by basculating bars with increasing difficulties in the instruments used and in the tasks required. They were performed close to a hand bar to prevent falls. There was always a therapist supervising.
After recording a detailed medical history, a complete neurological and physical examination was performed. All patients then underwent walking evaluation with a 6‐Minute Walk Test (6MWT) and a 10‐Meter Walk Test (10MWT), chosen as primary outcome measures (OMs). As secondary OMs we decided to assess a subjective evaluation of walking ability with the Walk12 scale, foot strength evaluation of plantar and dorsiflexion with a dynamometer (Citec Technics, Groningen, The Netherlands), balance with the Berg Balance Scale (BBS) and SPPB, and a subjective evaluation of the quality of life through the Medical Outcomes Study Short Form 36 (SF‐36). Disability was evaluated with the CMT Neuropathy Score version 1 and not with its modified form as the trial started before changes were made to the scale. The CMT Neuropathy Score was assessed only at the T0 evaluation as, in a previous 2‐year study of our group 7, no significant changes in the score were observed 8.
Sample size calculation and statistical methods
Power analysis was based on the 10MWT. Hence, the control arm was assumed to have an improvement of 0.5 s in the time needed to walk 10 m (SD, 1 s) and the minimum improvement of clinical interest to detect in the TreSPE arm was set to a minimum of 0.85 s for a total improvement after treatment of ≥1.35 s forthe TreSPE arm. With these assumptions and for a statistical power of 80% and a level of significance of 5% (two‐tailed), a minimum of 23 patients per group were required. Assuming an attrition rate of 10%, at least 26 patients per group were needed.
Mean and SD or median (range) were reported for continuous characteristics. Groups were compared at T0 using independent‐samples Student's t‐test for all quantitative characteristics and chi‐squared test for gender. Linear mixed model with random intercept and corrected per center was used to statistically test the longitudinal change at T1 and T2 of all clinical scales. To assess whether the two treatments had a different effect on longitudinal performances, the interaction term treatment * time was considered in the model. Normality of model residuals was checked graphically. As a skewed distribution was observed for three scales (BBS, SF‐36 limitation of physical role and SF‐36 limitation of emotional role), a linear mixed model was performed after a rank transformation of the scale. P < 0.05 was considered statistically significant. Stata Statistical Software (Release 14. StataCorp LP, College Station, TX, USA). was used for the computation.
Results
A total of 53 participants (32 females; 60.4%) with a mean age (range) of 52.1 (19–69) years were recruited and blindly randomized into the SPE (n = 26) and TreSPE (n = 27) groups. We evaluated 48 patients at T1 and 42 patients at T2 (Fig. 1).
Figure 1.
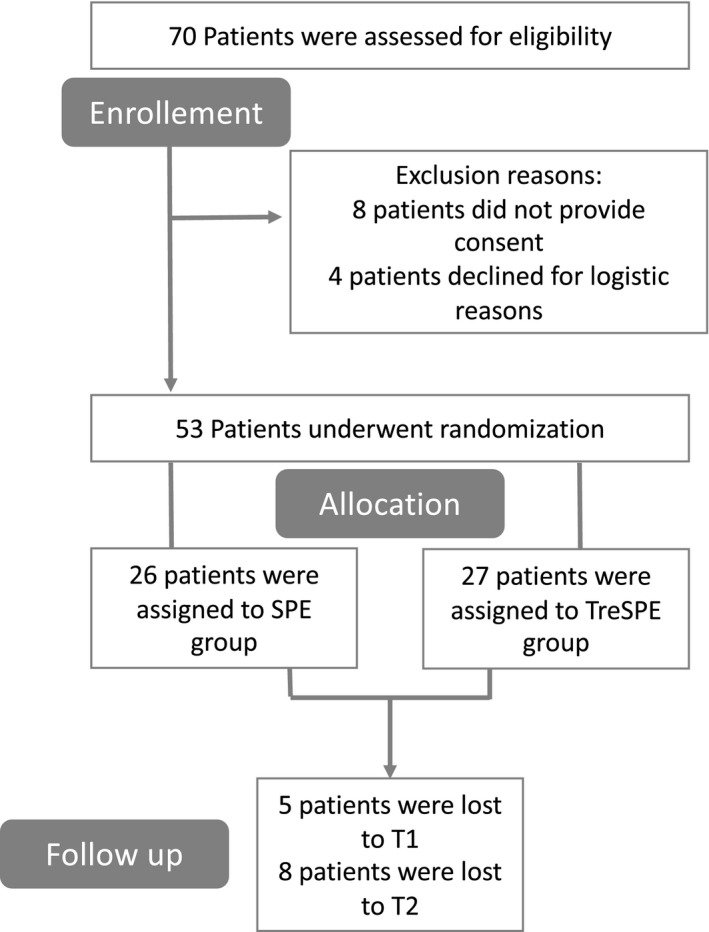
Flow Diagram of a multicenter study to evaluate the safety and efficacy of a rehabilitative treadmill training in CMT1A.
We had a low rate of drop outs; between T0 and T1, four patients were unable to reconcile work tasks with treatment sessions and one patient had family problems; at T2, three patients presented other intercurrent diseases (one patient presented a fracture in the lower limbs and two patients were unable to provide reasonable explanations).
As the intention of the TreSPE study was to test the efficacy of the aerobic exercise in a group of patients with CMT1A, we did not recruit a healthy control group to compare the T0 data.
Baseline demographic and clinical characteristics stratified for treatment arm
No differences were detected between the two groups (Table 1).
Table 1.
Baseline demographic and clinical characteristics
| SPE (n = 26) | TreSPE (n = 27) | P value | |
|---|---|---|---|
| Age (years) | 51.8 (9.6) | 49.9 (13.7) | 0.55 |
| Females | 17 (65.4) | 15 (57.7) | 0.78 |
| Disease duration (years) | 32.2 (19.5) | 28.2 (17.2) | 0.43 |
| CMTNS | 10.1 (3.5) | 10.1 (4.3) | 0.97 |
| BMI | 25.2 (5.6) | 24.5 (3.7) | 0.60 |
| 10MWT (s) | 8.3 (2.3) | 8.4 (2.8) | 0.88 |
| Walk12 | 30.8 (11.3) | 28.1 (10.4) | 0.39 |
| 6MWT (m) | 433 (79.7) | 427.4 (96.7) | 0.82 |
| BBS | 49.5 (6.7) | 47.6 (8.5) | 0.38 |
| Mobility | 9 (1.9) | 8.7 (2.6) | 0.63 |
| Left foot dorsiflexion | 45.1 (36) | 47.6 (36.8) | 0.80 |
| Right foot dorsiflexion | 44.9 (35.8) | 38.8 (33.2) | 0.53 |
| Left foot plantar flexion | 71.7 (51.6) | 74.2 (61.3) | 0.88 |
| Right foot plantar flexion | 72.5 (51.3) | 76.6 (57.4) | 0.79 |
| SF‐36 physical activity | 63.5 (25.4) | 63.7 (27.9) | 0.97 |
| SF‐36 physical role | 53.8 (40.4) | 67.6 (43.2) | 0.24 |
| SF‐36 pain | 59.8 (26.6) | 63.1 (27) | 0.66 |
| SF‐36 general health | 49.7 (18.7) | 57.2 (20.6) | 0.17 |
| SF‐36 vitality | 47.3 (17.2) | 58.5 (17.8) | 0.025 |
| SF‐36 social activity | 60.9 (22.7) | 70.8 (24.7) | 0.14 |
| SF‐36 emotional role | 51.2 (41.4) | 70.3 (40.7) | 0.095 |
| SF‐36 mental health | 60 (19.5) | 70.1 (20) | 0.068 |
6MWT, 6‐Minute Walk Test; 10MWT, 10‐Meter Walk Test; BBS, Berg Balance Scale; BMI, body mass index; CMTNS, CMT Neuropathy Score; SPE, stretching and proprioceptive exercise; SF‐36, Medical Outcomes Study Short Form 36; TreSPE, treadmill training plus SPE. Data are given as n (%).
Longitudinal assessment
Almost all patients showed an improvement in at least one OM at T1 and T2 without differences between the two groups (Table 2). Furthermore, 18/23 in the SPE and 18/24 in the TreSPE group [all: 36/47 (76.6%)] at T1 and 15/19 in the SPE and 19/21 in the TreSPE group [all: 34/40 (85%)] at T2 had a consistent improvement (>25% of change) in at least one performance.
Table 2.
Differences from baseline (T0) after 3 months (T1) and 6 months (T2) of treatment for clinical scales
| Scale | All sample | SPE | TreSPE | P value for difference between groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1–T0 | P value | T2–T0 | P value | T1–T0 | P value | T2–T0 | P value | T1–T0 | P value | T2–T0 | P value | ||
| 6MWT | 17.7 (6.9) | 0.01 | 21.4 (9.8) | 0.03 | 19.5 (10.8) | 0.07 | 27.8 (15.3) | 0.068 | 15.9 (8.9) | 0.075 | 15.7 (12.7) | 0.22 | 0.82 |
| 10MWT | −0.27 (0.10) | 0.008 | −0.19 (0.15) | 0.18 | −0.34 (0.14) | 0.016 | −0.23 (0.21) | 0.26 | −0.21 (0.15) | 0.16 | −0.17 (0.20) | 0.41 | 0.75 |
| Walk12 | 0.22 (1.00) | 0.82 | 1.79 (1.23) | 0.15 | −0.51 (1.5) | 0.73 | 0.75 (2.1) | 0.72 | 0.90 (1.3) | 0.50 | 2.45 (1.41) | 0.083 | 0.79 |
| BBS | 1.46 (0.51) | 0.013 | 0.05 (0.68) | 0.10 | 1.3 (0.85) | 0.09 | −1.05 (0.97); | 0.035 | 1.6 (0.58) | 0.01 | 1.05 (0.92) | 0.17 | 0.023 |
| 0 (0–2) | 0 (−2 to 1) | 0 (−9 to 11) | −1 (−9 to 14) | 1 (−1 to 10) | 0.5 (−11 to 10) | ||||||||
| SPPB | 0.40 (0.15) | 0.026 | 0.10 (0.24) | 0.66 | 0.30 (0.25) | 0.17 | −0.25 (0.36) | 0.57 | 0.48 (0.18) | 0.077 | 0.41 (0.33) | 0.21 | 0.41 |
| Foot dorsiflexion strength | −1.15 (2.54) | 0.66 | −3.96 (3.13) | 0.24 | −1.84 (3.70) | 0.62 | −7.21 (5.17) | 0.16 | −0.52 (3.51) | 0.88 | −0.55 (3.74) | 0.88 | 0.13 |
| Foot plantar flexion strength | 7.72 (4.2) | 0.066 | 10.1 (5.16) | 0.05 | 9.86 (5.92) | 0.096 | 6.97 (5.69) | 0.22 | 5.74 (6.13) | 0.35 | 12.05 (8.07) | 0.14 | 0.57 |
6MWT, 6‐Minute Walk Test; 10MWT, 10‐Meter Walk Test; BBS, Berg Balance Scale SPE, stretching and proprioceptive exercise; TreSPE, treadmill training plus SPE.
Data are given as median (interquartile range) and mean (standard error), estimated from linear mixed model or repeated‐measures anova on ranks (for limitation of physical role and emotional role), BBS, Short Physical Performance Battery (SPPB), etc.
Statistically sgnificant P values are enphasized in bold.
Concerning the primary OMs, the 6MWT showed a similar improvement at T1 in both groups (Table 2), whereas at T2 a further slight improvement was observed in the SPE group, and the TreSPE arm was stable. No significant differences were detected in trend over time between the two groups (P for interaction time * treatment = 0.82). Globally, a significant improvement during follow‐up (P = 0.029) with an estimated mean change of 17.7 m at T1 [standard error (SE), 6.9; P = 0.01] and 21.4 m at T2 (SE, 9.8; P = 0.03) for the whole series of participants was observed. For the 10MWT no significant differences between patients with SPE and TreSPE were revealed (P for interaction time * treatment = 0.75). However, at T1, patients in the SPE group showed a greater improvement than those in the TreSPE group. At T2, similar values were observed. The whole cohort showed a significant change at T1, whereas the T2 performance was not significantly different from T0.
Performances on Walk12 did not significantly change during follow‐up in both groups with no significant differences between the two groups of treatment on performances over time (P for interaction = 0.79) although a greater change was observed at T2 among patients with TreSPE.
With regard to the balance assessment based on the BBS, a significant difference was detected between the SPE and TreSPE groups. In fact, although at T1 both groups showed an improvement, at T2 patients in the TreSPE group seemed to maintain the improvement, whereas patients in the SPE group surprisingly worsened. Considering the whole cohort, at T1 there was a significant mean change from T0, whereas at T2 the mean change was 0.05. On the SPPB, whereas at T1 both groups increased their mean performance, only the TreSPE arm maintained its level at T2. No significant differences were found between the two groups over follow‐up (P for interaction test = 0.41). For the whole cohort, at T1 we found a significant mean change from T0, whereas at T2 a non‐significant mean change from T0 of 0.10 (SE, 0.24) was observed. Regarding strength assessment, we found that overall patients improved their foot plantar strength at T2, at the limit of statistical significance, without a significant difference between the two groups (P for interaction test = 0.57).
Health‐related quality of life measure: Medical Outcomes Study Short Form 36
We did not observe any significant changes during follow‐up for either the entire population or each group, except for the physical activity parameter, where a decrease of scores was assessed after the conclusion of treatment (T2–T0; mean decrease, 8.1; SE, 3.5) (Table 3). For all subscales, no significant differences were noticed in the scores over time when comparing the two treatments.
Table 3.
Differences from baseline (T0) and after 3 months (T1) and 6 months (T2) of treatment for Medical Outcomes Study Short Form 36 (SF‐36) subscales
| SF‐36 domain | All sample (n = 53) | SPE (n = 26) | TreSPE (n = 27) | P value for difference between groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1–T0 | P value | T2–T0 | P value | T1–T0 | P value | T2–T0 | P value | T1–T0 | P value | T2–T0 | P value | ||
| SF‐36 physical activity | −2.6 (2.6) | 0.31 | −8.1 (3.5) | 0.021 | −3.9 (3.6) | 0.28 | −8 (4.9) | 0.10 | −1.4 (3.7) | 0.70 | −8.2 (5) | 0.099 | 0.81 |
| (−5 to 5) | (−15 to 5) | (−10 to 10) | (−15 to 2.5) | (−5 to 5) | (−5 to 5) | ||||||||
| SF‐36 limitation of physical role | −1.5 (5.6) | 0.54 | −1 (7.2) | 0.97 | 0 (6.5) | 0.92 | −2.5 (10.6) | 0.99 | −3.1 (8) | 0.67 | 1.1 (11) | 0.80 | 0.96 |
| (0–0) | (0–0) | (−25 to 0) | (−37.5 to 25) | (0–0) | (0–0) | ||||||||
| SF‐36 physical pain | 1.4 (3) | 0.44 | −2.9 (3.6) | 0.42 | 3.4 (5) | 0.41 | 1.6 (5.7) | 0.78 | −0.6 (4.4) | 0.90 | −6.9 (4.3) | 0.11 | 0.57 |
| (−10 to 12.5) | (−16 to 1) | (−12 to 22) | (−20.5 to 22) | (−5 to 10) | (−12.5 to 0) | ||||||||
| SF‐36 general health | −3.1 (2.1) | 0.14 | −4.1 (2.6) | 0.11 | −1.7 (2.9) | 0.52 | −4.1 (3.8) | 0.28 | −4.5 (3.3) | 0.17 | −3.9 (3.6) | 0.27 | 0.71 |
| (−10 to 5) | (−14 to 6) | (−13 to 10) | (−16 to 7.5) | (−10 to 0.5) | (−9 to 6) | ||||||||
| SF‐36 vitality | 0.17 (2) | 0.93 | −2.9 (2.5) | 0.24 | 1.1 (2.8) | 0.69 | −1.5 (3.5) | 0.67 | −0.8 (3) | 0.78 | −4.3 (3.5) | 0.21 | 0.83 |
| (−5 to 10) | (−5 to 5) | (−5 to 10) | (−7.5 to 5) | (−5 to 5) | (−5 to 5) | ||||||||
| SF‐36 social activity | −1.4 (3.1) | 0.64 | −3.2 (3.8) | 0.40 | 2.7 (4.2) | 0.52 | −0.8 (5) | 0.87 | −5.4 (4.5) | 0.23 | −5.8 (5.6) | 0.31 | 0.41 |
| (−12 to 12.5) | (−12 to 12.5) | (−12 to 25) | (−13 to 13) | (−6 to 0.5) | (−12 to 12.5) | ||||||||
| SF‐36 limitation of emotional role | 7.1 (5.7) | 0.22 | 2 (7) | 0.78 | 4.4 (7.9) | 0.40 | 3.4 (9.7) | 0.94 | 8.4 (7.8) | 0.40 | 1.5 (11.3) | 0.94 | 0.77 |
| (0–33) | (0–33) | (0–0) | (0–33.2) | (0–33) | (0–0) | ||||||||
| SF‐36 mental health | −1.8 (2.4) | 0.45 | −4.1 (2.8) | 0.15 | −2.9 (3.5) | 0.41 | −5.1 (3.5) | 0.15 | −0.8 (4.3) | 0.81 | −3.6 (4.3) | 0.39 | 0.91 |
| (−28 to 27) | (−32 to 31) | (−28 to 27) | (−28 to 35.5) | (−30 to 26) | (−32 to 31) | ||||||||
| SF‐36 PCS (standardized) | −1 (1.1) | 0.37 | −2.2 (1.3) | 0.10 | −0.4 (1.5) | 0.81 | −1.6 (2) | 0.40 | −1.6 (1.6) | 0.32 | −2.5 (1.8) | 0.17 | 0.85 |
| (−4.8 to 3.2) | (−4.5 to 2.6) | (−3.4 to 5) | (−6.4 to 4) | (−5.1 to 1.8) | (−2.5 to 2) | ||||||||
| SF‐36 MCS (standardized) | 0.55 (1.35) | 0.69 | −0.58 (1.56) | 0.71 | 0.6 (1.9) | 0.74 | −0.2 (1.9) | 0.93 | 0.4 (1.9) | 0.83 | −1.2 (2.3) | 0.62 | 0.95 |
| (−3.5 to 7.3) | (−4.7 to 5) | (−3.2 to 7.9) | (−4.9 to 5.7) | (−3.7 to 4.2) | (−3.7 to 4.5) | ||||||||
MCS, mental composite score; PCS, physical composite score; SPE, stretching and proprioceptive exercise; TreSPE, treadmill training plus SPE. Data are given as mean (standard error), estimated from linear mixed model, and 25th–75th percentiles.
Statistically sgnificant P values are enphasized in bold.
Discussion
We chose to select only patients with CMT1A, as it is the most common variant and shares clinical features with other types of CMT disease. The observation that all participants improved in at least one OM at the different time‐points unequivocally confirms the importance of rehabilitation in patients with CMT disease. In fact, walking performances are better in both patients undergoing the TreSPE and SPE protocols. Our results also demonstrate that the strict rules of evidence‐based medicine may be applied to a rehabilitative trial in CMT disease. We ensured the blinding of the assessors in order to reduce the risk of bias 3. Furthermore, the fact that, in the present study, only four Italian centers specializing in CMT disease were involved guaranteed the correct execution of both the assessment and execution of the two treatments.
We found a significant improvement in walking and balance assessment in both groups. The observed amelioration at T2 in the 6MWT in the SPE group may support that SPE is sufficient to improve walking ability in patients with CMT disease and confirms the validity and sensitivity of this OM 8, 9, 10. The fact that we did not observe differences between the two groups at different follow‐up times may suggest that treadmill training does not add improvement to the conventional treatment. However, we showed that balance improved at the first follow‐up in the TreSPE group only, which may be explained because staying upright requires adaptive control of dynamic balance and adapts to a continuous perturbation in gait. Nardone et al. found more impaired static and dynamic control of balance when neuropathy affects the small afferent fibers in addition to the large afferent fibers, and suggested that diminished somatosensory input from the smaller fibers plays a critical role in the modulation of the support phase of gait, rather than muscle weakness or foot deformity 11. It is nevertheless possible that the power of the study was not sufficient to capture a further improvement caused by treadmill training and, despite CMT disease being characterized by lower‐limb disability, patients selected in the present study were not sufficiently compromised to require the aid of an adjunctive therapy in addition to the conventional therapy. It is interesting to note that the quality of Life did not improve despite some significant reported changes.
Preliminary experiments on patients with CMT disease showed that lung‐function tests were not affected 12, suggesting that patients may undergo aerobic exercises without fearing secondary effects. However, some authors suggest that it could be useful to educate the patient on lifestyle modifications and energy conservation techniques along with the progression of the disease, and low‐to‐moderate‐intensity exercise should be regularly encouraged as it may entail systemic health benefits 5, 6, 13. As the tendency to deteriorate was shown after a 6‐month follow‐up, some authors suggest undergoing two periods of rehabilitation per year in order to prevent the regression of the improvements obtained. Likewise, a home‐based resistance training exercise program focused on exercises specific to activities of daily living may improve performance in patients with CMT disease, although the results are variable 14, 15. However, patients with CMT disease should be part of a multidisciplinary plan of care to manage each impairment to its fullest and to achieve maximum functional benefit 6. Treadmill training, which to our knowledge has never been used in this population, has been proven to be well tolerated and not burdened by OW. OW is another common finding in neuromuscular disorders 13 that may require adaptation and utilization of energy conservation techniques. Although there is conflicting evidence on the OW in CMT disease, recent findings suggest that it may not be a common occurrence. Exercise intolerance and undue fatigue are common complaints in CMT disease. Reduced physical ability is due directly to the disease, but it is also due to physical deconditioning 16. The authors suggest that low‐to‐moderate‐intensity exercise should be regularly encouraged as it should have overall systemic health benefits and low‐intensity exercise appears to be more beneficial for patients with neuromuscular disease when compared with high‐resistance or high‐intensity exercise 6. Accordingly, our results suggest that this is a crucial point when designing rehabilitation programs and suggesting patterns of activities of daily living, because it means that patients should not limit themselves to prevent additional weakness. We had a low drop‐out rate and we did not find deterioration in motor performances or in quality of life.
The main limitation of the trial is a lack of control group or a usual care group to account for natural fluctuations in disease progression and heterogeneity of CMT disease. At the first session, we set the exercise intensity using the initial treadmill speed but, as the training period progressed, the work load required to exercise to maximum capacity may have changed. Moreover, given the strict inclusion criteria, patients were recruited who could tolerate the protocol, thus excluding the most compromised patients. It could be very interesting to treat more severely affected patients.
Conclusions
Rehabilitation treatment produces an objective benefit in people with CMT disease, both proprioceptive and stretching exercise as well as treadmill training. Moreover, we recommend advising patients with CMT disease to undergo rehabilitation treatment to prevent secondary impairments, maintain articular range of movement, avoid pain and contractures, and maximize remaining abilities.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
Acknowledgements
This work was supported by Telethon‐UILDM (grant number GUP09013). It was presented at the XVI S.I.R.N. congress (7–9 April 2016), the 44th S.I.M.F.E.R. congress (23–26 October 2016), the 6th International Charcot–Marie–Tooth and Related Neuropathy Consortium Meeting (8–10 September 2016) and the 2017 PNS Annual Meeting – Peripheral Nerve Society (8–12 July 2017). We thank all of the patients who participated in the trial.
Appendix 1. TreSPE study group.
Marina Grandis (Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa; IRCCS Ospedale Policlinico San Martino, Genoa, Italy), Giovanni Maggi (IRCCS Ospedale Policlinico San Martino, Genoa, Italy), Riccardo Zuccariono (Servizio di Riabilitazione Malattie Neuromuscolari, Ospedale ‘La Colletta’, Arenzano, Genoa, Italy), Lucio Marinelli (Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa; IRCCS Ospedale Policlinico San Martino, Genoa, Italy), Carlo Trompetto (Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa; IRCCS Ospedale Policlinico San Martino, Genoa, Italy), Deborah Scorsone (IRCCS Ospedale Policlinico San Martino, Genoa, Italy), Angelo Montesano (Fondazione Don Carlo Gnocchi Onlus, Milan, Italy), Davide Cattaneo (Fondazione Don Carlo Gnocchi Onlus, Milan, Italy), Eleonora Casati (Fondazione Don Carlo Gnocchi Onlus, Milan, Italy), Nicola Smania (Neuromotor and Cognitive Rehabilitation Research Center, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy), Annalisa Brugnera (Neuromotor and Cognitive Rehabilitation Research Center, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy), Carla Fontana (Neuromotor and Cognitive Rehabilitation Research Center, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy) and Daniele Munari (Neuromotor and Cognitive Rehabilitation Research Center, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy).
The copyright line for this article was changed on 23 October 2019 after original online publication.
Contributor Information
L. Mori, Email: laura.mori@unige.it.
TreSPE study group:
Marina Grandis, Giovanni Maggi, Riccardo Zuccariono, Lucio Marinelli, Carlo Trompetto, Deborah Scorsone, Angelo Montesano, Davide Cattaneo, Eleonora Casati, Nicola Smania, Annalisa Brugnera, Carla Fontana, and Daniele Munari
References
- 1. Sman AD, Hackett D, Fiatarone Singh M, Fornusek C, Menezes MP, Burns J. Systematic review of exercise for Charcot‐Marie‐Tooth disease. J Peripher Nerv Syst 2015; 20: 347–362. [DOI] [PubMed] [Google Scholar]
- 2. Lencioni T, Rabuffetti M, Piscosquito G, et al Postural stabilization and balance assessment in Charcot‐Marie‐Tooth 1A subjects. Gait Posture 2014; 40: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villamar M, Contreras V, Kuntz R, Fregni F. The reporting of blinding in physical medicine and rehabilitation randomized controlled trials: a systematic review. J Rehabil Med 2013; 45: 6–13. [DOI] [PubMed] [Google Scholar]
- 4. Corrado B, Ciardi G, Bargigli C. Rehabilitation management of the Charcot–Marie–Tooth Syndrome. Medicine (Baltimore) 2016; 95: e3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anens E, Emtner M, Hellström K. Exploratory study of physical activity in persons with Charcot‐Marie‐Tooth Disease. Arch Phys Med Rehabil 2015; 96: 260–268. [DOI] [PubMed] [Google Scholar]
- 6. McCorquodale D, Pucillo EM, Johnson NE. Management of Charcot‐Marie‐Tooth disease: improving long‐term care with a multidisciplinary approach. J Multidiscip Healthc 2016; 9: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pareyson D, Reilly MM, Schenone A, et al Ascorbic acid in Charcot–Marie–Tooth disease type 1A (CMT‐TRIAAL and CMT‐TRAUK): a double‐blind randomised trial. Lancet Neurol 2011; 10: 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mori L, Prada V, Signori A, et al Outcome measures in the clinical evaluation of ambulatory Charcot Marie Tooth 1A subjects. Eur J Phys Rehabil Med 2019; 55: 47–55. [DOI] [PubMed] [Google Scholar]
- 9. Padua L, Pazzaglia C, Pareyson D, et al Novel outcome measures for Charcot‐Marie‐Tooth disease: validation and reliability of the 6‐min walk test and StepWatch™ Activity Monitor and identification of the walking features related to higher quality of life. Eur J Neurol 2016; 23: 1343–1350. [DOI] [PubMed] [Google Scholar]
- 10. Solari A, Laurà M, Salsano E, Radice D, Pareyson D, CMT‐TRIAAL Study Group . Reliability of clinical outcome measures in Charcot‐Marie‐Tooth disease. Neuromuscul Disord 2008; 18: 19–26. [DOI] [PubMed] [Google Scholar]
- 11. Nardone A, Corna S, Turcato AM, Schieppati M. Afferent control of walking: are there distinct deficits associated to loss of fibres of different diameter? Clin Neurophysiol 2014; 125: 327–335. [DOI] [PubMed] [Google Scholar]
- 12. Carter GT. Phrenic nerve involvement in Charcot‐Marie‐Tooth. Muscle Nerve 1995; 18: 1215–1216. [PubMed] [Google Scholar]
- 13. Vinci P, Esposito C, Perelli SL, Antenor JAV, Thomas FP. Overwork weakness in Charcot‐Marie‐Tooth disease. Arch Phys Med Rehabil 2003; 84: 825–827. [DOI] [PubMed] [Google Scholar]
- 14. Chetlin RD, Gutmann L, Tarnopolsky M, Ullrich IH, Yeater RA. Resistance training effectiveness in patients with Charcot‐Marie‐Tooth disease: recommendations for exercise prescription. Arch Phys Med Rehabil 2004; 85: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 15. Ramdharry GM, Pollard A, Anderson C, et al A pilot study of proximal strength training in Charcot‐Marie‐Tooth disease. J Peripher Nerv Syst 2014; 19: 328–332. [DOI] [PubMed] [Google Scholar]
- 16. El Mhandi L, Millet GY, Calmels P, et al Benefits of interval‐training on fatigue and functional capacities in Charcot–Marie–Tooth disease. Muscle Nerve 2008; 37: 601–610. [DOI] [PubMed] [Google Scholar]


