Abstract
Blindness due to corneal diseases is a common pathology affecting up to 23 million individuals worldwide. The tissue‐engineered anterior human cornea, which is currently being tested in a Phase I/II clinical trial to treat severe corneal trophic ulcers with preliminary good feasibility and safety results. This bioartificial cornea is based on a nanostructured fibrin–agarose biomaterial containing human allogeneic stromal keratocytes and cornea epithelial cells, mimicking the human native anterior cornea in terms of optical, mechanical, and biological behavior. This product is manufactured as a clinical‐grade tissue engineering product, fulfilling European requirements and regulations. The clinical translation process included several phases: an initial in vitro and in vivo preclinical research plan, including preclinical advice from the Spanish Medicines Agency followed by additional preclinical development, the adaptation of the biofabrication protocols to a good manufacturing practice manufacturing process, including all quality controls required, and the design of an advanced therapy clinical trial. The experimental development and successful translation of advanced therapy medicinal products for clinical application has to overcome many obstacles, especially when undertaken by academia or SMEs. We expect that our experience and research strategy may help future researchers to efficiently transfer their preclinical results into the clinical settings.
Keywords: advanced therapy medicinal products (ATMPs), clinical translation, cornea, preclinical research, regulatory issues, tissue‐engineered anterior human cornea (TEAHC), tissue engineering
List of abbreviations
- AEMPS
Spanish Agency of Medicines and Medical Devices
- AIAT
Andalusian Initiative for Advanced Therapies
- ARVO
Association for Research in Vission and Ophthalmology
- ATMPs
advanced therapy medical products
- EPXMA
electron‐probe X‐ray microanalysis
- EST
expressed sequence tag
- FDR
False Discovery Rate
- GCP
Good Clinical Practice
- GMP
good manufacturing practice
- GO
Gene Ontology
- ICH
International Conference Harmonization
- IP
Intellectual property
- IRB
Institutional Review Board
- OCT
optical coherence tomography
- PCR
Polimerase Chain Reaction
- SME
Small or medium Enterprise
- TEAHC
tissue‐engineered anterior human cornea
- UV
ultraviolet
1. INTRODUCTION
Tissue engineering and regenerative medicine (TERM) have achieved important advancements in the last decades, bringing new therapeutic possibilities by improving restoration of tissues and biological functions using therapeutic cells and tissues (Harrison, St‐Pierre, & Stevens, 2014). Multiple models of advanced therapy medicinal products (ATMPs) have been generated in the laboratory, and tissue engineering and regenerative medicine technology has been applied to various tissues and organs, offering a paradigm shift from conventional donor organ or tissue transplantation. However, the number of patients treated so far suggests an important imbalance between scientific innovation and clinical translation (Martin, Ireland, Baldomero, & Passweg, 2015). The development of new ATMPs is not straightforward, as many challenges have been observed during the past years: for example, traditional approaches may not be applicable, and the characterization of the product itself may pose some problems (Celis et al., 2015; Cuende & Izeta, 2010).
Cornea transplantation—keratoplasty—is the most frequent transplant worldwide (Whitcher, Srinivasan, & Upadhyay, 2001). However, despite overall good success rates, results are suboptimal in high‐risk patients with inflammation and severe pathologies (e.g., chemical burns, autoimmune diseases, previously rejected grafts, or peripheral thinning), and transplants long‐term survival is approximately 60% at 10 years (Coster & Williams, 2005). Additionally, a severe shortage of good quality donor corneas is seen in many countries with only one cornea available for 70 needed (Gain et al., 2016; Wong, Kam, Chen, & Young, 2017). Hence, there is a need to develop alternative solutions when facing this disease, such as corneal bioengineering.
For over a decade now, our group has been developing an organotypic substitute of the anterior human cornea. The human cornea is a transparent, 0.5‐mm thick, nonvascular organ whose main functions are to (a) protect the inner eye tissues against external agents and (b) to provide an essential refractive power to allow focusing of light into the eye. It is composed of five layers: the epithelium, Bowman's layer, the stroma, the Descemet membrane, and the endothelium (Meek & Knupp, 2015). Our allogeneic tissue‐engineered anterior human cornea (TEAHC) is a two‐layer construct composed of stromal keratocytes immersed in a fibrin–agarose scaffold and corneal epithelial cells seeded onto the surface of the scaffold (Miguel Alaminos et al., 2006). To improve biomechanical properties, the TEAHC is subjected to a plastic compression (nanostructuration), based on methods previously described, without affecting cell viability (De La Cruz Cardona et al., 2011; Hadjipanayi et al., 2011; Ionescu et al., 2011; Scionti et al., 2014).
Traditional corneal transplantation is the only widely accepted treatment to restore sight in patients suffering from severe corneal blindness; however, shortage of good quality donor corneas is prevalent (Gain et al., 2016). Furthermore, in high‐risk clinical cases (chemical burns, inflammatory/autoimmune diseases, previously rejected grafts, peripheral thinning, etc.), donor corneal transplantation is not an option due to complications and high failure rates (Yu et al., 2014). Tissue‐engineered corneas like TEAHC present numerous advantages over eye bank native corneas: controlled manufacturing avoiding the need for expensive individual screening tests, high volume production with readily available bioengineered corneal substitutes, or specific tailoring of the corneal substitute's biomechanical, optical, or functional properties.
In this report, we provide details of our successful experience in the development and clinical translation of a tissue engineering product for the treatment of eye surface disorders. From its preclinical development planning, and complementary research as advised by the Spanish Agency of Medicines and Medical Devices (AEMPS), to the adaption of production protocols to obtain a clinical‐grade product, and eventually the design of a small‐scale Phase I/II clinical trial that is currently ongoing (NCT01765244, registered January 9, 2013; EudraCT 2010‐024290‐40; Miguel González‐Andrades et al., 2017). Although not the first (Pellegrini et al., 2018), this is a significant development in the field of tissue engineering providing the roadmap for other ATMPs developers in the area to navigate over the complex scientific and regulatory hurdles on the way to human clinical trials.
2. METHODS
2.1. Generation of a bioengineered anterior lamellar human cornea (TEAHC)
A bioengineered substitute of the anterior human cornea was generated at the Tissue Engineering Group of the University of Granada using fibrin–agarose nanostructured sheets as previously described (Gonzalez‐Andrades et al., 2009). Prior local IRB approval of the production validation protocol was obtained, as it involved human cadaveric samples. Briefly, small biopsies of the human scleral limbus were obtained according to the protocols established by the Spanish National Organization for Human Transplantation. For the use of human sclero‐corneal limbi, which are normally by‐products of the corneal transplant surgery or donor corneas with suboptimal quality for keratoplasty, a written approval consent form was obtained from the legal representatives. Limbal explant methods were used to generate primary cell cultures of epithelial cells, whereas the stromal keratocytes were isolated from fragments of corneal stroma attached to the sclero‐corneal limbus. Both cell types are allogeneic but not necessarily from the same donor. Epithelial and stromal cell cultures were then isolated and expanded in specific culture media using a sequential culture technique. Keratocytes were immersed within a hydrogel consisting of human plasma obtained from blood donors and 0.1% Type VII agarose. Fibrin was obtained from frozen plasma of human blood donors (provided and certified by the “Centro de Transfusión, Tejidos y Células,” Granada, under current national regulations; Real Decreto‐ley 9/2014). Tranexamic acid was used to prevent fibrinolysis, and calcium chloride was added to induce jellification of the biomaterial. Cornea epithelial cells were then seeded onto the surface of the constructed stromal model. When epithelial cells reached confluence, stratification and differentiation of the epithelial cell layer is promoted using air–liquid culture techniques on cell culture inserts with 0.4 μm porous membranes (Transwell, Corning‐Costar, Corning, NY, USA). Once generated, the TEAHC was subjected to plastic compression (nanostructuration, patent P200930625) in order to improve the biomechanical properties of the product (Figure 1a). This protocol was then adapted to fulfill all regulatory requirements for ATMP manufacturing according to good manufacturing practice (GMP) standards (clinical‐grade therapeutic product), as described below.
Figure 1.
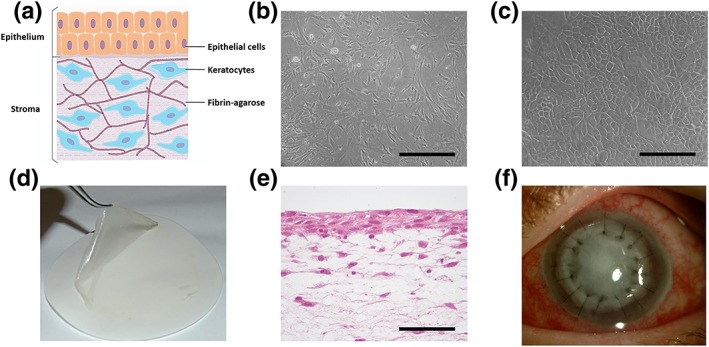
Primary cell cultures and tissue‐engineered anterior human cornea (TEAHC) generated by tissue engineering. (a) Schematic structure of the TEAHC. (b) Phase‐contrast microscopic images of cultured stromal keratocytes. (c) Phase‐contrast microscopic images of cultured corneal epithelial cells. (d) Macroscopic image of the artificial cornea after being subjected to nanostructuration. (e) Histological analysis of the artificial cornea stained with hematoxylin and eosin. (f) TEAHC implanted in the corneal lesion using interrupted 10–0 nylon sutures. Scale bar: 100 μm [Colour figure can be viewed at http://wileyonlinelibrary.com] [Colour figure can be viewed at http://wileyonlinelibrary.com]
2.2. In vitro product characterization
The first step of the characterization process is identifying the features of both isolated cell types in culture as well as in the final product itself. For histological analysis, TEAHC‐bioengineered corneas were fixed in 4% formaldehyde, dehydrated in an ethanol series, and embedded in paraffin. Cross sections were cut, 4 μm thick, stained with hematoxylin and eosin, and examined with a light microscope. On the other hand, phase‐contrast microscopy was used as a method for morphological evaluation of primary cultured cells. Under these conditions, stromal keratocytes display an elongated spindle‐like shape and a good proliferation rate in culture. In contrast, cultured corneal epithelial cells exhibit a typical cobblestone morphology (Figure 1b,c; Miguel Alaminos et al., 2006).
Product identity is essentially founded in our previously published studies with artificial corneas (Garzón et al., 2014; Gonzalez‐Andrades et al., 2009). Briefly, these studies showed that the TEAHC had the appearance of a thin translucent membrane (Figure 1d), structurally analogue to native corneal tissue (Figure 1e). Corneal epithelial cells had the ability to form a normal stratified layer of differentiated cells with well‐developed intercellular junctions (desmosomes, tight, and gap junctions) due to air‐contact stimuli. These data were consistent with an appropriate process of cell and tissue differentiation as determined by immunohistochemistry and mRNA microarrays (Gonzalez‐Andrades et al., 2009). Regarding the latter, total RNA corresponding to bioengineered corneas and primary cultures of epithelial cells and keratocytes (2 samples of each type) was extracted using the Qiagen RNeasy System (Qiagen, Mississauga, Ontario, Canada), following the manufacturer's recommendations, and comprehensive genome‐wide gene expression analysis was carried out to quantify the global gene expression in each sample. Results of the genome‐wide analysis are publically available at the public repository Gene Expression Omnibus ref. GSE86584.
TEAHC samples were compared against both epithelial cells and keratocytes. Overall, considering TEAHC as the baseline, 3,205 genes were found to be differentially expressed (DEGs), 1,200 of them being upregulated in TEAHC. DEGs were obtained applying a cut threshold of false discovery rate <0.05 and a fold change value of ±2. Figure 5a shows the hierarchical clustering of the 3,205 DEGs.
Figure 5.
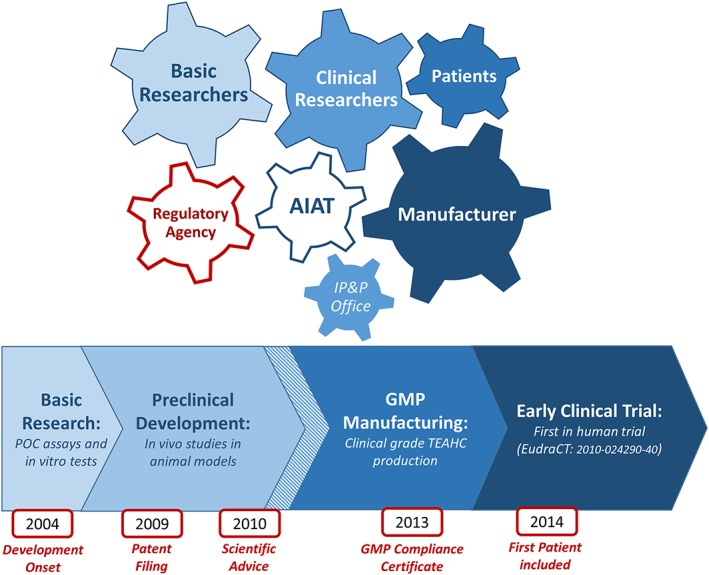
Product development and clinical translation process of the tissue‐engineered anterior human cornea (TEAHC). The translation of an advanced therapy medicinal product from basic research into a clinical‐grade product applicable in a human clinical trial is a complex, integrated activity that requires multidisciplinary expertise involving basic researchers, clinicians, manufacturing facilities, technology transfer offices, regulatory agencies, and patient organizations. The whole clinical translational process is facilitated by entities like the Andalusian Initiative for Advanced Therapies that promotes communication among strategic stakeholders and coordinates the activities of the research teams involved in the development of the TEAHC [Colour figure can be viewed at http://wileyonlinelibrary.com]
First, we analyzed the gene expression of a set of genes of specific interest in corneal epithelium development. TEAHC showed similar expression of genes JUP (PKG3, plakoglobin 3), DSG3 (desmoglein 3), DSP (desmoplakin), TJP2 (ZO‐2, zonula occludens 2), SPRR1 (Small Proline Rich Protein 1B), and GJA4 (CX37, connexin 37), as compared with primary cultures of corneal epithelial cells, thus confirming the immunohistochemistry findings and indicating the presence of efficient adherent, tight, and gap cell–cell junctions in the artificial corneal epithelium. Gene expression of other important genes involved in the morphogenesis of an epithelium such as KLK7, KRT16, KRT18, KRT24, or FGF7 are also shown (Figure 5b).
Concerning potency, as corneal epithelium acts as a potent ultraviolet (UV) light filter, we evaluated the TEAHC capacity to act as a shield against potential oxidative injury to intraocular tissues. The TEAHC stroma showed proper UV light‐absorption capabilities (Ionescu et al., 2010). Moreover, the TEAHC also resembled the biomechanical behavior—in terms of viscoelasticity, resistance and elasticity—and optical transparency of the native cornea with a stable, resilient, and elastic matrix (De La Cruz Cardona et al., 2011; Ionescu et al., 2011; Scionti et al., 2014).
Additionally, it is necessary to ensure that the primary cell cultures are viable, both after several passages (two passages for keratocytes, epithelial cells are obtained from limbal explant methods) and at the moment of its use. In this regard, we used electron‐probe X‐ray microanalysis (EPXMA) as a very sensitive method to determine cell viability on subcultured cornea cell cultures on pioloform‐covered plated gold grids as previously published (Garzón, Carriel, et al., 2012; M. Alaminos et al., 2007). To determine total ion content of each cell type—epithelial cells and keratocytes—we used the peak‐to‐local background ratio method with reference to standards composed of 20% dextran containing known amounts of inorganic salts. All determinations were performed on the central area of the cell nucleus. The intracellular concentration of each ion is associated to cell viability, therefore the K/Na ratio above 3 of both cell types indicated a high viability after the expansion passages (Table 1). This microanalysis was only performed in the primary cell culture, as the application of EPXMA in 3‐D tissues is not possible with the current technology (M. Alaminos et al., 2008, 2007; Garzón, Carriel et al., 2012; Rodriguez‐Morata et al., 2008).
Table 1.
Electron‐probe X‐ray microanalysis of cell viability
| Ions | Epithelial cells | Keratocytes |
|---|---|---|
| Na | 79.5 ± 32.2 | 40.1 ± 19.5 |
| Mg | 16.8 ± 4.5 | 18.9 ± 5.3 |
| P | 216.0 ± 22.1 | 284.3 ± 44.1 |
| S | 53.5 ± 12.3 | 62.3 ± 17.4 |
| Cl | 182.0 ± 58.3 | 143.5 ± 32.6 |
| K | 289.7 ± 45.4 | 439.7 ± 73.3 |
| Ca | 9.7 ± 5.4 | 17.7 ± 10.7 |
| K/Na | 3.6 | 11.0 |
Note. Intracellular concentrations of the major cell ions in primary cultures of cornea epithelial cells and keratocytes as determined by electron‐probe X‐ray microanalysis. Values correspond to average ± standard deviation (in mmol/kg dry weight), except for the dimensionless K/Na ratio.
Finally, we further analyzed cell function, viability, and tumorigenic potential of the TEAHC by determining mRNA expression levels of a few specific genes, following AEMPS recommendations. As shown in Figure 2c, all samples expressed high levels of the cell proliferation marker PCNA, especially the primary cell cultures, and low levels of the pro‐apoptotic gene CASP9. Crystallin gene LDHA, involved in corneal transparency, was highly expressed by both cell cultures and the TEACH (Figure 2c; Miguel Alaminos et al., 2006). These results complemented our aforementioned data regarding intercellular junctions and other differentiation‐related genes. Expression levels of TERT, MRAS, and C‐MYC genes were not different among conditions (Figure 2c).
Figure 2.
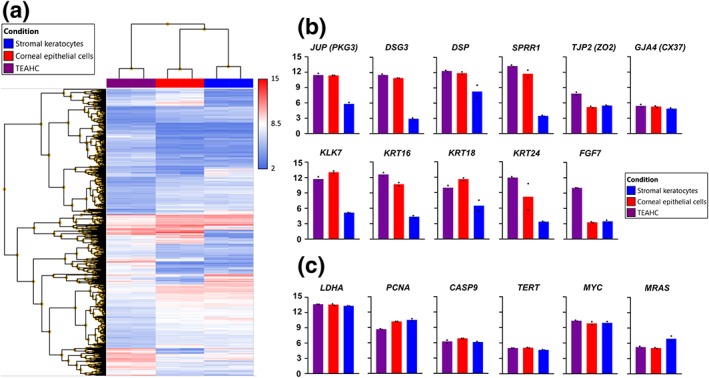
Microarray profiling of mRNA expression in primary cell cultures and tissue‐engineered anterior human cornea (TEAHC). (a) Hierarchical clustering of the 3,205 differentially expressed genes (condition FDR F‐test <0.05 and fold change ±2) in TEAHC compared with epithelial cells and keratocytes. (b) Selected genes related to corneal epithelium development and morphogenesis. (c) Selected genes related to corneal transparency (LDHA), proliferation (PCNA), apoptosis (CASP9), and malignant transformation (TERT, C‐MYC, and MRAS) [Colour figure can be viewed at http://wileyonlinelibrary.com]
2.3. In vivo evaluation in a rabbit model
The rabbit eye is a gold‐standard model for ophthalmic research because of its relatively large size and its practicality in ophthalmic surgical procedures. Currently, the albino New Zealand rabbit is one of the most accepted animal models for evaluating the biocompatibility of intraocular implants and lenses. In vivo analysis of the ATMP was performed by grafting this bioengineered tissue on the eye surface of New Zealand rabbits by anterior lamellar keratoplasty. Initial in vivo results presented for the scientific advice request to the AEMPS were completed and further confirmed with data from new in vivo studies carried out to increase the number of New Zealand rabbits to a total of 17. In each animal, the left eye was operated and the right eye was left as control. First, animals were deeply anesthetized by intramuscular injection of xylazine (5 mg/kg) and ketamine (25 mg/kg), and proparacaine drops were then instilled as local anesthesia during and after the surgical procedure. Then, one third of the total thickness of the central cornea was microsurgically removed from the left cornea. To do so, a partial thickness trephination was performed with a keratotome, followed by intrastromal manual dissection with a sharp crescent and a blunt spatula. After the native anterior cornea was removed, the TEAHC‐bioengineered cornea was trimmed to the diameter of the corneal defect with a trephine and surgically implanted at the defect site using 10/0 nylon stitches. The animals were followed for up to 12 months after implantation, and the following clinical parameters were evaluated: presence of edema, neovascularization, infection, inflammation, and tumorigenesis. For evaluation, the animals were examined using a slit lamp and by optical coherence tomography (OCT). This research was approved by the institutional experimentation committee (approval numbers 01‐09‐15‐313 and 25‐06‐2018‐099), and all animals were treated according to the national and international rules of animal welfare, including the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
The TEAHC proved to be manageable and suturable to the recipient bed, showing adequate strength and elasticity for surgical manipulation (Carriel, Garzón, Alaminos, & Campos, 2011). As preliminary evidence of efficacy, we observed recovery of corneal integrity and a progressive improvement of transparency levels, especially after 6 months of treatment (Figure 3a–d). In addition, OCT evaluation of the grafted TEAHC showed a proper structural integrity of the whole cornea, although an interface between the native cornea and the TEAHC graft was still detectable after 6 months of the procedure. Corneal tissue above and below this interface was similar in the OCT analysis (Figure 3e,f).
Figure 3.
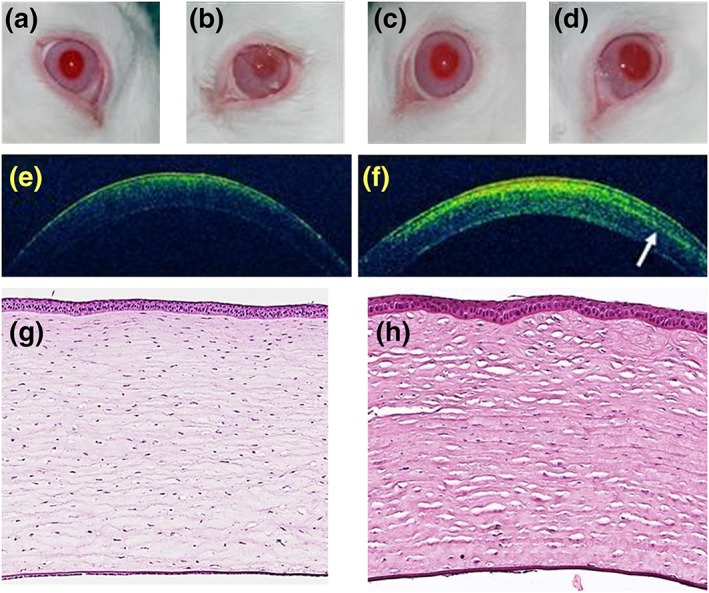
In vivo and histological evaluation of tissue‐engineered anterior human cornea (TEAHC) grafted on the eye surface of laboratory rabbits. (a) Control nonoperated cornea, (b) grafted TEAHC after 3 weeks, (c) grafted TEAHC at 3 months, (d) grafted TEAHC after 6 months, (e) optical coherence tomography (OCT) analysis of the control nonoperated cornea, and (f) OCT analysis of the grafted TEAHC after 6 months. The white arrow shows a heterogeneity area at the central stroma. (g) Histological study of native control and (h) TEAHC grafted in vivo in laboratory rabbits for 6 months using hematoxylin and eosin staining [Colour figure can be viewed at http://wileyonlinelibrary.com]
Histological analysis of the TEAHC showed that the graft was properly integrated in the animal cornea 6 months after implantation, and cells kept their adequate corneal differentiation status. More precisely, the corneal epithelium showed optimum stratification and cohesiveness, whereas the corneal stroma exhibited adequate structural conformation based on collagen lamellae. Although stroma was very similar to native control, our analysis showed that most apical stromal lamellae—corresponding to the grafted areas—were slightly more irregular and heterogeneous than the most basal layers, which were very similar to control samples (Figure 3g,h).
Regarding in vivo biodistribution, the histological analysis of biopsies of grafted animals showed no local migration to surrounding tissues, nor any sign of systemic cell distribution. Given that the cornea is an avascular tissue, and the topical route of administration of the product, no further safety results were requested by the competent authority. Finally, the favorable toxicological profile of the TEAHC was confirmed, as all animals survived with no toxic effects derived from the corneal implant. Regarding local tolerance, an initial inflammatory process was detected at the anterior surface of the eye, which tended to disappear 3 to 6 months after the surgical procedure (Figure 3b–d). Yet, no local infection, granuloma, tumorigenesis, or systemic side effects were detected during the follow‐up period. As described above, the TEAHC showed complete absence of tumors or predisposition to it, neither in the macroscopic analysis, the histological study, nor the RNA expression levels assays.
2.4. Production under GMP conditions
Current regulation related to the development of a clinical trial in advanced therapies establishes that the medicinal product to be tested must be manufactured as a clinical‐grade product in GMP facilities following all the regulations applicable to medicinal products and ATMPs. Therefore, the whole manufacturing process must be transferred from the basic research laboratory to the pharmaceutical facility, and the regulatory agency must approve both the GMP facility and the pharmaceutical grade production process. Production of the TEAHC is performed in the Unidad de Producción Celular e Ingeniería Tisular of Hospital Universitario Virgen de las Nieves, which belongs to the network of the Andalusian Initiative for Advanced Therapies (AIAT). This production is based on methods previously developed and reported in the basic research laboratory, including some modifications and additional studies in order to meet the regulatory requirements regarding clinical grade in ATMP manufacturing as described (Miguel Alaminos et al., 2006; De La Cruz Cardona et al., 2011; Garzón et al., 2014; Gonzalez‐Andrades et al., 2009; Ionescu et al., 2011; Scionti et al., 2014). Briefly, all materials and reagents used in the manufacturing process are certified for clinical‐grade production, except for the agarose that needs a previous conditioning phase. Also, standard in‐process controls, as well as final product quality tests, were implemented on the product according to the European Pharmacopeia as described hereunder (Figure 4). The sterility was demonstrated by microbiological control of cellular products using an automated blood culture system (Ph. Eur. 2.6.27) as well as gram and calcofluor staining. The Mycoplasma test was performed by conventional PCR in the culture supernatant (Ph. Eur. 2.6.7). Genetic fingerprint tests was based on PCR application of microsatellite and subsequent nucleotide sequencing (Ph. Eur. 2.6.21). The absence of adventitious virus was demonstrated by conventional culture in three cell lines (MRC‐5, Vero and RD) and subsequent observation of cytopathic effect. The absence of endotoxins was demonstrated by chromogenic endpoint method (Ph. Eur. 2.6.14). Finally, we demonstrated that the chromosome number and structure was normal by G‐bands karyotype analysis.
Figure 4.
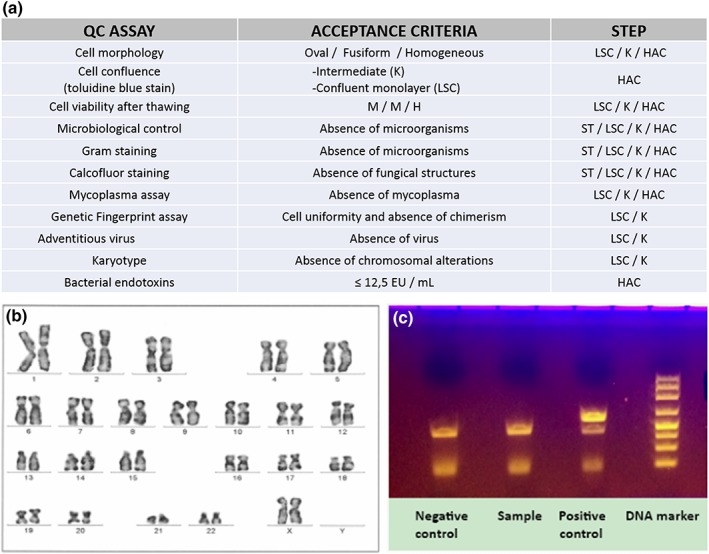
Clinical‐grade Production and quality controls in the production of the tissue‐engineered anterior human cornea (TEAHC) in compliance with good manufacturing practice. (a) Quality controls and acceptance criteria defined to release each product of the manufacturing process. H, high; HAC, human allogeneic cornea (TEAHC); K, keratocytes; LSC, limbal stem cells; M, medium. (b) Limbal cell karyotype included in the quality controls of the TEAHC; (c) Example of the absence of microorganism in mycoplasma assay quality control [Colour figure can be viewed at http://wileyonlinelibrary.com]
Keratocytes and corneal epithelial cells are kept in liquid nitrogen until obtaining quality control results. If the results are compliant, the cell lines will become available for manufacturing. Approximately 4 weeks before implant, the cell lines are thawed and the scaffold is manufactured. On the day of implantation, TEAHC is nanostructured and packaged in sterile containers immersed in culture medium.
Likewise, the translation of the basic research protocol into the GMP environment involved the implementation of a risk management plan in order to ensure the quality of the manufacturing process and ultimately the safety of the patient (International Conference Harmonization [ICH] Expert Working Group, 2005), a solid documentation system at different levels (involving Standard Operating Procedures, Master Batch Records, Working Instructions, Protocols, etc), meticulous testing of all starting materials (including human tissue samples), rigorous training of staff, and so forth. Cells and biomaterials applied in the TEAHC have a short half‐life in vitro, which influenced the choice of the packaging as well as transport methods. Taking this into account, and after carrying out a stability study, we concluded that the TEAHC was stable for up to 6 hr after being packed in a solid sterile refrigerated container.
2.5. Scientific advice request to the AEMPS
With the purpose of speeding up the clinical development of ATMPs, the AIAT provides specialized preclinical support and assessment from a regulatory point of view. Seeking to initiate a first in human clinical trial, a scientific advice request was carried out to the AEMPS in a face‐to‐face meeting. All available in vitro and in vivo preclinical data was presented and discussed, together with the ATMP manufacturing procedure and clinical trial design, previously also submitted in an electronic dossier. An updated scientific dossier was electronically submitted after incorporating new preclinical data following AEMPS requests in the aforementioned meeting.
3. RESULTS AND DISCUSSION
This article provides a complete description of the in vitro and in vivo studies, including the adaptation of the manufacturing process to GMP, for the development and translation of a tissue‐engineered product to the clinic, allowing its authorization in an early, first‐in‐human clinical trial. We believe that this successful clinical translation experience of a tissue‐engineered product could serve as guidance for other researchers developing this type of ATMP.
3.1. Challenges in ATMP clinical translation
One of the first steps of the translation process is to identify and classify the future medicinal product. The TEAHC belongs to the category of tissue‐engineered ATMPs as defined by Regulation (EC) 1394/2007 and Commission Directive 2009/120/EC (“Commission Directive 2009/120/EC of 14 September 2009 amending Directive 2001/83/EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use as regards advanced therapy medicinal products,” 2009; “REGULATION (EC) No 1394/2007 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004,” 2007), which are defined as medicinal products for human use containing substantially manipulated cells or tissues and intended to regenerate, repair, or replace human tissue to achieve a desired physiological function. As such, specific guidelines should be followed as much for nonclinical studies as for manufacturing, quality controls, and clinical development. This regulatory context can be challenging, as it is a well‐known fact that medicinal product standards may not always be compatible with the typical framework of cell‐based products, as some of them are of autologous origin, produced in very small batch sizes, or produced using materials with high variability (in some cases impossible to standardize). Additionally, the burden generated by meeting the standards of GMP in production stands as an added complication in this particular context, as most of the research and development of ATMPs is performed mainly by academia, hospitals, and SMEs (as is the case here; Pirnay et al., 2013).
The translation of an ATMP from basic research into a clinical‐grade product applicable in a human clinical trial is therefore a complex, integrated activity that requires professional and multidisciplinary expertise. Clinical translation is indeed a challenging goal that a research group cannot handle alone. At this point, involvement of strategic stakeholders is crucial, including but not limited to regulatory experts, technology transfer offices, clinicians, manufacturing facilities, regulatory agencies, and patient organizations. The AIAT is a publicly funded institution that facilitated communication among all the stakeholders, coordinating the activities of a multidisciplinary team and providing the regulatory expertise required for development of the TEAHC. Early collaboration with researchers performing high‐quality basic research, clinicians to identify unmet needs, and the people involved in the manufacturing process is key in the whole translational process (Figure 5). A nonprofit academia entity like the AIAT represented in this particular case an opportunity to speed up the development process of this particular ATMP facilitating the patient access to this technology. (Cuende, 2013)
3.2. Experimental design of the TEAHC
One of the main objectives of nonclinical studies is to define the pharmacological and toxicological effects of the experimental product. The goals of these studies include providing information for a safe and efficacious product dosage in future clinical trials (Celis et al., 2015). In this regard, the present manuscript describes all the experimental studies carried out for the efficient clinical translation of a bioengineered cornea, including exhaustive preclinical studies.
To help guiding the initial development and characterization of the product, some important issues should dictate the experimental design: the intended clinical application; the physical, chemical, mechanical, and degradation properties; the technical demands of the product (form, function, fixation and formation), and its biocompatibility and interactions with cells and tissues (Lu et al., 2015). Thus, in this report, we have showed a successful step‐by‐step process to translate in vitro and in vivo data of a potential new therapy consisting of a bioengineered corneal substitute for application as a topical engraftment in patients with severe corneal damage. For product identification, we used the microscopic assessment of both cells morphology, complemented with the results of immunohistochemistry. Complementarily, we used relevant data published by the basic research group including light, transmission, and scanning electron microscopy and histological images of TEAHC, with and without in vitro differentiation induction. These results were used as a proof of identity, purity, and potency characteristics of the final product: adequate structural, biomechanical behavior (viscoelasticity, resistance, elasticity), optical properties (transparency, UV light absorption, scattering, absorbance), epithelial stratification, differentiation, and intercellular junctions formation (De La Cruz Cardona et al., 2011; Garzón et al., 2014; Gonzalez‐Andrades et al., 2009; Ionescu et al., 2011, 2010; Scionti et al., 2014). These findings were confirmed by comprehensive gene expression analyses showing that the ATMP expresses numerous genes whose functions were related to normal corneal function and development, especially those related to epithelial cell‐cell junctions, basement membrane, organelles synthesis, and other epithelial functions. Most importantly, we used these highly sensitive microarray analyses to demonstrate that the gene functions that were associated to the novel ATMP were typical of cornea differentiation and development and were devoid of malignant transformation gene functions. Additionally, EPXMA demonstrated that both cells types included in the final ATMP were highly viable at the moment of use in the bioengineered cornea, with K/Na ratios above 3, which are typical of viable human cells kept in culture, and other types of cornea cells such as the rabbit cornea endothelial cells (M. Alaminos et al., 2007; Garzón, Carriel et al., 2012; Garzón et al., 2012; M. Martin‐Piedra et al., 2013). These findings were confirmed by the normal intracellular concentrations of the rest of elements analyzed in cultured keratocytes and epithelial cells, especially the high concentrations of chlorine (M. Alaminos et al., 2007; Garzón, Carriel et al., 2012; Garzón, Pérez‐Köhler, et al., 2012). Previous reports demonstrated that this highly sensitive technology is much more accurate than standard methods such as trypan blue and can be used for a precise analysis of cell viability in numerous cell types (M. A. Martin‐Piedra et al., 2014; Vico et al., 2015).
One of the most important issues that must be addressed before the first use in humans is the safety of the administration of therapeutic product. On the one hand, it has been observed that occurrence of cell abnormalities appears to be mainly related to the manufacturing process as opposed to patient‐specific factors; it is therefore important to determine whether the manufacturing process leads to chromosomal abnormalities during preclinical development (Barkholt et al., 2013). Consequently, all ATMP need to be analyzed by karyotyping to demonstrate the total absence of chromosomal abnormalities before clinical use. This should be taken into account to determine the required number of cells of each type, because karyotyping usually requires a high number of cells. Additionally, in vivo studies carried out on our TEAHC corneal substitute revealed that it was not associated to the development of any local infection, granuloma, or systemic side effects during the follow‐up period. No alterations that could lead to a malignant or tumorigenic transformation of the product cells were found.
Overall, the in vitro characterization results presented in this article are consistent with an adequate primary pharmacology of the medicinal product, and these findings were confirmed in vivo by a full integration and biocompatibility of the TEAHC corneal substitute in rabbits.
3.3. Adapting the ATMPS development process
Every small step of the preclinical research and product development plan needs to be established and adaptions have to be applied at different levels in the ATMP research process. Major gaps in ATMP development should be overcome by strategic rational customization of the experimental design. Regarding dosage selection for example, it is important to note that the intended objective is to evaluate a three‐dimensional artificial tissue, not a cell suspension. For this reason, the number of cells to be implanted with the TEAHC corresponds to the number required for the generation of a human corneal substitute, in terms of functional confluence of cells allowing differentiation as a stratified epithelium, with approximately 500,000 cells per layer per construct (Miguel Alaminos et al., 2006). Additionally, it is necessary to consider that the TEAHC could vary in size as it should be large enough to cover the lesion, therefore the total number of cells to be implanted will depend on the size of the corneal defect (or keratectomy) in each patient. Another main issue that required adaptive reformulation of the experimental strategy is biodistribution. In general, conventional pharmacokinetic studies (commonly used for chemical drugs) are not applicable to this type of products. According to the Guideline on human cell‐based medicinal products, “conventional ADME (Absorption, distribution, metabolism & excretion) studies are usually not relevant for this type of products. However, studies should be carried out to demonstrate tissue distribution, viability, trafficking, growth, phenotype and any alteration of phenotype due to factors in the new environment” (Committee for Medicinal Product for Human Use [CHMP], 2008). In fact, biodistribution was one of the main concerns raised by AEMPS during the scientific advice meeting. In our case, the in vivo assays were used to evaluate biodistribution, measured as migration and persistence in the relevant animal model (EMEA/CHMP/410869/2006). However, given that cornea is an avascular tissue, the animal model used, and the route of administration through topical engraftment, it was agreed that physical evaluation of the eye surface, further confirmed by histological study and OCT, confirming no migration to surrounding tissues or signs of systemic cell distribution would be sufficient in this specific product. The fact that the TEAHC was implanted on the central cornea has two important advantages as compared with other medicinal products. First, this specific superficial location allows for an easy clinical monitoring of the grafted tissue because of its accessibility for exploration and sampling. Second, the fact that the cornea is naturally free from vascularization hinders the possibility of cell distance migration through the bloodstream. Probably, other types of ATMPs will require much stricter controls to guarantee biosafety and proper biodistribution. Regarding the toxicity profile and local tolerance, clinical trial design was founded in the safety issues identified in the animal model using the same route (local implant on the corneal surface by anterior lamellar keratoplasty) and frequency (a graft per patient) of administration.
In parallel with preclinical product development, the manufacturing protocol was adjusted to produce a clinical‐grade TEAHC. The basic research production process was not essentially modified, yet raw materials, product manipulation, facility, and staff had to meet specific GMP‐compliant requirements as described previously. After successful definition and validation of the entire GMP production methods, the manufacturing process needs to be authorized by the competent authority. The basis for this approval is the investigational medicinal product dossier, which serves as the primary document describing manufacturing methods and control assays of the product.
We carefully considered available options to reduce materials from xenogeneic origin; however we believe that using a xeno‐free culture media may not have led to an easier regulatory approval at the time. The fetal bovine serum used had a certificate of suitability issued by the European Directorate for the Quality of Medicines (Council of Europe, 2018), therefore our procedure fully complied with the regulatory recommendations for the use of fetal bovine serum, ruling out any possibility of bovine spongiform encephalopathy transmission and ensuring patient safety (EMA/CAT/80183/2014; EMA/CHMP/BWP/457920/2012 rev.1.; Regulation (EC) 999/2001 of the European Parliament and of the Council).
However, there is nowadays an increasing pressure form regulatory agencies to incorporate nonxenogenic culture media in clinical‐grade manufacturing procedures, and our group is at the moment working on xeno‐free strategies for cell expansion.
A Phase I trial is designed to assess the safety of the investigational product and to identify any possible side effects. For a conventional clinical Phase I trial with a new product, usually healthy adult volunteers are recruited; however, due to the nature of this tissue‐engineered product, this traditional set‐up is not appropriate: no healthy volunteers could be recruited as corneal transplantation is only indicated in preexisting severe corneal defects. For obvious reasons, intentional creation of such defects would be unethical. Therefore, the selected approach for the trial was to recruit directly the patients that would also gain most benefit from this innovative therapy. In addition, in order to take the most conservative approach, trial selection criteria limited participation to patients with severe corneal defects in whom other conventional therapies were ineffective (including donor keratoplasty). The methodological design of the clinical trial should be established as early as possible, as the intended route, dose, and frequency of administration are key aspects to take into account in the experimental design of the preclinical development of the product according to European regulations (ICH Topic M3(R2), CPMP/ICH/286/95.30). Furthermore, it is advisable to establish an independent data safety monitoring committee that may provide an unbiased point of view in the evaluation of the safety data of the study participants. Especially in early clinical trials testing products like the TEAHC, which may potentially cause serious impairments, this committee helps the sponsor and principal investigators with their expertise, ensuring patient safety is warranted anytime during the trial. Figure 5 illustrates the development process pathway that successfully paved the way toward the start‐up of the early clinical trial testing the product in 2014 (Figure 1f; Miguel González‐Andrades, Mata, et al., 2017). This is a Phases I and II, randomized, controlled, open‐label clinical trial, currently ongoing in 11 Spanish hospitals (Miguel González‐Andrades, Mata, et al., 2017), to evaluate the safety and feasibility, as well as clinical efficacy evidence, of the TEAHC in adults with severe trophic corneal ulcers or its sequelae. The initial phase of the trial (n = 5) was completed, and preliminary data have already been presented (M. González‐Andrades et al., 2017).
3.4. Scientific advice with regulators
Early dialogue with regulators is encouraged for ATMPs development in order to speed up the progress of research, optimize the available resources, and speed up clinical use evaluation. The European Medicines Agency as well as the National Competent Authorities can give scientific advice to medicine developers, ensuring the appropriate tests and studies are performed and no major objections are likely to be raised during the evaluation of human clinical trials or marketing authorizations applications. Scientific advice is particularly useful in ATMPs development, as the information contained in the EU guidelines or guidance documents may be insufficient in certain relevant aspects, or it may be recommendable to deviate from the available guidance in some specific cases. Scientific advice can be requested at any stage of development of a medicine, and the guidance received is not legally binding to any future authorization applications for the product concerned. Accordingly, a scientific advice was requested to the AEMPS in 2010 to provide guidance over the TEAHC preclinical development plan. As a result, further preclinical studies as well as specific questions regarding GMP production were requested to complete the clinical trial application dossier. Concerning measures to avoid graft rejection, the high biocompatibility of fibrin–agarose scaffold was proved in several in vivo models (oral mucosa, Fernández‐Valadés‐Gámez et al., 2016; skin, Carriel et al., 2012; peripheral nerve, Carriel et al., 2013), where no signs of rejection or inflammation were found. The TEAHC is manufactured with cells (epithelial/corneal fibroblasts) from different allogeneic donors, which may imply an increased risk of graft rejection. However, HLA‐matching is generally not required in donor corneal transplantation, owing to the immune privilege of the cornea. Nevertheless, in our clinical trial testing the TEAHC (Gonzalez‐Andrades et al., 2017), the potential risk of graft rejection is addressed by per protocol administration of steroid eye drops over the first year after receiving the implant.
3.5. IP protection
Previous researches account for influence of commercialization criteria (e.g., IP landscape, market size, competitors, reimbursement, and adoption by users) in the development of the product (e.g., preclinical testing against competing products). The more valuable their studies become in supporting a marketing application, the higher the chance they withstand in raising necessary funding or partnering with an existing company (Lu et al., 2015). Nowadays, there is an intense research, development, and commercialization activity in the field of corneal substitutes that can be easily justified by cost–benefit improvement and higher intervention accessibility to a larger group of people (Ghezzi, Rnjak‐Kovacina, & Kaplan, 2015). The TEAHC was patented in an early stage of development for two main reasons: on the one hand, having a firm grasp on the importance of intellectual property from the outset can reduce future chances of financial loss (Juetten, 2016), and on the other hand, the bioengineered tissue that we have developed has shown important properties to be a future successful ATMP. Proactive and early intellectual property protection of the product as well as early market research are highly recommended for the clinical translation of an ATMP. In this sense, the patent of the TEAHC was requested in parallel to its preclinical development (PCT/ES2010/070569).
4. CONCLUSIONS
The successful development of ATMPs for clinical application has to overcome many obstacles such as the challenge in the interpretation of ATMPs regulation and guidelines, the heavy workload generated by the standards of GMP and GCP, and the financial burden of the whole drug development process. These difficulties become almost unbridgeable when undertaken by academia or SMEs. At this point, clinical translation of ATMPs can be facilitated by research platforms like the AIAT promoting the collaboration among strategic stakeholders, as well acting as a counseling entity for the implementation of specific regulations and guidelines. Furthermore, a strategy should be set‐up for the experimental development of the product, allowing the necessary adjustments according to a rational application of the guidelines at the specific target intended for its clinical use. Despite the long list of constraints, the experience reported in this article proves that, with strategic planning and support, clinical translation in the academic context can ultimately excel providing patients the access to this innovative type of medicines.
AUTHOR CONTRIBUTIONS
G. I., S. Q. M. C., M. A. J. I., C. A., and A. M.: performed experiments and collected the data for product development. S. G. S., P. M., J. P., L. M. A., R. G. A., P. M., and C. G.: were involved in adaptation of product manufacturing to comply with G. M. P. G. A. M., M. S., G. G. M. C., and C. N.: contributed to conceptual design of early phase clinical trial. R. S. L., M. A. J., L. N. L., S. P. R., and O. R. I.: conception and drafting of the manuscript. All authors contributed to critical revision of the manuscript and read and approved the final version.
CONFLICT OF INTERESTS
Dr. González‐Andrades, Dr. Alaminos, and Dr. Campos are inventors of issued patents P200930625 and P200930943, broadly relevant to the work. The remaining authors declare that they have no competing interests.
ETHICS APPROVAL
This research was approved by the institutional experimentation committee (approval numbers 01‐09‐15‐313 and 25‐06‐2018‐099), and all animals were treated according to the national and international rules of animal welfare, including the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated and/or analyzed during the current study are available in the Gene Expression Omnibus (GEO) public repository, ref. GSE86584 https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE86584
FUNDING INFORMATION
This study was supported by the Spanish National Plan for Scientific and Technical Research and Innovation (I + D + I) from the Spanish Ministry of Economy and Competitiveness (Carlos III Institute of Health), grants FIS PI14/0955 and FIS PI17/0391 (both cofinanced by ERDF‐FEDER, European Union); by the Spanish Ministry of Health, Social Policy and Equity, grant EC10‐285; and by preclinical research funds from the Regional Ministry of Health through the Andalusian Initiative for Advanced Therapies.
ACKNOWLEDGEMENTS
We thank the Andalusian Public Foundation Progress and Health, through the Andalusian Initiative for Advanced Therapies, for assuming the roles and responsibilities of sponsoring this clinical trial. We thank Dr. Manuel de la Rosa and Dr. Salvador Arias Santiago for providing insight and expertise that assisted the research.
Rico‐Sánchez L, Garzón I, González‐Andrades M, et al. Successful development and clinical translation of a novel anterior lamellar artificial cornea. J Tissue Eng Regen Med. 2019;13:2142–2154. 10.1002/term.2951
Rico‐Sánchez Laura and Garzón Ingrid contributed equally to this work.
Contributor Information
Natividad Cuende, Email: terapias.avanzadas@juntadeandalucia.es.
Antonio Campos, Email: acampos@ugr.es.
REFERENCES
- Alaminos, M. , González‐Andrades, M. , Muñoz‐Ávila, J. I. , Garzón, I. , Sánchez‐Quevedo, M. C. , & Campos, A. (2008). Volumetric and ionic regulation during the in vitro development of a corneal endothelial barrier. Experimental Eye Research, 86(5), 758–769. 10.1016/j.exer.2008.02.003 [DOI] [PubMed] [Google Scholar]
- Alaminos, M. , Sanchez‐Quevedo, M. , Muñoz‐Avila, J. I. , Garcia, J. M. , Crespo, P. V. , Gonzalez‐Andrades, M. , & Campos, A. (2007). Evaluation of the viability of cultured corneal endothelial cells by quantitative electron probe X‐ray microanalysis. Journal of Cellular Physiology, 211(3), 692–698. 10.1002/jcp.20976 [DOI] [PubMed] [Google Scholar]
- Alaminos, M. , Sánchez‐Quevedo, M. D. C. , Muñoz‐Ávila, J. I. , Serrano, D. , Medialdea, S. , Carreras, I. , & Campos, A. (2006). Construction of a complete rabbit cornea substitute using a fibrin‐agarose scaffold. Investigative Ophthalmology and Visual Science, 47(8), 3311–3317. 10.1167/iovs.05-1647 [DOI] [PubMed] [Google Scholar]
- Barkholt, L. , Flory, E. , Jekerle, V. , Lucas‐Samuel, S. , Ahnert, P. , Bisset, L. , … Salmikangas, P. (2013). Risk of tumorigenicity in mesenchymal stromal cell‐based therapies—Bridging scientific observations and regulatory viewpoints. Cytotherapy, 15(7), 753–759. 10.1016/j.jcyt.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Carriel, V. , Garrido‐Gómez, J. , Hernández‐Cortés, P. , Garzón, I. , García‐García, S. , Sáez‐Moreno, J. A. , … Alaminos, M. (2013). Combination of fibrin‐agarose hydrogels and adipose‐derived mesenchymal stem cells for peripheral nerve regeneration. Journal of Neural Engineering, 10(2), 026022 10.1088/1741-2560/10/2/026022 [DOI] [PubMed] [Google Scholar]
- Carriel, V. , Garzón, I. , Alaminos, M. , & Campos, A. (2011). Evaluation of myelin sheath and collagen reorganization pattern in a model of peripheral nerve regeneration using an integrated histochemical approach. Histochemistry and Cell Biology, 136(6), 709–717. 10.1007/s00418-011-0874-3 [DOI] [PubMed] [Google Scholar]
- Carriel, V. , Garzón, I. , Jiménez, J. M. , Oliveira, A. C. X. , Arias‐Santiago, S. , Campos, A. , … Alaminos, M. (2012). Epithelial and stromal developmental patterns in a novel substitute of the human skin generated with fibrin‐agarose biomaterials. Cells, Tissues, Organs, 196(1), 1–12. 10.1159/000330682 [DOI] [PubMed] [Google Scholar]
- Celis, P. , Ferry, N. , Hystad, M. , Schüßler‐Lenz, M. , Doevendans, P. A. , Flory, E. , … Salmikangas, P. (2015). Advanced therapy medicinal products: How to bring cell‐based medicinal products successfully to the market ‐ Report from the CAT‐DGTI‐GSCN Workshop at the DGTI Annual Meeting 2014. Transfusion Medicine and Hemotherapy, 42(3), 194–199. 10.1159/000382107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster, D. J. , & Williams, K. A. (2005). The impact of corneal allograft rejection on the long‐term outcome of corneal transplantation. American Journal of Ophthalmology, 140(6), 1112–1122. 10.1016/j.ajo.2005.07.024 [DOI] [PubMed] [Google Scholar]
- Council of Europe . (2018). European Directorate for the quality of Medicines & Health Care. Retrieved February 7, 2019, from https://www.edqm.eu/
- Cuende, N. (2013). Andalusian initiative for advanced therapies: Fostering synergies. Stem Cells Translational Medicine, 2(4), 243–245. 10.5966/sctm.2013-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuende, N. , & Izeta, A. (2010). Clinical translation of stem cell therapies: A bridgeable gap. Cell Stem Cell, 6(6), 508–512. 10.1016/j.stem.2010.05.005 [DOI] [PubMed] [Google Scholar]
- De La Cruz Cardona, J. , Ionescu, A. M. , Gómez‐Sotomayor, R. , González‐Andrades, M. , Campos, A. , Alaminos, M. , & Pérez, M. D. M. (2011). Transparency in a fibrin and fibrin‐agarose corneal stroma substitute generated by tissue engineering. Cornea, 30(12), 1428–1435. 10.1097/ICO.0b013e31821bdfd4 [DOI] [PubMed] [Google Scholar]
- EMA/CAT/80183/2014 (n.d.). Guideline on the quality, non‐clinical and clinical aspects of gene therapy medicinal products. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐quality‐non‐clinical‐clinical‐aspects‐gene‐therapy‐medicinal‐products_en.pdf
- EMA/CHMP/BWP/457920/2012 rev.1 (n.d.). Guideline on the use of bovine serum in the manufacture of human biological medicinal products https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐quality‐non‐clinical‐clinical‐aspects‐gene‐therapy‐medicinal‐products_en.pdf.
- EMEA/CHMP/410869/2006 (n.d.). Guideline on human cell‐based medicinal products. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐human‐cell‐based‐medicinal‐products_en.pdf
- Fernández‐Valadés‐Gámez, R. , Garzón, I. , Liceras‐Liceras, E. , España‐López, A. , Carriel, V. , Martin‐Piedra, M.‐Á. , … Fernández‐Valadés, R. (2016). Usefulness of a bioengineered oral mucosa model for preventing palate bone alterations in rabbits with a mucoperiostial defect. Biomedical Materials (Bristol), 11(1), 015015 10.1088/1748-6041/11/1/015015 [DOI] [PubMed] [Google Scholar]
- Gain, P. , Jullienne, R. , He, Z. , Aldossary, M. , Acquart, S. , Cognasse, F. , & Thuret, G. (2016). Global survey of corneal transplantation and eye banking. JAMA Ophthalmology, 134(2), 167–173. 10.1001/jamaophthalmol.2015.4776 [DOI] [PubMed] [Google Scholar]
- Garzón, I. , Carriel, V. , Marín‐Fernández, A. B. , Oliveira, A. C. , Garrido‐Gómez, J. , Campos, A. , … Alaminos, M. (2012). A combined approach for the assessment of cell viability and cell functionality of human fibrochondrocytes for use in tissue engineering. PLoS ONE, 7(12), e51961 10.1371/journal.pone.0051961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón, I. , Martín‐Piedra, M. A. , Alfonso‐Rodríguez, C. , Gonźalez‐Andrades, M. , Carriel, V. , Martínez‐Gómez, C. , … Alaminos, M. (2014). Generation of a biomimetic human artificial cornea model using wharton's jelly mesenchymal stem cells. Investigative Ophthalmology and Visual Science, 55(7), 4073–4083. 10.1167/iovs.14-14304 [DOI] [PubMed] [Google Scholar]
- Garzón, I. , Pérez‐Köhler, B. , Garrido‐Gómez, J. , Carriel, V. , Nieto‐Aguilar, R. , Martín‐Piedra, M. A. , … Alaminos, M. (2012). Evaluation of the cell viability of human Wharton's jelly stem cells for use in cell therapy. Tissue Engineering Part C: Methods, 18(6), 408–419. 10.1089/ten.tec.2011.0508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi, C. E. , Rnjak‐Kovacina, J. , & Kaplan, D. L. (2015). Corneal tissue engineering: Recent advances and future perspectives. Tissue Engineering Part B: Reviews, 21(3), 278–287. 10.1089/ten.teb.2014.0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Andrades, M. , Garzon, I. , Gascon, M. , Muñoz‐Avila, J. I. , Sanchez‐Quevedo, M. C. , Campos, A. , & Alaminos, M. (2009). Sequential development of intercellular junctions in bioengineered human corneas. Journal of Tissue Engineering and Regenerative Medicine, 3(6), 442–449. 10.1002/term.178 [DOI] [PubMed] [Google Scholar]
- González‐Andrades, M. , Martinez‐Atienza, J. , Campos, A. , Arias‐Santiago, S. , González Gallardo, C. , Mataix, B. , … Alaminos, M. (2017). Preliminary results of a multicenter randomized clinical trial evaluating the safety and feasibility of an allogeneic nanostructured artificial anterior human cornea. Cytotherapy, 19(5), Supplement), S26 10.1016/j.jcyt.2017.02.052 [DOI] [Google Scholar]
- González‐Andrades, M. , Mata, R. , Del Carmen González‐Gallardo, M. , Medialdea, S. , Arias‐Santiago, S. , Martínez‐Atienza, J. , … Cuende, N. (2017). A study protocol for a multicentre randomised clinical trial evaluating the safety and feasibility of a bioengineered human allogeneic nanostructured anterior cornea in patients with advanced corneal trophic ulcers refractory to conventional treatment. BMJ Open, 7(9), e016487 10.1136/bmjopen-2017-016487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipanayi, E. , Ananta, M. , Binkowski, M. , Streeter, I. , Lu, Z. , Cui, Z. F. , … Mudera, V. (2011). Mechanisms of structure generation during plastic compression of nanofibrillar collagen hydrogel scaffolds: Towards engineering of collagen. Journal of Tissue Engineering and Regenerative Medicine, 5(7), 505–519. 10.1002/term.343 [DOI] [PubMed] [Google Scholar]
- Harrison, R. H. , St‐Pierre, J.‐P. , & Stevens, M. M. (2014). Tissue engineering and regenerative medicine: A year in review. Tissue Engineering Part B: Reviews, 20(1), 1–16. 10.1089/ten.teb.2013.0668 [DOI] [PubMed] [Google Scholar]
- ICH Expert Working Group . (2005). Quality risk management Q9. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, (November), 1–19. 10.1007/s11095-007-9511-1 [DOI]
- Ionescu, A. M. , Alaminos, M. , de la Cruz Cardona, J. , Durán, J. D. D. G. L. , González‐Andrades, M. , Ghinea, R. , … del Mar Pérez, M. (2011). Investigating a novel nanostructured fibrin‐agarose biomaterial for human cornea tissue engineering: Rheological properties. Journal of the Mechanical Behavior of Biomedical Materials, 4(8), 1963–1973. 10.1016/j.jmbbm.2011.06.013 [DOI] [PubMed] [Google Scholar]
- Ionescu, A. M. , de la Cruz Cardona, J. , Gonzalez‐Andrades, M. , Alaminos, M. , Campos, A. , Hita, E. , & del Mar Perez, M. (2010). UV absorbance of a bioengineered corneal stroma substitute in the 240‐400 nm range. Cornea, 29(8), 895–898. 10.1097/ICO.0b013e3181ca3650 [DOI] [PubMed] [Google Scholar]
- Juetten, M. (2016). Startups are personal, Part VIII: Protect your intellectual property from the start. Forbes.
- Lu, L. , Arbit, H. M. , Herrick, J. L. , Segovis, S. G. , Maran, A. , & Yaszemski, M. J. (2015). Tissue engineered constructs: Perspectives on clinical translation. Annals of Biomedical Engineering, 43(3), 796–804. 10.1007/s10439-015-1280-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, I. , Ireland, H. , Baldomero, H. , & Passweg, J. (2015). The survey on cellular and engineered tissue therapies in Europe in 2012. Tissue Engineering Part A, 21(1–2), 1–13. 10.1089/ten.TEA.2014.0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Piedra, M. , Garzon, I. , Oliveira, A. , Alfonso‐Rodriguez, C. , Sanchez‐Quevedo, M. C. , Campos, A. , & Alaminos, M. (2013). Average cell viability levels of human dental pulp stem cells: An accurate combinatorial index for quality control in tissue engineering. Cytotherapy, 15(4), 507–518. 10.1016/j.jcyt.2012.11.017 [DOI] [PubMed] [Google Scholar]
- Martin‐Piedra, M. A. , Garzon, I. , Oliveira, A. C. , Alfonso‐Rodriguez, C. A. , Carriel, V. , Scionti, G. , & Alaminos, M. (2014). Cell viability and proliferation capability of long‐term human dental pulp stem cell cultures. Cytotherapy, 16(2), 266–277. 10.1016/j.jcyt.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Meek, K. M. , & Knupp, C. (2015). Corneal structure and transparency. Progress in Retinal and Eye Research, 49, 1–16. 10.1016/j.preteyeres.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini, G. , Ardigò, D. , Milazzo, G. , Iotti, G. , Guatelli, P. , Pelosi, D. , & De Luca, M. (2018). Navigating market authorization: The path holoclar took to become the first stem cell product approved in the European Union. Stem Cells Translational Medicine, 7(1), 146–154. 10.1002/sctm.17-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirnay, J. P. , Vanderkelen, A. , De Vos, D. , Draye, J. P. , Rose, T. , Ceulemans, C. , … Verbeken, G. (2013). Business oriented EU human cell and tissue product legislation will adversely impact Member States' health care systems. Cell and Tissue Banking, 14, 525–560. 10.1007/s10561-013-9397-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Morata, A. , Garzon, I. , Alaminos, M. , Garcia‐Honduvilla, N. , Sanchez‐Quevedo, M. C. , Bujan, J. , & Campos, A. (2008). Cell viability and prostacyclin release in cultured human umbilical vein endothelial cells. Annals of Vascular Surgery, 22(3), 440–448. 10.1016/j.avsg.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Scionti, G. , Moral, M. , Toledano, M. , Osorio, R. , Durán, J. D. G. , Alaminos, M. , … Lõpez‐Lõpez, M. T. (2014). Effect of the hydration on the biomechanical properties in a fibrin‐agarose tissue‐like model. Journal of Biomedical Materials Research ‐ Part A, 102(8), 2573–2582. 10.1002/jbm.a.34929 [DOI] [PubMed] [Google Scholar]
- Vico, M. , Rodriguez‐Morata, A. , Garzon, I. , Campos, F. , Jaimes‐Parra, B. , Perez‐Kohler, B. , … Sanchez‐Quevedo, M. C. (2015). Cell viability evaluation of transdifferentiated endothelial‐like cells by quantitative electron‐probe X‐ray microanalysis for tissue engineering. Histology and Histopathology, 30(11), 1333–1340. 10.14670/HH-11-629 [DOI] [PubMed] [Google Scholar]
- Whitcher, J. P. , Srinivasan, M. , & Upadhyay, M. P. (2001). Corneal blindness: A global perspective. Bulletin of the World Health Organisation, 79(3), 214–221. [PMC free article] [PubMed] [Google Scholar]
- Wong, K. H. , Kam, K. W. , Chen, L. J. , & Young, A. L. (2017). Corneal blindness and current major treatment concern‐graft scarcity. International Journal of Ophthalmology, 10(7), 1154–1162. 10.18240/ijo.2017.07.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, A. L. , Kaiser, M. , Schaumberger, M. , Messmer, E. , Kook, D. , & Welge‐Lussen, U. (2014). Perioperative and postoperative risk factors for corneal graft failure. Clinical Ophthalmology (Auckland, N.Z.), 8, 1641–1647. 10.2147/OPTH.S65412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Gene Expression Omnibus (GEO) public repository, ref. GSE86584 https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE86584


