Abstract
Aim
Anastomotic leakage (AL) is one of the most feared complications after rectal resection. This study aimed to assess a combination of biomarkers for early detection of AL after rectal cancer resection.
Method
This study was an international multicentre prospective cohort study. All patients received a pelvic drain after rectal cancer resection. On the first three postoperative days drain fluid was collected daily and C‐reactive protein (CRP) was measured. Matrix metalloproteinase‐2 (MMP2), MMP9, glucose, lactate, interleukin 1‐beta (IL1β), IL6, IL10, tumour necrosis factor alpha (TNFα), Escherichia coli, Enterococcus faecalis, lipopolysaccharide‐binding protein and amylase were measured in the drain fluid. Prediction models for AL were built for each postoperative day using multivariate penalized logistic regression. Model performance was estimated by the c‐index for discrimination. The model with the best performance was visualized with a nomogram and calibration was plotted.
Results
A total of 292 patients were analysed; 38 (13.0%) patients suffered from AL, with a median interval to diagnosis of 6.0 (interquartile ratio 4.0–14.8) days. AL occurred less often after partial than after total mesorectal excision (4.9% vs 15.2%, P = 0.035). Of all patients with AL, 26 (68.4%) required reoperation. AL was more often treated by reoperation in patients without a diverting ileostomy (18/20 vs 8/18, P = 0.03). The prediction model for postoperative day 1 included MMP9, TNFα, diverting ileostomy and surgical technique (c‐index = 0.71). The prediction model for postoperative day 2 only included CRP (c‐index = 0.69). The prediction model for postoperative day 3 included CRP and MMP9 and obtained the best model performance (c‐index = 0.78).
Conclusion
The combination of serum CRP and peritoneal MMP9 may be useful for earlier prediction of AL after rectal cancer resection. In clinical practice, this combination of biomarkers should be interpreted in the clinical context as with any other diagnostic tool.
Keywords: Anastomotic leakage, rectal resection, early detection, biomarkers, drain fluid
What does this paper add to the literature?
Anastomotic leakage (AL) is one of the most feared complications after rectal resection. Early detection is of paramount importance in order to minimize postoperative morbidity and mortality. This prospective cohort study showed that a combination of serum CRP and peritoneal MMP9 may be useful for early prediction of AL after rectal cancer resection.
Introduction
With the introduction of minimally invasive techniques, the short‐term outcomes of rectal surgery have improved over the last decades 1, 2. Despite these advances, the incidence of anastomotic leakage (AL) has not been reduced 3. Moreover, standardized recovery programmes have shortened hospital stay, with the downside that AL can become clinically apparent after discharge resulting in readmission and delayed management 4. Nowadays, 20% of AL is diagnosed after discharge, with a mean time to diagnosis of 6–15 days 5, 6.
The current diagnostic strategy, consisting of on‐demand CT scanning, fails to detect AL at an early stage as half of all leakages require reoperation 7, 8. Delayed reintervention after false‐negative CT scanning is associated with increased mortality and prolonged hospital stay 9. In addition, delay in diagnosis of 2.5 days is associated with an increase in mortality from 24% to 39% 10. Hence, early detection is of paramount importance in order to minimize postoperative morbidity and mortality.
Biomarkers in drain fluid have previously been proposed as an innovative strategy for early detection of AL. Elevated peritoneal levels of inflammatory cytokines and lactate as well as decreased pH seemed to be associated with AL, and measurement of such parameters is thus of interest for early detection of AL 11. Furthermore, promising results were shown for lipopolysaccharide‐binding protein (LBP) and Enterococcus faecalis in drain fluid 12, 13. However, implementation in clinical practice is lagging behind as previous studies were based on small sample sizes and lacked any estimation of predictive accuracy.
A systematic review concluded that a combination of biomarkers yielded improved predictive accuracy compared with separate analysis of biomarkers 14. Therefore, we aimed to assess a combination of biomarkers for prediction of AL after rectal cancer resection and to determine its predictive accuracy.
Method
Patients
This study was designed as an international multicentre prospective cohort study. Ten hospitals in the Netherlands and Belgium participated in the study. Patients were included between August 2015 and October 2017. The medical ethics committees of the Erasmus MC University Medical Centre in the Netherlands and of the University Hospital Leuven in Belgium approved this study. Ethical approval was also obtained in the other participating hospitals. This study was registered at http://www.ISRCTN.org (study ID 84052649).
Patients aged 18 years and above who underwent partial mesorectal excision (PME) or total mesorectal excision (TME) with construction of a colorectal or coloanal anastomosis were eligible for inclusion. Pregnant women and patients who underwent an emergency procedure were excluded. In addition, patients in whom no drain fluid was obtained or who underwent surgery for an indication other than adenocarcinoma were excluded. All patients gave written informed consent. The follow‐up ended at the first outpatient clinic visit after hospital discharge.
Collection and storage of drain fluid
All patients received a pelvic drain during surgery. Drain fluid was collected every morning on the first three postoperative days. Drain fluid was collected respecting rules of sterility with a syringe including a needle and deposited in a 10‐ml ethylenediaminetetraacetic acid (EDTA) tube. The drain fluid reservoir was replaced after the collection of drain fluid. The EDTA tube was transported to the laboratory and the drain fluid samples were centrifuged (at 1955g) for 10 min at 4°C. Subsequently, the supernatant was removed. Drain fluid was aliquotted in five cryotubes of 2 ml and stored at −80°C until further analysis. C‐reactive protein (CRP) was measured in peripheral blood samples at the hospitals’ clinical laboratories on the first three postoperative days.
Drain fluid analysis
Samples were thawed, vortexed and centrifuged for 1 min at 10 000g and 4°C before analysis. All biomarkers were measured in duplicate and the means were taken for further analysis. Matrix metalloproteinases (MMP2 and MMP9) and cytokines [interleukin 1‐beta (IL1β), IL6, IL10 and tumour necrosis factor alpha (TNFα)] were measured using ProcartaPlex® Multiplex Immunoassay (Thermo Fisher Scientific, Bleiswijk, The Netherlands) on a Luminex Magpix machine. High‐sensitivity assays were used for cytokine measurement. Levels of α‐amylase, glucose and lactate were measured using Roche/Hitachi cobas c systems from Roche Diagnostics (Indianapolis, Indiana, USA). LBP was measured with enzyme linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer's instruction. Escherichia coli and E. faecalis were measured using a semi‐quantitative real‐time PCR strategy. Prior to DNA isolation, 500 μl of drain fluid was spiked with 5 μl of Phocine Herpes Virus (PhHV) as an internal control from the European Virus Archive (EVAg). Samples were spun for 5 min at 8000g and the pellets resuspended in 180 μl buffer (20 mm Tris, 2 mm EDTA, 1% Tween 80 and 50 mg/ml lysozyme). The samples were incubated at 37°C with shaking at 600 rpm for 30 min after which 25 μl of proteinase K was added followed by a 2‐h incubation at 56°C at 700 rpm. DNA extraction was performed using a Macherey‐Nagel NucleoSpin® Tissue kit (Bioké, Leiden, the Netherlands). Template DNA was eluted in elution buffer in a total volume of 100 μl. Subsequently, primers for E. coli and E. faecalis were added in accordance with the previously published protocol 15. The StepOnePlus Real‐Time PCR System (Applied Biosystems, Bleiswijk, the Netherlands) was used for RT‐PCR. Threshold cycles (Ct) were corrected for differences in extraction efficiency using the threshold cycle of the internal control PhHV.
Clinical data assessment
Patient characteristics (age, gender, body mass index, medication use, bowel preparation, smoking, alcohol abuse, American Society of Anesthesiologists score, previous abdominal surgery, indication for surgery, preoperative radiotherapy, preoperative chemotherapy, location of lesion) and surgical characteristics (surgical procedure, surgical technique, conversion, construction of anastomosis, configuration of anastomosis, diverting ileostomy) were prospectively registered. Creation of the anastomosis was registered as ‘stapler’ or ‘manual’. Manual anastomosis was performed using interrupted coloanal sutures with a hand‐sewn technique. If the anastomosis was constructed with a stapler and additional manual sutures were added this was registered as stapled. Transanal TME was defined as follows: part of a TME being performed with transanal assistance. This includes a semi‐rigid platform with rigid instruments to perform a down‐to‐up TME.
AL was the primary outcome of interest, being defined as a clinically manifest insufficiency of the anastomosis leading to a clinical state requiring treatment (i.e. grade B/C) 7. AL was confirmed by either endoscopy, CT scan and/or contrast enema or reoperation. Fistulas communicating with the anastomosis on CT scan were classified as AL together with presacral abscesses if extravasation of the colonic contrast was visible on radiological imaging. In addition, postoperative indicators (time to discharge, postoperative complications with their respective treatment strategies, readmission, reoperation and mortality) were prospectively registered. Elective stoma reversals were not registered as reoperation.
Statistical analysis
Continuous variables were described as median ± interquartile range (IQR) and compared with the Mann–Whitney U‐test. Categorical variables were described as percentages and compared with the chi‐square test or Fisher's exact test when needed. Comparisons of biomarkers were corrected for multiple testing using Holm's method per postoperative day 16. A multiple imputation procedure was performed to impute missing data based on 10 completed datasets. For each postoperative day, multivariate penalized logistic regression models were constructed including clinically relevant baseline characteristics (age, gender, nonsteroidal anti‐inflammatory drugs, corticosteroids, diverting Ileostomy, surgical procedure, approach) and all biomarkers. Prediction models for each postoperative day were built including covariates with a P‐value < 0.1. Internal validation using the bootstrap method was done to obtain corrected estimates of model performance to avoid overfitting. Model performance was estimated by Harrell's concordance index (the c‐index). The c‐index measures how adequate the model is at discriminating between the outcome of interest, and represents the probability that, in a randomly selected pair of patients, the model assigns a higher risk to the patient who is truly high risk compared with the patient who is truly low risk. A c‐index of 0.5 indicates no association between prediction and true outcome and a value of 1.0 indicates perfect association. A c‐index of more than 0.75 is considered clinically useful 17. A calibration plot of the model with the best c‐index was built showing the relationship between the observed and predicted probability of the outcome. The observed and expected rates are similar in a well‐calibrated model. The final model was visualized using a nomogram and captured in an online calculator (https://www.evidencio.com/models/show/1537). Two‐sided P‐values < 0.05 were considered statistically significant. Analyses were performed using spss v.22 (IBM Corp., Armonk, New York, USA) and the NLME, LATTICE, ARM, AOD and RMS packages in r v.3.3.3 (http://www.r-project.org).
Results
Study population
A total of 310 patients were included. Nine patients were excluded because no drain fluid was obtained, and nine were excluded due to another surgical indication than rectal adenocarcinoma. In the end, 292 patients were eligible for analysis.
Table 1 represents baseline characteristics of the study population. The median time of follow‐up was 28.0 days (IQR 17.0–35.0). The median time to discharge was 7.0 days (IQR 5.0–11.0). In total, 42 (14.4%) patients were readmitted to the hospital and 38 (13.0%) underwent reoperation. Infection at the drain insertion site was reported in three (1.0%) patients. No other complications of the pelvic drain were reported. Two (0.7%) patients died. One patient died of AL and the other patient died 2 days after hospital discharge of an unknown reason as no autopsy was performed.
Table 1.
Patient and surgical characteristics of patients with and without anastomotic leakage (AL).
| Total no. of patients (n = 292) | No AL (n = 254, 87.0%) | AL (n = 38, 13.0%) | Missing | P‐value | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age (years), median ± IQR | 63.0 (57.0–71.0) | 63.5 (57.5–71.0) | 60.5 (53.8–68.5) | 0 (0.0%) | 0.135 |
| Gender | |||||
| Male | 193 (66.1%) | 167 (65.7%) | 26 (68.4%) | 0 (0.0%) | 0.745 |
| Female | 99 (34.0%) | 87 (34.4%) | 12 (31.6%) | ||
| BMI (kg/m2), median ± IQR | 25.8 (23.5–28.7) | 25.8 (23.3–28.7) | 25.9 (24.2–29.2) | 1 (0.3%) | 0.546 |
| Corticosteroids | 17 (5.8%) | 14 (5.5%) | 3 (7.9%) | 2 (0.7%) | 0.475* |
| NSAIDs | 8 (2.7%) | 7 (2.8%) | 1 (2.6%) | 2 (0.7%) | 1.000* |
| Bowel preparation | 244 (83.6%) | 209 (82.3%) | 35 (92.1%) | 25 (8.6%) | 1.000* |
| Smoking | 38 (13.0%) | 31 (12.2%) | 7 (18.4%) | 10 (3.4%) | 0.304* |
| Alcohol abuse | 39 (13.4%) | 32 (12.6%) | 7 (18.4%) | 10 (3.4%) | 0.378 |
| ASA score | |||||
| I | 45 (15.4%) | 37 (14.6%) | 8 (21.1%) | 2 (0.7%) | 0.468* |
| II | 181 (62.0%) | 156 (61.4%) | 25 (65.8%) | ||
| III | 62 (21.2%) | 57 (22.4%) | 5 (13.2%) | ||
| IV | 2 (0.7%) | 2 (0.8%) | 0 (0.0%) | ||
| Previous abdominal surgery | 100 (34.2%) | 90 (35.4%) | 10 (26.3%) | 1 (0.3%) | 0.263 |
| Clinical tumour stage | |||||
| T1 | 14 (4.8%) | 13 (5.1%) | 1 (2.6%) | 44 (15.1%) | 0.871* |
| T2 | 72 (24.7%) | 62 (24.4%) | 10 (26.3%) | ||
| T3 | 144 (49.3%) | 123 (48.4%) | 21 (55.3%) | ||
| T4 | 18 (6.2%) | 16 (6.3%) | 2 (5.3%) | ||
| Clinical nodal stage | |||||
| N0 | 101 (34.6%) | 86 (33.9%) | 15 (39.5%) | 0.600 | |
| N ≥ 1 | 139 (47.6%) | 119 (46.9%) | 17 (44.7%) | ||
| Preoperative radiotherapy | 155 (53.1%) | 135 (53.1%) | 20 (52.6%) | 1 (0.3%) | 0.933 |
| Short course | 58 (37.4%) | 52 (38.5%) | 6 (30.0%) | ||
| Long course | 89 (57.4%) | 77 (57.0%) | 12 (60.0%) | ||
| Preoperative chemotherapy | 102 (34.9%) | 87 (34.3%) | 15 (39.5%) | 1 (0.3%) | 0.540 |
| Location of lesion from anal verge (cm), median ± IQR | 10.0 (6.0–13.0) | 10.0 (6.0–14.0) | 9.0 (5.0–12.0) | 16 (5.5%) | 0.169 |
| Surgical characteristics | |||||
| Procedure | |||||
| PME | 61 (20.9%) | 58 (22.8%) | 3 (7.9%) | 0 (0.0%) | 0.035 |
| TME | 231 (79.1%) | 196 (77.2%) | 35 (92.1%) | ||
| Surgical technique | |||||
| Open | 11 (3.8%) | 10 (3.9%) | 1 (2.6%) | 0 (0.0%) | 0.736* |
| Laparoscopic | 161 (55.1%) | 142 (55.9%) | 19 (50.0%) | ||
| Transanal | 120 (41.1%) | 102 (40.2%) | 18 (47.4%) | ||
| Conversion | 8 (2.7%) | 8 (3.1%) | 0 (0.0%) | 0 (0.0%) | 0.598* |
| Construction of anastomosis | |||||
| Manual | 43 (14.7%) | 39 (15.4%) | 4 (10.5%) | 2 (0.7%) | 0.423 |
| Stapler | 247 (84.6%) | 213 (83.9%) | 34 (89.5%) | ||
| Configuration of anastomosis | |||||
| Side‐to‐side | 4 (1.4%) | 4 (1.6%) | 0 (0.0%) | 31 (10.6%) | 0.861* |
| Side‐to‐end | 173 (59.2%) | 147 (57.9%) | 26 (68.4%) | ||
| End‐to‐end | 79 (27.1%) | 70 (27.6%) | 9 (23.7%) | ||
| End‐to‐side | 5 (1.7%) | 5 (2.0%) | 0 (0.0%) | ||
| Diverting ileostomy | 158 (54.1%) | 140 (55.1%) | 18 (47.4%) | 0 (0.0%) | 0.371 |
Bold values indicate significance.
ASA, American Society of Anesthesiologists; BMI, body mass index; IQR, interquartile range.
*Fisher's exact test.
Anastomotic leakage
A total of 38 (13.0%) patients suffered from AL. No differences in patient characteristics were observed for patients with and without AL. The incidence of AL was no different for patients with and without diverting ileostomy (11.4% vs 14.9%, P = 0.371). AL occurred less often after PME than after TME (4.9% vs 15.2%, P = 0.035) (Table 1).
AL was clinically manifest as a presacral abscess in five patients. The median time to diagnosis was 6.0 days (IQR 6.0–14.8). Patients with AL had a significantly longer hospital stay (16.0 days vs 6.0 days, P ≤ 0.001). Production of drain fluid was no different for patients with and without AL (day 1, 155 ml vs 180.0 ml, P = 0.664; day 2, 97.5 ml vs 100.0 ml, P = 0.435; day 3, 60.0 ml vs 90.0 ml, P = 0.141).
In 30 (78.9%) patients AL was confirmed by a CT scan, in 5 (13.2%) by proctoscopy and in 1 (2.6%) patient by reoperation. Of all patients with AL, 26 (68.4%) required reoperation whereas 12 (31.6%) were treated more conservatively (antibiotics, drainage or Endo‐sponge). AL was more often treated by reoperation in patients without a diverting ileostomy (18/20 vs 8/18, P = 0.03).
Biomarkers
Table 2 compares the levels of biomarkers for patients with and without AL per postoperative day. Table 3 represents outcomes of multivariate penalized logistic regression analyses per postoperative day. Prediction models for each postoperative day were built including covariates with a P‐value < 0.1 in the multivariate analysis. The prediction model for postoperative day 1 included MMP9, TNFα, diverting ileostomy and surgical technique. The prediction model for postoperative day 2 only included CRP. The prediction model for postoperative day 3 included both CRP and MMP9.
Table 2.
Comparison of biomarker levels for patients with and without anastomotic leakage (AL).
| AL | Postoperative day 1 | Postoperative day 2 | Postoperative day 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Q1 | Q3 | P‐value | n | Median | Q1 | Q3 | P‐value | n | Median | Q1 | Q3 | P‐value | ||
| MMP2 × 105 (pg/ml) | Y | 37 | 0.7 | 0.5 | 1.2 | 1.000 | 31 | 1.0 | 0.6 | 1.4 | 1.000 | 31 | 1.1 | 0.7 | 2.1 | 1.000 |
| N | 248 | 0.7 | 0.4 | 1.1 | 236 | 1.0 | 0.6 | 1.4 | 230 | 1.2 | 0.7 | 1.7 | ||||
| MMP9 × 105 (pg/ml) | Y | 37 | 3.2 | 0.9 | 10.8 | 0.450 | 31 | 1.7 | 0.4 | 7.0 | 1.000 | 32 | 2.0 | 0.5 | 5.0 | 0.011 |
| N | 247 | 2.0 | 0.9 | 4.1 | 235 | 1.0 | 0.5 | 2.0 | 231 | 0.6 | 0.3 | 1.5 | ||||
| Glucose (mm) | Y | 37 | 2.0 | 0.4 | 3.8 | 0.252 | 38 | 0.2 | 0.1 | 2.4 | < 0.001 | 35 | 0.3 | 0.1 | 3.1 | 0.011 |
| N | 247 | 3.4 | 1.5 | 4.6 | 241 | 2.4 | 0.1 | 4.7 | 238 | 2.9 | 0.1 | 5.0 | ||||
| Lactate (mm) | Y | 37 | 10.5 | 6.9 | 14.5 | 1.000 | 38 | 12.5 | 9.1 | 19.7 | 1.000 | 37 | 11.3 | 8.1 | 19.2 | 0.444 |
| N | 248 | 9.1 | 6.2 | 13.2 | 242 | 11.1 | 6.4 | 17.3 | 243 | 9.2 | 5.2 | 14.9 | ||||
| CRP (mg/ml) | Y | 36 | 69.5 | 35.0 | 105.8 | 0.459 | 36 | 152.5 | 83.5 | 215.5 | < 0.001 | 37 | 170.0 | 113.8 | 290.5 | < 0.001 |
| N | 241 | 50.0 | 30.9 | 80.0 | 213 | 86.0 | 47.1 | 135.9 | 215 | 78.0 | 41.0 | 125.0 | ||||
| IL1β (pg/ml) | Y | 37 | 61.1 | 31.7 | 263.5 | 0.341 | 32 | 138.1 | 46.7 | 536.8 | 0.011 | 31 | 190.0 | 28.6 | 3271.1 | < 0.001 |
| N | 247 | 47.1 | 19.6 | 132.3 | 236 | 39.8 | 13.4 | 151.5 | 232 | 30.3 | 9.3 | 142.9 | ||||
| IL6 (pg/ml) | Y | 36 | 69717.7 | 19267.2 | 76184.2 | 1.000 | 32 | 73454.5 | 23889.4 | 76334.1 | 1.000 | 31 | 46178.8 | 17483.5 | 76070.8 | 0.301 |
| N | 246 | 51635.3 | 23484.4 | 76070.8 | 236 | 41860.7 | 17483.5 | 75786.4 | 232 | 24738.2 | 11239.9 | 68858.8 | ||||
| IL10 (pg/ml) | Y | 37 | 249.7 | 141.3 | 594.6 | 0.584 | 32 | 176.3 | 84.4 | 630.8 | 0.080 | 31 | 128.3 | 38.6 | 554.2 | 0.072 |
| N | 247 | 204.8 | 109.7 | 405.5 | 236 | 99.1 | 51.8 | 220.8 | 232 | 62.4 | 30.0 | 136.4 | ||||
| TNFα (pg/ml) | Y | 37 | 37.3 | 23.6 | 128.8 | 0.156 | 32 | 23.1 | 12.9 | 67.2 | 1.000 | 31 | 45.2 | 14.2 | 79.7 | 0.036 |
| N | 246 | 30.1 | 16.4 | 59.1 | 237 | 21.5 | 12.0 | 39.8 | 231 | 17.7 | 9.9 | 34.0 | ||||
| Escherichia coli (Ct) | Y | 37 | 34.2 | 32.4 | 35.8 | 1.000 | 33 | 34.8 | 31.4 | 37.5 | 1.000 | 30 | 34.3 | 26.7 | 36.4 | 1.000 |
| N | 247 | 34.6 | 32.4 | 37.0 | 231 | 34.6 | 32.4 | 36.7 | 227 | 34.7 | 32.9 | 36.6 | ||||
| Enterococcus faecalis (Ct) | Y | 38 | 26.3 | 25.2 | 26.9 | 1.000 | 33 | 26.5 | 25.4 | 27.5 | 1.000 | 32 | 26.2 | 25.1 | 27.4 | 1.000 |
| N | 248 | 26.2 | 25.1 | 27.1 | 234 | 26.0 | 25.0 | 27.0 | 228 | 25.9 | 25.1 | 27.0 | ||||
| LBP (μg/ml) | Y | 38 | 3.6 | 1.9 | 5.0 | 1.000 | 34 | 5.5 | 3.5 | 6.6 | 1.000 | 31 | 6.2 | 4.6 | 7.0 | 1.000 |
| N | 248 | 3.2 | 2.2 | 4.4 | 237 | 5.1 | 4.0 | 6.1 | 231 | 5.6 | 4.5 | 6.7 | ||||
| Amylase (U/l) | Y | 36 | 36.0 | 14.3 | 84.8 | 0.584 | 38 | 30.5 | 13.5 | 47.0 | 1.000 | 37 | 24.0 | 17.5 | 45.0 | 1.000 |
| N | 243 | 24.0 | 13.0 | 41.0 | 243 | 28.0 | 18.0 | 45.0 | 244 | 25.0 | 15.0 | 37.0 | ||||
Bold values indicate significance.
CRP, C‐reactive protein; IL, interleukin; LBP, lipopolysaccharide‐binding protein; MMP, matrix metalloproteinase; n, number of patients; Q1, first quartile; Q3, third quartile; TNFα, tumour necrosis factor alpha.
Table 3.
Outcomes of multivariate penalized logistic regression for anastomotic leakage (AL) per postoperative day.
| Postoperative day 1 | Postoperative day 2 | Postoperative day 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI (lower) | 95% CI (upper) | P‐value | OR | 95% CI (lower) | 95% CI (upper) | P‐value | OR | 95% CI (lower) | 95% CI (upper) | P‐value | |
| MMP2 (pg/ml) | 1.011 | 0.962 | 1.063 | 0.661 | 1.020 | 0.955 | 1.090 | 0.556 | 1.025 | 0.986 | 1.066 | 0.216 |
| MMP9 (pg/ml) | 1.106 | 0.995 | 1.229 | 0.063* | 1.094 | 0.952 | 1.257 | 0.203 | 1.130 | 0.982 | 1.301 | 0.088‡ |
| Glucose (mm) | 0.939 | 0.751 | 1.176 | 0.585 | 0.916 | 0.728 | 1.152 | 0.453 | 0.920 | 0.734 | 1.152 | 0.466 |
| Lactate (mm) | 0.990 | 0.890 | 1.101 | 0.854 | 0.993 | 0.912 | 1.081 | 0.869 | 0.987 | 0.917 | 1.063 | 0.737 |
| CRP (mg/ml) | 1.068 | 0.981 | 1.162 | 0.128 | 1.057 | 1.005 | 1.111 | 0.030† | 1.064 | 1.013 | 1.118 | 0.013‡ |
| IL1β (pg/ml) | 1.002 | 0.992 | 1.012 | 0.702 | 0.999 | 0.992 | 1.006 | 0.787 | 1.001 | 0.999 | 1.003 | 0.522 |
| IL6 (pg/ml) | 0.980 | 0.847 | 1.135 | 0.787 | 1.052 | 0.899 | 1.230 | 0.529 | 0.939 | 0.788 | 1.119 | 0.481 |
| IL10 (pg/ml) | 0.999 | 0.980 | 1.019 | 0.954 | 1.044 | 0.940 | 1.159 | 0.424 | 1.110 | 0.959 | 1.284 | 0.161 |
| TNFα (pg/ml) | 1.037 | 0.998 | 1.077 | 0.062* | 0.991 | 0.969 | 1.014 | 0.453 | 1.011 | 0.996 | 1.026 | 0.136 |
| Escherichia coli (Ct) | 0.975 | 0.852 | 1.115 | 0.707 | 0.947 | 0.830 | 1.081 | 0.422 | 1.029 | 0.905 | 1.169 | 0.664 |
| Enterococcus faecalis (Ct) | 1.072 | 0.756 | 1.520 | 0.697 | 1.179 | 0.820 | 1.695 | 0.373 | 1.009 | 0.703 | 1.448 | 0.960 |
| LBP (μg/ml) | 0.913 | 0.704 | 1.184 | 0.492 | 0.910 | 0.708 | 1.170 | 0.462 | 0.888 | 0.686 | 1.149 | 0.365 |
| Amylase (U/l) | 1.000 | 0.998 | 1.003 | 0.827 | 1.001 | 0.998 | 1.003 | 0.648 | 1.000 | 0.999 | 1.001 | 0.547 |
| Age | 0.978 | 0.944 | 1.013 | 0.222 | 0.973 | 0.936 | 1.010 | 0.154 | 0.986 | 0.948 | 1.027 | 0.503 |
| Gender | 0.931 | 0.415 | 2.088 | 0.862 | 0.866 | 0.367 | 2.044 | 0.743 | 0.698 | 0.275 | 1.772 | 0.449 |
| NSAIDs | 0.719 | 0.131 | 3.953 | 0.704 | 0.454 | 0.051 | 4.042 | 0.479 | 1.018 | 0.172 | 6.028 | 0.985 |
| Corticosteroids | 1.066 | 0.263 | 4.315 | 0.928 | 1.132 | 0.252 | 5.093 | 0.871 | 1.087 | 0.267 | 4.430 | 0.908 |
| Diverting ileostomy | 0.478 | 0.205 | 1.116 | 0.088* | 0.485 | 0.195 | 1.208 | 0.120 | 0.575 | 0.219 | 1.511 | 0.261 |
| Procedure | 2.829 | 0.888 | 9.014 | 0.079* | 2.540 | 0.787 | 8.193 | 0.119 | 2.229 | 0.603 | 8.239 | 0.229 |
| Surgical technique | 0.756 | 0.151 | 3.787 | 0.733 | 0.804 | 0.152 | 4.252 | 0.797 | 1.047 | 0.197 | 5.557 | 0.957 |
CI, confidence interval; CRP, C‐reactive protein; IL, interleukin; LBP, lipopolysaccharide‐binding protein; MMP, matrix metalloproteinase; NSAID, nonsteroidal anti‐inflammatory drug; OR, odds ratio.
*These variables were added to the prediction model of postoperative day 1.
†These variables were added to the prediction model of postoperative day 2.
‡These variables were added to the prediction model of postoperative day 3.
The prediction model of postoperative day 1 had a c‐index of 0.71 whereas the prediction model of postoperative day 2 had a c‐index of 0.69. These prediction models were thus lacking discrimination and therefore were not considered to be clinically useful. On the contrary, the prediction model of postoperative day 3, including CRP and MMP9, had a c‐index of 0.78. This c‐index indicated that for 78% of the time the model assigned a higher probability to a patient with AL than a patient without AL. For the prediction model of postoperative day 3, a nomogram was constructed facilitating the calculation of the individual risk of AL after rectal cancer resection based on CRP and MMP9 on postoperative day 3 (Fig. 1). An online calculator was built for this nomogram at https://www.evidencio.com/models/show/1537.
Figure 1.
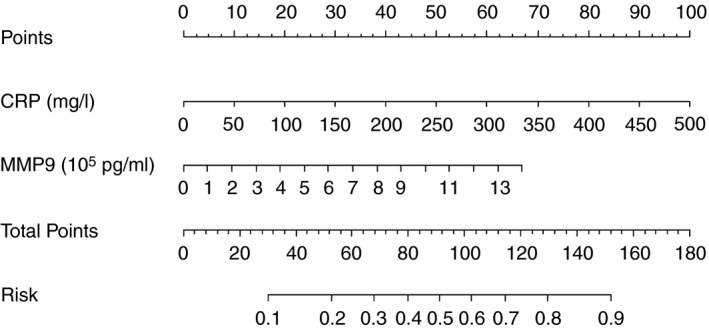
Nomogram of the prediction model of postoperative day 3 (c‐index = 0.78). This nomogram can estimate the risk of anastomotic leakage after rectal resection on postoperative day 3 with serum C‐reactive protein (CRP) and peritoneal matrix metalloproteinase 9 (MMP9).
Calibration was determined to estimate model performance with a calibration plot. In a calibration plot the predicted probability is plotted against the corresponding observed probability in the dataset. Ideally, this depicts a diagonal line and calibration is quantified by the mean absolute error. Figure 2 shows the calibration plot of the prediction model of postoperative day 3 (mean absolute error = 0.025).
Figure 2.
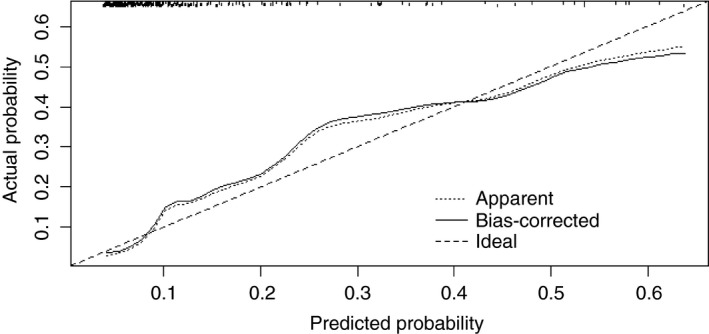
Calibration plot for nomogram predicting anastomotic leakage with serum C‐reactive protein and peritoneal matrix metalloproteinase 9 on postoperative day 3. This plot represents the relationship between predicted probability from the nomogram and observed probability in the dataset. The bootstrap method was used to obtain corrected probabilities.
Discussion and conclusions
This international multicentre prospective cohort study showed that a combination of serum CRP and peritoneal MMP9 may be useful for early prediction of AL after rectal cancer resection. The combination of these biomarkers can estimate the individual risk of AL after rectal cancer resection on the third postoperative day, which was 3 days earlier than the median time to diagnosis (6 days).
As with any other biomarker in clinical practice, this tool only assesses the risk of AL; it requires confirmation through additional imaging. However, this tool might enable timely intervention and subsequently minimize morbidity and mortality. For example, if this tool shows that a patient has high risk of AL on the third postoperative day and AL is subsequently confirmed by additional imaging even before the leak becomes clinically apparent, early reintervention could minimize the consequences of AL. So, this tool facilitates decision‐making for surgeons even before clinical symptoms occur 18.
Serum CRP is already a useful negative predictor for AL after anterior resection 19, 20. Nevertheless, serum CRP monitoring lacks specificity and positive predictive value (PPV) for AL, because the CRP level also rises due to other inflammatory complications 21. Previous research on biomarkers for AL showed that local biomarkers from peritoneal fluid were more specific than systemic biomarkers 22. The present study showed that peritoneal MMP9 was predictive for AL, and therefore this biomarker has additional value in prediction of AL over serum CRP alone. Furthermore, the c‐index of 0.78 of this combination showed adequate discrimination, which is important in a diagnostic setting where the classification of patients into different groups is of major interest.
MMP9 is a matrix metalloproteinase which plays a role in the degradation of extracellular matrix proteins, especially collagen, and is actively involved in the inflammation reaction and wound healing process 23, 24. Previously, experimental studies have investigated the association between MMPs and colorectal AL. MMPs negatively affect anastomotic healing 25, 26 whereas MMP inhibitors provided enhanced breaking strength of colonic anastomoses 27. The most pronounced collagen loss provoked by MMP9 was seen in the suture‐holding zone of colonic anastomoses 28. In addition, in an experimental model of bacterial peritonitis anastomotic MMP9 activity was increased 3 days after operation 29. Translation to clinical research obtained similar results. Patients with elevated levels of MMP1, MMP2 and MMP9 in perioperative biopsies from the colon more often had AL 30. Actually, peritoneal MMP9 had already been evaluated as biomarker for AL. Contradictory literature exists for colorectal resection 31, 32, but for rectal resection a pilot study showed that peritoneal MMP9 levels measured 4 h after surgery were increased in patients who developed AL 33. However, it remains unknown whether this association represents a causal relationship or is a consequential effect of AL.
In rectal cancer surgery, diversion is commonly applied to protect the anastomosis from leakage 34. However, the incidence of AL was no different for patients with and without a diverting ileostomy (11.4% vs 14.9%). Nevertheless, in patients without a diverting ileostomy, AL was more often treated by reoperation than in patients with a diverting ileostomy (18/20 vs 8/18, P = 0.03). These results suggest that a diverting ileostomy allows less invasive treatment strategies. Accordingly, it was previously shown from population‐based data of the Dutch ColoRectal Audit (DCRA) that a high tendency towards stoma construction in rectal cancer surgery did not reduce the incidence of AL 35.
The reported incidence of AL of 13.0% is high compared with several previous studies (3.0–11.1%) 36, 37. We hypothesize that the prospective design and inclusion of only rectal resections contributed to this relatively high incidence of AL. Another explanation is that the definition of AL varies and that some atypical presentations of leakages such as presacral abscesses or rectovaginal fistulas are not always included. In addition, the Dutch Snapshot study reported a comparable incidence of 13.4% within 30 days postoperation 4.
Over the last decade, our research group has been involved in the search for a reliable biomarker for AL after colorectal resection. In a clinical trial (the APPEAL study), we demonstrated that PCR in drain fluid for E. faecalis could be predictive for AL after colorectal resection 13. However, the relatively low PPV of 30.2% on the third postoperative day indicated a substantial number of false positives. Therefore, the present study was conducted with the aim of obtaining a combination of biomarkers with increased predictive accuracy. In addition, the previous study showed that an increase of one standard deviation in the average level of LBP on postoperative day 1 is associated with an increased risk of leakage of 1.6 12. LBP is an acute phase protein that binds to lipopolysaccharide (LPS) to elicit an immune response to Gram‐negative bacteria 38. However, the present study did not confirm these results, possibly due to different drain locations as the previous study obtained drain fluid from intra‐abdominal drains whereas the present study used pelvic drains which were positioned extraperitoneally. Furthermore, the different microbiome of patients with colon and rectal cancer may be another explanation because the previous study also included colonic resections 39, 40. This previous study showed promising results for drain fluid analysis on the first three postoperative days. Therefore, we decided to limit drain fluid collection to this interval.
The GRECCAR 5 trial has shown that pelvic drainage after rectal excision for rectal cancer does not reduce AL 41. On the other hand, pelvic drainage was not found to be detrimental 42. In this study, only three (1.0%) patients suffered from infection at the drain insertion site, which could be managed without invasive treatment strategies. So the opportunity for early detection of AL after rectal resection with innovative drain fluid analysis might justify pelvic drainage after rectal resection.
Measurements of MMP9 can easily be implemented as Luminex is a commonly used method in clinical laboratories. It is a fast method and relatively cheap. However, there were some limitations. First of all, dislocation of the drain may have influenced drain fluid composition 43. Secondly, intra‐operative spillage could have affected drain fluid composition by eliciting an inflammatory response. In addition, the emerging transanal technique may have an effect on pelvic contamination, although no evidence for this exists.
Since prediction models tend to perform better on data on which the model was constructed, external validation is essential before implementing prediction models in clinical practice 44. Furthermore, a phase II diagnostic study is required to confirm that this tool truly predicts AL in a time‐changing direction which runs from the diagnostic test forward to diagnosis 45. In this manner, the effect on time to diagnosis can be assessed prospectively. In the end, the effect of early detection on morbidity and mortality requires phase III diagnostic research.
This international multicentre prospective cohort study showed that a combination of serum CRP and peritoneal MMP9 may be useful for earlier prediction of AL after rectal cancer resection. Nevertheless, it is important to mention that this tool should never replace clinical observations, implying that the outcomes of this tool should be interpreted in the clinical context as with any other diagnostic tool.
Conflicts of interest
All authors declare no conflict of interest.
Acknowledgements
We would like to thank all patients who participated in this study. We are grateful to everyone in the department of surgery and laboratories of all participating hospitals: Havenziekenhuis, Rotterdam, the Netherlands, IJsselland Ziekenhuis, Capelle aan den Ijssel, the Netherlands, Reinier de Graaf Gasthuis, Delft, the Netherlands, Isala, Zwolle, the Netherlands, VU University Medical Center, Amsterdam, the Netherlands, University Medical Center Utrecht, Utrecht, the Netherlands, Jeroen Bosch Ziekenhuis, Den Bosch, the Netherlands, OLVG Oost, Amsterdam, the Netherlands, University Hospital Leuven, Leuven (Mrs I. Terrasson), Belgium and University Hospital Antwerpen, Antwerp, Belgium. We would also like to thank all co‐workers at the Department of Immunology, Gastroenterology and Clinical Chemistry of the Erasmus MC University Medical Center. This study was funded by Medtronic.
AD’H and JFL contributed equally.
References
- 1. Arezzo A, Passera R, Scozzari G, Verra M, Morino M. Laparoscopy for rectal cancer reduces short‐term mortality and morbidity: results of a systematic review and meta‐analysis. Surg Endosc 2013; 27: 1485–502. [DOI] [PubMed] [Google Scholar]
- 2. Bonjer HJ, Deijen CL, Abis GA et al A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372: 1324–32. [DOI] [PubMed] [Google Scholar]
- 3. Frouws MA, Snijders HS, Malm SH et al Clinical relevance of a grading system for anastomotic leakage after low anterior resection: analysis from a national cohort database. Dis Colon Rectum 2017; 60: 706–13. [DOI] [PubMed] [Google Scholar]
- 4. Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ, Dutch Snapshot Research Group . Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross‐sectional study. Ann Surg 2017; 266: 870–7. [DOI] [PubMed] [Google Scholar]
- 5. Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis 2017; 32: 549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sparreboom CL, van Groningen JT, Lingsma HF et al Different risk factors for early and late colorectal anastomotic leakage in a nationwide audit. Dis Colon Rectum 2018; 61: 1258–66. [DOI] [PubMed] [Google Scholar]
- 7. Rahbari NN, Weitz J, Hohenberger W et al Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010; 147: 339–51. [DOI] [PubMed] [Google Scholar]
- 8. Shogan BD, Carlisle EM, Alverdy JC, Umanskiy K. Do we really know why colorectal anastomoses leak? J Gastrointest Surg 2013; 17: 1698–707. [DOI] [PubMed] [Google Scholar]
- 9. Marres CCM, van de Ven AWH, Leijssen LGJ, Verbeek PCM, Bemelman WA, Buskens CJ. Colorectal anastomotic leak: delay in reintervention after false‐negative computed tomography scan is a reason for concern. Tech Coloproctol 2017; 21: 709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. den Dulk M, Noter SL, Hendriks ER et al Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur J Surg Oncol 2009; 35: 420–6. [DOI] [PubMed] [Google Scholar]
- 11. Wright EC, Connolly P, Vella M, Moug S. Peritoneal fluid biomarkers in the detection of colorectal anastomotic leaks: a systematic review. Int J Colorectal Dis 2017; 32: 935–45. [DOI] [PubMed] [Google Scholar]
- 12. Komen N, Slieker J, Willemsen P et al Acute phase proteins in drain fluid: a new screening tool for colorectal anastomotic leakage? The APPEAL study: analysis of parameters predictive for evident anastomotic leakage. Am J Surg 2014; 208: 317–23. [DOI] [PubMed] [Google Scholar]
- 13. Komen N, Slieker J, Willemsen P et al Polymerase chain reaction for Enterococcus faecalis in drain fluid: the first screening test for symptomatic colorectal anastomotic leakage. The Appeal‐study: analysis of parameters predictive for evident anastomotic leakage. Int J Colorectal Dis 2014; 29: 15–21. [DOI] [PubMed] [Google Scholar]
- 14. Su'a BU, Mikaere HL, Rahiri JL, Bissett IB, Hill AG. Systematic review of the role of biomarkers in diagnosing anastomotic leakage following colorectal surgery. Br J Surg 2017; 104: 503–12. [DOI] [PubMed] [Google Scholar]
- 15. Komen N, Morsink MC, Beiboer S et al Detection of colon flora in peritoneal drain fluid after colorectal surgery: can RT‐PCR play a role in diagnosing anastomotic leakage? J Microbiol Methods 2009; 79: 67–70. [DOI] [PubMed] [Google Scholar]
- 16. Holm S. Sequentially rejective multiple test procedures. Umea: Institute of Mathematics and Statistics, University of Umea, 1977. [Google Scholar]
- 17. Taylor JMG, Ankerst DP, Andridge RR. Validation of biomarker‐based risk prediction models. Clin Cancer Res 2008; 14: 5977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rencuzogullari A, Benlice C, Valente M, Abbas MA, Remzi FH, Gorgun E. Predictors of anastomotic leak in elderly patients after colectomy: nomogram‐Based Assessment from the American College of Surgeons National Surgical Quality Program Procedure‐Targeted Cohort. Dis Colon Rectum 2017; 60: 527–36. [DOI] [PubMed] [Google Scholar]
- 19. Reynolds IS, Boland MR, Reilly F et al C‐reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis 2017; 19: 812–8. [DOI] [PubMed] [Google Scholar]
- 20. Warschkow R, Beutner U, Steffen T et al Safe and early discharge after colorectal surgery due to C‐reactive protein: a diagnostic meta‐analysis of 1832 patients. Ann Surg 2012; 256: 245–50. [DOI] [PubMed] [Google Scholar]
- 21. Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta‐analysis of use of serum C‐reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 2014; 101: 339–46. [DOI] [PubMed] [Google Scholar]
- 22. Sparreboom CL, Wu Z, Dereci A et al Cytokines as early markers of colorectal anastomotic leakage: a systematic review and meta‐analysis. Gastroenterol Res Pract 2016; 2016: 3786418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)‐9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 2013; 139: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg 2001; 38: 72–140. [DOI] [PubMed] [Google Scholar]
- 25. Chowcat NL, Savage FJ, Hembry RM, Boulos PB. Role of collagenase in colonic anastomoses: a reappraisal. Br J Surg 1988; 75: 330–4. [DOI] [PubMed] [Google Scholar]
- 26. Savage FJ, Lacombe DL, Hembry RM, Boulos PB. Effect of colonic obstruction on the distribution of matrix metalloproteinases during anastomotic healing. Br J Surg 1998; 85: 72–5. [DOI] [PubMed] [Google Scholar]
- 27. Syk I, Agren MS, Adawi D, Jeppsson B. Inhibition of matrix metalloproteinases enhances breaking strength of colonic anastomoses in an experimental model. Br J Surg 2001; 88: 228–34. [DOI] [PubMed] [Google Scholar]
- 28. Agren MS, Andersen TL, Mirastschijski U et al Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery 2006; 140: 72–82. [DOI] [PubMed] [Google Scholar]
- 29. de Hingh IH, de Man BM, Lomme RM, van Goor H, Hendriks T. Colonic anastomotic strength and matrix metalloproteinase activity in an experimental model of bacterial peritonitis. Br J Surg 2003; 90: 981–8. [DOI] [PubMed] [Google Scholar]
- 30. Stumpf M, Klinge U, Wilms A et al Changes of the extracellular matrix as a risk factor for anastomotic leakage after large bowel surgery. Surgery 2005; 137: 229–34. [DOI] [PubMed] [Google Scholar]
- 31. Baker EA, Leaper DJ. Profiles of matrix metalloproteinases and their tissue inhibitors in intraperitoneal drainage fluid: relationship to wound healing. Wound Repair Regen 2003; 11: 268–74. [DOI] [PubMed] [Google Scholar]
- 32. Kostic Z, Panisic M, Milev B, Mijuskovic Z, Slavkovic D, Ignjatovic M. Diagnostic value of serial measurement of C‐reactive protein in serum and matrix metalloproteinase‐9 in drainage fluid in the detection of infectious complications and anastomotic leakage in patients with colorectal resection. Vojnosanit Pregl 2015; 72: 889–98. [DOI] [PubMed] [Google Scholar]
- 33. Pasternak B, Matthiessen P, Jansson K, Andersson M, Aspenberg P. Elevated intraperitoneal matrix metalloproteinases‐8 and ‐9 in patients who develop anastomotic leakage after rectal cancer surgery: a pilot study. Colorectal Dis 2010; 12: e93–8. [DOI] [PubMed] [Google Scholar]
- 34. Huser N, Michalski CW, Erkan M et al Systematic review and meta‐analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 2008; 248: 52–60. [DOI] [PubMed] [Google Scholar]
- 35. Snijders HS, van Leersum NJ, Henneman D et al Optimal treatment strategy in rectal cancer surgery: should we be cowboys or chickens? Ann Surg Oncol 2015; 22: 3582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jayne D, Pigazzi A, Marshall H et al Effect of robotic‐assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 2017; 318: 1569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nikolian VC, Kamdar NS, Regenbogen SE et al Anastomotic leak after colorectal resection: a population‐based study of risk factors and hospital variation. Surgery 2017; 161: 1619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen KF, Chaou CH, Jiang JY et al Diagnostic accuracy of lipopolysaccharide‐binding protein as biomarker for sepsis in adult patients: a systematic review and meta‐analysis. PLoS ONE 2016; 11: e0153188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flemer B, Lynch DB, Brown JM et al Tumour‐associated and non‐tumour‐associated microbiota in colorectal cancer. Gut 2017; 66: 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Youssef O, Lahti L, Kokkola A et al Stool microbiota composition differs in patients with stomach, colon, and rectal neoplasms. Dig Dis Sci 2018; 63: 2950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Denost Q, Rouanet P, Faucheron JL et al To drain or not to drain infraperitoneal anastomosis after rectal excision for cancer: the GRECCAR 5 randomized trial. Ann Surg 2017; 265: 474–80. [DOI] [PubMed] [Google Scholar]
- 42. Zhang HY, Zhao CL, Xie J et al To drain or not to drain in colorectal anastomosis: a meta‐analysis. Int J Colorectal Dis 2016; 31: 951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jansson K, Strand I, Redler B, Magnuson A, Ungerstedt U, Norgren L. Results of intraperitoneal microdialysis depend on the location of the catheter. Scand J Clin Lab Invest 2004; 64: 63–70. [DOI] [PubMed] [Google Scholar]
- 44. Bleeker SE, Moll HA, Steyerberg EW et al External validation is necessary in prediction research: a clinical example. J Clin Epidemiol 2003; 56: 826–32. [DOI] [PubMed] [Google Scholar]
- 45. Sackett DL, Haynes RB. The architecture of diagnostic research. BMJ 2002; 324: 539–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


