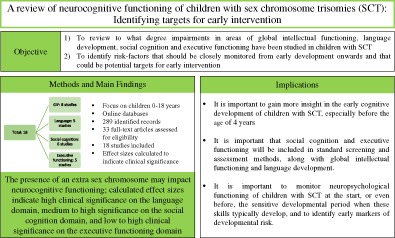
Abstract
Sex chromosome trisomies (SCT) are among the most common chromosomal duplications in humans. Due to recent technological advances in non‐invasive screening, SCT can already be detected during pregnancy. This calls for more knowledge about the development of (young) children with SCT. This review focused on neurocognitive functioning of children with SCT between 0 and 18 years, on domains of global intellectual functioning, language, executive functioning, and social cognition, in order to identify targets that could benefit from early treatment.
Online databases were used to identify peer‐reviewed scientific articles using specific search terms. In total 18 studies were included. When applicable, effect sizes were calculated to indicate clinical significance.
Results of the reviewed studies show that although traditionally, the focus has been on language and intelligence (IQ) in this population, recent studies suggest that executive functioning and social cognition may also be significantly affected already in childhood.
These findings suggest that neuropsychological screening of children diagnosed with SCT should be extended, to also include executive functioning and social cognition. Knowledge about these neurocognitive risks is important to improve clinical care and help identify targets for early support and intervention programs to accommodate for the needs of individuals with SCT.
Keywords: neurocognitive functioning, sex chromosome trisomies, XXX, XXY, XYY
With regard to the first aim, the reviewed studies collectively gave the following results. On the domain of global intellectual functioning (GIF), seven eight studies report outcomes in children between the ages of 4–18 years, with three studies focusing on children from the age of four years, and four five studies studying school‐aged children. To our knowledge, there were no studies that examined GIF in children with SCT before the age of four years. On the domain of language development, five seven studies reported outcomes in children between the ages of 2–18 years. To our knowledge, there were no studies that examined language development in children with SCT before the age of two years. Of the seven studies, two studies used only parent reports, the other three studies used either a performance task or a combination of parent report and performance tasks.
1. INTRODUCTION
Chromosome trisomies are genetic variations caused by a spontaneous error during early cell division.1 Sex chromosome trisomies (SCT), trisomies involving the X or Y chromosomes, are among the most common chromosomal duplications in humans,2 with an estimated prevalence ranging from 1‐650 to 1‐1000 live births.3, 4, 5 SCT can lead to a 47,XXY (Klinefelter syndrome) or 47,XYY (XYY syndrome) karyotype in males, and a 47,XXX (Trisomy X syndrome) karyotype in females.
Although SCT are relatively common genetic variations, they are also one of the most frequently underdiagnosed chromosomal conditions; up to 75% of individuals with SCT are never diagnosed.6 This high percentage may be explained by several factors. First, physical characteristics are relatively subtle.7, 8 Secondly, individuals may be treated for symptoms without knowledge of the underlying genetic condition. Finally, cognitive as well as behavioral symptoms are variable,9, 10 ranging from severe impairments in some individuals, with other individuals functioning on an average or above average level. The subtle physical characteristics, and the variability of symptoms often does not prompt to genetic testing. There are certain moments in life when the developing brain is especially sensitive to environmental influences regarding the development of specific neurocognitive functions.11 It is possible that when the genetic diagnosis is not made or delayed, the so called “window of opportunity” to explicitly support specific developmental stages passes, which could result in more severe cognitive and/or behavioral difficulties.12
Focusing on the neurocognitive underpinnings of behavior rather than behavioral symptoms itself is important as behavioral problems may arise as a consequence of different information processing deficits. Also, cognitive deficits may serve as early predictors of behavioral problems in later life, and may function as markers for children at risk for neurodevelopmental problems.
Over the last decade, the technology to detect genetic variations in unborn children has advanced significantly; one advantage being that they can be non‐invasive, for example by screening maternal blood. These advanced technological developments and the increased possibility to detect SCT during the pregnancy could lead to more individuals being diagnosed on the genetic, instead of the behavioral level.13 This calls for more knowledge about the development of (young) children with SCT, so children can get the appropriate support as early as possible when needed. The identification of a profile of neurocognitive risks, and knowledge about the mechanisms underlying these risks, could help improve early screening for neurobehavioral problems in young children with SCT and help identify targets for early, tailored support and intervention programs, which in turn could hopefully optimize outcomes in later life. Although some of these neurocognitive mechanisms are still “under construction” in early childhood, and for that reason are more apparent in late childhood or adolescence, precursors of some of these mechanisms can already be measured in early childhood.
Through a narrative review of the literature we evaluated evidence for cognitive impairments on the domains of global intellectual functioning (GIF), language development, executive functioning, and social cognition in children with SCT. Earlier reviews have focused on the development of individuals with SCT over the life‐span, primarily during adolescence and adulthood. In contrast, in this review, neurocognitive functioning of children with SCT was reviewed, with a focus on early development. As the domains of GIF, language development, social cognition, and executive functioning (EF) are vulnerable domains based on studies in adolescents and adults, and may be key factors that could drive the emotional and behavioral problems that can be found in individuals with SCT,14 it is important to monitor possible developmental risk in these domains already early in life. For that reason our first aim was to review to what degree impairments in areas of GIF, language development, social cognition, and EF have been studied in children with SCT, and identify possible gaps in research that future research should focus on. Secondly, in addition to identifying the type of impairments, we also aimed to determine the degree of impairment, to establish clinical significance and identify risk‐factors that should be closely monitored from early development onwards or that should be included in standard clinical neuropsychological screening to identify potential targets for support and intervention. Knowledge about the functioning of children with SCT in these domains is important to be able to identify children who are at risk for lowered adaptive functioning, academic challenges, and psychopathology, and whom thus may be in need of close monitoring and early support or intervention.
2. METHOD
2.1. Search strategy
A structured approach was used to identify and review articles. The online database Web of Knowledge was used to identify eligible peer‐reviewed scientific articles that were published before July 1, 2018. An overview of the used search terms can be found in Figure 1. The Web of Knowledge categories filter was used to include publications in the following categories: Behavior sciences, education, genetics heredity, language and linguistics, neurosciences, pediatrics, psychiatry, and psychology (clinical, developmental, and multidisciplinary). Using the same search strategy, the online database PubMed was consulted, but no additional relevant articles were identified. Finally, reference lists from identified papers were consulted to trace additional papers.
Figure 1.
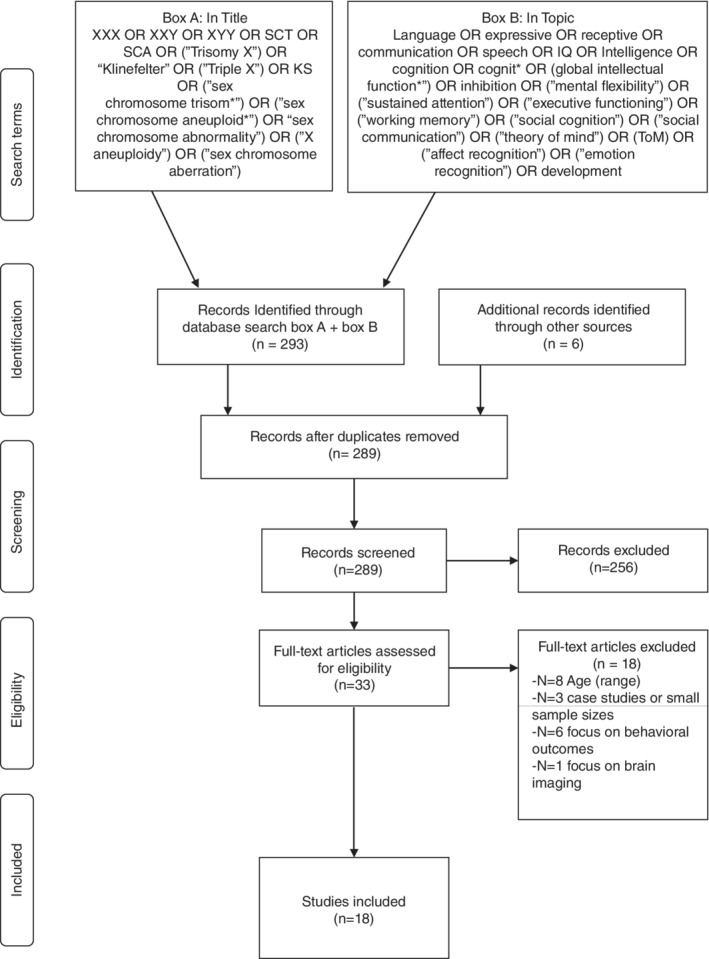
PRISMA flow chart of search strategy and included studies
2.2. Study selection
After removing duplicates using the EndNote automatic duplicate removal function, the retrieved articles were scanned for relevance by author 1. Titles and abstracts were assessed by authors 1 and 2 before assessing full texts of studies and discrepancies were resolved via consensus. The inclusion criteria specified that to be eligible for the review (a) Participants in the studies were aged between 0 and 18 years, or when the study included a broader age range, the effect of age was assessed, (b) Studies were published in international peer‐reviewed journals and available as a full‐text article written in English, (c) Studies included ≥15 participants, (d) The main focus of the study was on global intellectual functioning, language development, social cognition, or executive functioning. In addition, studies were included regardless of recruitment strategy, including newborn screening studies, as well as studies that included prenatally diagnosed participants, and postnatal follow‐up studies. Ascertainment bias plays a role in much of the literature on SCT. By including studies regardless of recruitment strategy (and thus clinical ascertainment) we aimed to describe as much of the variability on the reviewed domains, even though these outcomes may not be fully representative for the entire SCT population. This means that clinical ascertainment is also part of this review. Table 1 gives an overview of the sample ascertainment of the included studies. Also, studies were included when children with SCT were compared to a (matched)‐control group, or when validated instruments were used to compare children with SCT with a normed reference group, an overview of study design of the included studies can be found in Table 1. Finally, studies were included regardless of used instrument type, including both parent report and performance‐based tests.
Table 1.
Ascertainment and study design of included studies
| Authors | Included karyotypes | Prenatal diagnosed (%) | Study design |
|---|---|---|---|
| Ross et al, 2008 | XXY | 60 | Cross‐sectional, comparison with normed reference group |
| Ross et al, 2009 | XXY | 55 | Cross‐sectional, comparison with age‐matched controls |
| XYY | 29 | ||
| Cordeiro et al, 2012 | XXY | 56 | Cross‐sectional, comparison with normed reference group |
| XYY | 33 | ||
| Bruining et al, 2009 | XXY | 51 | Cross‐sectional, comparison with normed reference group |
| Ratcliffe, 2009 | XXY | 100 | Cross‐sectional, comparison with controls and siblings |
| XYY | 95 | Cross‐sectional, comparison with social class matched controls | |
| XXX | 100 | Cross‐sectional, comparison with female controls and siblings | |
| Rovet et al, 1995; 1996 | XXY | 100 | Cytogenetic survey followed by longitudinal follow‐up comparison with sibling controls |
| Netley, 1986 | XXY | N/Aa | Summary of several cytogenetic surveys with longitudinal follow‐up, comparison group differed between groups, including family member, unrelated controls, or a normed reference group |
| XXX | |||
| XYY | |||
| Zampini et al, 2018 | XXX/XXY | 100 | Cross‐sectional, comparison with controls |
| Haka‐Ikse et al, 1978 | XXY | 100 | Cytogenetic survey followed by longitudinal follow‐up comparison with normed reference group |
| Bishop et al, 2011 | XXX | 51 | Cross‐sectional, comparison with sibling controls |
| XXY | 100 | ||
| XYY | 36 | ||
| Lee et al, 2015 | XXY/XXX | 100 | Cross‐sectional, comparison with controls matched on chronological age and maternal education level |
| Van Rijn & Swaab, 2015 | XXX/XXY | 53 | Cross‐sectional, comparison with controls |
| Samango‐Sprouse et al, 2018 | XXY (NL) | 55 | Cross‐sectional, comparison with normed reference group |
| XXY (United States) | 91 | ||
| Ross et al, 2015 | XYY | 35 | Cross‐sectional, comparison with controls matched on chronological age |
| Van Rijn et al, 2014a | XXX/XXY | 53 | Cross‐sectional, comparison with controls |
| Van Rijn et al, 2018 | XXY | 24 | Cross‐sectional, comparison with normed reference group |
| Van Rijn et al, 2014b | XXX/XXY | 49 | Cross‐sectional, comparison with controls |
Percentage prenatal diagnosed is not explicitly stated in this summary overview.
In total, 18 publications met our criteria. For each publication, participant characteristics, study design, and results were summarized in a spreadsheet, which were the basis for the tables in this manuscript. As this is a narrative review, a formal meta‐analysis or methodological appraisal was not conducted. However, to indicate the clinical significance of the outcomes reported in the included studies, effect sizes were calculated when applicable.
3. RESULTS
3.1. Global intellectual functioning
Eight studies met our inclusion criteria regarding GIF. Main findings of the included studies, in addition to used instruments and studied populations can be found in Table 2.
Table 2.
Included studies global intellectual functioning
| Authors | N | Age | Comparison | Subdomain(s) | Instrument(s) | Results |
|---|---|---|---|---|---|---|
| Ross et al, 2008 | 47 XXY |
4‐9;11 years 10‐17;8 years |
Normed scores | GCA | DAS | Older boys < younger boys |
| Ross et al, 2009 |
93 XXY 21 XYY |
4‐18 years | Control group | GCA | DAS | XXY = XYY < controls |
| VP | XXY = XYY < controls | |||||
| NVP | XXY = XYY < controls | |||||
| Spatial cluster | XXY < XYY = controls | |||||
| Cordeiro et al, 2012 |
95 XXY 29 XYY |
4‐18 years | Normed scores | VIQ‐PIQ Gap | DAS, WASI or WISC |
XXY VIQ < PIQ XYY VIQ < PIQ |
| Bruining et al, 2009 | 47 XXY | 6‐19 years | Normed scores | FSIQ | WISC or WASI | XXY < controls |
| PIQ | XXY < controls | |||||
| VIQ | XXY < controls | |||||
| Ratcliffe, 1999 |
19 XXY 19 XYY 16 XXX |
6‐8 years | Control group | PIQ | WISC |
XXY < controls XYY < controls XXX < controls |
| VIQ |
XXY < controls XYY < controls XXX < controls |
|||||
| Rovet et al, 1995; 1996 | 21‐29 XXY | 6‐18 years | Control group | PIQ | WISC or WASI | XXY < controls |
| VIQ | XXY < controls | |||||
| VIQ‐PIQ Gap | XXY VIQ < PIQ | |||||
| Netley, 1986 |
73 XXY 32 XXX 28 XYY |
Mxxy = 10.3 years Mxxx = 10.5 years Mxyy = 9.5 years |
Normed scores | FSIQ | WISC or WASI |
XXY < controls XXX < controls XYY n.s. |
| PIQ |
XXY n.s. XYY n.s. XXX < controls |
|||||
| VIQ |
XXY < controls XXX < controls XYY n.s. |
|||||
| VIQ‐PIQ Gap | XXX VIQ < PIQ |
Abbreviations: DAS, Differential Ability Scales; FSIQ, full scale intelligence quotient; GCA, General Conceptual Ability; n.s., no significant differences; IQ, intelligence; NVP, Nonverbal Performance; PIQ, performance intelligence quotient; WASI, Wechsler Abbreviated Scale of Intelligence; VIQ, verbal intelligence quotient; VP, Verbal Performance; WISC, Wechsler Intelligence Scale for Children.
Ross et al15 studied 47 boys with XXY aged 4‐18 years and compared scores to a normed reference group. The 4‐to‐9‐year olds showed relative strengths on the non‐verbal reasoning subtests (ie, matrices, sequential and quantitative reasoning) and on the spatial subtests (ie, recall of design, pattern constructions), in contrast to subtests on the verbal cluster (ie, word definitions, similarities). The 10‐18‐year olds showed low average scores on the verbal and non‐verbal reasoning subtests, whereas they had average scores on the spatial cluster subtests. When comparing the younger and older subgroups, it appeared that the older children performed worse on the matrices subtest, and had slightly lower general conceptual ability than the younger boys.
A second study by Ross et al16 included 93 boys with XXY, 21 boys with XYY, and 36 matched control boys, aged 4‐18 years. General conceptual ability was lower in the XXY and XYY groups, compared to controls. Overall, performance was similar in XXY and XYY boys, with the exception of nonverbal spatial cognitive abilities, which were better (ie, not different from controls) in boys with XYY.
A cohort of boys aged 4‐18 years was included in the study of Cordeiro et al.17 Results of GIF were obtained for 95 boys with XXY and 29 boys with XYY. Results showed a wide range of intellectual abilities, with a total intelligence (IQ) ranging from extremely/very low to very superior/high. There were no significant differences between the XXY and XYY groups; in both groups, verbal intelligence quotient (VIQ) was significantly lower than performance intelligence quotient (PIQ).
The wide variability of intellectual abilities was also found in a study by Bruining et al.18 Forty‐seven boys with XXY aged between 6 and 19 years participated. Total IQ and PIQ scores ranged from extremely low to superior, whereas VIQ scores ranged from extremely low to high average.
In the Edinburgh cohort, 19 boys with XXY, 19 boys with XYY, and 16 girls with XXX were followed from birth until the ages of 16 to 27. Intelligence was tested between the ages of 6 and 8 years. The XYY boys scored slightly, but significantly, lower than controls matched on social class and sibling controls, especially in the verbal domains. The XXY boys, as well as the XXX girls, scored significantly lower than controls and siblings in both the verbal and the performance domains, and showed a wide variability in scores.19
In the Toronto cohort, boys with XXY were followed from birth until the age of 20 years. Intelligence was measured over time at several age intervals, with the sample size ranging from 21 to 29 participants. Results showed that scores on the performance domain were only lower in boys with XXY when compared to controls at the youngest age interval (ie, 6‐8 years), whereas scores on the verbal domain were lower in boys with XXY at all ages, except when they were 15‐17 years. Boys with XXY had poorer verbal scores compared to performance scores at all ages.20, 21
Netley22 summarized results of several longitudinal studies, including data from the Boston, Denver, Edinburgh, Japan, Toronto, and Winnipeg cohorts. In total 73 boys with XXY, 32 girls with XXX, and 28 boys with XYY participated and were compared to normed scores. Results showed that boys with XXY scored lower on the verbal, but not performance domains, whereas girls with XXX scored lower on both the verbal and performance domain, with better performance than verbal scores. Finally, no significant differences in GIF were found in boys with XYY.
3.2. Language development
Five studies met our inclusion criteria regarding language development in children with SCT. Main findings of the included studies, in addition to used instruments and studied populations can be found in Table 3. When applicable, effect sizes were calculated to indicate the clinical significance.
Table 3.
Included studies language domain and calculated effect sizes
| Authors | N | Age | Comparison | Subdomain(s) | Instrument(s) + Type(s) | Results | Effect sizes |
|---|---|---|---|---|---|---|---|
| Zampini et al, 2018 | 15 XXX /XXY | 24 months | Control group | Vocabulary size | CDI (P) | XXX/XXY < controls | d = 2.18*** |
| Verbal productions | Structured‐play session (O) | XXX/XXY < controls | d range = .99‐1.44*** | ||||
| Number of Utterances | XXX/XXY < controls | d range = 1.76‐2.08*** | |||||
| Pointing gestures | XXX/XXY > controls | d = 1.03*** | |||||
| Haka‐Ikse et al, 1978 | 25 XXY | 36‐72 months | Normed scores | Language difficulties | YDS (P) | >50% | N/A |
| Ross et al, 2008 | 47 XXY |
4‐9; 11 years 10‐17; 8 years |
Normed scores | Complex levels of language processing | TLC‐E (C) |
XXY < controls; Older boys < younger boys |
d = 1.45*** |
| Expressive vocabulary | EOWPVT (C) | n.s. | |||||
| Receptive vocabulary | ROWPVT (C) | n.s. | |||||
| Semantic fluency | DKEFs (C) | n.s. | |||||
| Phonetic fluency | n.s. | ||||||
| Phonological processing | CTOPP (C) | n.s. | |||||
| Ross et al, 2009 |
93 XXY 21 XYY |
4‐18 years | Control group | Receptive vocabulary | ROWPVT (C) | XYY < XXY < controls |
d xxy = 1.15*** d xyy = 1.85*** |
| Complex levels of language processing | TLC‐E (C) | XXY = XYY < controls |
d xxy = 1.63*** d xyy = 1.33*** |
||||
| Expressive vocabulary | EOWPVT (C) | XXY = XYY < controls |
d xxy = .96*** d xyy = 1.17*** |
||||
| Phonetic fluency | DKEFs (C) | XXY = XYY < controls |
d xxy = .97*** d xyy = 1.08*** |
||||
| Phonological processing | CTOPP (C) | Inconclusive results | |||||
| Semantic fluency | DKEFs (C) | n.s. | |||||
| Bishop et al, 2011 |
58 XXX 19 XXY 58 XYY |
4‐17 years | Control group | Structural and pragmatic difficulties | CCC (P) |
XXX 44%‐68% XXY 50% XYY 38%‐85% |
N/A |
Note: *** High clinical significance; ** Moderate clinical significance; * Low clinical significance; N/A, not applicable; n.s., no significant differences.
Abbreviations: C, Performance Task Child; CCC, Children's Communication Checklist; CDI, MacArthur Communicative Development Inventories; CTOPP, Comprehensive Test of Phonological Processing; DKEFs, Delis‐Kaplin Executive Function system; EOWPVT, Expressive One‐Word Picture Vocabulary Test; O, Observation; P, Parent Report; ROWPVT, Receptive One Word Picture Vocabulary Test; TLC‐EL, Test of Language Competence—Expanded Edition; YDS, Yale Developmental Schedules.
Zampini et al,23 studied 15 boys and girls with an extra X chromosome at the age of 24 months. Parents from children with an extra X reported that their child produced significantly less words than parents of control children. In addition, 60% of the children with an extra X were at risk for language impairments. In a semi‐structured play session between children and their parent, spontaneous utterances, verbal productions, and gestures of the child were coded and classified. During this play session, children with an extra X showed less verbal utterances, and more simple vocal productions. In addition—possibly to compensate—the extra X group showed more pointing gestures. When comparing the boys and girls in the extra X group, no significant differences were found, indicating that, although less pronounced in girls, the language difficulties could be similar in XXX and XXY.
This early risk for language problems was also found in a study by Haka‐Ikse et al,24 who studied 25 boys with XXY between the ages of 3 and 6 years, and used the revised Yale Developmental Schedules to assess performance on several domains including language. This study showed that already at preschool age, boys with XXY show a mild developmental delay in language development; with more than half of the children experiencing problems with language.
Two studies used more extensive language assessments and included measures for expressive language, receptive language, phonological processing, phonemic fluency, semantic fluency, and complex levels of language processing (ie, semantics, syntax, and pragmatics). The first study found age‐appropriate development of expressive and receptive vocabulary, as well as normal verbal fluency development in 47 boys with XXY aged 4‐18 years.15 More complex levels of language processing, however, were impaired. When comparing 4‐to‐9‐year olds with 10‐to‐18‐year olds, it appeared that the older group had significantly more difficulties with these complex levels of language processing. The second study compared boys between the ages of 4‐18 years with XXY (N = 93), XYY (N = 21), and controls matched on age.16 Results showed that both boys with XXY and XYY perform significantly worse than controls on measures of expressive and receptive language, with the XYY boys performing worse than the XXY boys. In addition, phonetic fluency was lower in XXY and XYY boys compared to controls, whereas semantic fluency and phonological processing were unimpaired. Finally, complex levels of language processing were impaired in both boys with XXY and XYY. The authors conclude that although boys with XXY and XYY both experience language difficulties, these difficulties appear to be more severe in boys with XYY.
Bishop et al25 relied solely on parent reports. This study included children between the ages of 4 and 16 years, and compared children who were diagnosed prenatally vs children who were diagnosed postnatally. More than half of the children with SCT received language therapy, compared to 10% of the sibling controls. Rates of language therapy were significantly higher among children who were diagnosed postnatally (68%) than children diagnosed prenatally (44%); and more common in boys with XYY (88%) than boys with XXY (47%) or girls with XXX (41%). Parents reported a similar profile of impairments across the SCT groups; however impairments appeared to be greater in boys than in girls, and in children with a postnatal diagnosis compared to children with a prenatal diagnosis.
3.3. Executive functioning
Five studies met our inclusion criteria regarding EF in children with SCT. Main findings of the included studies, in addition to used instruments and studied populations can be found in Table 4. When applicable, effect sizes were calculated to indicate the clinical significance.
Table 4.
Included studies executive functioning domain and calculated effect sizes
| Authors | N | Age | Comparison | Subdomain(s) | Instrument(s) + Type(s) | Results | Effect sizes |
|---|---|---|---|---|---|---|---|
| Lee et al, 2015 |
15 XXY 15 XXX |
5‐18 years | Control group | Daily life executive functioning | BRIEF (P) | XXX/XXY > controls† | N/A |
| Ross et al, 2008 | 47 XXY | 4‐18 years | Normed scores | Sustained attention—omissions | C(K)CPT (C) | XXY > controls† | N/A |
| Sustained attention—variability | XXY > controls† | N/A | |||||
| Sustained attention—reaction time | XXY > controls† | N/A | |||||
| Inhibition | DKEFS‐CWIT (C) | n.s. | |||||
| Mental flexibility | n.s. | ||||||
| Ross et al, 2009 |
93 XXY 21 XYY |
4‐18 years | Control group | Sustained attention—omissions | C(K)CPT (C) | XXY > XYY = controls† | d xxy = .83*** |
| Sustained attention—variability | XXY = XYY > controls† |
d xxy = .80*** d xyy = .86*** |
|||||
| Sustained attention—reaction time | XXY = XYY > controls† |
d xxy = 1.02*** d xyy = 1.04*** |
|||||
| Sustained attention—commissions | n.s. | ||||||
| Inhibition | DKEFS‐CWIT (C) | XYY < XXY < controls | d xyy = 1.09*** | ||||
| Mental flexibility | XYY < XXY < controls | d xyy = 1.71*** | |||||
| Van Rijn & Swaab, 2015 | 40 XXX/XXY | 9‐18 years | Control group | Sustained attentional control | ANT (C) | XXX/XXY < controls | d = .33* |
| Inhibition | XXX/XXY < controls | d = .38* | |||||
| Mental flexibility | XXX/XXY < controls | d = .45* | |||||
| Visual working memory | XXX/XXY < controls | d = .68** | |||||
| Focused attention | n.s. | ||||||
| Verbal working memory | n.s. | ||||||
| Daily life executive functioning | DEX (P) | XXX/XXY < controls | d = 1.37*** | ||||
| Samango‐Sprouse et al, 2018 |
44 XXY (NL) 54 XXY (United States) |
8‐18 years | Normed scores | Sustained attention; % significant impaired | ANT (C) | 19%‐57% | N/A |
| Inhibition; % significant impaired | 26%‐28% | N/A | |||||
| Mental flexibility; % significant impaired | 35%‐36% | N/A |
Note: *** High clinical significance; ** Moderate clinical significance; * Low clinical significance; N/A, not applicable; n.s., not significant; † higher scores denote more problems.
Abbreviations: ANT, Amsterdam Neuropsychological Tasks; BRIEF, Behavior Rating Inventory of Executive Function; C, Performance Task Child; C(K)CPT, Conners' (Kiddie) Continuous Performance Test; DEX, Dysexecutive Questionnaire; DKEFS‐CWIT; Delis‐Kaplin Executive Functioning Color‐Word Interference Test; P, Parent Report.
One study used parent report to assess difficulties with EF and showed that parents with children aged 5‐18 years with an extra X chromosome (N = 30) reported more difficulties than parents with typically developing children on all domains (ie, inhibition, ability to shift behavior, emotional control, working memory, planning/organizing, initiating behavior, and organization of materials). In addition, a cross‐sectional study with the same group of participants showed age‐effects in the extra X group; although there appeared to be developmental stability (ie, difficulties did not differ across the age‐groups) on most domains, difficulties on initiating and planning/organizing domains, became more pronounced with increased age.26
Four studies used performance‐based tasks to examine processing speed, sustained attention, response inhibition, and inhibitory control. In the first study age‐appropriate performance on cognitive inhibition tasks was found in 47 boys with XXY.15 When comparing 4‐to‐9‐year olds with 10‐to‐18‐year olds, it appeared that younger, but not older boys had difficulties with sustained attention. The second study compared boys with XXY (N = 93) or XYY (N = 21) with age‐matched controls between the ages of 4 and 18 years.16 Results showed significantly more difficulties with sustained attention in the XXY group, but not the XYY group. However, both the XXY and the XYY group had increased reaction times, and showed more variability during the sustained attention task. On inhibition tasks, the XYY, but not the XXY group displayed significantly more difficulties in both inhibiting a cognitive response, and switching between rules within the task, indicating more problems with mental flexibility in boys with XYY. The third study used both computerized performance‐based tasks as well as parent reports to assess EF in 23 boys with XXY and 17 girls with XXX all aged between 9 and 18 years.27 This study found no significant differences between the extra X groups and a group of controls on information processing speed, focused attention, or verbal working memory. However, significant group differences were found on measures of sustained attentional control, inhibition, mental flexibility, visual working memory, and daily life EF (as reported by parents). The results for XXY boys and XXX girls were not significantly different, although processing speed was lower in girls with XXX. Finally, differences between children who were diagnosed prenatally vs children with a postnatal diagnosis were not found. The fourth study used the same computerized tasks as the previous study to measure sustained attentional control, inhibition, and mental flexibility in two groups of boys with XXY from the Netherlands (N = 44) and from the United States (N = 54).28 Developmental risk was calculated as a percentage of children that scored in the significantly impaired range (ie, Z > 2.0). Results showed that 19%‐23% experienced significant and clinically relevant difficulties with sustained attention. However difficulties with attention regulation (ie, stability of reaction times) occurred in 22% of the US boys, and 57% of the Dutch boys. The authors note that time of diagnosis was a significant predictor for attention regulation, and that 46% of the Dutch boys received a prenatal diagnosis, compared to 91% of the US boys. On the inhibition task, 26%‐28% of the children experienced significant and clinically relevant difficulties, and on the mental flexibility task 35%‐36% experienced significant and clinically relevant difficulties, showing a developmental risk for several EF.
3.4. Social cognition
Six studies met our inclusion criteria regarding social cognition in children with SCT. Main findings of the included studies, in addition to used instruments and studied populations can be found in Table 5. When applicable, effect sizes were calculated to indicate the clinical significance.
Table 5.
Included studies social cognition domain and calculated effect sizes
| Authors | N | Age | Comparison | Subdomain(s) | Instrument(s) + Type(s) | Results | Effect sizes |
|---|---|---|---|---|---|---|---|
| Ross et al, 2015 | 18 XYY | 4‐14 years | Control group | Social cognition | SRS (P) | XYY > controls† | d = .68** |
| Cordeiro et al, 2012 |
102 XXY 40 XYY |
4‐18 years | Normed scores | Social cognition | SRS (P) | XYY > XXY > controls† |
d xxy = .93*** d xyy = 1.80*** |
| Van Rijn et al, 2014a | 60 XXX/XXY | 9‐18 years | Control group | Social cognition | SRS (P) | XXX/XXY > controls† | d = 1.61*** |
| Van Rijn et al, 2018 | 70 XXY | 8‐60 years | Normed scores | Pattern recognition—reaction time % impaired | ANT (C) | 17% | N/A |
| Pattern recognition—accuracy % impaired | 9% | N/A | |||||
| Face processing—reaction time % impaired | 26% | N/A | |||||
| Face processing—accuracy % impaired | 13% | N/A | |||||
| Facial emotion recognition—reaction time % impaired | 33% | η2 = .40*** | |||||
| Facial emotion recognition—accuracy % impaired | 13% |
η2 = .16** |
|||||
| Samango‐Sprouse et al, 2018 |
44 XXY (NL) 54 XXY (United States) |
8‐18 years | Normed scores | Face processing—% impaired | ANT (C) | 23%‐25% | N/A |
| Facial emotion recognition—% impaired | 16%‐44% | N/A | |||||
| Van Rijn et al, 2014b | 46 XXX/XXY | 9‐18 years | Control group | Theory of Mind—egocentric role taking | SCST (C) | XXX/XXY < controls | d = .85*** |
| Theory of Mind—subjective role taking | XXX/XXY < controls | d = 1.03*** | |||||
| Theory of Mind—self‐reflective role taking | XXX/XXY < controls | d = .69** | |||||
| Theory of Mind—mutual role taking | XXX/XXY < controls | d = .83*** | |||||
| Facial affect identification—angry faces | KDEF (P) | XXX/XXY < controls | d = 3.30*** |
Note: *** High clinical significance; ** Moderate clinical significance; * Low clinical significance; N/A, not applicable; n.s., not significant; † higher scores denote more problems.
Abbreviations: ANT, Amsterdam Neuropsychological Tests; C, Performance Task Child; KDEF, Karolinska Directed Emotional Faces; P, Parent Report; SCST, Social Cognitive Skills Tests; SRS, Social Responsiveness Scale.
Three studies used parent reports to assess social cognition in children with SCT. The first study included 18 boys with XYY between the ages of 4 and 14 years.29 The XYY boys had higher scores than controls, indicating more difficulties with social cognition. A second study included children and adolescents with XXY (N = 102) and XYY (N = 40) aged 4‐to‐18 years.17 Parents of boys with XXY and XYY reported more impairments with social cognition, than parents in the normative sample. Parents of XYY boys also reported more impairments than parents of XXY boys. In addition, parents of the XXY and XYY groups both reported more variability in scores compared to the normative sample, indicating a wide range of social cognitive abilities in boys with SCT. The third study included 60 boys and girls with an extra X chromosome, between the ages of 9 and 18 years.30 Parents of children with an extra X chromosome reported more difficulties in social cognition compared to parents of typically developing children. No significant differences were found in the reported difficulties between boys and girls with an extra X chromosome, indicating similar impairments in social cognition.
Three studies were identified that used child‐assessments to measure social cognition skills, such as theory of mind (ToM) and (facial) emotion recognition. The first study involved 70 boys and men with XXY, and although age ranged from 8 to 60 years, the effect of age was assessed.31 Social cognition was assessed using computerized tasks of pattern identification, face recognition, and facial emotion recognition. Accuracy in performance in the XXY group differed from the control group specifically when stimuli were of a more social nature (ie, during facial emotion recognition). The XXY group on average needed more time to identify facial expressions, although performance accuracy did not increase with more time. The results were independent of age, suggesting that the difficulties with emotion recognition are already apparent during childhood. The second study used the same computerized tasks to study face processing and emotion recognition skills in in two groups of boys with XXY from the Netherlands (N = 44) and from the United States (N = 54).28 Developmental risk was calculated as a percentage of children that scored in the significantly impaired range (ie, Z > 2.0). Results showed that 23%‐25% of the children experienced significant and clinically relevant difficulties with face processing. In addition, 16%‐44% of the children experienced significant and clinically relevant difficulties with emotion recognition (ie, identifying sad, happy, or angry emotions) The third study tested a group of 46 boys and girls with an extra X chromosome, between the ages of 9 and 18 years.32 Measures included assessments of ToM and emotion recognition. Children with an extra X chromosome performed more poorly on the ToM task than the control group. In addition, on average children with an extra X chromosome showed difficulties in the ability to identify emotional faces which was expressed in the reduced accuracy, rather than reaction times, and most prominent for angry faces. No differences were found in the performance of the XXX vs the XXY group, nor in the performance of children in the prenatal follow‐up vs the referred group.
4. DISCUSSION
The aim of this review was two‐fold. The first aim was to review to what degree impairments in areas of global intellectual functioning, language development, social cognition, and EF have been studied in children with SCT, and identify possible gaps in research that future research should focus on. The second aim, was to establish clinical significance of these impairments and identify risk‐factors that should be closely monitored from early development onwards or that should be included in standard clinical neuropsychological screening to identify potential targets for support and intervention.
With regard to the first aim, the reviewed studies collectively gave the following results. On the domain of GIF, seven studies report outcomes in children between the ages of 4 and 18 years, with three studies focusing on children from the age of 4 years, and four studies studying school‐aged children. To our knowledge, there were no studies that examined GIF in children with SCT before the age of 4 years. On the domain of language development, five studies reported outcomes in children between the ages of 2 and 18 years. To our knowledge, there were no studies that examined language development in children with SCT before the age of 2 years. Of the seven studies, two studies used only parent reports, the other three studies used either a performance task or a combination of parent report and performance tasks. On the domain of executive functioning, five studies reported outcomes in children between the ages of 4 and 18 years. To our knowledge, there are no studies to date that assess (precursors of) EF in children with SCT before the age of 4 years. In addition, all studies included children with XXY; two studies also included girls with XXX, and one study also included boys with XYY. Finally, one study used parent report, with the other four studies using performance‐based tasks or a combination of both. On the domain of social cognition, six studies reported outcomes in children between the ages of 4 and 18 years. To our knowledge, there are no studies to date that assess (precursors of) social cognition in children with SCT before the age of 4 years. In addition, until the age of 8 years, and in XXX and XXY groups only, social cognition has not been tested with performance‐based measures, but has solely been assessed with parent reports. To this date, no studies have reported child‐data on social cognition in boys with XYY. Taken together, although GIF and language have received relatively much attention, there is a great need for more studies in areas of EF and social cognition in children with SCT. Also, research should rely more on performance‐based measures in addition to parent report. Finally, we stress the importance of following children over time. Longitudinal studies are needed to keep an eye on the developmental trajectory, and could help determine which difficulties in early life are predictive of outcomes in later life.
With regard to the second aim, the researched studies collectively gave the following result. On the domain of global intellectual functioning, from the age of 4 years there appears to be a general finding that the GIF of children with SCT is variable, and ranges from impaired to above average with mean GIF in the average to low‐average range. There might be to be some differences between the three karyotypes, with XXX girls showing reductions in both VIQ and PIQ, XXY boys showing reduced VIQ compared to PIQ, and XYY boys functioning variably. On the domain of language development, it appears that language difficulties can already be detected during the toddler‐age, and can be persistent throughout adolescence. Difficulties with language development have not only been reported by parents, but have also been observed during language assessments. All calculated effect sizes indicated high clinical significance; stressing the need for early detection and support programs on the domain of language. Especially complex levels of language, such as semantics, syntax, and pragmatics seem to be impaired. In addition, one study reported that older children appear to experience more difficulties than younger children. It is possible that children experience more (severe) difficulties, or that problems become more apparent during a certain age because of different task demands. A possible explanation for this is the phenomenon of “growing into deficit”; which occurs when age increases, while the expected rate of progress stays behind, resulting in a growing deficit (as compared with typically developing peers), and a growing impact on daily life.33 The reported language difficulties appear to be somewhat similar in girls with XXX and boys with XXY. Only one study compared boys with XXY and XYY, with XYY boys experiencing more difficulties in receptive vocabulary, but performing similarly with XXY boys on other areas of language development. On the domain of executive functioning, two studies indicated that parents of children with SCT report more difficulties with executive functioning. For one of these studies, we were able to calculate an effect size, which indicated high clinical significance. The studies that used performance‐based tasks report somewhat variable outcomes, partially depending on the included participant groups. All five studies included boys with XXY and have reported poorer performance and/or more difficulties when compared to controls, effect sizes were calculated for two of these studies, with one study indicating high clinical significance on the subdomain of sustained attention, inhibition, and mental flexibility, whereas the other study, which included slightly older children, indicated low to moderate clinical significance on these domains. Two studies included girls with XXX (in combination with boys with XXY) and reported poorer performance and/or more difficulties when compared to controls on the subdomains of sustained attentional control, inhibition, mental flexibility, and visual working memory, effect sizes indicated low to moderate clinical significance. One study included boys with XYY and reported more variability and longer reaction times on tasks that measure sustained attention. Effect sizes indicated high clinical significance. On the domain of social cognition, three studies indicated that parents of children with SCT report more difficulties with social cognition. Calculated effect sizes for all three studies indicated high clinical significance. One study that used a performance‐based task reported difficulties in boys with XXY on the subdomain of Theory of Mind; with effect size indicating high clinical significance. Three of the studies that included boys with XXY reported difficulties with facial emotion recognition, with effect sizes indicating high clinical significance. One study included girls with XXX (in combination with boys with XXY) and reported poorer performance on facial effect identification, in particular when identifying angry faces. Calculated effect sized indicates very high clinical significance.
In conclusion, from a developmental perspective it is important to monitor neuropsychological functioning of children with SCT at the start, or even before, the sensitive developmental period when these skills typically develop, and identify precursors and early markers of developmental risk. Considering the increased prevalence of (characteristics of) behavioral and neurodevelopmental disorders, such as ADHD, autism spectrum disorders, anxiety, and depression in the SCT population,14, 34, 35 more knowledge of developmental neurocognitive risk markers could lead to more timely, preventive support, hopefully reducing the risk for these behavioral and neurodevelopmental disorders in the future. In addition, the results of this review call for more studies on early neurocognitive vulnerabilities, which are expected based on the impact of the extra chromosome on the development of the brain.36 It is important to learn more about the involvement of genes on the sex chromosomes in order to identify how expression of these genes can lead to the behavioral phenotype of individuals with SCT and how different genes on different sex chromosomes can lead to the similarities and differences in the behavioral profile of children with XXX, XXY, and XYY. There is a specific need for more knowledge in areas in EF and social cognition, not only because more extensive research has shown these domains appear to be affected in adulthood,14 but also because these cognitive domains are crucial for behavioral and socio‐emotional development, adaptive functioning, and quality of life. Also, the results of this review illustrate that more attention should be given to timely screening for cognitive vulnerabilities, that these should be monitored during relevant developmental stages, and that interventions should be tailored to these risk profiles.
Finally, it is also important to gain more insight in the karyotype‐specific profiles of neurocognitive functioning, as the presence of an extra X or Y may have similar ánd different effects on development of brain areas involved in social cognition and language, and therefore could have effect on neurocognitive development. This may help in understanding expected neurodevelopmental profiles and related, tailored, intervention options.
Recruitment strategy will always lead to variance in the SCT phenotype with overestimation of some difficulties (eg, because these difficulties led to genetic screening in postnatally diagnosed individuals), whereas other difficulties may be underestimated (eg, because prenatally diagnosed individuals may have benefited from early preventive support, such as speech therapy). For that reason, it is difficult to assess the full spectrum of strengths and weaknesses in individuals with SCT when using only one strategy. By including all studies regardless of the used recruitment strategy, we have attempted to balance bias, even though the described outcomes may not be fully representative for the total population children with SCT.
To conclude, this review of studies shows that the presence of an extra sex chromosome, may have impact on neurocognitive functioning of children with SCT, and identified that domains of language development, executive functioning, and social cognition should be closely monitored in these children. In addition, it is important to gain more insight in the early development of children with SCT population, especially before the age of 4 years on the domains of social cognition and executive functioning. Finally, it is important that social cognition and EF will be included in the standard screening and assessment methods, as this review showed that social cognition and EF in addition to language development, are domains that require close monitoring, and are targets for early support and intervention programs. With more knowledge about the development of young children with SCT, existing evidence‐based (preventive) intervention programs can be tailored to the SCT profile in hopes of reducing these difficulties, and by reducing these neurocognitive underpinnings of behavior, could possibly prevent neurobehavioral problems in later life.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
This work was supported by a personal grant (to SvR, grant number 016.165.397) from the Netherlands Organization for Scientific Research (NWO).
Urbanus E, van Rijn S, Swaab H. A review of neurocognitive functioning of children with sex chromosome trisomies: Identifying targets for early intervention. Clin Genet. 2020;97:156–167. 10.1111/cge.13586
Data Availability Statement: Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Funding information Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Number: 016.165.397
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Leggett V, Jacobs P, Nation K, et al. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Dev Med Child Neurol. 2010;52(2):119‐129. 10.1111/j.1469-8749.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hong DS, Reiss AL. Cognitive and neurological aspects of sex chromosome aneuploidies. Lancet Neurol. 2014;13(3):306‐318. 10.1016/s1474-4422(13)70302-8. [DOI] [PubMed] [Google Scholar]
- 3. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevelance of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metabol. 2003;88(2):622‐626. [DOI] [PubMed] [Google Scholar]
- 4. Groth KA, Skakkebaek A, Høst C, et al. Klinefelter syndrome—a clinical update. J Clin Endocrinol Metabol. 2013;98(1):20‐30. [DOI] [PubMed] [Google Scholar]
- 5. Morris JK, Alberman E, Scott C, Jacobs P. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet. 2008;16:163‐170. [DOI] [PubMed] [Google Scholar]
- 6. Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of inidcation for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17(4):363‐368. [DOI] [PubMed] [Google Scholar]
- 7. Lenroot RK, Blumenthal JD, Wallace GL, Clasen LS, Lee NR, Giedd JN. A case‐control study of brain structure and behavioral characteristics in 47,XXX syndrome. Genes Brain Behav. 2014;13(8):841‐849. 10.1111/gbb.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Otter M, Schrander‐Stumpel CT, Curfs LM. Triple X syndrome: a review of the literature. Eur J Hum Genet. 2010;18(3):265‐271. 10.1038/ejhg.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giltay J, Maiburg M. Klinefelter syndrome: clinical and molecular aspects. Expert Rev Mol Diagn. 2010;10:765‐776. [DOI] [PubMed] [Google Scholar]
- 10. Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47,XXX). Orphanet J Rare Dis. 2010;5(8). 10.1186/1750-1172-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1–2):3‐18. 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 12. Wigby K, D'Epagnier C, Howell S, et al. Expanding the phenotype of triple X syndrome: a comparison of prenatal versus postnatal diagnosis. Am J Med Genet A. 2016;170(11):2870‐2881. 10.1002/ajmg.a.37688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samango‐Sprouse CA, Keen C, Sadeghin T, Gropman A. The benefits and limitations of cell‐free DNA screening for 47,XXY (Klinefelter syndrome). Prenat Diagn. 2017;37(5):497‐501. 10.1002/pd.5044. [DOI] [PubMed] [Google Scholar]
- 14. Van Rijn S. A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47,XYY). Curr Opin Psychiatry. 2018;32:79‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross JL, Roeltgen DP, Stefanatos G, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A. 2008;146A(6):708‐719. 10.1002/ajmg.a.32232. [DOI] [PubMed] [Google Scholar]
- 16. Ross JL, Zeger MP, Kushner H, et al. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Dev Disabil Res Rev. 2009;15(4):309‐317. 10.1002/ddrr.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cordeiro L, Tartaglia N, Roeltgen D, Ross J. Social deficits in male children and adolescents with sex chromosome aneuploidy: a comparison of XXY, XYY, and XXYY syndromes. Res Dev Disabil. 2012;33(4):1254‐1263. 10.1016/j.ridd.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruining H, Swaab H, Kas M, et al. Psychiatric characteristics in a self‐selected sample of boys with Klinefelter syndrome. Pediatrics. 2009;123(5):e865‐e870. 10.1542/peds.2008-1954. [DOI] [PubMed] [Google Scholar]
- 19. Ratcliffe S. Long term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999;80:192‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rovet J, Netley C, Bailey J, Keenan M, Stewart D. Intelligence and achievement in children with extra X aneuploidy: a longitudinal perspective. Am J Med Genet. 1995;60:356‐363. [DOI] [PubMed] [Google Scholar]
- 21. Rovet J, Netley C, Keenan M, Bailey J, Stewart D. The psychoeducational profile of boys with Klinefelter syndrome. J Learn Disabil. 1996;29(2):180‐196. [DOI] [PubMed] [Google Scholar]
- 22. Netley CT. Summary overview of behavioural development in individuals with neonatally identified X and Y aneuploidy In: Ratcliffe SG, Paul N, eds. Prospective Studies on Children with Sex Chromosome Aneuploidy. New York, NY: Alan R. Liss, Inc; 1986:293‐306. [PubMed] [Google Scholar]
- 23. Zampini L, Draghi L, Silibello G, et al. Vocal and gestural productions of 24‐month‐old children with sex chromosome trisomies. Int J Lang Commun Disord. 2018;53(1):171‐181. 10.1111/1460-6984.12334. [DOI] [PubMed] [Google Scholar]
- 24. Haka‐Ikse K, Stewart D, Cripps MH. Early development of children with sex chromosome aberrations. Pediatrics. 1978;62(5):761‐766. [PubMed] [Google Scholar]
- 25. Bishop DV, Jacobs PA, Lachlan K, et al. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child. 2011;96(10):954‐959. 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee NR, Anand P, Will E, et al. Everyday executive functions in down syndrome from early childhood to young adulthood: evidence for both unique and shared characteristics compared to youth with sex chromosome trisomy (XXX and XXY). Front Behav Neurosci. 2015;9:264 10.3389/fnbeh.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rijn S, Swaab H. Executive dysfunction and the relation with behavioral problems in children with 47,XXY and 47,XXX. Genes Brain Behav. 2015;14(2):200‐208. 10.1111/gbb.12203. [DOI] [PubMed] [Google Scholar]
- 28. Samango‐Sprouse CA, Stapleton E, Chea S, et al. International investigation of neurocognitive and behavioral phenotype in 47,XXY (Klinefelter syndrome): predicting individual differences. Am J Med Genet. 2018;176:877‐885. [DOI] [PubMed] [Google Scholar]
- 29. Ross JL, Tartaglia N, Merry DE, Dalva M, Zinn AR. Behavioral phenotypes in males with XYY and possible role of increased NLGN4Y expression in autism features. Genes Brain Behav. 2015;14(2):137‐144. 10.1111/gbb.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Rijn S, Stockmann L, Borghgraef M, et al. The social behavioral phenotype in boys and girls with an extra X chromosome (Klinefelter syndrome and Trisomy X): a comparison with autism spectrum disorder. J Autism Dev Disord. 2014;44(2):310‐320. 10.1007/s10803-013-1860-5. [DOI] [PubMed] [Google Scholar]
- 31. van Rijn S, de Sonneville L, Swaab H. The nature of social cognitive deficits in children and adults with Klinefelter syndrome (47,XXY). Genes Brain Behav. 2018. [DOI] [PubMed] [Google Scholar]
- 32. van Rijn S, Stockmann L, van Buggenhout G, van Ravenswaaij‐Arts C, Swaab H. Social cognition and underlying cognitive mechanisms in children with an extra X chromosome: a comparison with autism spectrum disorder. Genes Brain Behav. 2014;13(5):459‐467. 10.1111/gbb.12134. [DOI] [PubMed] [Google Scholar]
- 33. Rourke BP, Bakker DJ, Fisk JL, et al. Child Neuropsychology. New York, NY: Guilford Press; 1983. [Google Scholar]
- 34. Ross JL, Roeltgen DP, Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics. 2012;129(4):769‐778. 10.1542/peds.2011-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tartaglia NR, Ayari N, Hutaff‐Lee C, Boada R. Attention‐deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. J Dev Behav Pediatr. 2012;33(4):309‐318. 10.1097/DBP.0b013e31824501c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Printzlau F, Wolstencroft J, Skuse DH. Cognitive, behavioral, and neural consequences of sex chromosome aneuploidy. J Neurosci Res. 2017;95(1–2):311‐319. 10.1002/jnr.23951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


