Abstract
We examined the efficacy, safety, and tolerability of ONO‐4474 in Japanese patients with osteoarthritis (OA) of the knee. In this multicenter, placebo‐controlled, randomized, double‐blind, parallel‐group comparative study, patients with moderate to severe OA who were refractory to nonsteroidal anti‐inflammatory drugs were orally administered 100 mg of ONO‐4474 twice daily for 28 days. The primary end point was knee pain during walking, assessed by visual analog scale over 24 hours (VAS24). Treatment‐emergent adverse events (TEAEs) and adverse drug reactions were reported for safety. In total, 110 patients were randomized (1:1) to receive placebo or ONO‐4474. The mean (standard deviation) change in VAS24 scores at week 4 was −26.9 (25.0) mm in the ONO‐4474 group and −19.5 (19.6) mm in the placebo group. The difference (ONO‐4474 group − placebo group) in posterior mean change in VAS24 at week 4 was −5.8 (posterior standard deviation, 4.4; 95% confidence interval, −14.3 to 2.8) mm. TEAEs were reported in 41.8% of patients in the ONO‐4474 group and 18.2% of patients in the placebo group. The most common TEAEs in the ONO‐4474 group related to the musculoskeletal system and the peripheral and central nervous systems were myalgia (7.3%), arthralgia (5.5%), dizziness (3.6%), and hypoesthesia (3.6%). Four patients from the ONO‐4474 group and 1 patient from the placebo group discontinued treatment because of AEs; however, none were judged to be serious, and all patients recovered or were recovering after discontinuation. ONO‐4474 is a novel tropomyosin receptor kinase inhibitor that has an analgesic effect in patients with OA.
Keywords: analgesic, nerve growth factor, nociceptive pain, osteoarthritis, phase 2 randomized controlled trial, tropomyosin receptor kinase inhibitor
Nonsteroidal anti‐inflammatory drugs (NSAIDs) and opioids are the main therapies prescribed for osteoarthritis (OA) pain. However, caution is advised, as the long‐term use of NSAIDs significantly increases the risk of gastrointestinal, cardiovascular, and renal adverse effects, especially in elderly patients.1, 2 In Japan, opioids are not generally prescribed due to concerns about opioid abuse and addiction, and physicians often report difficulty in the safe administration of opioids because of the potential for abuse.1, 2 Additionally, adverse events (AEs) such as nausea, vomiting, constipation, and dizziness are common. Thus, more effective and safer analgesics for the long‐term treatment of moderate to severe pain in OA are needed.
Nerve growth factors (NGFs) have recently been identified as important mediators of pain. These endogenous ligands are substrates for tropomyosin receptor kinase (Trk), a receptor‐type tyrosine kinase expressed in cells of the peripheral and central nervous systems.3 Increased expression of NGF has been reported in cartilage and synovial membrane tissue in patients with OA.4, 5, 6 Several clinical trials have demonstrated the analgesic effects of anti‐NGF antibodies.7, 8, 9, 10 For example, tanezumab,9 fulranumab,7 and fasinumab8 have shown potent analgesic effects in patients with moderate to severe OA of the knee and hip. However, the long half‐life of anti‐NGF antibodies presents a challenge for dose titration, which may be necessary in managing adverse effects. Furthermore, concerns were raised in 2010 regarding events of unanticipated joint deterioration in anti‐NGF‐treated patients that were initially reported to be osteonecrosis; however, subsequent analyses showed that these events instead were a result of a rapidly progressive form of OA. Although there was a dose‐response relationship with anti‐NGF treatment and incidence of rapidly progressing OA, it is notable that the incidence of OA increased 3‐fold when anti‐NGF drugs were concomitantly administered with NSAIDs.11
ONO‐4474 is a selective and orally available TrkA, TrkB, and TrkC inhibitor with low blood‐brain barrier penetration.12 In preclinical studies, ONO‐4474 dose‐dependently inhibited NGF‐induced increases in vascular permeability, and dermal blood flow and hyperalgesia in rats12, 13 and monkeys (unpublished data). Furthermore, in a rat model of monosodium iodoacetate‐induced OA, ONO‐4474 demonstrated potent analgesic effects, comparable with the effects of morphine, at the dose required to control spontaneous motor activity.12, 13 Safety, tolerability, and pharmacokinetics were also investigated in a phase 1 study conducted in adult Japanese men, in which healthy volunteers underwent a 14‐day repeat oral administration of twice‐daily ONO‐4474 100 mg, 200 mg, or placebo (data not shown). Plasma ONO‐4474 trough concentrations reached steady state after the second dose at both dose levels. Moreover, 12 hours after the final dose of twice‐daily 100 mg, plasma ONO‐4474 concentrations exceeded or were comparable to the trough concentration at which ONO‐4474 showed an adequate pharmacodynamic response in a rat OA model or a monkey NGF‐induced pharmacodynamic response model. Although the incidence of AEs was higher in the ONO‐4474 group than in the placebo group, there were no deaths, serious AEs, or AEs leading to withdrawal of the study drug.
Based on the above findings, a twice‐daily 100‐mg dose of ONO‐4474 was selected for the present study involving OA patients. The objectives of this study were to determine the efficacy, safety, and tolerability of repeated oral dosing of ONO‐4474 in Japanese OA patients with insufficient response to NSAID therapy.
Methods
Ethics
This study was conducted in compliance with the ethical principles derived from the Declaration of Helsinki; the protocol stipulated by the Japanese Pharmaceutical and Medical Device Act; the standards stipulated in Article 14, Paragraph 3, Article 80‐2 of the Pharmaceutical Affairs Law; and Good Clinical Practice guidelines. This study was approved by the institutional review boards of the participating centers. All participants provided written informed consent before entering the study.
Study Design
This was a multicenter, placebo‐controlled, randomized, double‐blind, parallel‐group comparative study across 8 sites (listed in the Acknowledgments). The study design is shown in Figure S1. In brief, this consisted of 3 periods: a 2‐week screening period, followed by a 4‐week double‐blind treatment period and a 2‐week observational follow‐up period. ONO‐4474 was administered as 100 mg twice daily after meals, for a total daily dose of 200 mg. The dose was selected to exceed a trough plasma concentration of ONO‐4474 that demonstrated a sufficient pharmacologic effect in a preclinical rat OA model and NGF‐induced monkey model (unpublished data).
The randomization manager performed randomization using a permuted block method with a 1:1 ratio. The patient and provider were blinded, as the study drugs were sealed individually before allocation at the registration center, and patient eligibility was confirmed by the principal investigator or subinvestigator.
Patients
This early phase 2 study included adult Japanese patients with OA who met all of the inclusion criteria. Eligible patients were Japanese, aged 40 to <75 years; had a body mass index of 18.5 to <39.0 kg/m2; had knee symptoms for ≥6 months and were diagnosed with OA ≥6 months prior to the start of the screening period; had knee pain caused by OA for ≥4 days a week in ≥3 months before the start of the screening period; met the clinical criteria of OA as defined by the American College of Rheumatology Criteria14 at the time of screening; were classified as Kellgren‐Lawrence Grade 2‐4 according to X‐ray radiography at the time of screening; with insufficient analgesic effects of NSAIDs used continuously at a consistent dose and administered ≥14 days prior to the start of the screening period; and had average knee pain (visual analog scale over 24 hours [VAS24])15 during walking of ≥40 mm and <80 mm (100‐mm scale) when using an e‐patient diary at the start of the screening period. At the time of randomization, patients also had to have knee pain (VAS24) during walking of ≥50 mm and <90 mm. Additionally, for patients who discontinued pain medication during the screening period, an increase in the VAS24 of ≥10 mm was required between screening and randomization.
Patients who met any of the following exclusion criteria were not eligible: secondary OA due to distinct causes such as musculoskeletal diseases (eg, rheumatoid arthritis and fibromyalgia) and other rheumatic diseases; artificial joint replacement before or during the study period; history of or concurrent articular diseases accompanied by pain other than OA (eg, inflammatory arthritis, rapidly progressive OA, osteonecrosis, and osteoporotic bone fracture); arthroendoscopy within 3 months before screening; concurrent diseases accompanied by pain that may influence pain assessment (eg, hip OA, spinal degenerative disease, sciatica, headache, or external injury such as bone fracture); previous joint injection of hyaluronic acid 1 month before screening or a joint injection of an adrenocortical steroid 3 months prior; strong opioids, oral steroids, analgesics other than NSAIDs, or pharmaceutical‐grade chondroitin sulfate 28 days before screening; and history of mental illness/psychosis or related complications.
Prior and Concomitant Medications
Prohibited prior and concomitant medications for OA patients included any analgesic other than rescue treatments with acetaminophen. Conditionally allowed concomitant treatments included acetaminophen at a dose of 300 to 500 mg (total maximum daily dose of 1500 mg) for the treatment of knee pain from OA from the start of the screening period until the end of the follow‐up period and at follow‐up; pharmaceutical‐grade chondroitin sulfate for the purpose of pain relief was permitted from ≥29 days before screening until completion of all observations in the follow‐up period, if continued without any changes to the dose and mode of administration.
End Points
The primary end point was average knee pain during walking assessed by the VAS24. VAS24 was assessed once daily before bed using the e‐patient diary from the start of the screening period until the penultimate day of the follow‐up period. Efficacy was also analyzed using the Western Ontario and McMaster Universities OA Index (WOMAC) questionnaire (Japanese version 11 translated from English version 3.1), the Patient Global Assessment (PGA) score, and the frequency of rescue treatment use. WOMAC and PGA were assessed by VAS at weeks 0, 2, and 4 (or at time of discontinuation) and at follow‐up.
Safety end points included a physical examination, 12‐lead electrocardiogram (ECG) measurements, general laboratory tests (hematologic, blood biochemistry, and urine test), the Columbia‐Suicide Severity Rating Scale (C‐SSRS, Japanese version finalized July 10, 2015, translated from English version, dated January 14, 2009), a neurological exam, treatment‐emergent adverse events (TEAEs), and adverse drug reactions (ADRs). Physical examination, 12‐lead ECG, general laboratory tests, and neurological exam were assessed at weeks 0, 2, 4 (or at time of discontinuation) and at follow‐up. The C‐SSRS was assessed at weeks 2 and 4 and at follow‐up or at time of discontinuation. TEAEs and ADRs were assessed from week 0 until the end of the follow‐up period.
Sample Size Calculation
A sample size of 50 OA patients per group was planned: 100 patients were randomized in a 1:1 ratio to the ONO‐4474 group and the placebo group. If 10% of patients were excluded from the full analysis set, it was expected that at least 45 participants in each treatment group would be included in the VAS24 analysis at week 4.
A meta‐analysis was performed by Ono Pharmaceutical using the data from clinical studies of tramadol.16, 17, 18 A Bayesian approach was selected to allow flexible judgment based on 2 criteria of posterior probability, described as follows. The difference between the tramadol group and the placebo group at week 4 was −10.8 mm (2‐sided 95% confidence interval [CI], −13.4 mm to −8.2 mm). Assuming that the difference between the ONO‐4474 group and the placebo group (delta) is the same as the previously calculated difference (−10.8 mm) and that the standard deviation (SD) of each treatment group is 25.0 mm, a Bayesian posterior probability was evaluated based on the following 2 criteria: that the posterior probability of a delta value below 0 mm was 90% and that the posterior probability of a delta value below −8.2 mm was 50%. The probability of achieving the first criterion was approximately 78%, the probability of achieving the second criterion was approximately 69%, and the probability of achieving both criteria was approximately 69%. The assumption of a 25.0‐mm SD for each treatment group was based on data from Bellamy et al,19 which reported a VAS score SD of 23.0 mm, and later studies by DeLemos et al17 and Gana et al,18 in which we calculated the SD (∼26‐27 mm) based on their standard error values.
Statistical Analyses
The safety analysis set included all patients who received ONO‐4474 at least once. The full analysis set included all enrolled patients who were included in the safety analysis set and had evaluable efficacy data collected at least once after the start of the study treatment. Summary statistics of the number of patients, mean, and SD were calculated for baseline characteristics.
The primary efficacy analysis was conducted by calculating the posterior mean change in VAS24 from baseline and corresponding 95%CIs using an analysis of covariance for repeated measures model. Treatment groups, time points, and the interaction between time points and treatment groups were factors, with baseline values as the covariate. Nonstructural covariance was assumed for correlation among repeated measures data. The posterior probabilities for a difference between groups of <0 mm (criterion 1) and <−8.2 mm (criterion 2) were calculated. The least squares mean change from baseline was calculated for WOMAC and PGA efficacy end points. Statistical analyses were performed using SAS software version 9.3 (SAS Institute, Inc., Cary, North Carolina).
Results
Patient Baseline Characteristics and Disposition
A total of 153 patients were enrolled, of which 110 patients were randomized (1:1) to receive placebo or ONO‐4474 (n = 55 in each group). The patient disposition is shown in Figure 1. The baseline patient characteristics are shown in Table 1. The mean VAS24 at the time of registration in the ONO‐4474 group (72.8 ± 9.1 mm) was similar to that in the placebo group (71.2 ± 8.5 mm) (Table 2).
Figure 1.
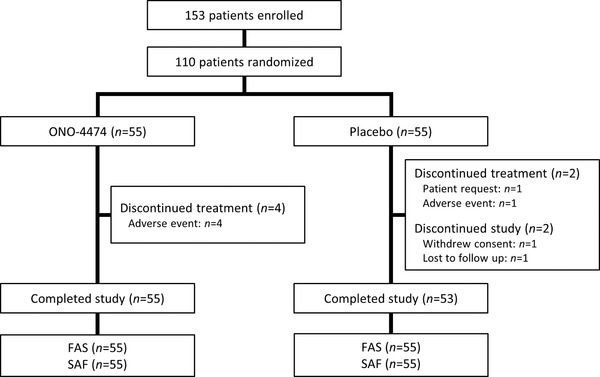
Patient disposition. Patients who discontinued treatment were still considered to have completed the study. FAS, full analysis set; SAF, safety analysis set.
Table 1.
Baseline Patient Characteristics
| Baseline Demographics | ONO‐4474 (n = 55) | Placebo (n = 55) |
|---|---|---|
| Sex, n (%) | ||
| Male | 18 (32.7) | 24 (43.6) |
| Female | 37 (67.3) | 31 (56.4) |
| Age, y | ||
| Mean (SD) | 61.3 (7.3) | 62.5 (8.9) |
| Body weight, kg | ||
| Mean (SD) | 67.38 (15.32) | 66.16 (14.49) |
| BMI, kg/m2 | ||
| Mean (SD) | 25.95 (4.16) | 25.60 (4.43) |
| Duration of OA, y | ||
| Mean (SD) | 4.72 (4.29) | 4.84 (5.80) |
| Kellgren‐Lawrence grade, n (%) | ||
| 2 | 19 (34.5) | 23 (41.8) |
| 3 | 28 (50.9) | 20 (36.4) |
| 4 | 8 (14.5) | 12 (21.8) |
| Use of rescue treatment in screening period, n (%) | ||
| Yes | 28 (50.9) | 23 (41.8) |
| No | 27 (49.1) | 32 (58.2) |
BMI, body mass index; OA, osteoarthritis; SD, standard deviation.
Table 2.
Efficacy Analysis of ONO‐4474
| Week 6 (2 Weeks After | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 2 | Week 4 | Treatment) | |||||
| ONO‐4474 | Placebo | ONO‐4474 | Placebo | ONO‐4474 | Placebo | ONO‐4474 | Placebo | |
| VAS24, mm | ||||||||
| N | 55 | 55 | 52 | 49 | 49 | 50 | 48 | 48 |
| Mean (SD) | 72.8 (9.1) | 71.2 (8.5) | 56.9 (21.1) | 56.7 (18.6) | 45.7 (23.6) | 51.8 (21.1) | 56.4 (20.5) | 55.9 (19.0) |
| Mean change from baseline (SD) | – | – | −16.1 (21.0) | −14.8 (16.7) | −26.9 (25.0) | −19.5 (19.6) | −16.4 (19.0) | −14.8 (16.9) |
| WOMAC, mm | ||||||||
| N | 55 | 54 | 51 | 52 | 50 | 52 | 50 | 49 |
| Mean (SD) | 60.3 (14.0) | 59.3 (13.5) | 48.5 (23.1) | 44.6 (18.7) | 38.1 (24.0) | 42.8 (18.7) | 47.1 (22.3) | 44.8 (19.3) |
| Mean change from baseline (SD) | – | – | −11.7 (16.7) | −14.3 (16.0)a | −21.3 (19.2) | −16.8 (16.0)a | −12.7 (16.8) | −14.2 (16.5)b |
| WOMAC (pain), mm | ||||||||
| N | 55 | 54 | 51 | 52 | 50 | 52 | 50 | 49 |
| Mean (SD) | 62.3 (12.4) | 61.0 (13.1) | 48.7 (22.3) | 45.0 (18.2) | 38.1 (23.4) | 42.6 (18.7) | 46.5 (22.0) | 45.4 (18.1) |
| Mean change from baseline (SD) | – | – | −13.6 (17.1) | −15.1 (18.3)a | −23.5 (21.1) | −18.0 (18.5)a | −15.0 (18.3) | −14.6 (18.3)b |
| PGA, mm | ||||||||
| N | 55 | 55 | 53 | 52 | 50 | 52 | 51 | 50 |
| Mean (SD) | 68.9 (11.7) | 65.7 (11.1) | 52.4 (25.8) | 50.7 (20.3) | 38.8 (25.3) | 44.9 (21.4) | 53.7 (22.0) | 49.0 (21.6) |
| Mean change from baseline (SD) | – | – | −17.2 (24.6) | −14.2 (20.0) | −29.6 (25.5) | −20.6 (21.7) | −14.9 (19.4) | −16.4 (22.8) |
PGA, Patient Global Assessment; SD, standard deviation; VAS24, visual analog scale over 24 hours; WOMAC, Western Ontario and McMaster Universities OA Index.
n = 51.
n = 48.
Efficacy Analysis
The change in VAS24 from baseline is shown in Figure 2. The difference (ONO‐4474 group − placebo group) in the posterior mean change in VAS24 from baseline was −5.8 (posterior SD, 4.4; 95%CI, −14.3 to 2.8) mm at week 4 (Table 3). The Bayesian posterior probability with a between‐group difference of <0 mm was 90.6%, which met the expected probability of 90%. However, the Bayesian posterior probability for a between‐group difference of <−8.2 mm was 29.2%, which did not meet the expected probability of 50%. Following treatment, the ONO‐4474 group VAS24 score was similar to that of the placebo group.
Figure 2.
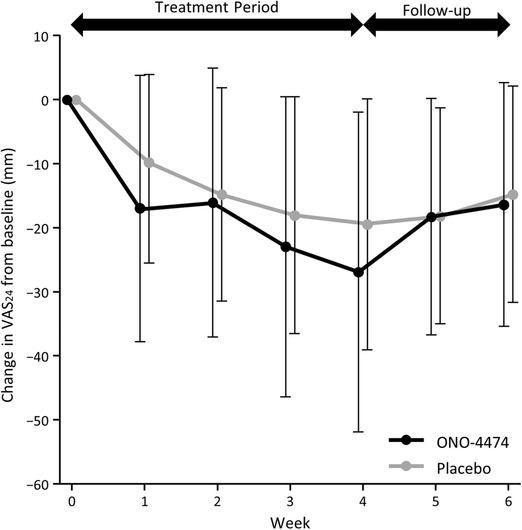
Change in VAS24 from baseline. VAS24, visual analog scale over 24 hours.
Table 3.
Mean Change and the Difference (ONO‐4474 − Placebo) in Mean Change From Baseline to Week 4 in VAS24, WOMAC, and PGA Scales
| ONO‐4474 | Placebo | |
|---|---|---|
| VAS24, mm | ||
| N | 49 | 50 |
| Posterior mean change (posterior SD) | −25.5 (3.1) | −19.8 (3.1) |
| Difference in posterior mean change (posterior SD) | −5.8 (4.4) | |
| 95%CI for difference in posterior mean | −14.3 to 2.8 | |
| Bayesian posterior probability with the difference <0 mm | 90.6% | |
| Bayesian posterior probability with the difference <−8.2 mm | 29.2% | |
| WOMAC, mm | ||
| N | 50 | 51 |
| LS mean change (SD) | −20.4 (2.4) | −16.8 (2.5) |
| Difference in LS mean change (SD) | −3.6 (3.5) | |
| 95%CI for difference in LS mean change | −10.5 to 3.3 | |
| WOMAC (pain), mm | ||
| N | 50 | 51 |
| LS mean change (SD) | −22.4 (2.7) | −18.3 (2.7) |
| Difference in LS mean change (SD) | −4.1 (3.8) | |
| 95%CI for difference in LS mean change | −11.7 to 3.5 | |
| PGA, mm | ||
| N | 50 | 52 |
| LS mean change (SD) | −27.9 (3.2) | −21.4 (3.2) |
| Difference in LS mean change (SD) | −6.4 (4.5) | |
| 95%CI for difference in LS mean change | −15.4 to 2.6 | |
CI, confidence interval; LS, least squares; PGA, Patient Global Assessment; SD, standard deviation; VAS24, visual analog scale over 24 hours; WOMAC, Western Ontario and McMaster Universities OA Index.
Covariance analysis was performed for repeated measures data with treatment groups, time points, and the interaction between time points and treatment groups as factors, and baseline values as the covariate. Nonstructural covariance was assumed for correlation among repeated measures data.
The differences (ONO‐4474 group − placebo group) in the least squares mean change in the total WOMAC score and WOMAC (pain) score from baseline were −3.6 (SD, 3.5; 95%CI, −10.5 to 3.3) and −4.1 (SD, 3.8; 95%CI, −11.7 to 3.5) mm, respectively, at week 4 (Table 3). The difference (ONO‐4474 group − placebo group) in the least squares mean change in PGA from baseline was −6.4 (SD, 4.5; 95%CI, −15.4 to 2.6) mm at week 4 (Table 3).
The number of patients with a VAS24 response of ≥30%, ≥50%, or ≥70% below baseline is shown in Figure 3. Overall, the responder rate tended to be higher in the ONO‐4474 group compared with the placebo group.
Figure 3.
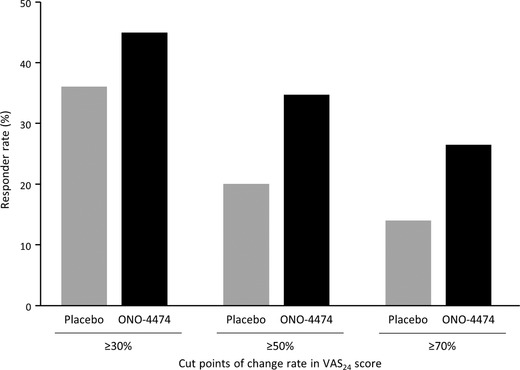
Treatment response rates at week 4. Responder rates are shown according to number of patients with VAS24 scores ≥30%, ≥50%, and ≥70% below baseline. VAS24, visual analog scale over 24 hours.
The rate of patients who received rescue medication during the treatment period (ONO‐4474, 34.5%; placebo, 41.8%) and the total amount (ONO‐4474: mean, 725.5; SD, 1320.9 mg; placebo: mean, 1285.5; SD, 2535.3 mg) and average milligrams per day of rescue medication (ONO‐4474: mean, 27.1; SD, 49.3 mg; placebo: mean, 48.1; SD, 95.2 mg) was greater in the placebo group than in the ONO‐4474 group.
Safety Analysis
TEAEs occurred in 23 patients (41.8%) in the ONO‐4474 group and in 10 patients (18.2%) in the placebo group (Table 4). ADRs occurred in 16 patients (29.1%) in the ONO‐4474 group and 7 patients (12.7%) in the placebo group (Table 4).
Table 4.
Summary of TEAEs and ADRs
| ONO‐4474 | Placebo | |
|---|---|---|
| Adverse Event | (n = 55) | (n = 55) |
| Total TEAEs, n (%) | 23 (41.8) | 10 (18.2) |
| Serious TEAEs, n (%) | – | 1 (1.8) |
| Discontinued treatment due to TEAEs, n (%) | 4 (7.3) | 1 (1.8) |
| Total ADRs, n (%) | 16 (29.1) | 7 (12.7) |
| Serious ADRs, n (%) | – | – |
| Discontinued treatment due to ADRs, n (%) | 3 (5.5) | 1 (1.8) |
ADR, adverse drug reaction; TEAE, treatment‐emergent adverse event.
The most frequent TEAE and ADR was myalgia, which occurred in 4 patients (7.3%) in the ONO‐4474 group (Table 5). The most frequent TEAEs and ADRs in the placebo group were hematuria and dyspepsia, both of which occurred in 2 patients (3.6%) (Table 5).
Table 5.
Most Common Adverse Events (>3%)
| TEAE | ADR | |||
|---|---|---|---|---|
| Adverse Event | ONO‐4474 | Placebo | ONO‐4474 | Placebo |
| SOC, Preferred Terma | (n = 55) | (n = 55) | (n = 55) | (n = 55) |
| Gastrointestinal disorders | ||||
| Constipation | 2 (3.6) | – | 2 (3.6) | – |
| Dyspepsia | – | 2 (3.6) | – | 2 (3.6) |
| Infections and infestations | ||||
| Viral upper respiratory tract infection | 3 (5.5) | 1 (1.8) | – | – |
| Investigations | ||||
| Blood urine present | 1 (1.8) | 2 (3.6) | 1 (1.8) | 2 (3.6) |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 3 (5.5) | 1 (1.8) | 2 (3.6) | 1 (1.8) |
| Myalgia | 4 (7.3) | – | 4 (7.3) | – |
| Nervous system disorders | ||||
| Dizziness | 2 (3.6) | – | 2 (3.6) | – |
| Hypoesthesia | 2 (3.6) | 1 (1.8) | 2 (3.6) | 1 (1.8) |
| Skin and subcutaneous tissue disorders | ||||
| Rash | 2 (3.6) | – | 2 (3.6) | – |
ADR, adverse drug reaction; SOC, system organ class; TEAE, treatment‐emergent adverse event.
Medical Dictionary for Regulatory Activities, Japanese Translation, version 20.0.
TEAEs related to the peripheral and central nervous systems included dizziness (3.6%) and hypoesthesia (3.6%) in the ONO‐4474 group, with 1 incidence (1.8%) of hypoesthesia occurring in the placebo group (Table S1). All were mild in severity.
TEAEs related to the musculoskeletal system included myalgia (7.3%; 5 events in 4 patients), arthralgia (5.5%; 3 events in 3 patients), and pain in the extremity (1.8%; 1 event in 1 patient) in the ONO‐4474 group (Table S1). Of the 5 myalgia TEAEs, 3 were mild and 2 were moderate in severity. Of the 3 arthralgia TEAEs, 1 was mild, 1 was moderate, and 1 was severe, with 2 patients requiring treatment. In the placebo group, 1 arthralgia TEAE occurred in 1 patient (1.8%). All TEAEs were ADRs, except for arthralgia in 1 patient, and were mild and resolved without further treatment.
Administration of the study drug was discontinued due to TEAEs in 4 patients (7.3%) in the ONO‐4474 group (2 events of myalgia, 2 events of arthralgia, and 1 event each of herpes zoster, headache, hypoesthesia, pruritus, and rash) and in 1 patient (1.8%) in the placebo group (1 event each of diarrhea, dyspepsia, and nausea).
One serious AE (aortic dissection) occurred in 1 patient (1.8%) in the placebo group, and a relationship with the study drug was assessed as “not related.” No clinically significant events regarding vital signs, 12‐lead ECG, laboratory tests, or C‐SSRS were observed, and no deaths occurred in this study.
Discussion
This study evaluated the efficacy, safety, and tolerability of the novel tropomyosin receptor kinase inhibitor ONO‐4474 as an analgesic in patients with moderate to severe OA and who were refractory to NSAID therapy. Twice‐daily administration of 100‐mg ONO‐4474 was well tolerated and was confirmed here, for the first time, to have an analgesic effect in patients with knee OA. However, a total daily dose of 200 mg of ONO‐4474 did not meet the expected efficacy of a weak opioid, as categorized in Japan.
The long‐term use of oral NSAIDs and opioids for the treatment of OA pain comes with significant safety issues, and therefore the development of a more suitable therapy is needed.1, 2 NSAIDs increase the risk of ADRs related to the gastrointestinal tract, cardiac vessels, and kidneys, particularly in elderly patients. However, opioid use comes with a significant concern of dependency and addiction, as well as other safety issues, including nausea, vomiting, constipation, and dizziness.
In a phase 3 study of combination tramadol and acetaminophen therapy, AEs were observed in 80.1% of participants after 2 weeks of administration.20 In comparison, the incidence of AEs in OA patients receiving ONO‐4474 was only 41.8% in this phase 2 study. Furthermore, 13.4% of patients withdrew after 2 weeks from the phase 3 tramadol and acetaminophen study (JNS013‐JPN‐04) because of AEs. In comparison, TEAEs, which are more commonly associated with oral NSAIDs and opioids, were not common in this phase 2 study, and the withdrawal rate of the study drug due to TEAEs was low (7.3%).
In this study, the primary efficacy end point was to determine the posterior mean change of VAS24 from baseline between ONO‐4474 treatment and placebo. The posterior probability that the difference between treatment groups was <0 mm was 90.6%, and this met the expected probability of 90%. This suggests that ONO‐4474 is likely to have an analgesic effect in patients with knee OA. However, the Bayesian posterior distribution calculation (29.2%) did not meet an expected probability of 50%, which is comparable (−8.2 mm) with that of a weak opioid. Furthermore, we observed that following the treatment period, the VAS24 score in the ONO‐4474 group was similar to the score in the placebo group, thus justifying the appropriateness of the primary end point evaluation. Similar changes were observed in WOMAC and PGA scores, suggesting that the analgesic effect of ONO‐4474 may lead to improvements in WOMAC and PGA scores.
The study limitations include the small sample size, short treatment duration, and lack of an active comparator. In the future, the clinical significance of twice‐daily ONO‐4474 at a higher total daily dose of 400 mg in Japanese patients with OA should be studied. It is hoped that this future study will clarify the efficacy and safety of ONO‐4474 relative to NSAIDs and weak opioids in the treatment of OA pain.
Conclusions
Based on the results of this small study, ONO‐4474 was well tolerated overall and may offer an improved safety profile compared with NSAIDs or weak opioids, although further research is necessary to confirm these findings. ONO‐4474 was confirmed to have an analgesic effect in patients with knee OA, as was observed by the change of VAS24 after 4 weeks of treatment. These findings suggest that ONO‐4474 may be a tolerable and effective analgesic for the treatment of moderate to severe knee pain in OA patients.
Conflicts of Interest
N.I. received research funding from Astellas Pharma Inc., AbbVie GK, Asahi Kasei Corporation, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Kaken Pharmaceutical Co., Ltd., Medical Corporation Sanjinkai, Medical Corporation Toukoukai, Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., and Zimmer Biomet G.K.; and lecture fees from Astellas Pharma Inc., Eli Lilly Japan K.K., Daiichi Sankyo Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Bristol‐Myers Squibb Company. S.O., R.H., and K.Y. are employees of Ono Pharmaceutical Co., Ltd.
Supporting information
Figure S1
Table S1
Acknowledgments
We acknowledge the following investigators who contributed to the data collection for this study: Dr. Koichi Nakamura, Medical Director, Clinical Research Hospital, Tokyo; Dr. Takafumi Yoshida, Executive Medical Director, Applied Bio‐Pharmatech Kurume Clinical Pharmacology Clinic; Dr. Hirotaka Nagashima, Director, Shinjuku Research Park Clinic; Dr. Kenichi Murase, Director, Nishikasai Orthopedic Surgery and Rheumatology Clinic; Dr. Shunji Matsuki, Deputy Director, Clinical Research Center, Souseikai Fukuoka Mirai Hospital; Dr. Masaharu Hori, Clinical Pharmacology Center, Souseikai Nishikumamoto Hospital; Dr. Masanari Omata, Director, Ooimachi Orthopaedic Surgery Clinic; and Dr. Manabu Nakayama, Director, Yokohama Motomachi Clinic. We thank Dr. James Graham of Edanz Medical Writing for providing medical writing support.
Funding
Funding for these studies was provided by Ono Pharmaceutical Co., Ltd.
Data Sharing
Data for this study cannot be made publicly available.
Author Contributions
N.I., R.H., and K.Y. designed the study. All authors analyzed and interpreted the data, discussed the results, and provided commentary on the manuscript. All authors approved the final version of this manuscript.
References
- 1. O'Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non‐opioid and opioid analgesics. Am J Geriatr Pharmacother. 2012;10(6):331‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarberg B, Tenzer P. Complexities in the pharmacologic management of osteoarthritis pain. Curr Med Res Opin. 2013;29(5):539‐548. [DOI] [PubMed] [Google Scholar]
- 3. Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25(11):1386‐1403. [DOI] [PubMed] [Google Scholar]
- 4. Iannone F, De Bari C, Dell'Accio F, et al. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford). 2002;41(12):1413‐1418. [DOI] [PubMed] [Google Scholar]
- 5. Ramos YFM, den Hollander W, Bovée JVMG, et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PLoS One. 2014;9(7):e103056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 2014;66(11):3018‐3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanga P, Katz N, Polverejan E, et al. Efficacy, safety, and tolerability of fulranumab, an anti‐nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain. 2013;154(10):1910‐1919. [DOI] [PubMed] [Google Scholar]
- 8. Tiseo PJ, Kivitz AJ, Ervin JE, Ren H, Mellis SJ. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double‐blind, placebo‐controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155(7):1245‐1252. [DOI] [PubMed] [Google Scholar]
- 9. Schnitzer TJ, Ekman EF, Spierings EL, et al. Efficacy and safety of tanezumab monotherapy or combined with non‐steroidal anti‐inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis. 2015;74(6):1202‐1211. [DOI] [PubMed] [Google Scholar]
- 10. Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage. 2015;23(suppl 1):S8‐S17. [DOI] [PubMed] [Google Scholar]
- 11. Hochberg MC. Serious joint‐related adverse events in randomized controlled trials of anti‐nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage. 2015;23(suppl 1):S18‐S21. [DOI] [PubMed] [Google Scholar]
- 12. Nagaura T, Yasuhiro T, Oda K, et al. Selective and peripheral‐specific Trk inhibitor shows potent analgesic effect comparable to morphine in rat osteoarthritis model without CNS toxicity. American College of Rheumatology 2014 ACR/ARHP Annual Meeting; 2014. Abstract 267.
- 13. Yasuhiro T, Oda K, Nagaura T, Mitsui K, Katsumata S, Hirota Y. A pan‐Trk inhibitor with low brain penetration inhibits NGF‐induced pharmacological responses, and exerts analgesic effect comparable to morphine in rat osteoarthritis pain model. Society for Neuroscience, Neuroscience 2015 Meeting; 2015. Abstract 239.01.
- 14. Salehi‐Abari I. 2016 ACR revised criteria for early diagnosis of knee osteoarthritis. Autoimmune Dis Ther Approaches. 2016;3(1):118. [Google Scholar]
- 15. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S240‐S252. [DOI] [PubMed] [Google Scholar]
- 16. Babul N, Noveck R, Chipman H, Roth SH, Gana T, Albert K. Efficacy and safety of extended‐release, once‐daily tramadol in chronic pain: a randomized 12‐week clinical trial in osteoarthritis of the knee. J Pain Symptom Manage. 2004;28(1):59‐71. [DOI] [PubMed] [Google Scholar]
- 17. DeLemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended‐release once‐daily in the treatment of osteoarthritis of the knee and/or hip: a double‐blind, randomized, dose‐ranging trial. Am J Ther. 2011;18(3):216‐226. [DOI] [PubMed] [Google Scholar]
- 18. Gana TJ, Pascual ML, Fleming RR, et al. Extended‐release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double‐blind, placebo‐controlled clinical trial. Curr Med Res Opin. 2006;22(7):1391‐1401. [DOI] [PubMed] [Google Scholar]
- 19. Bellamy N, Kean WF, Buchanan WW, Gerecz‐Simon E, Campbell J. Double blind randomized controlled trial of sodium meclofenamate (Meclomen) and diclofenac sodium (Voltaren): post validation reapplication of the WOMAC Osteoarthritis Index. J Rheumatol. 1992;19(1):153‐159. [PubMed] [Google Scholar]
- 20. Matsushita T, Yabuki S, Ushida T, et al. Phase 3 clinical study of a tramadol hydrochloride/acetaminophen combination tablet in patients with chronic osteoarthritis pain or chronic low back pain: a randomized withdrawal, double‐blind, parallel‐group, placebo‐controlled study. Rinsho Seikei Geka. 2011;46:825‐853. [In Japanese] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1


