Abstract
Objective
To determine the effects on the vaginal microbiota of an oral probiotic preparation administered from early pregnancy.
Design
Randomised, double blind, placebo‐controlled trial.
Setting
Four maternity units in the UK.
Population
Women aged 16 years or older recruited at 9–14 weeks' gestation.
Methods
Participants were randomly allocated to receive oral capsules of probiotic containing Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 each at 2.5 × 109 colony‐forming units (CFUs) or placebo once daily from recruitment until the end of pregnancy.
Main outcome measure
Rates of bacterial vaginosis (BV, defined as Nugent score ≥7) at 18–20 weeks' gestation compared by logistic regression adjusted for possible confounders.
Results
The primary analysis included 78% (238/304) of participants who initially consented (probiotic group 123, placebo group 115). Of these participants, 95% (227/238) reported an intake of 93% or more of the required number of capsules. The rates of BV did not differ between groups at 18–20 weeks' gestation (15% (19/123) in the probiotic group vs. 9% (10/115) in the placebo group, adjusted odds ratio 1.82, 95% confidence interval 0.64–5.19). There were also no differences between the groups in the proportion of women colonised with the probiotic strains, Escherichia coli, group B streptococci or other vaginal microbiota. There were no differences in the alpha diversity or composition of the bacterial communities between or within the probiotic and placebo groups at 9–14 and 18–20 weeks’ gestation.
Conclusions
Oral probiotics taken from early pregnancy did not modify the vaginal microbiota.
Tweetable abstract
The oral probiotic preparation used in this study does not prevent BV in pregnant women.
Keywords: Bacterial vaginosis, Lactobacillus reuteri, Lactobacillus rhamnosus, probiotic
Tweetable abstract
The oral probiotic preparation used in this study does not prevent BV in pregnant women.
Introduction
Bacterial vaginosis (BV), in which the normally dominant lactobacilli of the vagina are replaced with anaerobic bacteria, is associated with adverse pregnancy outcomes including preterm birth (PTB)1, 2, 3, 4. Antibiotics are effective in eradicating BV during pregnancy5 and are recommended for use in symptomatic pregnant women.6 The consequences of antibiotic use during pregnancy have been poorly studied,7 but there are concerns that antibiotic‐related changes in the vaginal microbiota8 are associated with short‐ and long‐term morbidity in infancy and later years.9
Probiotics might be a safe and effective alternative to antibiotics in restoring the imbalance of the vaginal microbiota found in BV. Probiotics are defined as ‘live micro‐organisms that confer a health benefit on the host when administered in adequate amounts’.10 Limited evidence suggests that they have several beneficial roles including the ability to displace and kill pathogens, and modulate the body's immune response.11, 12 Lactobacilli, primarily the strains that produce higher levels of hydrogen peroxide, appear to protect against BV13, 14, 15 but the importance of hydrogen peroxide in this is unclear.16
Evidence in non‐pregnant women suggests that oral probiotics can colonise the vagina, restore its microbiota in the presence of microbial imbalance, and eradicate or reduce the incidence of urogenital infections.17, 18, 19, 20, 21 Several commercial probiotic products are marketed for the restoration and maintenance of a ‘healthy vaginal microbiota’ in both non‐pregnant and pregnant women22, 23 but evidence of colonisation or benefit in pregnant women is lacking.
We therefore performed a randomised trial of a commercially available probiotic preparation in pregnant women to assess its biological effects on the vaginal microbiota. The constituents of the preparation have been shown to colonise the vagina in some non‐pregnant women. We hypothesised that the probiotic preparation containing Lactobacillus spp. taken orally from early pregnancy (9–14 completed weeks) would colonise the vagina and reduce the prevalence of BV. The probiotic preparation selected has been shown to be safe when taken during pregnancy.14, 15
Methods
A pragmatic randomised, double‐blind, placebo‐controlled trial with microbiome analysis was carried out to determine the biological effects of oral probiotics on the vaginal microbiota and the feasibility of conducting a full‐scale trial of oral probiotic supplementation of women from early pregnancy to delivery in preventing PTB. The study received ethics approval from the UK National Research Ethics Committee (15/LO/1549) and was registered on ClinicalTrials.gov (NCT02692820). We followed CONSORT guidelines24 to produce this report, and analysis was conducted using an analysis plan agreed in advance with the independent trial steering and data monitoring committee.
Participants
Women in early pregnancy were recruited from the maternity departments of four East London hospitals during their routine dating ultrasound scan appointment. All participants provided written informed consent. Eligible women were aged 16 years and above at consent and between 9 and 14 weeks’ gestation as confirmed by the dating ultrasound scan. We excluded those unable to provide written informed consent or who had a poor understanding of verbal or written English. There were no other exclusion criteria. Maternal demographic, medical and obstetric history, and antibiotic and probiotic use data were collected.
Interventions
The treatment group received oral probiotic capsules containing 2.5 billion CFUs each of L. rhamnosus GR‐1and L. reuteri RC‐14, while the placebo group received identical capsules containing excipients alone (Chr. Hansen, Hørsholm, Denmark). The contents of both capsules are stable at room temperature, and enough capsules were provided to each participant at the time of enrolment to last until 42 weeks’ gestation. We cultured the lactobacilli from a sample of the probiotic capsules before the start of the trial to ensure they were viable and could be detected by our culture techniques. Participants were instructed to take one capsule daily until delivery and self‐report compliance at each study visit.25
Outcomes
Our primary outcome was the rate of BV at 18–20 weeks’ gestation, measured using the Nugent score (Nugent score ≥7 shows presence of BV). Secondary outcomes were vaginal colonisation rates at 18–20 weeks’ gestation of the probiotic or other Lactobacillus spp., two common neonatal pathogens (Escherichia coli and group B streptococcus), and the composition of the vaginal microbiota.
Study plan
Research midwives approached potentially eligible women attending their routine dating ultrasound scan appointment. After confirming eligibility, informed consent was obtained and baseline data recorded. A double‐headed vaginal swab was collected, and the research midwife dispensed randomised packs of placebo or probiotic capsules. Vaginal swab samples were obtained again at two time points, 18–20 weeks’ and 34–36 weeks’ gestation, during scheduled routine antenatal appointments. At each visit, participants were asked to report on antibiotic use and any side effects from the intervention.
Sample size
A sample size of 366 was calculated to estimate the proportion of recruited women who would complete the study. Based on studies carried out on non‐pregnant women treated orally with the same lactobacillus strain,20 this sample size would allow us to detect with at least 80% power a decrease in BV of at least 50%, assuming a baseline risk of 25%, following treatment with the Lactobacillus spp. intervention.26
Randomisation
The random allocation sequence was generated based on permuted blocks of random block sizes of four, six, and eight, stratified by participating site and without adaptive or minimisation strategies. Allocation was done on a 1:1 ratio. The sequence was given to a trial support company, Sharp Clinical Services (SCS, Crickhowell, Wales), which labelled and packaged the probiotic and placebo capsules into identical tamper‐proof boxes for the study. Only the trial statistician and SCS were aware of the allocation sequence.
Blinding and allocation concealment
The probiotic and placebo capsules, made of hard hypromellose, were provided in identical plastic tubes by the manufacturer and shipped directly to SCS. The allocation sequence was used to label the tubes with details of the products’ expiry date, storage instructions, and directions for use. These were then packaged into tamper‐proof boxes. Participants, investigators, and analysing microbiologists were blinded to the study grouping.
Microbiology methodology
The double‐headed swab was separated in the laboratory. One swab was used to prepare a glass slide for Gram stain, and the other was extracted into 3 ml of Brain Heart Infusion broth (Unipath Ltd, Basingstoke, UK) containing 10% glycerol and 0.005% cysteine hydrochloride. The swab used to prepare the slide was stored at −70 °C and later used in the vaginal microbiota DNA profiling.
Gram‐stained glass slides prepared from the swabs were examined by microscopy and scored between 0 and 10 based on bacterial morphotypes according to the Nugent method.27 Stored samples were thawed and diluted through a series of 10‐fold dilutions in saline before being inoculated onto a range of selective and non‐selective culture media (Unipath). These included Mann Rogosa Sharpe (MRS) agar incubated for 48 h at 35 °C in 10% CO2, Columbia blood agar incubated aerobically at 37 °C for 48 h, Fastidious Anaerobe agar with 5% horse blood and kanamycin incubated anaerobically at 36 °C for 48 to 72 h, Sabouraud's agar plates incubated micro‐aerophilically at 36 °C for 24–48 h, and MacConkey agar incubated aerobically at 37 °C for 48 h. Colony counts from serial dilutions allowed an estimate of the numbers of different species and strains identified. These cultures allowed identification of Lactobacillus spp., Enterobacteriaceae, and group B streptococci. Isolates were identified using matrix‐assisted laser desorption/ionisation‐time of flight (MALDI‐TOF) analysis.28
Vaginal bacterial community profiling
DNA was extracted from thawed samples by means of the GenElute Bacterial Genomic DNA kit (Sigma‐Aldrich, Poole, UK), modified to optimise lysis of Gram‐positive bacteria, following the manufacturer's instructions. From each DNA extract, variable regions V1 and V2 of the 16S rRNA gene were amplified by PCR using fusion primers incorporating template specific primers 27F‐YM (AGAGTTTGATYMTGGCTCAG) and 338R‐R (TGCTGCCTCCCGTAGRAGT) and MiSeq adapters and barcodes to achieve a double indexing system. Amplicons were purified and normalised using the SequalPrep Normalization Plate Kit (ThermoFisher Scientific, Dartford, UK). Sequencing was performed at the Barts and The London Genome Centre using an Illumina MiSeq instrument, with a 2 × 250 flow cell for paired‐end sequencing. Sequence reactions were spiked with 10% 12.5 pm PhiX DNA. Reads were filtered by quality score using DADA229 to remove sequences with an expected error over 2 bp. The forward and reverse sequences were truncated at 250 and 200 bp, respectively. The filtered sequences were analysed using the mothur pipeline according to the SOP available at https://www.mothur.org/wiki/MiSeq_SOP. Sequences were clustered into operational taxonomic units (OTUs) at a sequence dissimilarity distance of 0.015 using an average neighbour algorithm and then classified using a Naïve Bayesian classifier implemented in mothur with the Human Oral Microbiome Database release 14.51 reference dataset. The alpha diversity of the samples was estimated by calculating the Inverse Simpson index and compared between groups by the Wilcoxon test. A dissimilarity theta‐YC matrix between the samples was constructed with the same subsampling approach averaging over 1000 replicas. The beta diversity between subject groups was then compared using the amova test.
Statistical analysis
We used descriptive analysis to report baseline characteristics of trial participants by allocated group. Continuous variables were reported as means and standard deviations, and ordinal/categorical variables were reported as absolute and relative frequencies. To assess the microbiological effects of probiotics on vaginal microbiota, we compared the proportion of women with BV and the proportion of women with vaginal colonisation of the intervention species at 18–20 weeks’ gestation between the groups. Nugent score was dichotomised as BV (score ≥7) and no BV (score 0–6).
We estimated treatment effect using binary logistic regression model adjusting for possible confounders, as this approach has known benefits including enhancement of power.30 A covariate was considered a confounding factor if the difference between adjusted and unadjusted model coefficients for the intervention variables varied by more than 10%. In such cases, the odds ratio (OR) was shown along with the list of confounders used for adjustment. Otherwise, we reported the model without adjusting for confounders. Mean Nugent score and its 95% confidence interval (CI) were also plotted by group from baseline to 34–36 weeks’ gestation to compare evolution of the marker during pregnancy.
All analysis was carried out using STATA software version 14.31
Patient and public involvement
Patient representatives provided input into the design, protocol development, and conduct of the study. Prior to this trial, a qualitative study was conducted to obtain input from women.32 The available core outcome set for PTB33 had an influence on data collected. We are planning to disseminate findings in the form of a newsletter and PPI feedback session, following primary publication of these results through our patient network.34
Results
We screened 1301 pregnant women between 3 May and 1 July 2016 for their eligibility to participate in the study. A total of 997 women were excluded from the trial either because they did not meet the inclusion criteria (270/1301) or they declined to participate. Those who declined to participate did so mostly because of an unwillingness to take the study intervention (138/727) and not being interested in research study participation (250/727). After exclusions, 304 women were recruited and randomised to the probiotic (152 women) and placebo (152 women) groups. At 18–20 weeks’ gestation, 123 women remained in the probiotic group and 115 in the placebo group. Complete data for primary analysis were therefore available for 238 (78%) randomised women (Figure 1).
Figure 1.
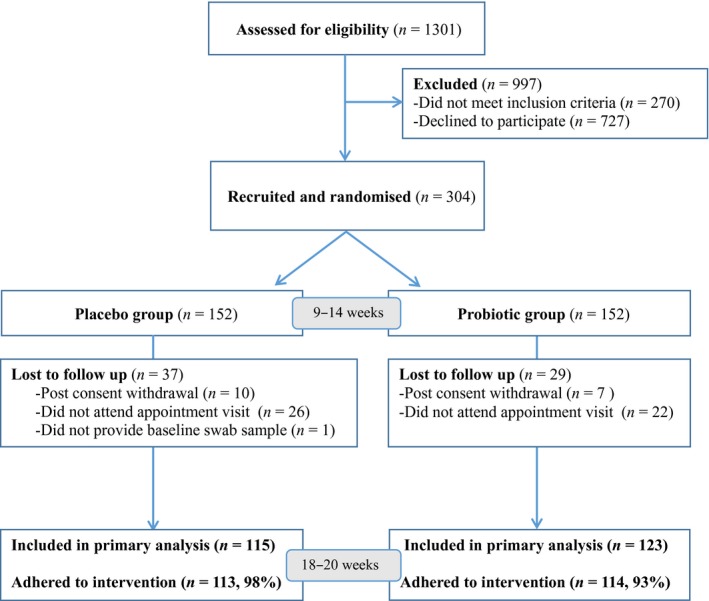
Flow chart showing numbers of participants at each stage of the trial.
There were no differences in characteristics of participants between the two groups at baseline (Table 1). In a random sample of probiotic capsules tested for Lactobacilli, the mean count was 2 × 109 for L. rhamnosus GR‐1 and 1 × 109 for L. reuteri RC‐14. No other bacteria were detected. Self‐reported compliance to the intervention at 18–20 weeks’ was 93% (114/123) in the probiotic group and 98% (113/115) in the placebo group. No serious adverse events related to the study interventions were reported.
Table 1.
Baseline characteristics* and outcome at 18–20 weeks’ gestation of participants allocated to placebo or probiotics
| Characteristic/Outcome | Placebo group (n = 152) Mean (SD) or n (%) | Probiotic group (n = 152) Mean (SD) or n (%) |
|---|---|---|
| Maternal characteristics | ||
| Age (years) | 31.3 (5.2) | 31.1 (5.4) |
| Body mass index | 25.1 (4.2) | 25.4 (5.0) |
| Ethnicity | ||
| White | 74 (48.7) | 64 (42.1) |
| Black | 18 (11.8) | 27 (17.8) |
| Asian | 51 (33.6) | 50 (32.9) |
| Mixed/Other | 9 (5.9) | 11 (7.2) |
| Participant born preterm | 6 (3.9) | 6 (3.9) |
| Supplements/Medication use at baseline | ||
| Antibiotics | 9 (5.9) | 7 (4.6) |
| Other probiotics | 3 (2.0) | 6 (3.9) |
| Microbiological assessment at baseline | ||
| Bacterial vaginosis (Nugent score ≥7) | 18 (11.8) | 27 (17.8) |
| Lactobacillus colonisation | ||
| reuteri | 2 (1.3) | 1 (0.7) |
| rhamnosus | 6 (3.9) | 5 (3.3) |
| Others | 95 (62.5) | 93 (61.2) |
| Escherichia coli | 39 (25.7) | 39 (25.7) |
| Group B Streptococcus | 23 (15.1) | 17 (11.2) |
| Placebo group (n = 115) | Probiotic group (n = 123) | |
|---|---|---|
| Microbiological assessment at 18–20 weeks | ||
| Bacterial vaginosis (Nugent score ≥7)** | 10 (8.7) | 19 (15.4) |
| Lactobacillus colonisation | ||
| reuteri | 0 | 0 |
| rhamnosus | 6 (5.2) | 6 (4.9) |
| Others | 67 (58.3) | 74 (60.2) |
| Escherichia coli | 29 (25.2) | 29 (23.6) |
| Group B Streptococcus | 9 (7.8) | 5 (4.1) |
There were no significant differences between the groups.
P = 0.11.
At 18–20 weeks’ gestation, the rate of BV in the probiotic group was 15% (19/123) compared with 9% (10/115) in the placebo group. There were no differences between the groups in the rates of vaginal colonisation with L. rhamnosus GR‐1 (5%, 6/123 and 5%, 6/115), E. coli (23.6%, 29/123 and 25.2%, 29/115) or group B streptococci (4%, 5/123 and 8%, 9/115). Vaginal colonisation with L. reuteri RC‐14 was not detected in either group (Table 1). After adjusting for Nugent score at baseline, there were no differences between the groups in the odds of BV (adjusted OR 1.82, 95% CI 0.64–5.19; P = 0.26).
Of the women who provided a swab sample at 18–20 weeks’ gestation, vaginal samples were available in 70% (167/238) of women at 34–36 weeks’ gestation (72% (83/115) in the placebo group and 68% (84/123) in the probiotic group. The mean Nugent scores at 9–14, 18–20, and 34–36 weeks’ gestation in the probiotic group were 1.74 (95% CI 1.08–2.40), 1.54 (95% CI 0.87–2.20), and 1.74 (95% CI 1.10–2.38), respectively. In the placebo group, the mean Nugent scores at the same time points were 1.17 (95% CI 0.67–1.67), 0.86 (95% CI 0.37–1.34), and 1.33 (95% CI 0.82–1.83), respectively (Figure 2). The rates of BV in the groups over the same period were 17% (14/84), 13% (11/84), and 13% (11/84) in the probiotic group and 6% (5/83), at all three time points in the placebo group.
Figure 2.
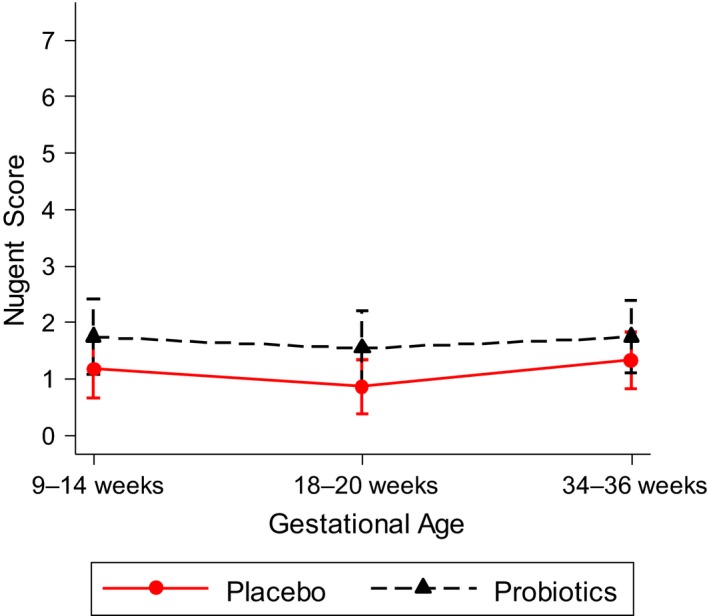
Mean Nugent scores with 95% confidence intervals from 9–14 to 34–36 weeks’ gestation of participants in the trial.
Although the secondary outcomes did not differ between the two groups, this trial was not powered to assess these adequately. There was one miscarriage in the placebo group and three in the probiotic group, all occurring between 9–14 weeks’ and 18–20 weeks’ gestation. There were nine and eight PTBs in the placebo and probiotic groups, respectively, giving PTB rates of 8.2% and 6.7%; these rates are consistent with the 7.7% PTB rate reported for England and Wales in 2015.35 In each group, the mean duration of pregnancy was 39 weeks, and in those who delivered preterm, 36 weeks.
Participants (n = 152) who attended at all time‐points were selected for the microbiome analysis. PCR amplification at baseline failed in two participants in each group, and the 18–20 weeks’ gestation swabs from three participants were unavailable, leaving 147 participants who provided 299 samples (75 in each group at baseline, and 74 and 75 in the probiotic and placebo groups, respectively, at 18–20 weeks’). The 16S rRNA genes were successfully amplified and sequenced from these, and 6714 sequence reads were sub‐sampled from each sample. The composition of the vaginal community was as expected, with subsets dominated by Lactobacillus crispatus, Lactobacillus iners or Lactobacillus jensenii. A further group was characterised by a diverse community, which included anaerobic species and Gardnerella vaginalis (Figure 3).
Figure 3.
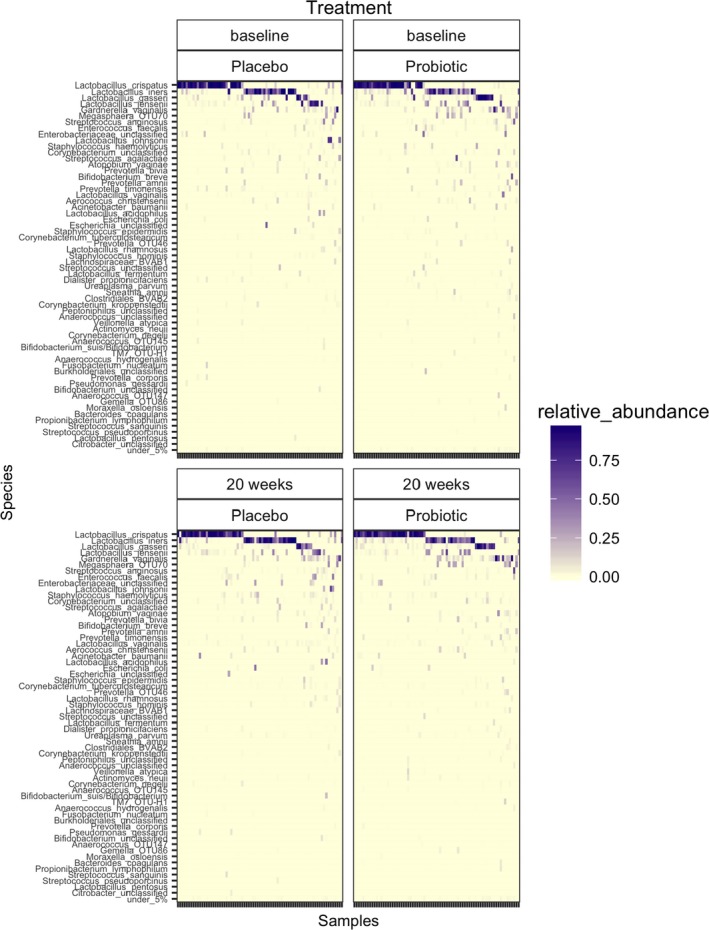
Heat map showing the composition of the vaginal microbiota at baseline and at 18–20 weeks’ in placebo and probiotic groups.
There were no significant differences (Wilcoxon) in alpha diversity (Inverse Simpson index) or composition (amova) of the bacterial community in the probiotic and placebo groups at either 9–14 or 18–20 weeks’ gestation (Figure 3). The stability of the microbiota within subjects assessed by determining the theta‐YC distance between the baseline and 18–20 weeks’ gestation samples was not significantly different between the probiotic and placebo groups (Wilcoxon).
Discussion
The aim of this trail was to compare the effects on the vaginal microbiota of oral probiotic capsules containing L. rhamnosus GR‐1 and L. reuteri RC‐14 versus placebo taken orally from early pregnancy until delivery. The biological effects were measured by Nugent scoring and culture of vaginal samples collected at three time points during pregnancy.
Main findings
The oral administration of L. rhamnosus GR‐1 and L. reuteri RC‐14 daily from early pregnancy did not affect the prevalence of BV or common neonatal pathogens, or alter the composition of the vaginal microbiota at 18–20 weeks’ gestation. Previous observational studies and small‐scale randomised controlled trials in non‐pregnant populations have suggested that oral administration of probiotics can alter the vaginal microbiota in a significant number of women where microbial imbalance exists.18, 19, 20, 21 It is on this basis that several commercial probiotic products are marketed for the restoration and maintenance of a healthy vaginal microbiota in both non‐pregnant and pregnant women.19, 20 Our findings have not shown any effect on the vaginal microbiota with the probiotic preparation used in this trial.
The microbiota at human colonisation sites is resistant to perturbation; this resistance is referred to as colonisation resistance.36, 37 It is likely that stable colonisation with desirable probiotic strains will require a reduction in colonisation resistance. Little is known about modulation of vaginal colonisation resistance at the present time.
Strengths and weakness of the study
This study was a large and pragmatic multicenter, randomised trial carried out in pregnant women to assess the effect of probiotic supplementation on the vaginal microbiota. We recruited women in early pregnancy and the time to our primary endpoint was long enough to observe an effect of the intervention on the vaginal microbiota.15, 19, 20, 38 The large sample size and participant retention ensured internal validity, while the multicentre recruitment and broad inclusion criteria enhanced external validity. We used probiotics that are safe during pregnancy and have been reported to colonise the vagina in some non‐pregnant women within 2 weeks of treatment.19 Before the start of the study, we confirmed the viability of the lactobacilli in the probiotic capsules and that our laboratory techniques would detect them in culture. Antibiotic prescribing during pregnancy is common in the UK39 and we took account of antibiotic exposure as a potential confounding variable. We did not take account of vaginal douching, which may alter the vaginal microbiota with adverse health consequences.40
The study has some limitations. The trial was underpowered as a result of the lower than anticipated event rate, so we can't be certain of the magnitude of effect observed. Secondly, we recruited and randomised fewer women than planned, with only 23% (304/1031) of eligible women agreeing to participate in the trial. The 2‐month recruitment phase of the trial was devised to estimate the feasibility of recruiting to a full‐scale trial of oral probiotic supplementation of women from early pregnancy to prevent PTB. This response rate is, however, not unusual in trials on apparently healthy participants who may not have an expressed health need,41 particularly in pregnancy, where there may be impediments to participation, mainly due to perceived risk to the fetus.42 Thirdly, 77% (997/1301) of eligible women were excluded from the trial mainly due to refusal to participate. Fourthly, we had a higher than expected number lost to follow up, which tends to affect the ability to detect a difference. However, as the direction of mean effect was opposite to that hypothesised, our negative finding merits consideration. Finally, adherence to the trial intervention, although over 90% in both groups, was reliant on self‐reporting because participants rarely remembered to bring in unused capsules for a count at their scheduled antenatal visits. However, it has been shown that self‐reported compliance is a reliable substitute for capsule count, particularly in trials where there is regular in‐person follow up.25
Comparison with other studies
Results of clinical trials in non‐pregnant women have reported positive effects of probiotics on BV.43 However, conclusive evidence has been hampered by inadequacies in trial design (for example sample size), variations in the interventions strains that were used, in the outcome measures, mode of administration, and control of confounding factors such as use of antibiotics and topical antimicrobials. Our findings support those recently reported by Gille et al.44 In a randomised, controlled trial, 320 women at <12 weeks’ gestational age were allocated to once‐daily oral capsules of L. rhamnosus GR‐1 or L. reuteri RC‐14 or placebo for 8 weeks. No differences were found in the prevalence of BV assessed by Nugent score between probiotic and placebo groups before (placebo group 5.4% versus probiotic group 2.8%, P = 0.376) and after the intervention period (placebo group 1.5% versus probiotic group 2.2%, P = 1.000).
Implications for clinical practice and research
The current study was part of a trial assessing the feasibility of conducting a definitive trial on PTB prevention with probiotics. PTB is the major determinant of adverse newborn outcome. The rate of PTB is rising globally45 and current strategies to stem its growth are ineffective.46 The association between BV and PTB, and the reported benefits of probiotic therapy on BV, mainly in non‐pregnant women, have raised interest in the role of probiotics in the prevention of PTB.47 The observed benefit of dietary probiotic intake on the risk of spontaneous PTB48 supports this concept. Although the probiotic supplements used in this trial did not affect vaginal microbiota during pregnancy, we believe that the notion of using probiotics to reduce the risk of PTB merits further investigation. Future work should first focus on identifying the probiotic strain(s) and mode of administration that have a beneficial effect on the vaginal microbiota during pregnancy before their role in the prevention of PTB can be assessed.
Conclusion
Our study did not show evidence of an effect of oral probiotics on the vaginal microbiota in pregnancy.
Disclosure of interests
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare no conflict of interest. Completed disclosure of interests forms are available to view online as supporting information.
Contribution to authorship
The trial was conceived by MM and MW, and designed by SH and KK. ZD coordinated the trial with supervision from JA and JD. JZ and BMF generated the randomisation sequence and analysed the results. AW and MM carried out the microbiological assessment for the primary outcome. EP and WW carried out the DNA sequencing. ST, CC, and BMT provided overall support to the conduct and delivery of the trial. JA wrote the first draft, which was further developed by SH and KK. All authors contributed to the preparation and approval of the final manuscript.
Details of ethics approval
The National Research Ethics Service Committee London ‐ City Road & Hampstead (reference 15/LO/1549), approved this study on 26 October 2015. All participants provided informed consent to take part in the trial.
Data sharing
The research team will consider reasonable requests for sharing of patient level data. Requests should be made to KK or SH. Consent for data sharing has been obtained and will be provided in an anonymised format, which will render the risk of identification low.
Funding
This report summarises independent research funded by Barts Charity (grant reference 1082/2243) under its Large Programme Grant funding stream. The study was supported by the NIHR CRN portfolio with recruitment and follow up of participants, and was sponsored by Queen Mary University of London. As agreed with Chr Hansen as part of the condition of support for the trial, the final report was provided to them for review before submission. Neither the funders nor sponsors had any role in design, conduct, data collection, management, analysis or interpretation of data, writing of the report or the decision to submit the paper for publication.
Supporting information
Acknowledgements
We thank the principal investigators, research midwives, and researchers: Umme Ali, Sarah Davenport, Bashir Dawlatly, Laurie Deeks, Melonie Gouldbourne, Sarah Hanks, Sal Higgins, Vivian Holmes, Joanne Hutchinson, Prudence Jones, Rehan Khan, Zandile Maseko, Sharon Mcduell, Yvonne Muwalo, Vincent Oon, Maryam Parisaei, Alice Rossi, Amrithpaul Sandhu, and Amy Thomas, as well as the women who participated in the trial and contributed time and effort to make this study a success. We thank Epigenesys who designed and maintained our database and the NIHR CRN for their support during the set‐up and conduct of the study.
Husain S, Allotey J, Drymoussi Z, Wilks M, Fernandez‐Felix BM, Whiley A, Dodds J, Thangaratinam S, McCourt C, Prosdocimi EM, Wade WG, de Tejada BM, Zamora J, Khan K, Millar M. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double‐blind, placebo‐controlled trial with microbiome analysis. BJOG 2020;127:275–284.
Linked article This article is commented on by DA Eschenbach, pp. 285–6 in this issue. To view this commentary visit https://doi.org/10.1111/1471-0528.15751.
Trial registry: ClinicalTrials.gov (NCT02692820).
References
- 1. DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015;112:11060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009;116:1315–24. [DOI] [PubMed] [Google Scholar]
- 3. Leitich H, Kiss H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2007;21:375–90. [DOI] [PubMed] [Google Scholar]
- 4. Wilks M, Wiggins R, Whiley A, Hennessy E, Warwick S, Porter H, et al. Identification and H2O2 production of vaginal lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. J Clin Microbiol 2004;42:713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2013;(1):CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. BASHH (2012) UK national guideline for the management of bacterial vaginosis 2012. British Association for Sexual Health and HIV. http://www.bashh.org.
- 7. Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M. A review of antibiotic use in pregnancy. Pharmacotherapy 2015;35:1052–62. [DOI] [PubMed] [Google Scholar]
- 8. Stokholm J, Schjorring S, Eskildsen CE, Pedersen L, Bischoff AL, Folsgaard N, et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin Microbiol Infect 2014;20:629–35. [DOI] [PubMed] [Google Scholar]
- 9. Kuperman AA, Koren O. Antibiotic use during pregnancy: how bad is it? BMC Med 2016;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. FAO/WHO Expert Consultation Group . Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Geneva: WHO; 2007. [Google Scholar]
- 11. Yeganegi M, Watson CS, Martins A, Kim SO, Reid G, Challis JR, et al. Effect of Lactobacillus rhamnosus GR‐1 supernatant and fetal sex on lipopolysaccharide‐induced cytokine and prostaglandin‐regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol 2009;200:532.e1–8. [DOI] [PubMed] [Google Scholar]
- 12. Piewngam P, Zheng Y, Nguyen TH, Dickey SW, Joo HS, Villaruz AE, et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018;562:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner‐Hanssen P, et al. Hydrogen peroxide‐producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058–63. [DOI] [PubMed] [Google Scholar]
- 14. Krauss‐Silva L, Moreira ME, Alves MB, Braga A, Camacho KG, Batista MR, et al. A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: preliminary results. Trials 2011;12:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic‐supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double‐blind, placebo‐controlled study. Br J Nutr 2010;103:1792–9. [DOI] [PubMed] [Google Scholar]
- 16. Tachedjian G, O'Hanlon DE, Ravel J. The implausible ‘in vivo’ role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 2018;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Othman M, Neilson JP, Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database Syst Rev 2007;(1):CD005941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petricevic L, Unger FM, Viernstein H, Kiss H. Randomized, double‐blind, placebo‐controlled study of oral lactobacilli to improve the vaginal flora of postmenopausal women. Eur J Obstet Gynecol Reprod Biol 2008;141:54–7. [DOI] [PubMed] [Google Scholar]
- 19. Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B. Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol 2001;30:49–52. [DOI] [PubMed] [Google Scholar]
- 20. Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, et al. Oral use of Lactobacillus rhamnosus GR‐1 and L. fermentum RC‐14 significantly alters vaginal flora: randomized, placebo‐controlled trial in 64 healthy women. FEMS Immunol Med Microbiol 2003;35:131–4. [DOI] [PubMed] [Google Scholar]
- 21. Vujic G, Jajac Knez A, Despot Stefanovic V, Kuzmic VV. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double‐blind, randomized, placebo‐controlled study. Eur J Obstet Gynecol Reprod Biol 2013;168:75–9. [DOI] [PubMed] [Google Scholar]
- 22. Gy‐Na‐Tren ‐ Homeopathic Treatment for Feminine Health. [https://www.natren.com/gy-na-tren-feminine-health.html]. Accessed 5 December 2017.
- 23. OptiBac ‘For women’. [https://www.optibacprobiotics.co.uk/shop/for-women?gclid=EAIaIQobChMI5ZK15Iv_1gIVEY0bCh1ewgF0EAQYBCABEgLtDvD_BwE]. Accessed 5 December 2017.
- 24. Schulz KF, Altman DG, Moher D, Group C . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaunitz AM, Portman D, Westhoff CL, Archer DF, Mishell DR Jr, Foegh M. Self‐reported and verified compliance in a phase 3 clinical trial of a novel low‐dose contraceptive patch and pill. Contraception 2015;91:204–10. [DOI] [PubMed] [Google Scholar]
- 26. Kane SP. Sample Size Calculator. ClinCalc. [http://clincalc.com/Stats/SampleSize.aspx]. Accessed 1 July 2017.
- 27. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Husain SM, Wilks M, Mupita M, Reddy SP, Hennessy EM, Macfarlane AJ, et al. Diversity and stability of cultured vaginal lactobacilli in pregnant women from a multi‐ethnic urban UK population. J Appl Microbiol 2014;117:258–65. [DOI] [PubMed] [Google Scholar]
- 29. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high‐resolution sample inference from Illumina amplicon data. Nat Methods 2016;13:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kahan BC, Jairath V, Dore CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials 2014;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. StataCorp . 2014. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- 32. Rayment J, Lanlehin R, McCourt C, Husain SM. Involving seldom‐heard groups in a PPI process to inform the design of a proposed trial on the use of probiotics to prevent preterm birth: a case study. Res Involv Engagem 2017;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van‘t Hooft J, Duffy JM, Daly M, Williamson PR, Meher S, Thom E, et al. A core outcome set for evaluation of interventions to prevent preterm birth. Obstet Gynecol 2016;127:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moss N, Daru J, Lanz D, Thangaratinam S, Khan KS. Involving pregnant women, mothers and members of the public to improve the quality of women's health research. BJOG 2017;124:362–5. [DOI] [PubMed] [Google Scholar]
- 35. Office for National Statistics [GB]. Birth characteristics in England and Wales . 2015. Annual live births by sex, ethnicity and month, maternities by place of birth and with multiple births, and stillbirths by age of parents and calendar quarter. [https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2015]. Accessed 31 January 2019.
- 36. Lawley TD, Walker AW. Intestinal colonization resistance. Immunology 2013;138:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mullineaux‐Sanders C, Suez J, Elinav E, Frankel G. Sieving through gut models of colonization resistance. Nat Microbiol 2018;3:132–40. [DOI] [PubMed] [Google Scholar]
- 38. Nishijima K, Shukunami K‐i, Kotsuji F. Probiotics affects vaginal flora in pregnant women, suggesting the possibility of preventing preterm labor. J Clin Gastroenterol 2005;39:447–8. [DOI] [PubMed] [Google Scholar]
- 39. Petersen I, Gilbert R, Evans S, Ridolfi A, Nazareth I. Oral antibiotic prescribing during pregnancy in primary care: UK population‐based study. J Antimicrob Chemother 2010;65:2238–46. [DOI] [PubMed] [Google Scholar]
- 40. Fiscella K. The risk of low birth weight associated with vaginal douching. Obstet Gynecol 1998;92:913–7. [DOI] [PubMed] [Google Scholar]
- 41. Salisbury C, O'Cathain A, Thomas C, Edwards L, Gaunt D, Dixon P, et al. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. BMJ 2016;353:i2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palmer S, Pudwell J, Smith GN, Reid RL. Optimizing participation of pregnant women in clinical trials: factors influencing decisions about participation in medication and vaccine trials. J Obstet Gynaecol Can 2016;38:945–54. [DOI] [PubMed] [Google Scholar]
- 43. Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol 2017;168:782–92. [DOI] [PubMed] [Google Scholar]
- 44. Gille C, Boer B, Marschal M, Urschitz MS, Heinecke V, Hund V, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am J Obstet Gynecol 2016;215:608 e1–e7. [DOI] [PubMed] [Google Scholar]
- 45. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A‐B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72. [DOI] [PubMed] [Google Scholar]
- 46. Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns‐Smith S, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 2013;381:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang P, Chen YH, Yen CF, Chen HL. Psychiatric diagnoses, emotional‐behavioral symptoms and functional outcomes in adolescents born preterm with very low birth weights. Child Psychiatry Hum Dev 2015;46:358–66. [DOI] [PubMed] [Google Scholar]
- 48. Myhre R, Brantsaeter AL, Myking S, Gjessing HK, Sengpiel V, Meltzer HM, et al. Intake of probiotic food and risk of spontaneous preterm delivery. Am J Clin Nutr 2011;93:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


